Abstract
Bronchopulmonary dysplasia (BPD) is a common and serious complication of premature birth, characterized by a pronounced arrest of alveolar development. The underlying pathophysiological mechanisms are poorly understood although perturbations to the maturation and remodeling of the extracellular matrix (ECM) are emerging as candidate disease pathomechanisms. In this study, the expression and regulation of three members of the lysyl hydroxylase family of ECM remodeling enzymes (Plod1, Plod2, and Plod3) in clinical BPD, as well as in an experimental animal model of BPD, were addressed. All three enzymes were localized to the septal walls in developing mouse lungs, with Plod1 also expressed in the vessel walls of the developing lung and Plod3 expressed uniquely at the base of developing septa. The expression of plod1, plod2, and plod3 was upregulated in the lungs of mouse pups exposed to 85% O2, an experimental animal model of BPD. Transforming growth factor (TGF)-β increased plod2 mRNA levels and activated the plod2 promoter in vitro in lung epithelial cells and in lung fibroblasts. Using in vivo neutralization of TGF-β signaling in the experimental animal model of BPD, TGF-β was identified as the regulator of aberrant plod2 expression. PLOD2 mRNA expression was also elevated in human neonates who died with BPD or at risk for BPD, compared with neonates matched for gestational age at birth or chronological age at death. These data point to potential roles for lysyl hydroxylases in normal lung development, as well as in perturbed late lung development associated with BPD.
Keywords: lung development, extracellular matrix, bronchopulmonary dysplasia, lysyl hydroxylase
bronchopulmonary dysplasia (BPD) is a significant complication of premature birth that, since the first description of BPD by Northway and colleagues in 1967 (28), remains a key cause of morbidity and mortality in neonatal intensive care units worldwide. The diagnosis of clinical BPD currently relies exclusively on the degree of oxygen dependence at a defined postmenstrual age (21). A key pathophysiological feature of infants afflicted with BPD is a dysmorphic pattern of lung development, including an arrest of alveolarization, where secondary septation is limited, and thus the formation of the alveolar gas exchange units is impeded (19). The resultant general reduction in the gas exchange surface area of the lung has both immediate and long-term consequences for affected infants that extend beyond childhood (29). Interestingly, improvements in the medical management of premature infants have led to improved survival of extremely premature infants and, with that, a concomitant increase in the prevalence of BPD. However, the pathogenesis of BPD, in particular, the molecular basis of blunted septation and the consequent impaired alveolarization, is very poorly understood (20, 24).
An emerging area of interest in BPD pathogenesis is the possible role played by aberrant remodeling of the extracellular matrix (ECM). In particular, several studies have pointed to improper deposition and maturation of elastin and collagen fibers in the developing septa (1, 5, 6, 8, 23, 30, 43). This has been attributed to two possible phenomena. First, the abundance of total collagen and elastin may be altered in the injured lung. Abnormal collagen and elastin deposition has been reported in premature lambs (1, 6, 30), in mechanically ventilated newborn mice (5, 8), and mouse pups in which late lung development has been arrested by exposure to high oxygen levels (23, 49). Second, a growing body of evidence indicates that the cellular machinery that is responsible for the posttranslational processing of ECM components (7), in particular, the proteolytic processing and covalent cross-linking of ECM structures, is dysregulated in the lungs of patients with BPD, as well as in animal models of BPD. Notable among these ECM maturation systems is the lysyl oxidase family of amine oxidases, which catalyze the covalent cross-linking of lysine and hydroxylysine residues in collagen and elastin and thereby promote stability of the ECM. Recent reports have indicated that lysyl oxidase expression and activity are increased both in lungs of patients affected with BPD (23), as well as in two rodent models of BPD that rely on mechanical ventilation and hyperoxia to induce lung damage (5, 23). It has been proposed that an aberrantly active ECM cross-linking system, as would be expected with elevated activity of lysyl oxidases, would generate a lung matrix structure that was “over cross-linked” or too stable, which may resist the normal remodeling of the developing lung that must take place to allow new alveolar units to form (23).
Although the lysyl oxidases have been studied in BPD, lysyl oxidases do not act alone to covalently cross-link ECM structures in the lung and other organs. Lysyl oxidases require lysine or hydroxylysine residues in substrate ECM molecules, which serve as cross-linkable residues. Hydroxylysine residues are generated by another family of enzymes: the lysyl hydroxylase, or procollagen-lysine, 2-oxoglutarate 5-dioxygenase (PLOD) (EC 1.14.11.4) family (26). The lysyl hydroxylase family comprises three members, all of which are products of separate genes, designated PLOD1, PLOD2, and PLOD3, which are mixed-function oxygenases that catalyze the hydroxylation of peptidyllysine (usually in protocollagen, as well as other proteins with collagen-like domains) to peptidylhydroxylysine (26). The hydroxylysine residues generated serve as substrates for lysyl oxidase, which convert hydroxylysine to hydroxyallysine, which is a precursor for a covalent cross-link. Additionally, the hydroxylysine residues serve as acceptor sites for sugars, permitting glycosylation of ECM proteins (26). Indeed, PLOD3, in addition to lysine hydroxylation activity, also possesses glucosyltransferase and galactosyltransferase activities. Thus lysyl hydroxylases promote ECM structural stability and maturation by promoting inter- and intramolecular cross-links and the addition of carbohydrate moieties to ECM molecules (26).
These lysyl hydroxylases have been implicated in connective tissue disorders, exemplified by Ehlers-Danlos Syndrome type VI, where mutations in the PLOD1 gene cause a heritable disorder characterized by kyphoscoliosis, joint laxity, skin fragility, and muscle hypertonia (9, 50). PLOD2 is implicated in pathological processes, where increased PLOD2 expression in fibroblasts is associated with systemic sclerosis (dermal fibroblasts) (39) and fibrosis (fibroblasts isolated from keloid, hypertrophic scars, and the palmar fascia of patients with Dupuytren's disease) (46), and mutations in the PLOD2 gene are associated with Bruck's Syndrome, a recessively inherited ECM disorder presenting with skeletal changes comparable to osteogenesis imperfecta with contractures of the large joints (18). More recently, mutations in PLOD3 have been linked to a severe connective tissue disorder reminiscent of Stickler Syndrome (36). However, to date, no lysyl hydroxylase has been implicated in normal or pathological processes in the lung. Given the emerging importance of perturbed ECM remodeling in arrested lung development, the authors hypothesized that lysyl hydroxylase expression was deregulated during aberrant late lung development associated with BPD.
MATERIALS AND METHODS
Mouse model of BPD.
All animal procedures were approved by the Regierungspräsidium Gießen (which houses the functional equivalent of an Institutional Animal Care and Use Committee in Germany) under approval 22/2000, for animal studies conducted in Germany. An arrest of alveolarization was induced in mouse pups by exposure to normobaric hyperoxia (85% O2), exactly as described previously (3). Mouse pups were randomized to two groups, one group exposed to normobaric normoxia (21% O2) and the other group exposed to normobaric hyperoxia (85% O2), within 12 h of birth [postnatal day (P) 0.5]. This model has previously been described and carefully characterized by the investigators (3, 23) and other groups (10, 14, 27), where a pronounced arrest of lung development is seen in response to hyperoxia exposure.
Cells.
The A549 cell line was obtained from the American Type Culture Collection. Primary mouse lung fibroblasts and alveolar type II cells were isolated from the lungs of adult C57Bl/6J mice, and primary lung fibroblasts were isolated from human lungs, exactly as described previously (2, 25). Primary human lung microvascular endothelial cells and primary human pulmonary artery smooth muscle cells were purchased from Promocell and maintained as recommended by the manufacturer.
Gene and protein expression analysis.
By convention, mouse genes and proteins are indicated in lower case (for example, plod1 and Plod1, respectively), and human genes and proteins are indicated in upper case (for example PLOD1 and PLOD1, respectively). Real-time RT-PCR was undertaken exactly as described previously (2, 25), using mouse lung, human lung, and whole cell mRNA pools as a template, with the primers listed in Table 1. For TGF-β stimulation, cells were exposed to TGF-β1 (2 ng/ml final concentration; R&D Systems) for 18 h. This represents a dose well within the standard range (0.2–10.0 ng/ml) for in vitro TGF-β1 stimulation studies (3, 23). Immunoblotting was undertaken exactly as described previously, using the following primary antibodies: goat anti-Plod1 (SC-50062, 1:200; Santa Cruz Biotechnology), goat anti-Plod2 (SC-50067, 1:200; Santa Cruz Biotechnology), rabbit anti-Plod3 (11027–1-AP, 1:200; Protein Tech), and rabbit α-tubulin (SC-5286, 1:2,500; Santa Cruz Biotechnology). Immune complexes were detected with the following secondary antibodies: donkey anti-goat IgG horseradish peroxidase conjugate (SC-2020, 1:1,000; Santa Cruz Biotechnology) and goat anti-rabbit IgG horseradish peroxidase conjugate (31460, 1:3,000; Pierce), employing enhanced chemiluminescence.
Table 1.
Primers employed in this study
| Gene* | Application | Forward Primer† | Reverse Primer† |
|---|---|---|---|
| Human genes | |||
| PLOD1 | Expression analysis | 5′-AAGCCGGAGGACAACCTTTTA −3′ | 5′-CCGAAGAGAATGACCAGATCC-3′ |
| PLOD2 | Expression analysis | 5′-CATGGACACAGGATAATGGCTG-3′ | 5′-AGGGGTTGGTTGCTCAATAAAAA-3′ |
| PLOD3 | Expression analysis | 5′-GACCCGGTCAACCCAGAGA-3′ | 5′-CTCCACCAACTGTTCGAGCC-3′ |
| HPRT | Expression analysis | 5′-AAGGACCCCACGAAGTGTTG-3′ | 5′-GGCTTTGTATTTTGCTTTTCCA-3′ |
| PLOD2 promoter | Cloning | 5′-GCTAGCCCATCCTGAGGTAGGGTAAGT-3′ (NheI) | 5′-CTCGAGCCACGTCTGGACTGTTTGCTC-3′ (XhoI) |
| Mouse genes | |||
| plod1 | Expression analysis | 5′-GTTTTTCCTGCTGCTGCTGCG-3′ | 5′-CCGTGCTCCGCCAGGAACTG-3′ |
| plod2 | Expression analysis | 5′-TGGAGCACTACGCCAGTCAGGA-3′ | 5′-AGCCCGTCCGCTGCAAAGAC-3′ |
| plod3 | Expression analysis | 5′-ACACAGACCACCTACACCCAGACC-3′ | 5′-TCTGCTCGGTCAGCAGTGGGA-3′ |
| Hprt | Expression analysis | 5′-GATGATCTCTCAACTTTA-3′ | 5′-AGTCTGGCCTGTATCCAA-3′ |
| 18S rRNA | Expression analysis | 5′-AGGGGAGAGCGGGTAAGAGA-3′ | 5′-GGACAGGACTAGGCGGAACA-3′ |
| plod2 promoter | Cloning | 5′-GAGCTCCTTCAACCTCACCTACTCAGT-3′ (SacI) | 5′-CTCGAGGGAAGGCCTGTGGCGAGGACT-3′ (XhoI) |
By convention, mouse genes are indicated in lower case and human genes in upper case.
Engineered restriction sites are indicated in bold type, with the corresponding restriction enzymes indicated in parentheses.
Immunohistochemistry.
Lungs from mouse pups were pressure fixed at 20 cm H2O pressure, embedded in paraffin, and 3-μm sections were prepared from mouse and human lungs and were processed for the detection of lysyl hydroxylases exactly as described previously (2, 25). Lysyl hydroxylases were detected using the following primary antibodies: goat anti-Plod1 (SC-50062, 1:50; Santa Cruz Biotechnology), goat anti-Plod2 (SC-50067, 1:25; Santa Cruz Biotechnology), rabbit anti-Plod3 (11027–1-AP, 1:25; Protein Tech). Staining specificity was assessed by preadsorbing with a 100-fold molar excess of a competing peptide C-19 (SC-50062P; Santa Cruz Biotechnology) for Plod1, competing peptide N-15 (SC-50067P; Santa Cruz Biotechnology), and a glutathione-S-transferase (GST)-Plod3 fusion protein (Ag1480; Protein Tech) for Plod3. Immune complexes were detected with biotinylated Histostain secondary antibodies: biotinylated rabbit anti-mouse (95–6543B, “ready to use”; Invitrogen) and biotinylated rabbit anti-goat (A10518, 1:1,000; Invitrogen), followed by a Streptavidin-horseradish peroxidase complex colorimetric detection system.
Cloning of the mouse plod2 and human PLOD2 promoters.
The mouse plod2 promoter was cloned by PCR amplification of a 2,767 base-pair fragment using the forward and reverse primers: 5′-GAGCTCCTTCAACCTCACCTACTCAGT-3′ (forward; SacI) and 5′-CTCGAGGGAAGGCCTGTGGCGAGGACT-3′ (reverse; XhoI), containing built-in restriction sites (in bold type) and mouse lung genomic DNA as a template. The mouse plod2 promoter sequence has been deposited in the GenBank database under accession number FJ416599. Similarly, the human PLOD2 promoter was cloned by PCR amplification of a 3,196 base-pair fragment using forward and reverse primers: 5′-GCTAGCCCATCCTGAGGTAGGGTAAGT-3′ (forward; NheI) and 5′-CTCGAGCCACGTCTGGACTGTTTGCTC-3′ (reverse; XhoI), respectively, and human lung genomic DNA as a template. The human PLOD2 promoter sequence has been deposited in the GenBank database under accession number KC788822. Sequences were initially T/A cloned into pGEM T-Easy (Promega) and then subcloned into pGL3-basic (Promega) using the restriction sites built into the primers to create pGL3-plod2 (for mouse plod2 promoter) and pGL3-PLOD2 (for human PLOD2 promoter).
Dual luciferase reporter assay.
The dual luciferase reporter assay was performed exactly as described previously (2, 25), using the firefly luciferase-expressing pGL3-plod2 and pGL3-PLOD2 constructs described above, along with the Renilla luciferase-expressing pRLTK (Promega) normalization construct.
In vivo TGF-β neutralization experiments.
The inhibition of TGF-β signaling in the lungs of mice exposed to normobaric hyperoxia, using the pan-TGF-β-neutralizing IgG1 antibody 1D11 (R&D Systems), along with the isotype-matched nonimmune IgG1 antibody MOPC21 (Sigma) that has been described previously by the investigators (23) and other groups (27). This treatment partially normalizes lung structure in oxygen-injured lungs (27).
Human patient material.
All use of human material was approved by the Ethik-Kommission (the German equivalent of an institutional review board) of the University of Giessen School of Medicine under approval number 189/09. The harvesting of lung material from preterm and term neonates has been previously described in detail (23). The clinical characteristics of patients afflicted with BPD or at risk for the development of BPD are provided in Table 2. Additionally, two control patients groups are also described in Table 3, where one control group was age matched to the BPD group for chronological age at death (CAD), whereas the other control group was age matched to the BPD group for gestational age at birth (GAB).
Table 2.
Clinical characteristics of patients with BPD or at risk for the development of BPD
| Patient Number | Birth Weight, g | Sex | Gestational Age, wk | CAD, days | Duration FiO2 >0.50, days | Mechanical Ventilation, days | Cause of Death/autopsy Diagnosis and Medication |
|---|---|---|---|---|---|---|---|
| 1* | 720 | M | 29 | 62 | 13 | 62 (c, hf) | BPD, IRDS, Staphylococcus aureus sepsis. Drugs: surfactant, inotropes, tobramycin, flucloxacillin, cortisone |
| 2 | 1,055 | M | 32 | 18 | 18 | 18 (c) | BPD, ventricular septal defect, Edwards syndrome. Drugs: inotropes |
| 3* | 835 | M | 26 | 65 | 27 | 65 (c, hf) | BPD, cerebral bleeding, ductus arteriosus. Drugs: surfactant, inotropes, dexamethasone, theophylline |
| 4* | 930 | M | 26 | 99 | 98 | 99 (c, hf) | BPD, IRDS, pneumothorax, subependymal hemorrhage. Drugs: surfactant, inotropes, dexamethasone, tobramycin, penicillin, amphotericin |
| 5* | 1,250 | F | 28 | 34 | 34 | 34 (c, hf) | BPD, IRDS, Staphylococcus epidermidis sepsis. Drugs: surfactant, furosemide, amoxicillin, erythromycin |
| 6* | 1,220 | M | 31 | 35 | 35 | 35 (c, hf) | BPD, IRDS, right ventricular hypertrophy, anemia, rickets. Drugs: furosemide, amoxicillin, vancomycin |
| Median | 930 | 28 | 33 | 18 | 33 | ||
| Mean ± SE | 976 ± 77 | 28 ± 0.9 | 38 ± 8 | 27 ± 8 | 38 ± 9 | ||
| P value vs.† CAD (Table 3)‡ | 0.0016 | 0.0039 | 02789 (NS) | ||||
| P value vs. GAB (Table 3)† | 0.593 (NS) | 0.1776 (NS) | <0.0001 |
Inotropes included dopamine, dobutamine, and adrenaline.
Patient had clinically-defined BPD.
By unpaired Student t-test. In the case of chronological age at death (CAD), the postmenstrual ages at death, rounded to the nearest full week, were compared. BPD, bronchopulmonary dysplasia; c, conventional ventilation; hf, high-frequency ventilation; GAB, gestational age at birth; IRDS, infant respiratory distress syndrome; NS, not significant.
Table 3.
Clinical characteristics of control patients
| Patient Number | Birth Weight, g | Sex | Gestational Age, wk | CAD, days | Duration FiO2 >0.50, days | Mechanical Ventilation, days | Cause of Death/autopsy Diagnosis and Medication/material Available |
|---|---|---|---|---|---|---|---|
| Control group age matched to the BPD group (Table 2) for CAD | |||||||
| 7 | 1,625 | M | 33 | 3 | 0 | 0 | Congenital heart malformation. Drugs: atropine, prostaglandin A1 (r) |
| 8 | 2,350 | M | 35 | <1 | 0 | <1 h | Perinatal asphyxia. Drugs: atropine, adrenaline (h+r) |
| 9 | 1,740 | F | 34 | <1 | 0 | 0 | Intrauterine death (ventriculomegaly) (r) |
| 10 | 1,800 | M | 32 | 5 | 0 | 5 | Meningoencephalitis (h+r) |
| 11 | 1,190 | M | 31 | <1 | 0 | 0 | Placental abruption (h+r) |
| Median | 1,740 | 33 | |||||
| Means ± SE | 1,741 ± 186 | 33 ± 0.7 | |||||
| Control group matched to the BPD group (Table 2) for birth weight and GAB | |||||||
| 12 | 954 | M | 26 | <1 | 0 | 0 | Intracranial hemorrhage (h+r) |
| 13 | 914 | M | 27 | <1 | 0 | 0 | DiGeorge syndrome (22q11.2 deletion); intrauterine infection (h+r) |
| 14 | 826 | M | 29 | <1 | 0 | 0 | Hydrocephalus, Arnold Chiari malformation (h+r) |
| 15 | 852 | M | 27 | <1 | 0 | 0 | Hypoxic-ischemic encephalopathy (r) |
| 16 | 995 | M | 27 | <1 | 0 | 0 | Placental abruption (h+r) |
| 17 | 758 | M | 26 | <1 | 0 | 0 | Intrauterine infection (h+r) |
| Median | 914 | 27 | |||||
| Means ± SE | 929 ± 55 | 27 ± 0.4 | |||||
h, histological sections; r, RNA.
RESULTS
Lysyl hydroxylase expression was deregulated in the lungs of neonatal mice exposed to 85% O2.
The mRNA expression levels of plod1 (Fig. 1A), plod2 (Fig. 1B), and plod3 (Fig. 1C) in the lungs of mouse pups were regulated over the first 2 wk of normal lung development and were altered by exposure to 85% O2. Over the course of normal late lung development, the alveolarization process in mouse pups proceeds over the first 2 wk of life and is largely completed by P14, which is followed by a phase of microvascular maturation. During these first 2 wk of life, the bulk of the alveolar gas exchange units is formed. Our data reveal that, over this period, there is a pronounced downregulation of lysyl hydroxylase gene expression, where both plod1 (Fig. 1A) and plod3 (Fig. 1C) mRNA levels were reduced by 0.5 ΔCt units by P9.5 (vs. P2.5), and 5 days later the expression of all three genes, plod1 (Fig. 1A), plod2 (Fig. 1B), and plod3 (Fig. 1C) was dramatically downregulated by 3–4 ΔCt units, comparing P14.5 with P9.5 (comparing normoxia-treated groups only, for normally developing lungs). In sum, lysyl hydroxylase gene expression was progressively downregulated in normally developing lungs of mouse pups over the first 14 days of life. This trend is strongly impacted (and to a degree, reversed) by exposure to 85% O2, where levels of all three genes were comparable between the 21% O2 and 85% O2 groups at P2.5, but by P9.5 both plod1 (Fig. 1A) and plod3 (Fig. 1C) mRNA levels were increased in the lungs of mouse pups exposed to 85% O2. By P14.5, the levels of plod1 (Fig. 1A), plod2 (Fig. 1B), and plod3 (Fig. 1C) mRNA were all elevated by 2–4 ΔCt units in the 85% O2 group, compared with the 21% O2 group. The ability of 85% O2 to maintain elevated lysyl oxidase expression was also evident in immunoblots, where an increase in Plod1 and Plod2 protein levels was evident in the lungs of 85% O2-exposed mouse pups, compared with 21% O2-exposed littermates (Fig. 1D). In sum, although lysyl hydroxylase expression is gradually downregulated over the course of normal alveolarization, exposure to 85% O2 leads to abnormally elevated levels of lysyl hydroxylases, over the course of aberrant alveolarization in mouse pups.
Fig. 1.
Expression of lysyl hydroxylases during normal and aberrant late lung development. Expression levels of mRNA encoding plod1 (A), plod2 (B), and plod3 (C) were assessed by real-time RT-PCR in mRNA pools from lung homogenates from mouse pups at postnatal days (P) 2.5, 9.5, and 14.5, after exposure to 21% O2 (●) or 85% O2 (△) from P0.5. The 18S rRNA was used as a reference. Data reflect means ± SE (n = 5). The P values, indicated above pairs of data sets, were assessed by 1-way ANOVA with Tukey's post hoc test. D: protein expression of Plod1, Plod2, and Plod3 was assessed by immunoblot of protein extracts from lung homogenates from mouse pups over the course of late lung development, during exposure to 21% O2 or 85% O2 from P0.5. A single representative series is illustrated that is representative of at least 2 other series.
Lysyl hydroxylases have specific expression patterns in the developing mouse lung.
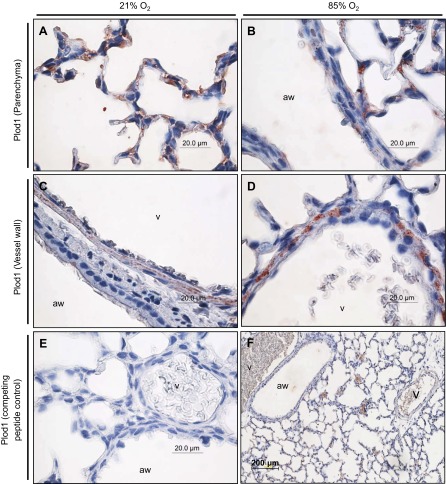
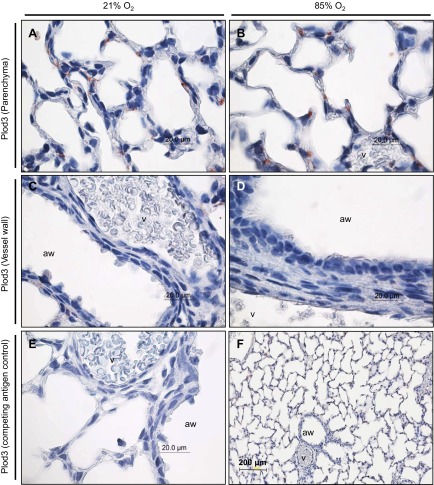
To assess where deregulated lysyl hydroxylase expression may impact late lung development, the localization of the three lysyl hydroxylases was determined by immunohistochemistry. Plod1 could be localized to the parenchyma, where Plod1 staining was observed in developing septa of mouse pups at P7 exposed to 21% O2 (Fig. 2A) and 85% O2 (Fig. 2B). Plod1 staining was also observed in the blood vessel walls, appearing to localize to the muscular layer of the vessel wall in the lungs of mouse pups exposed to 21% O2 (Fig. 2C) and 85% O2 (Fig. 2D). Staining for Plod1 in the lung vessel walls appeared to be more intense in the 85% O2-treated mouse pups (Fig. 2D) compared with the 21% O2-treated mouse pups (Fig. 2C) although the authors do not consider the relative intensity of immunohistochemical staining to be quantitative. Plod2 was also detected in the developing septa of mouse pups at P7 (Fig. 3, A and B), with staining again appearing more intense in the 85% O2-treated group (Fig. Fig. 3B) compared with the 21% O2-treated group (Fig. 3A). In contrast to Plod1, no Plod2 staining was evident in the vessel wall of mouse pups at P7 exposed to 21% O2 (Fig. 3C) and 85% O2 (Fig. 3D). Staining for Plod3 revealed a very specific and discrete expression pattern, specifically at the base of the developing septa (Fig. 4, A and B), which localizes Plod3 to a place of intense activity in the developing lung and, hence, in a position where Plod3 may influence alveolar development. No appreciable staining for Plod3 was evident in the muscular layer of the vessel walls in the lungs of mouse pups at P7 exposed to 21% O2 (Fig. 4C) and 85% O2 (Fig. 4D) although staining in the endothelium of the latter group was evident (Fig. 4D). Together, these data reveal that lysyl hydroxylases have discrete expression patterns in the developing mouse lung and that the detection of all three lysyl hydroxylases in the developing septa places all three members of this family of matrix cross-linking enzymes in a position to influence secondary septation and, hence, alveolarization.
Fig. 2.
Localization of Plod1 expression in the parenchyma (A and B) and vessel walls (C and D) of the lungs of mouse pups at P7.5, after exposure to 21% O2 or 85% O2 from P0.5. The airways (aw) and vessels (v) are indicated. E: staining for Plod1 in a neonatal mouse lung at P7.5, after exposure to 85% O2 from P0.5, after preadsorption of the primary anti-Plod1 antibody with a competing peptide, to demonstrate specificity. F: low magnification staining for Plod1 in a neonatal mouse lung at P7.5 after exposure to 85% O2 from P0.5.
Fig. 3.
Localization of Plod2 expression in the parenchyma (A and B) and vessel walls (C and D) of the lungs of mouse pups at P7.5, after exposure to 21% O2 or 85% O2 from P0.5. The airways and vessels are indicated. E: staining for Plod2 in a neonatal mouse lung at P7.5, after exposure to 85% O2 from P0.5, after preadsorption of the primary anti-Plod2 antibody with a competing peptide, to demonstrate specificity. F: low magnification staining for Plod2 in a neonatal mouse lung at P7.5 after exposure to 85% O2 from P0.5.
Fig. 4.
Localization of Plod3 expression in the parenchyma (A and B) and vessel walls (C and D) of the lungs of mouse pups at P7.5, after exposure to 21% O2 or 85% O2 from P0.5. The airways and vessels are indicated. E: staining for Plod3 in a neonatal mouse lung at P7.5, after exposure to 85% O2 from P0.5, after preadsorption of the primary anti-Plod3 antibody with a competing antigen, to demonstrate specificity. F: low magnification staining for Plod3 in a neonatal mouse lung at P7.5 after exposure to 85% O2 from P0.5.
TGF-β regulates mRNA levels of lysyl hydroxylases in constituent cells types of the developing lung.
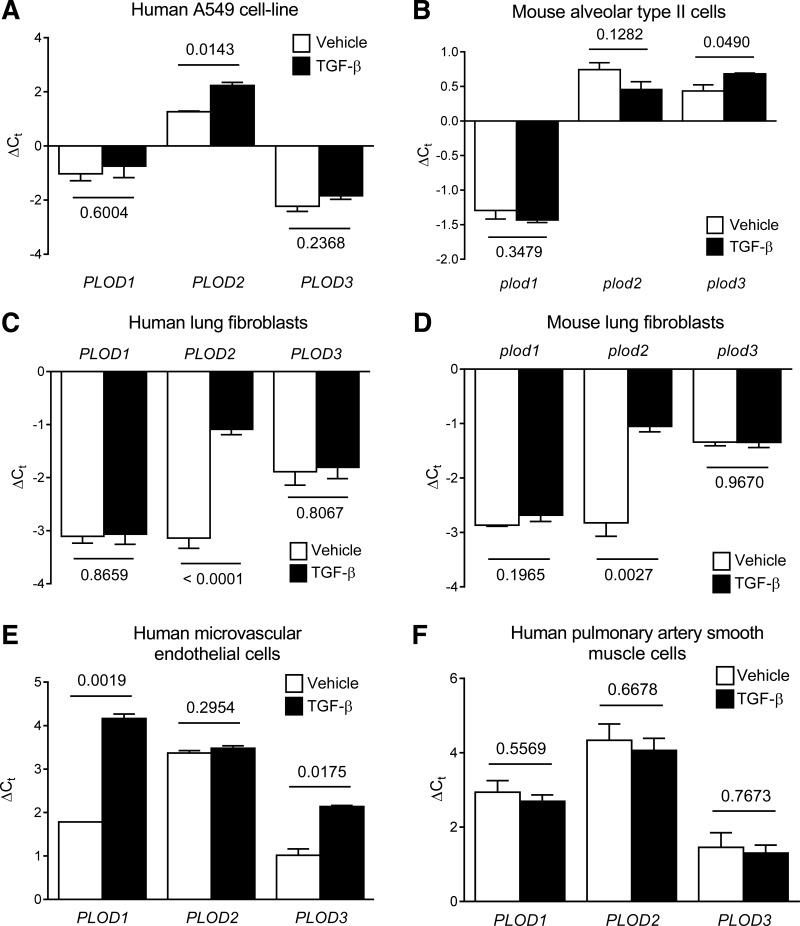
TGF-β is acknowledged as a key regulator of late lung development and alveolarization. TGF-β is also a potent profibrotic growth factor and has been ascribed a key role in the pathogenesis of BPD. Furthermore, we identified multiple Smad-binding elements (which confer TGF-β responsiveness) in the plod2 and PLOD2 promoters, by in silico analysis. For these reasons, the ability of TGF-β to impact lysyl hydroxylase expression in constituent cell types of the developing alveolus was assessed. TGF-β exhibited a broad spectrum of effects on lysyl hydroxylase gene expression, where an 18-h exposure of A549 cells, a commonly used model of the human lung epithelium, to a dose of 2 ng/ml TGF-β, upregulated PLOD2 expression (Fig. 5A). In contrast, in primary mouse alveolar type II cells, TGF-β was without effect on plod2 mRNA levels but did upregulate plod3 mRNA expression (Fig. 5B). TGF-β dramatically increased PLOD2 mRNA levels in primary human lung fibroblasts (Fig. 5C) and dramatically increased plod2 mRNA levels in primary mouse lung fibroblasts (Fig. 5D). TGF-β also upregulated PLOD3 mRNA levels in primary human lung microvascular endothelial cells (Fig. 5E) although TGF-β was without any impact on lysyl hydroxylase mRNA levels in primary human pulmonary artery smooth muscle cells (Fig. 5F). Thus TGF-β appears to be a mediator of plod2 and plod3 expression in constituent cells types of the alveolus.
Fig. 5.
Regulation of lysyl hydroxylase mRNA levels by TGF-β. PLOD1, PLOD2, and PLOD3 expression was assessed by real-time RT-PCR using the primers listed in Table 1, in mRNA pools from human A549 cells (A), as well as in primary human lung fibroblasts (C), microvascular endothelial cells (E), and pulmonary artery smooth muscle cells (F) after stimulation with vehicle alone or TGF-β (2 ng/ml; 16 h). The HPRT gene was used as a reference. Similarly, plod1, plod2, and plod3 expression was assessed by real-time RT-PCR using the primers listed in Table 1, in mRNA pools from primary mouse lung alveolar type II cells (B), and mouse lung fibroblasts (D) after stimulation with vehicle alone or TGF-β (2 ng/ml; 16 h). The hprt gene was used as a reference. Data reflect means ± SD (n = 3). The P values (above the horizontal line) represented compare vehicle- vs. TGF-β-treated groups and were assessed by unpaired Student's t-test.
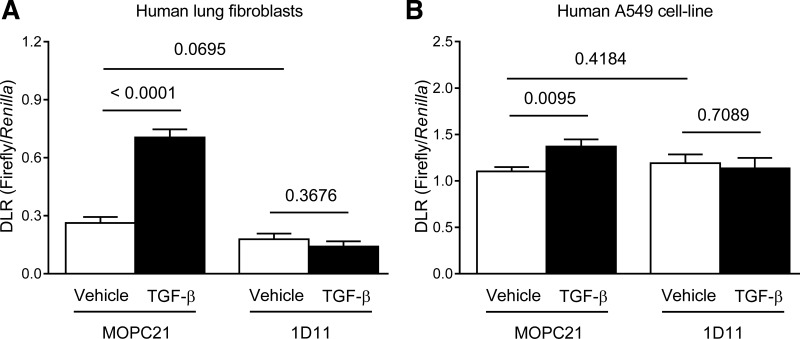
TGF-β regulated PLOD2 promoter activity in human lung fibroblasts and epithelial cells.
TGF-β increased the activity of the human PLOD2 promoter in human lung fibroblasts (Fig. 6A) and in A549 cells (Fig. 6B). The specificity of the effect of TGF-β was confirmed by the observation that a pan-TGF-β-neutralizing antibody (1D11) was able to abrogate the effects of TGF-β, while an isotype-matched control antibody (MOPC21) was not (Fig. 6, A and B). These data support the observation that TGF-β increased the abundance of PLOD2 mRNA in human lung fibroblasts (Fig. 5C) and in A549 cells (Fig. 5A) and support the contention that TGF-β is a regulator of PLOD2 expression in constituent cells types of the developing alveolus.
Fig. 6.
Regulation of PLOD2 promoter activity by TGF-β. The activity of the human PLOD2 promoter was assessed by dual luciferase assay (DLR) in primary human lung fibroblasts (A), as well as human lung A549 cells (B), as a transfectable model of the alveolar epithelium, after preincubation (30 min, 37°C; in stimulation medium) of TGF-β with a pan-TGF-β-neutralizing antibody (1D11) or an isotype-matched control (MOPC21) antibody. Data reflect means ± SD (n = 5). The P values (above the horizontal line) represented compare vehicle- vs. TGF-β-treated groups, or MOPC21 vs. 1D11 control groups and were assessed by unpaired Student's t-test.
Exposure to 85% O2 drove aberrant plod2 expression in the developing mouse lung via TGF-β.
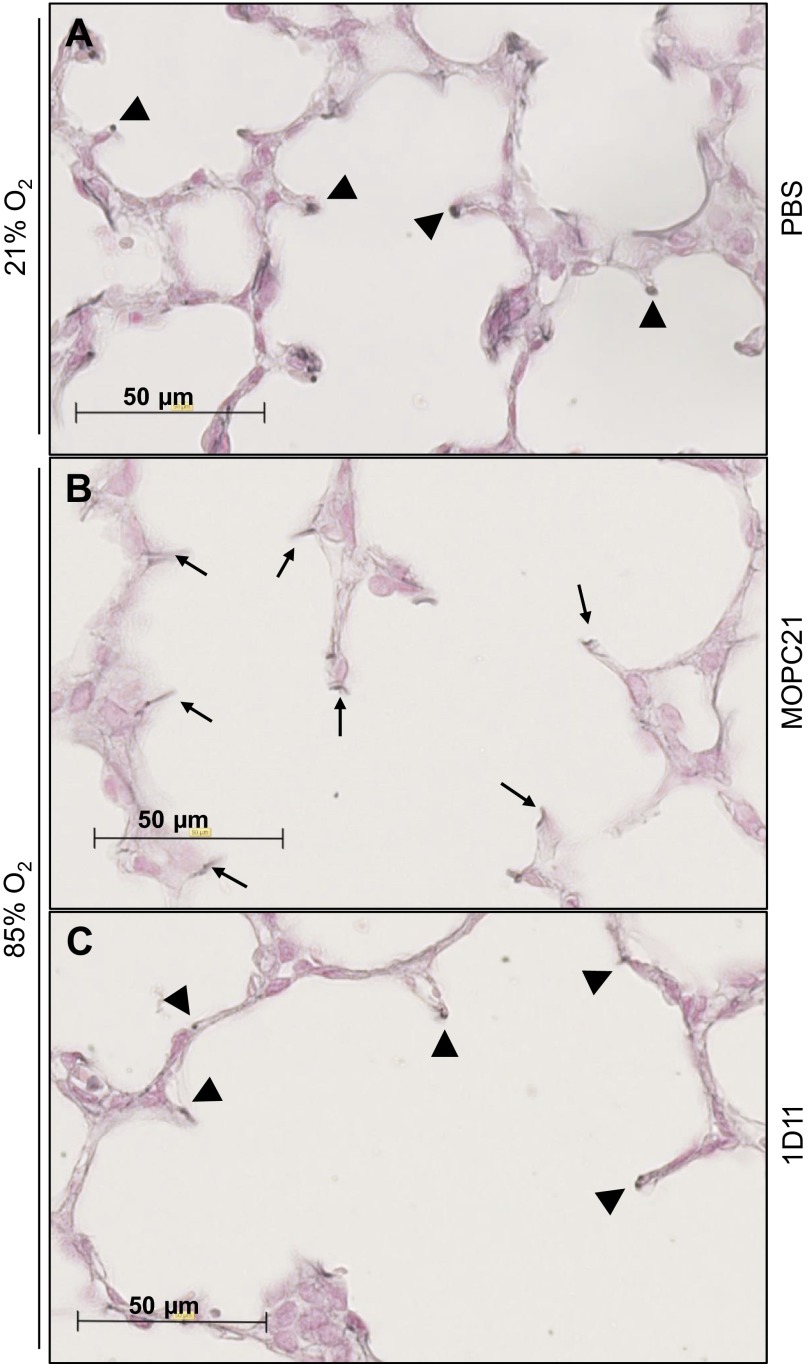
Neutralization of TGF-β signaling in vivo in mouse pups that were exposed to 85% O2 partially normalized elastin structures in the developing septa (Fig. 7C; arrowheads) because these structures largely resembled elastin structures in the developing septa of mouse lungs exposed to 21% O2 (Fig. 7A; arrowheads), compared with the perturbed elastin structures seen in the septa of developing lungs in mice that were exposed to 85% O2 that received a control MOPC21 antibody (Fig. 7B; arrows). These data support previous observations (23, 27) that neutralization of TGF-β signaling in the hyperoxia model of BPD partially restores normal structure to the developing lung.
Fig. 7.
Impact of in vivo neutralization of TGF-β signaling on hyperoxia-induced changes to elastin structure in the developing septa. Sections of developing mouse lungs at P10.5 were stained for elastin using Hart's stain, after treatment with phosphate-buffered saline (PBS) and exposure to 21% O2 (A), with a control MOPC21 antibody and exposure to 85% O2 (B), or treatment with a pan-TGF-β-neutralizing antibody and exposure to 85% O2 (C). Arrowheads indicate the normal, punctate elastin structures, whereas arrows indicate the disturbed “brush-like” elastin structures in the septa associated with aberrant late lung development.
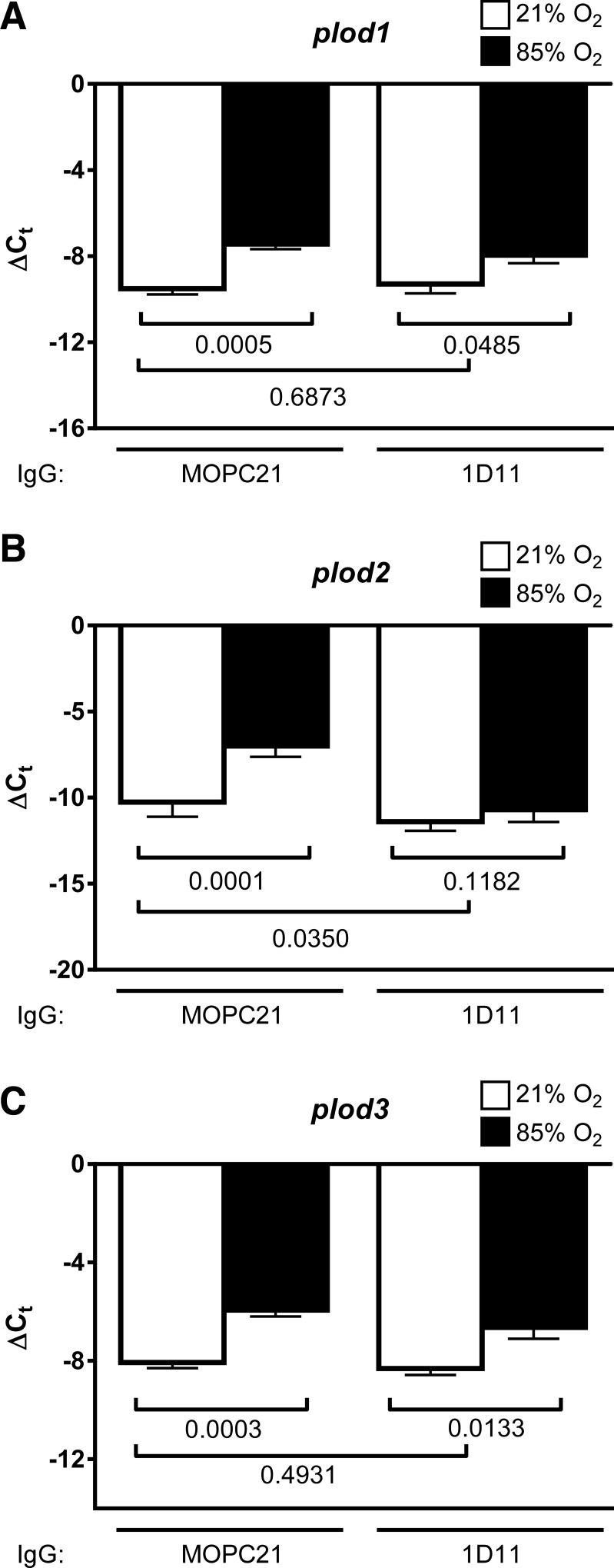
Neutralization of TGF-β signaling in vivo in mouse pups that were exposed to 85% O2 did not impact plod1 expression in the lungs of mouse pups (Fig. 8A); however, TGF-β neutralization did restore normal plod2 expression in the lungs of mouse pups (Fig. 8B). As with plod1, no impact of TGF-β neutralization on plod3 expression was noted (Fig. 8C). Although the authors do not consider the intensity of immunohistochemical staining to be quantitative, staining intensity for Plod2 in the developing septa of mouse pup lungs exposed to 85% O2 that received a TGF-β-neutralizing antibody (Fig. 9D) appeared less intense when compared with mouse pup lungs exposed to 85% O2 that received a control (nonimmune) MOPC21 antibody (Fig. 9B). Together, these data suggest that TGF-β mediated the effects of hyperoxia exposure on Plod2 expression in the aberrantly developing mouse lung.
Fig. 8.
Impact of in vivo neutralization of TGF-β signaling on hyperoxia-induced lysyl hydroxylase expression. Expression levels of mRNA encoding plod1 (A), plod2 (B), and plod3 (C) were assessed by real-time RT-PCR in mRNA pools from lung homogenates from mouse pups at P10.5, after exposure to 21% O2 (open bars) or 85% O2 (closed bars) from P0.5 on, with concomitant administration of a pan-TGF-β-neutralizing antibody (1D11) or an isotype-matched control (MOPC21) antibody. The 18S rRNA was used as a reference. Data reflect means ± SD (n = 6, per group). The P values (presented below the horizontal brackets) were assessed by 1-way ANOVA with Tukey's post hoc test.
Fig. 9.

Impact of in vivo neutralization of TGF-β signaling on hyperoxia-induced changes to Plod2 localization. Sections of developing mouse lungs at P10.5 were stained for Plod2 after treatment with a control MOPC21 antibody and exposure to 21% O2 (A) or 85% O2 (B), or after treatment with a pan-TGF-β-neutralizing antibody and exposure to 21% O2 (C) or 85% O2 (D).
Elevated PLOD2 expression is associated with BPD.
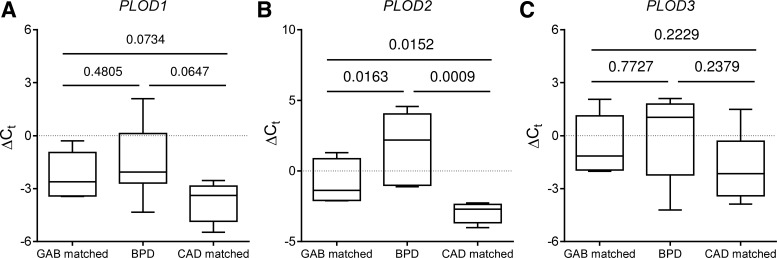
The mRNA levels of PLOD1 (Fig. 10A), PLOD2 (Fig. 10B), and PLOD3 (Fig. 10C) were assessed in mRNA pools from the lungs of patients that had died with BPD or at risk for BPD, as well as in two control groups, one control group age matched to the BPD group for gestational age at birth (GAB) and another control group age matched to the BPD group for chronological age at death (CAD). No appreciable differences were observed comparing the expression of PLOD1 (Fig. 10A) and PLOD3 (Fig. 10C) across all three groups. However, the mRNA levels of PLOD2 were appreciably elevated in the lungs of patients with or at risk for BPD, compared with both the GAB and CAD groups (Fig. 10C). These data demonstrate that the expression of lysyl hydroxylases, namely the PLOD2 gene, is deregulated in the lungs of infants with or at risk for BPD. All three Plod proteins were detected in the developing lungs of human neonates by immunohistochemistry, with Plod1 evident in the airway epithelium, vascular smooth muscle, and in the septa (Fig. 11, A and B). Plod2 was evident in the endothelium, airway epithelium, and developing septa (Fig. 11C), whereas Plod3 was evident in the septa (Fig. 11D). It is evident from these data that Plod2, which has emerged from this study as a candidate player in the pathogenesis of BPD, is present at sites of intensive ECM production and remodeling in the developing human lung.
Fig. 10.
Expression of lysyl hydroxylases in human neonates with normal lung development or in neonates afflicted with, or at risk for, bronchopulmonary dysplasia (BPD). PLOD1 (A), PLOD2 (B), and PLOD3 (C) expression was assessed by real-time RT-PCR using the primers listed in Table 1, in mRNA pools from patients afflicted with or at risk for BPD (Table 2), from control patients matched to the BPD group for gestational age at birth (GAB; Table 3), or from control patients matched to the BPD group for chronological age at death (CAD; Table 3). The HPRT gene was used as a reference. The bars represent the data range, the boxes represent the lower and upper quartiles, and the line within the quartile box indicates the median. No outliers were noted by Grubbs' test. The P values represented compare the 3 patient groups and were assessed by 1-way analysis of variance with the Newman-Keuls post hoc test (see Tables 2 and 3 for number of patients per group).
Fig. 11.

Plod expression in the developing lungs of human neonates. The tissue localization of Plod1 (A and B; patient 8), Plod2 (C; patient 10), and Plod3 (D; patient 8) was assessed by immunohistochemistry, and specificity controls were performed with competing peptides for Plod1 (B; inset) and Plod2 (C, inset), and with a competing antigen for Plod3 (D, inset). The airways, alveolar airspaces (a), endothelium (Endo), vascular smooth muscle (VSM), alveolar septa (s), and vessels are indicated. Staining patterns are representative for patterns observed in at least 2 other series.
DISCUSSION
The proper deposition and posttranslational modification and remodeling of the lung ECM is critical to normal lung development, including the formation of the alveolar air sacs, the principal gas exchange unit of the lung, which takes place during late lung development. Perturbations to the proper deposition, processing, and remodeling of the lung ECM are credited as being a key underlying cause of disturbed late lung development. Clinically, disturbed late lung development is exemplified in humans by BPD, also called chronic lung disease of early infancy. BPD results from the mechanical ventilation and/or oxygen supplementation of premature infants, which then exhibit a pronounced arrest of late lung development (19), where alveolar simplification is evident, as well as blunted lung microvascular development (41) and dysangiogenesis (13). The pathomechanisms at play in the lung of affected infants are not well understood. However, several histopathological observations have pointed to dramatically malformed matrix structures in patients with BPD, including collagen fibers in the alveolar septa that were described to be “thickened, tortuous, and disorganized” (43). These observations have also been made examining lungs of experimental animals where BPD has been modeled, including in ventilated preterm lambs (1, 6, 30), ventilated mouse pups (5, 8), and mouse pups exposed to elevated oxygen concentrations (23, 49).
To date, little information is available about how and why ECM structures are deformed in lungs in which alveolarization has been disturbed and whether these perturbed matrix structures play a causal role in arresting the alveolarization process. One theory put forward is that the matrix processing and maturations systems, which remodel the ECM structures, may be disturbed. Along these lines, deregulated expression and activity of matrix metalloproteinases, which remodel ECM structures, have been implicated in BPD. Another theory forwarded is that the ECM stabilization systems, which cross-link collagen and elastin polypeptides to permit the formation of stable collagen and elastin fibers, respectively, may be impacted in BPD. These systems include the concerted action of lysyl hydroxylases, which catalyzes the hydroxylation of peptidyllysine to peptidylhydroxylysine, and generate a substrate for lysyl oxidases, which convert hydroxylysine to hydroxyallysine, which forms part of the lysyl oxidase-generated covalent cross-links in ECM structures. Indeed, lysyl oxidases have been studied in the context of clinical and experimental BPD, where the expression of two members of the lysyl oxidase family, namely, Lox and LoxL1, is elevated in both patients that died with BPD, as well as in the lungs of mice in a BPD rodent model. These data make a strong case for a role for perturbed matrix cross-linking in arrested lung development.
In the present study, the authors addressed the expression and regulation of a second family of ECM cross-linking enzymes, that of the lysyl hydroxylases, or procollagen-lysine, 2-oxoglutarate 5-dioxygenases, which comprises three members in humans: PLOD1, PLOD2, and PLOD3. All three enzymes generate hydroxylysine residues from lysine residues in collagen peptides (as well as in other peptides with a collagen-like domain) and provide a key substrate for subsequent intra- and intermolecular cross-linking mediated by the lysyl oxidases. Additionally, PLOD3 catalyzes the glycosylation of collagen and other molecules.
No roles for any lysyl hydroxylase in lung physiology have been described to date although several lines of investigation point at possible pulmonary functions for lysyl hydroxylases. All three lysyl hydroxylases are abundantly expressed in the lung (33, 37). The data presented here reveal that the expression of all three lysyl hydroxylases was deregulated in a rodent model of BPD and that this deregulation took place over the critical period of late lung development when alveolarization takes place. Additionally, the localization studies reveal that all three lysyl hydroxylases are ideally positioned to influence late lung development because, in the developing mouse lung, Plod1, Plod2, and Plod3 were all detected in the developing septa.
Plod1 is dispensable in mice because plod1−/− mice are viable. However, Plod1 appears to play a role in the lung because the amounts of hydroxylysine residues (per collagen triple helix) in the lungs of plod1−/− mice were reduced by 14% compared with wild-type (plod1+/+) mice, and the amounts of hydroxylysylpyridinoline cross-links in the lungs of plod1−/− mice were decreased by 66% (42). In the present study, Plod1 was detected in the developing septa, as well as in the lung vessel walls, placing Plod1 in a position that could influence secondary septation and vascular development.
As with Plod1, no role for Plod2 has been described in the lung. However, Plod2 is widely implicated in fibrotic processes and, in particular, with TGF-β-driven synovial fibrosis associated with osteoarthritis (32), where TGF-β has also been demonstrated to drive Plod2 expression in dermal fibroblasts (44), and increased PLOD2 expression is seen in fibrotic skin lesions from patients with systemic sclerosis (45). Plod2 was detected in the present study in developing septal walls although not in the blood vessel walls. Additionally, Plod2 exhibited the highest expression levels of all three lysyl hydroxylases in human and mouse lung epithelial cells and was induced by TGF-β.
Curiously, Plod3 exhibited the most restricted pulmonary expression of all the lysyl hydroxylases and was localized to the base of the developing septa, which is a region of intense tissue remodeling during the alveolarization process. Plod3 is a bifunctional enzyme that catalyzes the formation of hydroxylysine in collagen polypeptides and can also transfer sugars to these residues by virtue of glucosyltransferase and galactosyltransferase activities. Unlike Plod1 and Plod2, Plod3 is present both intracellularly and is also secreted (47) and has a critical function in the glycosylation of type IV and type VI collagen that is undertaken by the galactosylhydroxylysyl glucosyltransferase (GGT) activity of Plod3, which regulates type IV and type VI collagen secretion and assembly (31, 40). Disrupting the GGT activity of Plod3 disrupts the localization of type IV collagen and, hence, formation of the basement membrane, leading to embryonic lethality of plod3−/− mice at embryonic day (E) 9.5 (34). The plod3−/− mouse embryos exhibited a 30% reduction in the number of hydroxylysine residues in the type IV and type V collagen fractions of mouse embryo lungs (and kidneys) although the levels of hydroxylysine in type I, type II, and type III collagen fractions of mouse embryo lungs (and kidneys) remained normal (34). These data point to a role for Plod3 in the formation of the lung epithelial basement membrane. Particularly interesting is that the lung exhibits the highest Plod3 protein abundance and the highest Plod3 GGT activity of any organ (38). As such, perturbations to Plod3 expression, such as those described to occur during the arrested lung development in the mouse lungs we describe in this study, may have a pronounced impact on secondary septation and alveolar development.
The ability of TGF-β to regulate lysyl hydroxylase activity in constituent cells of the lung alveolus was interesting, given that TGF-β signaling is dynamically regulated during lung alveolarization (3, 48). Furthermore, excessive TGF-β signaling has been associated with BPD (15) because elevated levels of TGF-β are detected in bronchoalveolar lavage fluid of patients with BPD (4, 22) and that TGF-β has been causally implicated in the pathogenesis of experimental BPD, where inhibition of TGF-β signaling by elafin (16, 17), curcumin (35), and neutralizing antibodies (23, 27) attenuated the deleterious impact of hyperoxia on lung structure and function, and other avenues for the attenuation of TGF-β signaling in fetal sheep lungs, such as antenatal glucocorticoids, are currently being explored (11, 12). Additionally, TGF-β signaling is upregulated in the lungs of mouse pups in which alveolarization has been arrested by exposure to hyperoxia (2). Consistent with the findings that Plod2 is upregulated in a TGF-β-dependent manner in synovial fibrosis (32), and in dermal fibroblasts (44), TGF-β dramatically upregulated PLOD2 expression and activated the PLOD2 promoter in primary human lung fibroblasts and A549 cells. Similarly, plod2 expression was upregulated by TGF-β in primary mouse lung fibroblasts. These data suggested that TGF-β may drive plod2 expression in the BPD rodent model, with the caveat that these studies were performed in cell lines or primary cells derived from adult, not neonatal mice. To address this, TGF-β signaling was neutralized in the lungs of hyperoxia-exposed mouse pups. TGF-β neutralization, which partially normalized alveolarization in the BPD model, normalized plod2 gene expression, identifying TGF-β as the mediator of aberrant plod2, but not plod1 or plod3 expression, in hyperoxia-exposed mouse pup lungs. These data add to a growing body of evidence that points to a role for TGF-β in driving perturbed ECM remodeling in animal models of BPD. It is important to note that observations were made using RNA pools from lung tissue homogenates, and, as such, the effects of TGF-β neutralization on lysyl hydroxylase expression in specific cellular compartments of the developing lung would not have been detected in this approach and cannot be ruled out.
In support of a role for PLOD2 in aberrant late lung development, PLOD2 expression was also elevated in patients with BPD or at risk for BPD, irrespective of whether the patient group was matched for gestational age at death or chronological age at death. As such, PLOD2 is an exciting candidate that warrants further study in the context of BPD. No plod2−/− mouse line is available, and neither is a specific Plod2 inhibitor available to address this idea further. It is important to note that the mouse lung expression, TGF-β neutralization, and clinical BPD studies examined changed in lysyl hydroxylase expression in whole lung mRNA pools. As such, compartment-specific roles for Plod1 and Plod3 in arrested late lung development cannot be ruled out at this stage. The development of specific inhibitors or floxed alleles for plod1 and plod3 would go a long way to discerning the roles for these enzymes in normal and aberrant late lung development.
GRANTS
This study was supported by the German Research Foundation through Grant Mo 1789/1 (to R. Morty) and Excellence Cluster 147 “Cardio-Pulmonary System” (to S. Herold, K. Mayer, I. Vadász, W. Seeger, and R. Morty) as well as the Federal Ministry of Higher Education, Research and the Arts of the State of Hessen LOEWE Program (to S. Herold, K. Mayer, I. Vadász, W. Seeger, and R. Morty), the German Center for Lung Research (Deutche Lungenforschungszentrum; all German authors), and U.S. National Heart, Lung, and Blood Institute Grant HL-094608 (to J. Roberts).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.J.W., P.T., E.S., W.S., and R.E.M. conception and design of research; T.J.W., P.T., E.S., G.N., S.B., K.M., and J.D.R. performed experiments; T.J.W., P.T., G.N., S.H., K.M., I.V., J.D.R., W.S., and R.E.M. analyzed data; T.J.W., S.H., I.V., W.S., and R.E.M. interpreted results of experiments; T.J.W., P.T., E.S., G.N., S.B., S.H., K.M., I.V., J.D.R., W.S., and R.E.M. approved final version of manuscript; E.S., G.N., and R.E.M. prepared figures; S.H., I.V., and R.E.M. drafted manuscript; K.M., I.V., and R.E.M. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the constructive discussions and provision of reagents by Richard D. Bland, Thomas J. Mariani, Robert Mecham, Richard A. Pierce, Dick Tibboel, and David Warburton.
REFERENCES
- 1.Albertine KH, Jones GP, Starcher BC, Bohnsack JF, Davis PL, Cho SC, Carlton DP, Bland RD. Chronic lung injury in preterm lambs. Disordered respiratory tract development. Am J Respir Crit Care Med 159: 945–958, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Alejandre-Alcázar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Pérez J, Wygrecka M, Eul B, Köbrich S, Hesse M, Schermuly RT, Seeger W, Eickelberg O, Morty RE. Hyperoxia modulates TGF-beta/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L537–L549, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Alejandre-Alcázar MA, Michiels-Corsten M, Vicencio AG, Reiss I, Ryu J, de Krijger RR, Haddad GG, Tibboel D, Seeger W, Eickelberg O, Morty RE. TGF-beta signaling is dynamically regulated during the alveolarization of rodent and human lungs. Dev Dyn 237: 259–269, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Been JV, Debeer A, van Iwaarden JF, Kloosterboer N, Passos VL, Naulaers G, Zimmermann LJ. Early alterations of growth factor patterns in bronchoalveolar lavage fluid from preterm infants developing bronchopulmonary dysplasia. Pediatr Res 67: 83–89, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Bland RD, Ertsey R, Mokres LM, Xu L, Jacobson BE, Jiang S, Alvira CM, Rabinovitch M, Shinwell ES, Dixit A. Mechanical ventilation uncouples synthesis and assembly of elastin and increases apoptosis in lungs of newborn mice. Prelude to defective alveolar septation during lung development? Am J Physiol Lung Cell Mol Physiol 294: L3–L14, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Bland RD, Xu L, Ertsey R, Rabinovitch M, Albertine KH, Wynn KA, Kumar VH, Ryan RM, Swartz DD, Csiszar K, Fong KS. Dysregulation of pulmonary elastin synthesis and assembly in preterm lambs with chronic lung disease. Am J Physiol Lung Cell Mol Physiol 292: L1370–L1384, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Bourbon J, Boucherat O, Chailley-Heu B, Delacourt C. Control mechanisms of lung alveolar development and their disorders in bronchopulmonary dysplasia. Pediatr Res 57: 38R–46R, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Brew N, Hooper SB, Allison BJ, Wallace MJ, Harding R. Injury and repair in the very immature lung following brief mechanical ventilation. Am J Physiol Lung Cell Mol Physiol 301: L917–L926, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Brinckmann J, Acil Y, Feshchenko S, Katzer E, Brenner R, Kulozik A, Kugler S. Ehlers-Danlos syndrome type VI: lysyl hydroxylase deficiency due to a novel point mutation (W612C). Arch Dermatol Res 290: 181–186, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Buczynski BW, Yee M, Paige Lawrence B, O'Reilly MA. Lung development and the host response to influenza A virus are altered by different doses of neonatal oxygen in mice. Am J Physiol Lung Cell Mol Physiol 302: L1078–L1087, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins JJ, Kunzmann S, Kuypers E, Kemp MW, Speer CP, Newnham JP, Kallapur SG, Jobe AH, Kramer BW. Antenatal glucocorticoids counteract LPS changes in TGF-β pathway and caveolin-1 in ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 304: L438–L444, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins JJ, Kuypers E, Nitsos I, Jane Pillow J, Polglase GR, Kemp MW, Newnham JP, Cleutjens JP, Frints SG, Kallapur SG, Jobe AH, Kramer BW. LPS-induced chorioamnionitis and antenatal corticosteroids modulate Shh signaling in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 303: L778–L787, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Paepe ME, Mao Q, Powell J, Rubin SE, DeKoninck P, Appel N, Dixon M, Gundogan F. Growth of pulmonary microvasculature in ventilated preterm infants. Am J Respir Crit Care Med 173: 204–211, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Gonzalez A, Alex Mitsialis S, Liu X, Kourembanas S. Vasculoprotective effects of heme oxygenase-1 in a murine model of hyperoxia-induced bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 302: L775–L784, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gauldie J, Galt T, Bonniaud P, Robbins C, Kelly M, Warburton D. Transfer of the active form of transforming growth factor-beta 1 gene to newborn rat lung induces changes consistent with bronchopulmonary dysplasia. Am J Pathol 163: 2575–2584, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilgendorff A, Parai K, Ertsey R, Jain N, Navarro EF, Peterson JL, Tamosiuniene R, Nicolls MR, Starcher BC, Rabinovitch M, Bland RD. Inhibiting lung elastase activity enables lung growth in mechanically ventilated newborn mice. Am J Respir Crit Care Med 184: 537–546, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilgendorff A, Parai K, Ertsey R, Juliana Rey-Parra G, Thebaud B, Tamosiuniene R, Jain N, Navarro EF, Starcher BC, Nicolls MR, Rabinovitch M, Bland RD. Neonatal mice genetically modified to express the elastase inhibitor elafin are protected against the adverse effects of mechanical ventilation on lung growth. Am J Physiol Lung Cell Mol Physiol 303: L215–L227, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyry M, Lantto J, Myllyharju J. Missense mutations that cause Bruck syndrome affect enzymatic activity, folding, and oligomerization of lysyl hydroxylase 2. J Biol Chem 284: 30917–30924, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr 23: 167–172, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jobe AH. What is BPD in 2012 and what will BPD become? Early Hum Dev 88, Suppl 2: S27–S28, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163: 1723–1729, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Kotecha S, Wangoo A, Silverman M, Shaw RJ. Increase in the concentration of transforming growth factor beta-1 in bronchoalveolar lavage fluid before development of chronic lung disease of prematurity. J Pediatr 128: 464–469, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Kumarasamy A, Schmitt I, Nave AH, Reiss I, van der Horst I, Dony E, Roberts JD, Jr, de Krijger RR, Tibboel D, Seeger W, Schermuly RT, Eickelberg O, Morty RE. Lysyl oxidase activity is dysregulated during impaired alveolarization of mouse and human lungs. Am J Respir Crit Care Med 180: 1239–1252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madurga A, Mizikova I, Ruiz-Camp J, Morty RE. Recent advances in late lung development and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 305: L893–L905, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Morty RE, Nejman B, Kwapiszewska G, Hecker M, Zakrzewicz A, Kouri FM, Peters DM, Dumitrascu R, Seeger W, Knaus P, Schermuly RT, Eickelberg O. Dysregulated bone morphogenetic protein signaling in monocrotaline-induced pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 27: 1072–1078, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Myllyla R, Wang C, Heikkinen J, Juffer A, Lampela O, Risteli M, Ruotsalainen H, Salo A, Sipila L. Expanding the lysyl hydroxylase toolbox: new insights into the localization and activities of lysyl hydroxylase 3 (LH3). J Cell Physiol 212: 323–329, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Nakanishi H, Sugiura T, Streisand JB, Lonning SM, Roberts JD., Jr TGF-β-neutralizing antibodies improve pulmonary alveologenesis and vasculogenesis in the injured newborn lung. Am J Physiol Lung Cell Mol Physiol 293: L151–L161, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease Bronchopulmonary dysplasia. N Engl J Med 276: 357–368, 1967 [DOI] [PubMed] [Google Scholar]
- 29.O'Reilly M, Sozo F, Harding R. The impact of preterm birth and bronchopulmonary dysplasia on the developing lung: long-term consequences for respiratory health. Clin Exp Pharmacol Physiol 40: 765–773, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Pierce RA, Albertine KH, Starcher BC, Bohnsack JF, Carlton DP, Bland RD. Chronic lung injury in preterm lambs: disordered pulmonary elastin deposition. Am J Physiol Lung Cell Mol Physiol 272: L452–L460, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Rautavuoma K, Takaluoma K, Sormunen R, Myllyharju J, Kivirikko KI, Soininen R. Premature aggregation of type IV collagen and early lethality in lysyl hydroxylase 3 null mice. Proc Natl Acad Sci USA 101: 14120–14125, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Remst DF, Blaney Davidson EN, Vitters EL, Blom AB, Stoop R, Snabel JM, Bank RA, van den Berg WB, van der Kraan PM. Osteoarthritis-related fibrosis is associated with both elevated pyridinoline cross-link formation and lysyl hydroxylase 2b expression. Osteoarthritis Cartilage 21: 157–164, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Ruotsalainen H, Sipila L, Kerkela E, Pospiech H, Myllyla R. Characterization of cDNAs for mouse lysyl hydroxylase 1, 2 and 3, their phylogenetic analysis and tissue-specific expression in the mouse. Matrix Biol 18: 325–329, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Ruotsalainen H, Sipila L, Vapola M, Sormunen R, Salo AM, Uitto L, Mercer DK, Robins SP, Risteli M, Aszodi A, Fassler R, Myllyla R. Glycosylation catalyzed by lysyl hydroxylase 3 is essential for basement membranes. J Cell Sci 119: 625–635, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Sakurai R, Li Y, Torday JS, Rehan VK. Curcumin augments lung maturation, preventing neonatal lung injury by inhibiting TGF-β signaling. Am J Physiol Lung Cell Mol Physiol 301: L721–L730, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salo AM, Cox H, Farndon P, Moss C, Grindulis H, Risteli M, Robins SP, Myllyla R. A connective tissue disorder caused by mutations of the lysyl hydroxylase 3 gene. Am J Hum Genet 83: 495–503, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salo AM, Sipila L, Sormunen R, Ruotsalainen H, Vainio S, Myllyla R. The lysyl hydroxylase isoforms are widely expressed during mouse embryogenesis, but obtain tissue- and cell-specific patterns in the adult. Matrix Biol 25: 475–483, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Salo AM, Wang C, Sipila L, Sormunen R, Vapola M, Kervinen P, Ruotsalainen H, Heikkinen J, Myllyla R. Lysyl hydroxylase 3 (LH3) modifies proteins in the extracellular space, a novel mechanism for matrix remodeling. J Cell Physiol 207: 644–653, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Seth P, Yeowell HN. Fox-2 protein regulates the alternative splicing of scleroderma-associated lysyl hydroxylase 2 messenger RNA. Arthritis Rheum 62: 1167–1175, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sipila L, Ruotsalainen H, Sormunen R, Baker NL, Lamande SR, Vapola M, Wang C, Sado Y, Aszodi A, Myllyla R. Secretion and assembly of type IV and VI collagens depend on glycosylation of hydroxylysines. J Biol Chem 282: 33381–33388, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol 67: 623–661, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Takaluoma K, Hyry M, Lantto J, Sormunen R, Bank RA, Kivirikko KI, Myllyharju J, Soininen R. Tissue-specific changes in the hydroxylysine content and cross-links of collagens and alterations in fibril morphology in lysyl hydroxylase 1 knock-out mice. J Biol Chem 282: 6588–6596, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Thibeault DW, Mabry SM, Ekekezie II, Zhang X, Truog WE. Collagen scaffolding during development and its deformation with chronic lung disease. Pediatrics 111: 766–776, 2003 [DOI] [PubMed] [Google Scholar]
- 44.van der Slot AJ, van Dura EA, de Wit EC, De Groot J, Huizinga TW, Bank RA, Zuurmond AM. Elevated formation of pyridinoline cross-links by profibrotic cytokines is associated with enhanced lysyl hydroxylase 2b levels. Biochim Biophys Acta 1741: 95–102, 2005 [DOI] [PubMed] [Google Scholar]
- 45.van der Slot AJ, Zuurmond AM, Bardoel AF, Wijmenga C, Pruijs HE, Sillence DO, Brinckmann J, Abraham DJ, Black CM, Verzijl N, DeGroot J, Hanemaaijer R, TeKoppele JM, Huizinga TW, Bank RA. Identification of PLOD2 as telopeptide lysyl hydroxylase, an important enzyme in fibrosis. J Biol Chem 278: 40967–40972, 2003 [DOI] [PubMed] [Google Scholar]
- 46.van der Slot AJ, Zuurmond AM, van den Bogaerdt AJ, Ulrich MM, Middelkoop E, Boers W, Karel Ronday H, DeGroot J, Huizinga TW, Bank RA. Increased formation of pyridinoline cross-links due to higher telopeptide lysyl hydroxylase levels is a general fibrotic phenomenon. Matrix Biol 23: 251–257, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Wang C, Ristiluoma MM, Salo AM, Eskelinen S, Myllyla R. Lysyl hydroxylase 3 is secreted from cells by two pathways. J Cell Physiol 227: 668–675, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, Rogers O, De Langhe S, Kemp PJ, Riccardi D, Torday J, Bellusci S, Shi W, Lubkin SR, Jesudason E. Lung organogenesis. Curr Top Dev Biol 90: 73–158, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol Lung Cell Mol Physiol 275: L110–L117, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Yeowell HN, Walker LC. Mutations in the lysyl hydroxylase 1 gene that result in enzyme deficiency and the clinical phenotype of Ehlers-Danlos syndrome type VI. Mol Genet Metab 71: 212–224, 2000 [DOI] [PubMed] [Google Scholar]