Abstract
Previous reports demonstrated that bleomycin-induced injury of lungs in mice can be improved by the administration of murine multipotent adult stem/progenitor cells (MSCs) from the bone marrow. Recently some of the beneficial effects of MSCs have been explained by the cells being activated by signals from injured tissues to express the inflammation modulating protein TNF-α-stimulated gene/protein 6 (TSG-6). In this study, we elected to test the hypothesis that targeting the early phase of bleomycin-induced lung injury with systemic TSG-6 administration may produce therapeutic effects such as preventing the deterioration of lung function and increasing survival by modulation of the inflammatory cascade. Lung injury in C57Bl/6J mice was induced by intratracheal administration of bleomycin. Mice then received intravenous injections of TSG-6 or sham controls. Pulse oximetry was used to monitor changes in lung function. Cell infiltration was evaluated by flow cytometry, cytokine expression was measured by ELISA assays, and lungs were assessed for histological attributes. The results demonstrated that intravenous infusion of TSG-6 during the early inflammatory phase decreased cellular infiltration into alveolar spaces. Most importantly, it improved both the subsequent decrease in arterial oxygen saturation levels and the survival of the mice. These findings demonstrated that the beneficial effects of TSG-6 in a model of bleomycin-induced lung injury are largely explained by the protein modulating the early inflammatory phase. Similar phase-directed strategy with TSG-6 or other therapeutic factors that MSCs produce may be useful for other lung diseases and diseases of other organs.
Keywords: lung injury, inflammation, pulmonary gas exchange, mesenchymal stromal cells
bleomycins are a family of antibiotics that are currently used as chemotherapeutic agents to treat germ-cell cancers, lymphomas, and malignancies of head and neck (6). Unfortunately, bleomycin chemotherapy is a frequent cause of interstitial pneumonitis, a complex lung disease that can progress into interstitial pulmonary fibrosis with a poor clinical prognosis and high rate of mortality (16, 40). Up to 46% of bleomycin-treated patients develop some form of lung toxicity leading to death in 3% of treated patients (43).
Bleomycin exerts its toxic effects by intercalating into double-stranded DNA to degrade the DNA and trigger the intrinsic apoptotic pathway (41). Within the initial 48 h after administration, bleomycin produces damage/necrosis of the alveolar epithelium, capillary congestion, perivascular permeability leading to edema, and the formation of hyaline membranes (10, 11, 46). Concomitantly, there is an increase in inflammatory cell infiltrates in the bronchoalveolar lavage fluid (BALF) (17, 46). There is also a marked influx of immune cells such as lymphocytes during this acute phase (11, 46). As the injury progresses, pulmonary fibrosis develops with an excessive deposition of extracellular matrix deposition in the lung interstitium (15, 17, 44). In the long term, there is compromised lung function with impaired transfer of oxygen and carbon dioxide gases.
Several reports demonstrated that bleomycin-induced injury of lungs in mice can be improved by administration of multipotent adult stem/progenitor cells referred to as mesenchymal stem or stromal cells (MSCs). Ortiz et al. (30, 31) demonstrated that murine bone marrow-derived MSCs decreased inflammation and collagen deposition in lung by expression of IL-1R antagonist. Rojas et al. (39) reported that the murine bone marrow-derived MSCs localized to lung and assumed the phenotypes of lung cells, but they also suppressed inflammation and triggered production of growth factors. In other models of acute lung injury, MSCs were shown to suppress proinflammatory cytokines, edema, and the influx of neutrophils (18, 22). They improved clearance of bacteria (9, 23). The beneficial effects of MSCs were largely explained by the ability of the cells to modulate immune and inflammatory reactions via several different mechanisms (32–34). Similar beneficial effects of MSCs were also observed in other disease models, such as sepsis (27), myocardial infarction (13, 19, 25), and acute kidney injury (47, 48).
Accordingly, a common theme in recent reports is that MSCs produce their beneficial effects by being activated by signals from injured tissues to express genes that modulate inflammatory and immune responses (18, 32). Among the anti-inflammatory factors MSCs are activated to express is TNF-α stimulated gene/protein-6 (TSG-6) (19). TSG-6 was first discovered in the early 1990s and was shown to have multiple anti-inflammatory effects (24, 50). It cross-links proinflammatory fragments of hyaluronan (2) and catalytically transfers a heavy chain from inter-α-inhibitor to hyaluronan, thereby inhibiting the cascade of proteases released by inflammation (2, 26). It also inhibits transport of leukocytes through endothelial cells (3). Administration of recombinant TSG-6 was shown to reproduce most of the beneficial effects of MSC administration in models of myocardial infarction (19), peritonitis (7), and corneal injury (29, 38). Also, Danchuk et al. (8) demonstrated that the beneficial effects of MSCs in a model of LPS-induced lung injury were partly explained by the secretion of TSG-6 by MSCs.
Here we tested the hypothesis that beneficial effects can be produced by administration of TSG-6 to target the early inflammatory phase of bleomycin-induced lung injury. In effect, administration of the protein during the early inflammatory phase of the injury might be more effective than administration of MSCs. Therapies with MSCs are complex because the cells undergo a lag period of 10–12 h before they are activated by signals from injured cells to express potentially therapeutic factors such as TSG-6, and after intravenous (iv) infusion they disappear from the lung after 24 h (19). We used a rapid and real-time assay for arterial oxygen saturation (SpO2) to follow the deleterious effects of bleomycin on gaseous exchange in the lung. The results demonstrated that administration of TSG-6 on 2 and 4 days after bleomycin exposure decreased the initial cellular infiltration into alveolar spaces and improved both the subsequent decrease in SpO2 levels and the survival of the mice.
MATERIALS AND METHODS
Animals.
Animal use protocol was approved by the Texas A&M Health Science Center Institutional Animal Care and Use Committee at Scott and White Hospital. Female 6- to 8-wk-old wild-type (C57BL/6J) mice and female transgenic mice with a CD44 gene knockout (B6.Cg-Cd44tm1Hbg/J) obtained from Jackson Laboratory were used for this study. The mice were kept on a 12-h light-dark cycle and fed and watered ad libitum.
Bleomycin-induced lung injury.
Mice were anesthetized with 4% isoflurane in 100% oxygen for 4 min to reach the level of deep anesthesia and placed on the intubation stand facing upward at a 45° tilt by using sterile elastic string positioned under the animal's front incisors. The tongue was retracted with forceps and the trachea was intubated with a 22-G plastic sterile iv catheter (Terumo). An external high-power light source was used to visualize the tracheal opening. Bleomycin sulfate from Streptomyces verticillus (Sigma-Aldrich) at 2.25 U/kg body wt in 0.9% sodium chloride (Sigma-Aldrich) or 0.9% sodium chloride alone (sham injury) was instilled through a catheter at volume 4 μl/g body wt in two sets. The dose per mouse varied from 0.036 to 0.047 U. Mice were kept on the board for an additional 90 s with the continued 4% isoflurane in 100% oxygen anesthesia via facemask.
Intravenous administration of recombinant human TSG-6 (R&D Systems) was performed on days 2 and 4 after bleomycin administration. Mice were anesthetized with 3% isoflurane, the tail vein was visualized by warming the tail, and a 28-G needle was used to inject rhTSG-6 (50 μg in 50–150 μl of sterile PBS) or sterile PBS (50–150 μl).
End point tissue, blood, and bronchoalveolar lavage fluid collection.
At the indicated time points after bleomycin administration, mice were anesthetized with 3% isoflurane in 100% oxygen for 3 min and euthanized by intraperitoneal injections of ketamine-xylazine solution at 80 and 8 mg/kg body wt, respectively. The rib cage was opened without damaging the lungs. Blood was collected from the right heart ventricle and placed in a tube containing activators of clotting (Terumo Medical, Somerset, NJ) for 20 min and then stored on ice until further processing. Care was used to prevent bleeding into the rib cage. Serum was separated by centrifuging the tube at 1,500 g for 10 min and stored at −80°C until further analysis. Immediately following the collection of blood, the trachea was cannulated with a 20-G plastic iv catheter (Exelint, Los Angeles, CA). Two fractions of BALF were obtained. The first fraction was obtained by flushing the lungs back and forth four times with a single volume of 800 μl of PBS containing Halt Protease Inhibitor Cocktail (Thermo Fisher Scientific, Rockford, IL). The second fraction was obtained by flushing the lung four additional times each with a volume of 800 μl of PBS without protease inhibitor cocktail. The first BALF fraction was centrifuged at 500 g for 10 min at 4°C to obtain a cell pellet, which was later combined with the cell pellet from the second fraction. The supernatant was then spun again at 10,000 g for 10 min and stored at −80°C until further analysis. The cell pellet from the second wash was obtained by centrifugation at 500 g for 10 min and combined with the cell pellet from the first fraction. To lyse erythrocytes, the combined pellets were incubated at room temperature with red blood cell lysis buffer (eBioscience, San Diego, CA) for 5 min, centrifuged at 500 g for 5 min at 4°C, resuspended with 10 ml of ice-cold PBS, and centrifuged again. The supernatant was discarded and the cells were then resuspended in PBS containing 1% bovine serum albumin (BSA) (Thermo Fisher Scientific), counted by use of disposable Improved Neubauer Hemocytometers (INCYTO, Cheonan-si, Chungcheongnam-do, South Korea), and used later for flow cytometry analysis.
After obtaining BALF, the lungs were excised, washed once in ice-cold PBS and immediately frozen and stored in −80°C until further processing.
Flow cytometry.
The cells from BALF were prepared as described in the previous section, counted, resuspended in 100 μl of PBS with 1% BSA, and incubated for 10 min at 4°C with anti-CD16/32 antibody at a concentration 0.5 μg per 1×106 cells in 100 μl (eBioscience) to block nonspecific binding to Fc-receptors. After being washed once with PBS-1% BSA, the cells were incubated for 20 min at room temperature with both phycoerythrin-Cy7-conjugated anti-mouse F4/80 antibody (eBioscience) and FITC-conjugated anti-Ly-6G antibody (BD Pharmingen, San Diego, CA). The antibodies were used at a concentration of 1 μg per 1×106 cells in 100 μl of PBS-1% BSA. Isotype-matching antibodies at similar concentrations obtained from the same manufacturers and single-color labeling were used as controls for the specificity of labeling. After two washes in PBS, the cells were again resuspended in PBS-1% BSA and analyzed by FC500 flow cytometer (Beckman Coulter, Brea, CA) to determine macrophage (F4/80-positive) and neutrophil (Ly-6G-positive) populations.
Cytokine ELISA in BALF and lung tissues.
BALF was prepared as described in the previous section and frozen lungs were homogenized with RIPA buffer (ThermoFisher Scientific). Appropriate dilutions were used in the assays. Lung protein concentration was determined by using Micro BCA Protein Assay Kit (ThermoFisher Scientific) according to the manufacturer's instructions. Cytokine concentrations were determined by using commercially available ELISA kits for detection of IL-6, TNF-α, IL-1β, CCL2/MCP-1, CCL3/MIP-1α, CXCL2/MIP-2, and CXCL1/KC (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Histology.
Mice were anesthetized with 3% isoflurane in 100% oxygen for 3 min and euthanized by intraperitoneal injections of ketamine-xylazine solution as described previously. The rib cage was opened and the blood flow was stopped by placing a permanent silk suture between atria and ventricles of the heart. The trachea was then cannulated with 20-G iv catheter and the lungs were excised, immediately washed with PBS, and fixed with 4% paraformaldehyde at pressure 20 cmH2O for 24 h. The lungs were then dehydrated with graded ethanol solutions and embedded in paraffin. Five-micrometer sections were cut with Leitz 1512 microtome (Leitz, Germany) and stained with a Masson TriChrome Kit (Richard-Allan Scientific, Kalamazoo, MI). The bright-field images were taken with Nikon Eclipse 80i upright microscope (Nikon, Kawasaki, Japan), and linear adjustments were made in Adobe Photoshop CS3 (Adobe Systems, San Jose, CA).
Pulse oximetry.
A portable mouse pulse oximeter (STARR Life Sciences) was used to monitor SpO2 and other physiological parameters (heart and breath rates, pulse distention) in free-roaming nonanesthetized mice. The collars of experimental animals were trimmed of fur at least a day before the beginning of pulse oximetry monitoring. At indicated experimental time points, mice were anesthetized with 3% isoflurane in 100% oxygen for 2 min and an extra-small MouseOX collar clip was placed on the animal's neck. Mice were allowed to recover from anesthesia (1–2 min) and pulse oximetry readings were recorded as a Windaq Waveform file at 15-Hz sample rate. After placing the collar clip, we observed two sequential behavioral states in mice: 1) an agitated state, characterized by frequent movements resulting in an unstable signal, and 2) a relatively calm state with improved signal quality. The measurements were continued to allow recording of 3–5 min of stable signal. Using Windaq Waveform Browser (DATAQ Instruments), we extracted data from only the calm state. An in-house Microsoft Excel (Microsoft) VBA-based script was then used to filter values associated with error-free signals implementing formulas provided by the manufacturer. With the same script, arithmetic means for each reading were obtained and later used for statistical analysis.
Statistical analysis.
Unpaired t-test or one-way ANOVA with Bonferroni's post hoc analysis was used to compare two or more groups, respectively. Null hypotheses were rejected at P values less than 0.05. Survival of animals between groups was compared by log-rank (Mantel-Cox) test. All statistical analyses were performed with GraphPad Prism software.
RESULTS
Functional oxygen saturation correlates with changes in cellular infiltrates and cytokines in BALF.
To monitor in real-time the bleomycin-induced injury to the lung, we used a pulse oximeter to assay SpO2 in mice (Fig. 1, A and B). Placing a collar clip on the necks of the mice agitated them and distorted the reading. However, with care in handling, the mice became calm so that reliable values with minimum associated errors were readily obtained. Intratracheal administration of 2.25 U/kg of bleomycin produced a progressive decrease in SpO2 below 90% reflecting hypoxemia beginning on day 8 (Fig. 1A).
Fig. 1.

Pulse oximetry in the bleomycin model of lung injury. A: dynamics of oxygen saturation (SpO2) levels in bleomycin-injured and uninjured (sham-injured with NaCl) mice over the course of 12 days following injury. Only bleomycin-injured mice dropped SpO2 levels below 90% (dashed line) that were significantly lower than in uninjured mice beginning on day 8. B: SpO2 curves extracted from Windaq files of bleomycin-injured and uninjured mice prior to the beginning of the experiment and 12 days after injury. Note visible changes in the SpO2 curve of injured animals (bleomycin day 12). C and D: although no significant changes from preinjury baseline recordings (day 0) were detected, heart rates of bleomycin-injured mice were significantly lower on day 12 and breath rates were significantly lower on days 4, 6, and 12 compared with uninjured mice. E: weight changes normalized to preinjury (day 0). Significant weight changes were noted on day 6 and constituted ∼20% weight loss in injured mice. P values were calculated between uninjured and bleomycin-injured groups at each time point by 1-tailed t-test. *P < 0.05, **P < 0.01.
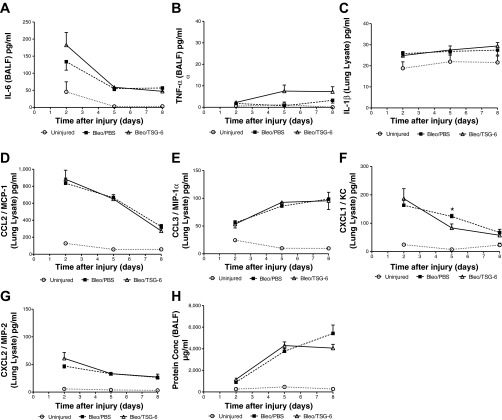
As expected, the decrease in SpO2 paralleled changes in the BALF. There were progressive increases in total cells and protein concentration (Fig. 2, A and D). Macrophages in the BALF showed an initial decrease and then increased on day 6 and day 12 (Fig. 2B). Neutrophils were more variable but showed increases vs. a control in the number of neutrophils from days 2 to 12 (Fig. 2C). As also expected, the major changes in cytokine and chemokine levels occurred earlier than the decrease in SpO2 (Fig. 3). Levels of the proinflammatory cytokines IL-6 and TNF-α in the BALF were increased on day 2 and then decreased. Similarly, the chemokines CCL2 and CXCL1 were increased on day 2 and then decreased.
Fig. 2.
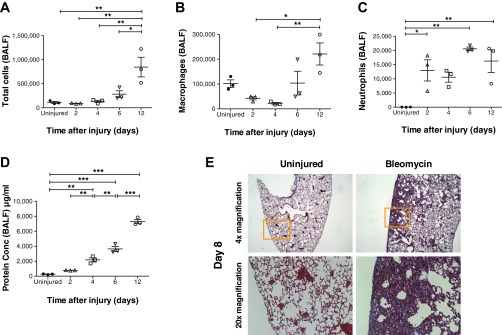
Temporal changes in cell composition of bronchoalveolar lavage fluid (BALF) and histological changes in the lungs following bleomycin injury. BALF was collected at 2, 4, 6, and 12 days following bleomycin injury and at 12 days from uninjured mice (A–D); for histology, lungs were collected at day 8 (E). A and D: total number of cells in BALF was counted with a disposable hemocytometer. Protein concentration (Conc) in BALF was determined by the BCA assay. Both total BALF cell number and protein concentration increased temporally. Both parameters were the highest by day 12 postinjury and were significantly higher than in the uninjured controls. Significant differences in total cells and protein concentration between groups were also observed at various time points as indicated in the figure. B and C: the cells were labeled with antibodies against macrophage (F4/80) and neutrophil (Ly6G) markers and analyzed by flow cytometry. Macrophages and neutrophils also increased temporally following bleomycin injury. However, on days 2 and 4, whereas total cells and macrophages were either similar to or lesser in number compared with uninjured mice, neutrophil numbers on those days were higher than the uninjured mice. E: representative images of Masson's trichrome-stained lung sections from bleomycin-injured and uninjured mice from day 8. Lower row represents magnification of area inside orange frame in upper row. Area of inflammation and fibrosis is clearly visible in bleomycin-injured lungs. Values are represented as arithmetic means ± SE (n = 3 mice per group). P values were calculated by 1-way ANOVA with a post hoc Bonferroni's multiple comparison analysis performed between all the groups. *P < 0.05, **P < 0.01, and ***P < 0.001.
Fig. 3.
Temporal changes in cytokine expression of BALF in the bleomycin model of lung injury. Cytokine expression levels on days 2, 4, 6, and 12 were assayed in BALF by use of ELISA. IL-6 (A), TNF-α (B), CCL2/MCP-1 (C), and CXCL1/KC (D) expression levels were the highest on day 2 and were significantly higher than those in uninjured mice. Expression levels of all the cytokines tested returned to close to uninjured levels by days 6 and 12. Significant differences in cytokine levels between groups on the individual days tested are indicated. Values are represented as arithmetic means ± SE (n = 3 mice per group). P values were calculated by 1-way ANOVA with a post hoc Bonferroni's multiple comparison analysis performed between all the groups. *P < 0.05, **P < 0.01, and ***P < 0.001.
In addition, the decrease in SpO2 roughly paralleled a decrease in body weight (Fig. 1E) and pathological changes in lung morphology (Fig. 2E). There were more variable decreases in heart rate and respiration rate (Figs. 1, C and D).
Intravenous administration of TSG-6 improved functional oxygen saturation and survival of bleomycin-injured mice.
We elected to test the potential effectiveness of TSG-6 in suppressing the early inflammatory phase in the bleomycin model. Day 8 postinjury was chosen for performing last end point assays in further experiments. This was based on the findings from the optimization of the bleomycin model (Figs. 1–3) in which SpO2 levels in bleomycin-injured mice were significantly different from uninjured mice on day 8 and the extent of inflammation paralleled SpO2 levels. Additionally, the bleomycin-injured mice were observed until day 21 for long-term survival.
A high (50 μg) and a low dose (12.5 μg) of TSG-6 were tested to determine the most effective dose. The functional SpO2 data on day 8 (Fig. 4, A and B) and the cellular infiltrates on day 5 (Fig. 4, C–H) suggested that the high 50-μg dose would be most beneficial in reducing early inflammation and improving function in the bleomycin lung injury model. Hence the high dose (50 μg) was used for the rest of the study. The TSG-6 protein (50 μg) was infused intravenously on day 2 and day 4 to target the early inflammatory stage. As expected, there was a decrease in early signs of inflammation by a decrease in total cells, macrophages, and neutrophils in BALF on day 5 (Fig. 5). There was also a decrease in the proinflammatory cytokines TNF-α on day 2 and IL-6 on day 3 (Fig. 6, A and B). However, IL-1β expression in lung tissues was increased in TSG-6-treated mice on day 2 (Fig. 6C). In addition, administration of TSG-6 increased the BALF levels of the chemokines CCL2, CCL3, CXCL1, and CXCL2 on day 2 (Fig. 6, D–G). Protein concentration levels were not significantly different between TSG-6- and PBS-treated mice (Fig. 6H) at all examined end points. These changes were accompanied by an improvement in both the SpO2 and survival of the bleomycin injured mice (Fig. 7, A and B). Furthermore, there was a significant improvement in percent change of initial weight on days 7 and 8 following TSG-6 treatment (Fig. 7E), whereas there were no significant changes in heart and breath rates between groups (Fig. 7, C and D). However, there was no significant reduction in fibrosis in TSG-6-treated lungs as measured by hydroxyproline content in the lungs on day 8 (data not shown), suggesting that there was no increase in fibrosis at this time.
Fig. 4.
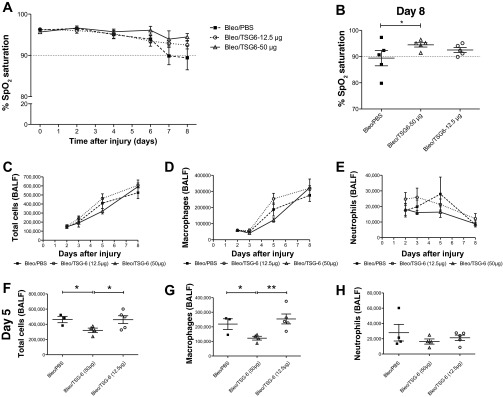
TNF-α-stimulated gene/protein 6 (TSG-6) dose response on SpO2 and cellular infiltrates. A: effect of TSG-6 dose response on the dynamics of SpO2 levels over the course of 8 days following bleomycin (Bleo) injury. A high dose (50 μg) and a low dose (12.5 μg) were administered on days 2 and 4 following bleomycin injury. B: dot plot represents the distribution of SpO2 levels on day 8. Significant differences between high dose of TSG-6 and PBS groups was observed on day 8. For the analysis of cellular infiltrates, BALF was collected on days 2, 3, 5, and 8 following bleomycin injury. From day 3 onward following injury of the lungs with bleomycin, total cells (C) and macrophages (D) in BALF increased in the low-dose TSG-6 mice compared with PBS control mice. F and G: the high dose of TSG-6 lowered these parameters compared with PBS-treated mice with statistically significant differences between these 2 groups observed only on day 5. E and H: the low dose of TSG-6 increased neutrophil numbers early on day 2, whereas the high dose of TSG-6 maintained the neutrophil infiltration lower than PBS mice at all time points. Values are represented as arithmetic means ± SE (n = 5–8 mice per group). For the SpO2 data, P value was calculated between PBS and TSG-6 groups by 1-tailed t-test. For the cellular infiltrates data, P values were calculated by 1-way ANOVA with a post hoc Bonferroni's multiple comparison analysis performed between all the groups. *P < 0.05, **P < 0.01.
Fig. 5.
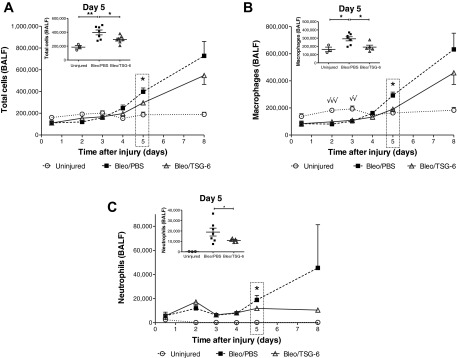
TSG-6 treatment reduces cellular infiltration in the lungs during the early inflammatory phase. BALF was collected at 12 h and on days 2, 3, 4, 5, and 8 following bleomycin injury. On days 2 and 4 alone, BALF was collected 4 h after TSG-6 (50 μg) administration. From day 4 onward following injury of the lungs with bleomycin, total cells (A), macrophages (B), and neutrophils (C) in BALF increased compared with uninjured mice. TSG-6 treatment lowered these parameters compared with PBS-treated mice with statistically significant differences between these 2 groups observed only on day 5. Insets represent changes on day 5 in individual mice from the 3 groups. Note that on days 2 and 3 following bleomycin injury, macrophages (B) were significantly lower in PBS and TSG-6 groups (√√P < 0.01, √√√P < 0.001) compared with uninjured mice. Values are represented as arithmetic means ± SE (n = 5–8 mice per group). P values were calculated by 1-way ANOVA with a post hoc Bonferroni's multiple comparison analysis performed between all the groups. P values represented in the larger panels only indicate significant differences between PBS and TSG-6 groups following bleomycin injury. P values represented in the insets indicate significant differences between all 3 groups. *P < 0.05 and **P < 0.01.
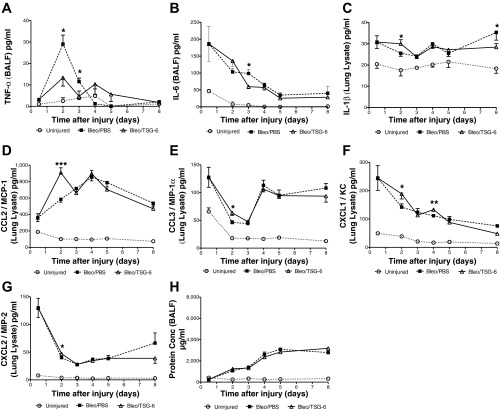
Fig. 6.
Temporal changes in cytokine/chemokine expression following TSG-6 treatment in the bleomycin model of lung injury. TSG-6 (50 μg) treatment significantly decreased TNF-α (A) expression on both days 2 and 3, whereas decreasing IL-6 (B) only on day 3 in comparison with PBS-treated mice. Surprisingly, TSG-6 initially increased IL-1β (C) expression on day 2 but decreased it significantly by day 8. TSG-6 treatment significantly increased expression of CCL2/MCP-1 (D), CCL3/MIP-1α (E), and CXCL2/MIP-2 (G) chemokines on day 2, whereas significantly increasing CXCL1/KC (F) on both days 2 and 4. No significant differences in protein concentration (H) were observed after TSG-6 treatment. Values are represented as arithmetic means ± SE (n = 5–8 mice per group). P values were calculated by 1-way ANOVA with a post hoc Bonferroni's multiple comparison analysis performed between all the groups. Only significant differences between bleomycin-injured (PBS and TSG-6) groups are indicated in the figure: *P < 0.05, **P < 0.01, and ***P < 0.001.
Fig. 7.
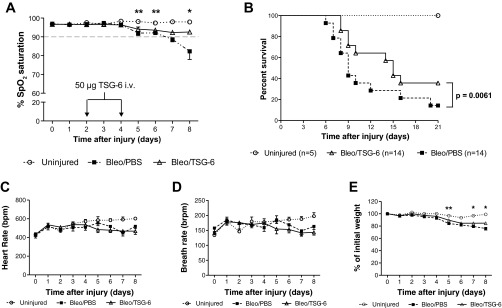
TSG-6 improves functional SpO2 and survival following bleomycin injury. A: effect of TSG-6 (50 μg) treatment on the dynamics of SpO2 levels over the course of 8 days following bleomycin injury (representative of 3 independent experiments). Significant differences between TSG-6 and PBS groups were observed on days 5, 6, and 8. Note that TSG-6 treatment prevented the drop of SpO2 levels below 90% threshold (dashed line) through day 8. P values represented in panel (A) only indicate significant differences between PBS and TSG-6 groups following bleomycin injury. B: Kaplan-Meier curves depicting survival proportions after administration of bleomycin. TSG-6 administration significantly improved survival compared with PBS-treated mice [P = 0.0363, log-rank (Mantel-Cox) test]. Dynamics of heart rate (C) and breath rate (D) following bleomycin injury and TSG-6 administration. No significant changes from preinjury baseline recordings (day 0) were detected. E: weight changes normalized to preinjury (day 0). Significant weight changes between bleomycin-injured (PBS and TSG-6) groups was noted on days 5, 7, and 8 and constituted ∼20% weight loss in injured mice. Values are represented as arithmetic means ± SE (n = 5–8 mice per group). P values were calculated by 1-way ANOVA with a post hoc Bonferroni's multiple comparison analysis performed between all the groups. *P < 0.05 and **P < 0.01.
The effects of TSG-6 are not observed in mice that do not express CD44.
Previous reports demonstrated that some of the anti-inflammatory effects of TSG-6 involved its interaction with CD44 either by directly binding to the receptor or in a complex with hyaluronan (7, 24, 51). Therefore we elected to test the hypothesis that the beneficial effects of TSG-6 observed in the bleomycin model would not be observed in transgenic mice that did not express CD44 (42). As expected, TSG-6 administration had no effect in bleomycin-treated mice on SpO2 (Fig. 8, A and B), or on BALF content of total cells, macrophages or neutrophils (Fig. 8, C–E). Also, administration of TSG-6 had no effect on the BALF levels of the cytokines IL-6, TNF-α, and IL-1β (Fig. 9, A–C). Similarly it had no effect on the BALF levels of the chemokines CCL2, CCL3, CXCL1, or CXCL2 (Fig. 9, D–G) and protein concentration (Fig. 9H).
Fig. 8.
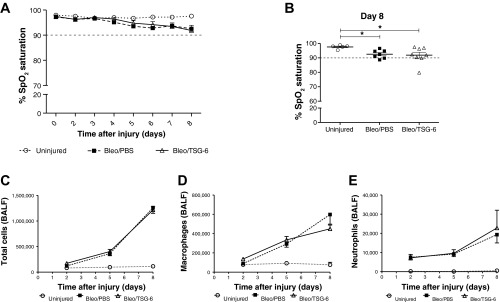
CD44 partly mediates beneficial effect of TSG-6 in vivo. A: effect of TSG-6 (50 μg) treatment on the dynamics of SpO2 levels over the course of 8 days following bleomycin injury (representative of 2 independent experiments) in CD44 knockout mice. No significant differences between TSG-6 and PBS groups were observed on any of the days tested. Note that SpO2 levels of both TSG-6 and PBS groups remained slightly above 90% threshold (dashed line) through day 8. B: dot plot represents the distribution of SpO2 levels on day 8 following bleomycin injury. Significant differences were observed between bleomycin-injured and uninjured mice, although, in the absence of CD44, TSG-6 failed to improve SpO2 levels over the PBS-treated group. C–E: BALF was collected in a similar manner as described above for the wild-type mice. No significant differences were observed between total cells, macrophages, and neutrophil numbers between TSG-6 and PBS-treated groups. Values are represented as arithmetic means ± SE (n = 5–8 mice per group). P values were calculated by 1-way ANOVA with a post hoc Bonferroni's multiple comparison analysis performed between all the groups. *P < 0.05.
Fig. 9.
Temporal changes in cytokine/chemokine expression following TSG-6 treatment in CD44 knockout mice. No significant changes were observed in CD44 knockout mice after TSG-6 (50 μg) treatment in any of the proinflammatory cytokines examined such as IL-6 (A), TNF-α (B), and IL-1β (C), or in the expression of chemokines such as CCL2/MCP-1 (D), CCL3/MIP-1α (E), and CXCL2/MIP-2 (G), or in protein concentration (H) levels. However, TSG-6 treatment significantly decreased CXCL1/KC (F) levels only on day 5 compared with the PBS group. Values are represented as arithmetic means ± SE (n = 5–8 mice per group). P values were calculated by 1-way ANOVA with a post hoc Bonferroni's multiple comparison analysis performed between all the groups. No P values are indicated on this figure since there were no statistical differences between the bleomycin-injured (PBS and TSG-6) groups in most of the cytokines/chemokines tested and at any of the time points examined.
DISCUSSION
The assay of SpO2 with pulse oximeter provided a useful measure of the deleterious effects of bleomycin on the lungs of mice. The data are obtained in a rapid, quantitative, and real-time manner without the need to euthanize the mice. Therefore this assay has many advantages both in following the natural progression of the injury and for testing potential therapies for lung injury.
The response of the lung to injury by bleomycin is complex and occurs in several relatively distinct phases (10, 11, 17, 41, 46). The beneficial effects previously observed with administration of MSCs may in fact reflect the plasticity of the cells in responding to injured tissues in a manner that is appropriate to the type and phase of the injuries (30, 31). For example, MSCs were shown to produce beneficial effects (4, 22, 32–34) in various injury models by expressing factors that enhance vascularization (VEGF and IL-6), enhance cell proliferation (TGF-β, KGF), modulate immune responses (indoleamine 2,3-dioxygenase in human MSCs, iNOS with mouse MSCs, CCL2, MMP-9), reduce reactive oxygen species and apoptosis (stanniocalcin-1), and are antibacterial (peptide LL-37).
The characterization of the bleomycin model gave us an exact timeline of cellular and morphological events that occurred after the injury. Hence it allowed us to target specific inflammatory and functional events to examine whether TSG-6 had positive effects on reducing cytokine storm and infiltration of inflammatory cells and finally whether these early reductions translated to functional improvement and improved survival. In the experiments described here, we elected to use iv administration of TSG-6 over intratracheal administration for several reasons: 1) clinical relevance, 2) increased risk of physical insult resulting from the repeated intubation required, and 3) oxygen-exacerbated damage to the lung during anesthesia (5, 11, 12, 36). Under the conditions employed in this study, we reached therapeutic effect in the lungs by iv infusion of 50 μg TSG-6 protein. This was demonstrated by an improvement in oxygen saturation, increased survival, and modulation of cytokines following bleomycin injury.
To test TSG-6 in the bleomycin model, we infused the protein during the early inflammatory phase marked by the appearance of neutrophils and proinflammatory cytokines (IL-6 and TNF-α) in the BALF (day 2 and day 4). The TSG-6 decreased the inflammatory phase as indicated by a decrease in BALF on day 5 of total cells, macrophages, and neutrophils. It also decreased proinflammatory cytokine TNF-α on day 2, and IL-6 on day 3. At the same time, administration of TSG-6 increased the levels in BALF of chemokines (CCL2/MCP-1, CCL3/MIP-1α, CXCL1/KC, and CXCL2/MIP-2) that attract macrophages to resolve inflammation. The potential beneficial effects of the increase in chemokines is consistent with the observations by Liang et al. (20) that mice with lung-specific overexpression of CCL2/MCP-1 were protected from bleomycin-induced lung injury because of increased recruitment of cells capable of clearing apoptotic cells.
However, our data on day 5 demonstrate that the early increases in MCP-1 after TSG-6 administration only translated to a modest increase in macrophage recruitment between days 2 and 5 but that these increases were still significantly lower than the number of macrophages recruited in the PBS-treated group. To explain this discrepancy, we speculate here that the macrophages recruited to alveolar spaces in TSG-6-treated animals possibly contained a larger proportion of alternatively activated or regulatory M2 macrophages. This could result in decrease of inflammation and subsequent decrease in the recruitment of proinflammatory monocytes from the circulation. In support of this theory, Roca et al. (37) have demonstrated that CCL2 (MCP-1) in the lungs can induce M2-type macrophage polarization. Furthermore, employing models of gastric aspiration and lung contusion, there is evidence for the protective effect of CCL2 and its receptor CCR2 in the pathogenesis of acute lung injury (ALI) by attenuating the inflammatory response, decreasing neutrophil infiltration, increasing macrophage recruitment, activation of the M2 polarized macrophages, and improved survival (35, 44a). In a pneumonia model of ALI, Amano et al. (1) also demonstrated that CCL2 was critical in decreasing neutrophil infiltration and increasing the phagocytosis of apoptotic neutrophils by alveolar macrophages. Thus this study might also help explain why there was decreased neutrophil recruitment on day 5 in our model following TSG-6 treatment despite elevated levels of MIP-2 and KC chemokines on day 2. We speculate that TSG-6 treatment of mice that were injured with bleomycin results in a complex interaction between different types of cells and their corresponding cytokines, which needs further investigation. However, the combined results from these studies lead us to conclude that the early elevated chemokine levels following TSG-6 treatment in our model could partly explain the overall beneficial effects that we observed such as resolution of inflammation and reduced neutrophil numbers due to either reduced recruitment of neutrophils or their increased phagocytosis, thus resulting in improved oxygenation and survival. In addition, decrease in levels of TNF-α by TSG-6 could have resulted in decreased levels of apoptosis in alveolar epithelium as indicated by Wang et al. (49), although apoptosis was not addressed in our study. Thus the cumulative effects of TSG-6 on the early inflammatory stage in part explained the subsequent increase in SpO2 and survival seen in treated mice compared with controls (Fig. 10). However, in this model, there was some mortality in bleomycin-injured mice treated with PBS, with the earliest occurrence beginning on day 6. Thus, had these mice survived to day 8, the average SpO2 levels would have been much lower and accordingly the numbers of cellular infiltrates in BALF would have been much higher compared with the bleomycin/TSG-6 group than what is represented. Although not explored here, more frequent or larger doses on TSG-6 may have been even more effective.
Fig. 10.
Modulatory effects of TSG-6 in bleomycin-injured lungs. The beneficial effects of TSG-6 on modulating the early inflammatory phase in bleomycin-injured lungs is indicated by 1) a decrease in proinflammatory cytokines IL-6 and TNF-α (days 2 and 3), 2) an increase in chemokines CCL2, CCL3, KC, and CXCL2 (days 2 and 4), and 3) a corresponding decrease in total cells and infiltrating cells such as macrophages and neutrophils (day 5). These early modulatory events promoted by TSG-6 lead to functional improvement by preventing the drop of SpO2 levels below 90% (day 8) and decreased mortality (weeks 1-3).
TSG-6 has been previously shown to have multiple interactions that can be anti-inflammatory (2, 3, 24, 26, 50). Particularly, in a recently published study by Choi et al. (7), it was shown that at least part of the inflammatory action of TSG-6 in the peritonitis model could be explained by its direct or indirect interaction with CD44 on resident macrophages. TSG-6 bound to CD44, either directly or in a complex with hyaluronan, to dissociate CD44 from TLR2 and thereby limit TLR2-driven NF-κB signaling. In CD44 knockout mice, bleomycin induced an unrelenting inflammatory response in a TLR2- and TLR4-dependent manner (14, 45). TSG-6 was also effective in wild-type mice but ineffective in CD44 knockout mice employed in a mouse model for sterile injury to the cornea (28). In the present study, we employed the CD44 knockout mouse model, similar to that used in the study by Choi et al. In these mice, CD44 expression is constitutively knocked out in all cell lineages and not restricted to only macrophages. Also, on the basis of previous publications (7, 29) we hypothesized that resident macrophages with functional CD44 could be important in mediating the effects of TSG-6 in bleomycin-injured lungs. Since TSG-6 was not effective in reducing inflammation and improving bleomycin-induced injury in the CD44 knockout mice, the results were consistent with the conclusion that TSG-6 decreased inflammation by interacting with functional CD44 on resident macrophages in a manner previously demonstrated in zymosan-induced peritonitis (7) and chemical injury to the cornea (29).
Toxic agents such as bleomycin trigger a cascade of destructive events in the lung. It seems unlikely that any single therapy can halt all of them. The results presented in this study demonstrate that the systemic administration of TSG-6 improved functional outcome and survival of bleomycin-injured mice by modulating inflammation at early stages. Hence these data suggest that it may be useful to target specific phases in the cascade with agents such as TSG-6 for the early inflammatory phase. Similar phase-specific strategies may be useful for other diseases of the lung.
GRANTS
The work was supported in part by grants from the NIH VCP01 HL 075161.
DISCLOSURES
D. J. Prockop is the chairman of the scientific advisory committee of Temple Therapeutics. None of the other authors have a conflict of interest.
AUTHOR CONTRIBUTIONS
A.M.F., N.B., and D.J.P. conception and design of research; A.M.F., N.B., X.T., A.T., and T.J.B. performed experiments; A.M.F., N.B., X.T., A.T., and T.J.B. analyzed data; A.M.F., N.B., X.T., and D.J.P. interpreted results of experiments; A.M.F. and N.B. prepared figures; A.M.F., N.B., and D.J.P. drafted manuscript; A.M.F., N.B., X.T., A.T., T.J.B., and D.J.P. edited and revised manuscript; A.M.F., N.B., and D.J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Bret Clough for assistance with lung histology.
REFERENCES
- 1.Amano H, Morimoto K, Senba M, Wang H, Ishida Y, Kumatori A, Yoshimine H, Oishi K, Mukaida N, Nagatake T. Essential contribution of monocyte chemoattractant protein-1/C-C chemokine ligand-2 to resolution and repair processes in acute bacterial pneumonia. J Immunol 172: 398–409, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Baranova NS, Nileback E, Haller FM, Briggs DC, Svedhem S, Day AJ, Richter RP. The inflammation-associated protein TSG-6 cross-links hyaluronan via hyaluronan-induced TSG-6 oligomers. J Biol Chem 286: 25675–25686, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao TV, La M, Getting SJ, Day AJ, Perretti M. Inhibitory effects of TSG-6 Link module on leukocyte-endothelial cell interactions in vitro and in vivo. Microcirculation 11: 615–624, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell 9: 11–15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cersosimo RJ, Mathews SJ, Hong WK. Bleomycin pneumonitis potentiated by oxygen administration. Drug Intell Clin Pharm 19: 921–923, 1985 [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Stubbe J. Bleomycins: towards better therapeutics. Nat Rev Cancer 5: 102–112, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood 118: 330–338, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danchuk S, Ylostalo JH, Hossain F, Sorge R, Ramsey A, Bonvillain RW, Lasky JA, Bunnell BA, Welsh DA, Prockop DJ, Sullivan DE. Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-alpha-induced protein 6. Stem Cell Res Ther 2: 27, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V, Matthay MA. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 67: 533–539, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hay J, Shahzeidi S, Laurent G. Mechanisms of bleomycin-induced lung damage. Arch Toxicol 65: 81–94, 1991 [DOI] [PubMed] [Google Scholar]
- 11.Hay JG, Haslam PL, Dewar A, Addis B, Turner-Warwick M, Laurent GJ. Development of acute lung injury after the combination of intravenous bleomycin and exposure to hyperoxia in rats. Thorax 42: 374–382, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingrassia TS, 3rd, Ryu JH, Trastek VF, Rosenow EC., 3rd Oxygen-exacerbated bleomycin pulmonary toxicity. Mayo Clin Proc 66: 173–178, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, Sobel BE, Delafontaine P, Prockop DJ. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun 354: 700–706, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11: 1173–1179, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Karlinsky JB. Glycosaminoglycans in emphysematous and fibrotic hamster lungs. Am Rev Respir Dis 125: 85–88, 1982 [DOI] [PubMed] [Google Scholar]
- 16.King TE., Jr Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med 172: 268–279, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Laurent GJ, McAnulty RJ, Corrin B, Cockerill P. Biochemical and histological changes in pulmonary fibrosis induced in rabbits with intratracheal bleomycin. Eur J Clin Invest 11: 441–448, 1981 [DOI] [PubMed] [Google Scholar]
- 18.Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: Mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells 29: 913–919, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5: 54–63, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang J, Jung Y, Tighe RM, Xie T, Liu N, Leonard M, Gunn MD, Jiang D, Noble PW. A macrophage subpopulation recruited by CC chemokine ligand-2 clears apoptotic cells in noninfectious lung injury. Am J Physiol Lung Cell Mol Physiol 302: L933–L940, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthay MA, Thompson BT, Read EJ, McKenna DH, Jr, Liu KD, Calfee CS, Lee JW. Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest 138: 965–972, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, Slutsky AS, Liles WC, Stewart DJ. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med 182: 1047–1057, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Milner CM, Higman VA, Day AJ. TSG-6: a pluripotent inflammatory mediator? Biochem Soc Trans 34: 446–450, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med 12: 459–465, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Nagyeri G, Radacs M, Ghassemi-Nejad S, Tryniszewska B, Olasz K, Hutas G, Gyorfy Z, Hascall VC, Glant TT, Mikecz K. TSG-6 protein, a negative regulator of inflammatory arthritis, forms a ternary complex with murine mast cell tryptases and heparin. J Biol Chem 286: 23559–23569, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15: 42–49, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh JG, Choi H, Lee RH, Roddy GW, Ylostalo JH, Wawrousek E, Prockop DJ. Identification of the HSPB4/TLR2/NF-κB axis in macrophage as a therapeutic target for sterile inflammation of the cornea. EMBO Mol Med 4: 435–448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh JY, Roddy GW, Choi H, Lee RH, Ylostalo JH, Rosa RH, Jr, Prockop DJ. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc Natl Acad Sci USA 107: 16875–16880, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 104: 11002–11007, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 100: 8407–8411, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther 17: 939–946, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prockop DJ, Kota DJ, Bazhanov N, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs). J Cell Mol Med 14: 2190–2199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther 20: 14–20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raghavendran K, Davidson BA, Mullan BA, Hutson AD, Russo TA, Manderscheid PA, Woytash JA, Holm BA, Notter RH, Knight PR. Acid and particulate-induced aspiration lung injury in mice: importance of MCP-1. Am J Physiol Lung Cell Mol Physiol 289: L134–L143, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Rinaldo J, Goldstein RH, Snider GL. Modification of oxygen toxicity after lung injury by bleomycin in hamsters. Am Rev Respir Dis 126: 1030–1033, 1982 [DOI] [PubMed] [Google Scholar]
- 37.Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem 284: 34342–34354, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roddy GW, Oh JY, Lee RH, Bartosh TJ, Ylostalo J, Coble K, Rosa RH, Jr, Prockop DJ. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-alpha stimulated gene/protein 6. Stem Cells 29: 1572–1579, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 33: 145–152, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossi SE, Erasmus JJ, McAdams HP, Sporn TA, Goodman PC. Pulmonary drug toxicity: radiologic and pathologic manifestations. Radiographics 20: 1245–1259, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Sakai TT, Riordan JM, Kumar NG, Haberle FJ, Elgavish GA, Glickson JD, Levy A. Studies on bleomycin-DNA and bleomycin-iron interactions. J Biomol Struct Dyn 1: 809–827, 1983 [DOI] [PubMed] [Google Scholar]
- 42.Sarthy V, Hoshi H, Mills S, Dudley VJ. Characterization of green fluorescent protein-expressing retinal cells in CD 44-transgenic mice. Neuroscience 144: 1087–1093, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sleijfer S. Bleomycin-induced pneumonitis. Chest 120: 617–624, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Starcher BC, Kuhn C, Overton JE. Increased elastin and collagen content in the lungs of hamsters receiving an intratracheal injection of bleomycin. Am Rev Respir Dis 117: 299–305, 1978 [DOI] [PubMed] [Google Scholar]
- 44a.Suresh MV, Yu B, Machado-Aranda C, Bender MD, Ochoa-Frongia L, Helinski JD, Davidson BA, Knight PR, Hogaboam CM, Moore BB, Raghavendran K. Role of macrophage chemoattractant protein-1 in acute inflammation after lung contusion. Am J Respir Cell Mol Biol 46: 797–806, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science 296: 155–158, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Thrall RS, Barton RW, D'Amato DA, Sulavik SB. Differential cellular analysis of bronchoalveolar lavage fluid obtained at various stages during the development of bleomycin-induced pulmonary fibrosis in the rat. Am Rev Respir Dis 126: 488–492, 1982 [DOI] [PubMed] [Google Scholar]
- 47.Togel F, Cohen A, Zhang P, Yang Y, Hu Z, Westenfelder C. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev 18: 475–485, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289: F31–F42, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Wang R, Alam G, Zagariya A, Gidea C, Pinillos H, Lalude O, Choudhary G, Oezatalay D, Uhal BD. Apoptosis of lung epithelial cells in response to TNF-alpha requires angiotensin II generation de novo. J Cell Physiol 185: 253–259, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Wisniewski HG, Vilcek J. TSG-6: an IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine Growth Factor Rev 8: 143–156, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Wisniewski HG, Vilcek J. Cytokine-induced gene expression at the crossroads of innate immunity, inflammation and fertility: TSG-6 and PTX3/TSG-14. Cytokine Growth Factor Rev 15: 129–146, 2004 [DOI] [PubMed] [Google Scholar]