Abstract
Acute lung injury (ALI) is a severe inflammatory condition whose pathogenesis is irrevocably linked to neutrophil emigration to the lung. Activation and recruitment of neutrophils to the lung is mostly attributable to local production of the chemokines. However, much of our understanding of neutrophil recruitment to the lung is based on studies focusing on early time points after initiation of injury. In this study, we sought to evaluate the extended temporal relationship between neutrophil chemotactic factor expression and influx of neutrophils into the lung after intratracheal administration of either LPS or bleomycin. In both models, results demonstrated two phases of neutrophil chemotactic factor expression; first, an early phase characterized by high levels of CXCL1/keratinocyte-derived chemokine, CXCL2/monocyte-inhibitory protein-2, and CXCL5/LPS-induced chemokine expression, and second, a late phase distinguished by increases in extracellular ATP. Furthermore, we show that strategies aimed at either enhancing ATP catabolism (ip ecto-5′-nucleotidase administration) or inhibiting glycolytic ATP production (ip 2-deoxy-d-glucose treatment) reduce extracellular ATP accumulation, limit vascular leakage, and effectively block the late, but not the early, stages of neutrophil recruitment to the lung after LPS instillation. In conclusion, this study illustrates that neutrophil recruitment to the lung is mediated by the time-dependent expression of chemotactic factors and suggests that novel strategies, which reduce extracellular ATP accumulation, may attenuate late neutrophil recruitment and limit lung injury during ALI.
Keywords: extracellular ATP, acute lung injury, chemokines, neutrophil, acute respiratory distress syndrome
acute lung injury (ALI) is an inflammatory condition whose pathogenesis is linked to the recruitment of neutrophils (19). Neutrophils are the first line of defense against acute challenges; however, these cells are equipped with an arsenal of materials, which, when released, can have deleterious effects on lung function (22, 33). Understanding the mechanisms leading to neutrophil recruitment is important for developing strategies that facilitate their entry when needed but restrict further influx when infiltration is no longer necessary.
Neutrophils possess several unique properties that help to minimize their destructive potential. First, neutrophils typically enter organs only when specific chemotactic factors are expressed (3, 36), and second, neutrophils have extremely short half-lives (∼3 h in lung) (7, 13), which help to minimize their time within tissues. These properties imply that sustained recruitment requires ongoing production of chemotactic factors (2). In mice, the major chemotactic factors contributing to neutrophil recruitment to the lung are thought to be the chemokines keratinocyte-derived chemokine (KC), monocyte-inhibitory protein-2 (MIP-2), and CXCL5 (30, 38, 39). The importance of these chemokines in neutrophil trafficking has been documented extensively in murine studies. However, one important limitation is that most of these studies focused exclusively on early time points after injury, and it is well recognized that chemokine expression is short-lived (1, 4, 9).
Emerging evidence now indicates that neutrophil chemotaxis is also mediated by factors other than chemokines (37). In particular, extracellular ATP (eATP) has been shown to play an important role in neutrophil mobilization (5, 17). eATP is released from cells in response to stress and is believed to serve as a danger signal that recruits inflammatory cells to the site of injury (11, 28). Furthermore, extracellular ATP, when released from neutrophils, helps to autoregulate both speed and direction of mobilization (5, 27, 28).
In this study, we sought to evaluate the extended temporal relationship between chemotactic factor expression and neutrophil trafficking to the lung. Using LPS- and bleomycin-induced acute lung injury models, we found that expression of the chemokines KC, MIP-2, and CXCL5 was largely restricted to early time points after injury and that eATP peaked later, when neutrophils were maximally recruited to the lung. Furthermore, we showed that, by inhibiting ATP accumulation, we can effectively block the late, but not the early, stages of neutrophil recruitment to the lung after LPS administration. Taken together, our findings indicate that neutrophil recruitment to the lung is regulated by complex mechanisms that involve expression of early (e.g., KC, MIP-2) and late (eATP) chemotactic factors.
MATERIALS AND METHODS
Mice.
Male C57BL/6 mice at 6–8 wk old were purchased from the Jackson Laboratory (Bar Harbor, ME). All mice were housed in the pathogen-free animal facilities of Thomas Jefferson University, Philadelphia, PA. Before studies were performed, animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Thomas Jefferson University at Philadelphia, PA.
Materials.
Lipopolysaccharide from Escherichia coli 0111:B4 (LPS), ecto-5′-nucelotidase, 2-deoxy-d-glucose (2-DG), ATP, and formyl-methionyl-leucyl-phenylalanine (FMLP) were purchased from Sigma-Aldrich (St. Louis, MO), whereas bleomycin sulfate was acquired from Enzo Life Sciences (Farmingdale, NY). Anti-CD39 and anti-CD73 antibodies were purchased from Abcam (Cambridge, MA) and Cell Signaling Technologies (Boston, MA), respectively.
Murine model of ALI.
ALI was induced by administering a one-time intratracheal instillation of either LPS (100 μg) or bleomycin (0.075 U). Delivery of LPS and bleomycin was performed using the tongue-pull maneuver in isoflurane-anesthetized mice as described previously (15). In addition, some mice receiving LPS were treated twice daily by intraperitoneal injection with either 2-DG or ecto-5′-ecnucleotidase at select time points after injury. For 2-DG studies, treatment was initiated immediately after LPS administration, and injections were performed every day until experimental endpoint. For ecto-5′-ecnucleotidase studies, treatment was initiated 24 h after LPS administration and continued until experimental endpoint.
Analysis of BAL fluid.
Bronchoalveolar lavage (BAL) was performed by cannulating the trachea with a blunt 22-gauge needle and lavaging both lungs with 1 ml of sterile PBS solution. Total cell counts were measured in BAL fluid using a TC20 automated cell counter (Bio-Rad Laboratories, Hercules, CA). The BAL fluid was centrifuged, and the supernatant was stored for further analysis. Differential cell counts were determined on resuspended cells after cytocentrifugation onto glass slides and staining with HEMA 3 (Fisher Scientific, Tustin, CA). BAL protein concentration was measured by Pierce BCA assay kit (Thermo Scientific, Rockford, IL) as previously described (15).
Immunohistochemistry.
Immunostaining was performed on paraffin lung sections after antigen retrieval using Retrievagen A (Target Retrieval Solution; Dako, Carpenteria, CA) at 100°C for 20 min and quenching endogenous peroxidases with 3% H2O2. Sections were subsequently blocked with 2% BSA in PBS followed by staining with primary anti-Gr-1 Ab (R&D Systems, Minneapolis, MN) overnight at 4°C. After slides were washed, secondary antibody was applied, and then sections were exposed to Vectastain ABC (Vector Laboratories, Burlingame, CA) followed by 3,39-diaminobenzidine (Vector Laboratories).
ELISA.
KC, MIP-2, and CXCL5 were quantified using commercially available DuoSet ELISA development kits (R&D Systems) according to the manufacturer's instructions. Briefly, Nalgene Nunc Maxisorp plates were coated overnight with antibodies to KC (2 μg/ml), MIP-2 (2 μg/ml), or CXCL5 (4 μg/ml). The following morning, plates were washed, and blocking was performed with 2% BSA for 1 h. Several dilutions of samples were added to the wells. Samples were incubated for 2 h, followed by washing and incubation with biotinylated secondary antibody (1:200 dilution in diluent buffer) for 1 h. This step was followed by addition of streptavidin-horseradish peroxidase conjugate for 20 min and incubation with substrate solution (1:1 volume of substrate A and B). The reaction was subsequently halted with stop solution, and plates were read at 450 nm (Biotek Instrument, Winooski, VT).
MPO assay.
Myeloperoxidase (MPO) assay (BioVision, Mountain View, CA) was performed according to manufacturer's protocol. Briefly, 50 μg of lung was homogenized in 200 μl of MPO assay buffer and centrifuged at 4°C for 5 min at 14,000 revolution/min. MPO activity was determined in 10 μl of sample by measuring activity over a 30- to 120-min period. All samples were measured at 450 nm using a colorimetric microplate reader.
Neutrophil isolation.
Mature neutrophils were isolated from the femur of mice as previously described (35). The marrow was flushed with Hank's buffered saline, and the suspension was layered on top of a Percoll gradient (72, 64, and 52%) and centrifuged at 750 g for 25 min. Neutrophils were recovered by aspiration, and Wright-Giemsa-staining of cytospun samples demonstrated that >95% of cells were morphologically consistent with neutrophils. Typical yields were ∼6–8 × 106 neutrophils/mouse, of which >96% were viable.
Measurement of eATP.
eATP was measured in BAL fluid using a commercially available colorimetric kit (BioVision) according to the manufacturer's instructions. All samples were placed immediately on ice after collection; ice-cold BAL fluid samples were centrifuged at 4°C, and eATP measurements were performed on the same day.
ATP chemotaxis assay.
Neutrophil chemotaxis assays were performed using a 96-well microchemotaxis plate (NeuroProbe, Gaithersburg, MD) in which the chambers were separated by 5 μM pore-size polyvinylpyrrolidone-free polycarbonate membranes (10). Briefly, chemoattractants (FMLP 0.5, 1, 10, and 100 nM; and ATP 0.5, 1, 5, and 10 μM) diluted in RPMI 1640 medium containing 0.01% BSA were placed in the bottom chamber, while 45 μl of neutrophil suspension (106 cells/ml) was pipetted onto the upper membrane. RPMI 1640 alone was used as negative control. Chambers were placed into the incubator for 45 min at 37°C and 5% CO2. Subsequently, filters were removed carefully, fixed with 70% ethanol, and stained with hematoxylin. The number of neutrophils that migrated to the lower side of the filter was counted in three random fields using a ×40 objective. Experiments were performed in triplicate for each variable and the means determined. The results are expressed as the number of neutrophils/field and are representative of three different experiments. In vivo chemotactic assays were performed by instilling 100 μl of 50 or 100 mM of ATP intratracheally and measuring BAL and lung neutrophil cell counts at select time points after instillation.
Real-time quantitative RT-PCR.
Gene transcript levels were quantified by real-time PCR. Total RNA was isolated using RNeasy Mini-Kit (Qiagen, Valencia, CA), according to the manufacturer's instructions, and quantified with the Nano-Drop spectrophotometer. One microgram of RNA was used to make cDNA using the GoScript Reverse Transcription System (Promega, Madison, WI). The primer sets for the PCR reaction contained 1 μM forward primer and 1 μM reverse primer with SYBR Green I GoTaq qPCR Master Mix (Promega). The primers sequences (forward primer, reverse primer) used were: KC primers forward, GCGAATTCACCATGATCCCAGCCACCCG: reverse, GCTCTAGATTACTTGGGGACACCTTTTAG; MIP-2 primers forward, CAGAATTCACTTCAGCCTAGCGCCAT: reverse, GCTCTAGAGTCAGTTAGCCTTGCCTTTG; CXCL5 primers forward CTCAGTCATAGCCGCAACCGAGC: reverse, CGCTTCTTTCCACTGCGAGTGC; CD39 primers forward, TACCACCCCATCTGGTCATT: reverse, GGACGTTTTGTT TGGTTGGT; CD73 primers forward, CAAATCCCACACAACCACTG: reverse, TGCTCACTTGGTCACAGGAC, respectively. The primer set was amplified using cycles of 94°C for 1 min, 58°C for 0.5 min, 72°C for 1 min. Murine hypoxanthine phosphoribosyltransferase (HPRT)-1 forward primer, GTTGGATACAGGCCAGACTTTGT and reverse primer, CACAGGACTAGAACACCTGC were used to control for the starting template. Q-PCR analyses were performed by using the iCycler thermocycler (Bio-Rad Laboratories), and values were determined using iCycler iQ Real-Time Detection System software (version 3.0a; Bio-Rad) and normalized to HPRT-1.
Western blot analysis.
Lung tissues were homogenized in ice-cold lysis buffer (PBS, 0.5% Triton X-100, pH 7.4) containing protease inhibitor (Roche Complete mini) and phosphatase inhibitor. After centrifugation (14,000 g, 30 min, 4°C), the supernatant was collected, and protein concentration was determined by Pierce BCA assay kit (Thermo Scientific). Protein samples (10 μg) were solubilized in 4× Laemmli sample buffer, heated at 65°C for 10 min, centrifuged at 3,000 g for 1 min, loaded on a 10% Tris·HCl-SDS-polyacrylamide gel, and run for 1 h at 120 V. Protein was transferred to a nitrocellulose membrane (Bio-Rad) and then blocked with Odyssey Blocking Buffer (Li-Cor Biosciences, Lincoln, NE) for 1 h at room temperature. After being blocked, the membrane was incubated overnight at 4°C with a specific polyclonal rabbit primary antibody to CD39 (Abcam) and CD73 (Cell Signaling, Danvers, MA) at a dilution of 1:1,000 in blocking buffer with 0.1% Tween-20 followed by incubation with donkey anti-rabbit secondary antibody (Li-Cor Biosciences) at a dilution of 1:1,000 in blocking buffer. After three washings with PBS, all immunoblots were visualized using the Odyssey infrared imaging system (Li-Cor Biosciences).
Statistical analysis.
Statistics were performed using GraphPad Prism 5.0 software. Comparisons between groups were performed using a one-way ANOVA, followed by Tukey's post hoc analysis. Statistical significance was achieved when P < 0.05 at 95% confidence interval.
RESULTS
Neutrophil infiltration into lung persists after declines in neutrophil chemokine expression.
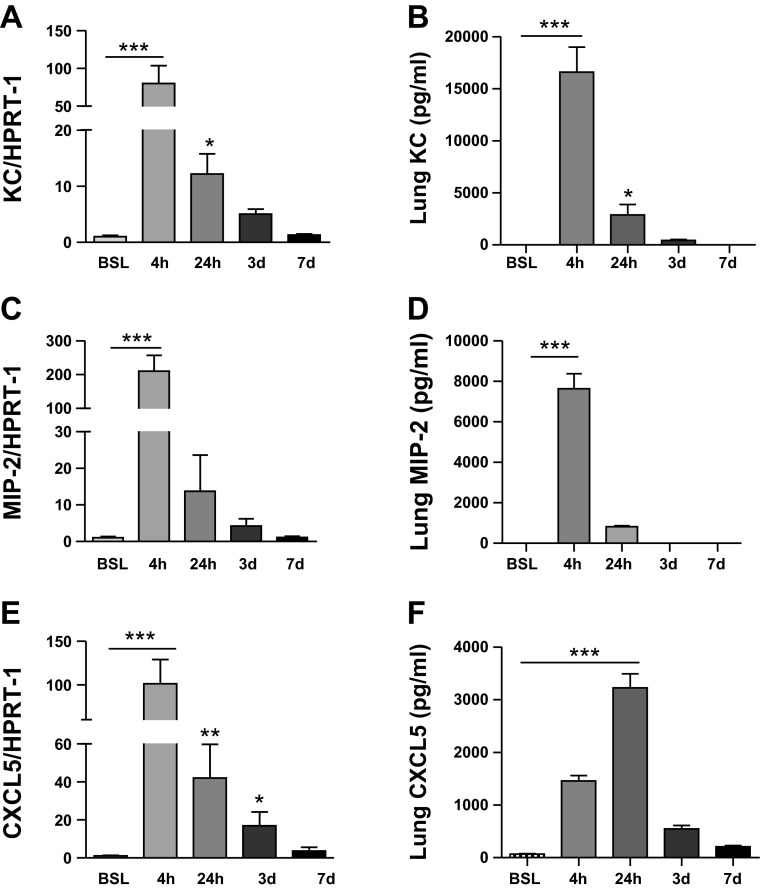
To better understand chemokine kinetics, we evaluated transcript and protein levels for KC, MIP-2, and CXCL5 at various time points after injury. Transcript levels were rapidly induced after LPS administration, peaking within 4 h of LPS delivery to the lung (Fig. 1). Moreover, chemokine protein concentrations declined within 24 h of LPS administration, and levels were undetectable or barely detectable in BAL fluid and lung at 3 days (Fig. 1). Together, these findings illustrate the transient nature of neutrophil chemokine expression in the lung after LPS injury.
Fig. 1.
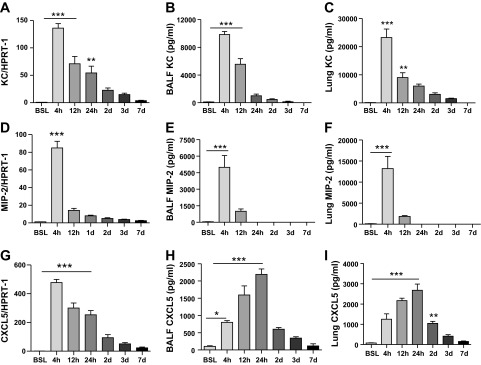
Time course of chemokine expression in lung after intratracheal LPS. Transcript and protein expression for keratinocyte-derived chemokine (KC) (A–C), monocyte-inhibitory protein-2 (MIP-2) (D–F), and CXCL5 (G–I) at baseline (BSL), 4, and 24 h, and 3 and 7 days after intratracheal LPS instillation. Transcript and protein levels peaked at 4 h for KC and MIP and at 24 h for CXCL5. Protein expression for all 3 chemokines was either undetectable or minimally detectable 3 days after intratracheal LPS administration. HPRT, hypoxanthine phosphoribosyltransferase; BALF, bronchoalveolar lavage fluid Data are expressed as means ± SE of n = 6 animals in each group, *P < 0.05, **P < 0.01, and ***P < 0.001.
To evaluate whether neutrophil recruitment was dependent on expression of these chemokines, neutrophil influx was examined at select time points after injury. As shown in Fig. 2, neutrophils increased markedly in BAL fluid within 4 h of LPS administration, and this correlated with the peak in KC and MIP-2 concentration (Fig. 1) in the lung. However, neutrophils further accumulated even after chemokine levels for KC, MIP-2, and CXCL5 had significantly declined in the lung. In fact, neutrophils did not peak until day 3 after LPS, a time point when all three chemokines were only marginally increased in the lung. BAL fluid protein concentration, a surrogate for lung injury, also peaked on day 3, providing additional evidence linking neutrophil infiltration to the development of ALI (Fig. 2).
Fig. 2.
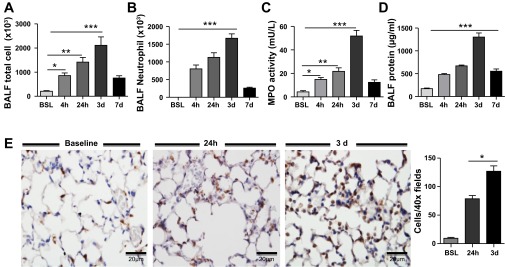
Time-dependent recruitment of neutrophils into the lung after intratracheal LPS. A and B: total immune cells and neutrophils in BAL fluid of control and LPS-injured lung. C: myeloperoxidase (MPO) activity in lung. D: total protein concentration in BAL fluid. E: neutrophil infiltration in the lung quantified by immunohistochemical staining for the granulocyte marker Gr-1. Gr-1+ cells (brown stain) peaked at 3 days after intratracheal LPS administration and declined thereafter. Morphometric analysis was performed on BSL, 24 h, and 3 days. Data are expressed as means ± SE of n = 6 animals in each group, *P < 0.05, **P < 0.01, and ***P < 0.001.
Levels of eATP peak in BAL fluid on day 3 after intratracheal LPS instillation.
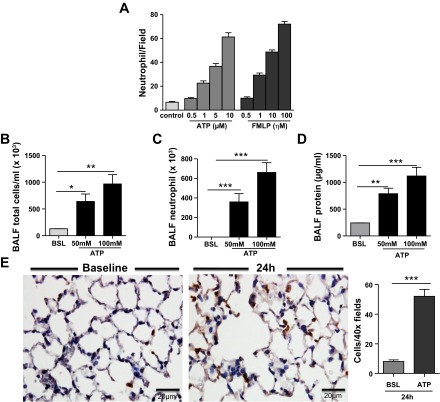
eATP is now identified as an important neutrophil chemotactic factor. To confirm the ability of eATP to promote neutrophil migration, we performed in vitro chemotaxis studies across a semipermeable membrane. As previously reported, eATP was found to be a potent inducer of neutrophil chemotaxis, as we detected a dose-dependent increase in neutrophil migration in our in vitro assay. Moreover, these findings were corroborated by showing a dose-dependent increase in neutrophil chemotaxis to the lung after intratracheal ATP administration (Fig. 3).
Fig. 3.
ATP stimulates neutrophil chemotaxis in vitro and in vivo. A: ATP showed neutrophil chemotaxis in a concentration-gradient manner. Chemoattractant formyl-methionyl-leucyl-phenylalanine (FMLP) has been used as a positive control. The results are expressed as the number of neutrophils/field and are representative of 3 different experiments. B: 24 h after intratracheal ATP instillation, total cell count (B), neutrophil cell count (C), and protein concentration (D) were significantly increased in BAL fluid. E: immunohistochemistry staining for the granulocyte marker Gr-1 (brown staining) further confirmed the increase in neutrophil recruitment into lung after intratracheal ATP administration. Data are expressed as means ± SE of n = 6 animals in each group, *P < 0.05, **P < 0.01, and ***P < 0.001 vs. control mice within group.
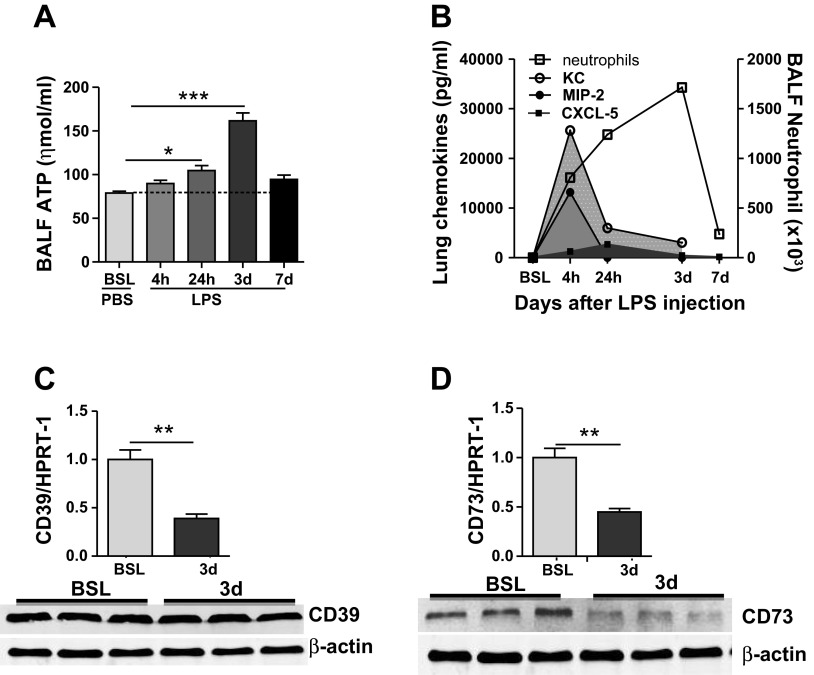
Because we found discordance between chemokine expression and late neutrophil recruitment to the lung, we sought to evaluate the temporal relationship between eATP and neutrophils in the lung after LPS administration. Results demonstrated that eATP was only marginally increased in the lung at early time points after LPS instillation (Fig. 4A), whereas levels of eATP increased significantly by day 3 when neutrophils were maximally recruited into the lung (Fig. 4B), and chemokine levels were significantly reduced. Increases in eATP on day 3 were associated with decreased transcript levels for ecto-apyrase (CD39) and decreased transcript and protein levels for ecto-5′-nucelotidase (CD73) (Fig. 4, C and D). These latter findings suggest that impaired ATP catabolism may contribute to its accumulation after LPS injury on day 3.
Fig. 4.
Extracellular ATP peaks in BAL fluid after LPS instillation. A: extracellular ATP increases within 4 h after intratracheal LPS administration, but levels did not peak until day 3. C and D: gene expression and protein expression for the ectonucleotidases; ecto-apyrase (CD39) and ecto-5′-nucleotidase (CD73). Transcript levels for ecto-apyrase were decreased on day 3, and transcript and protein levels of ecto-5′-nucleotidase were both diminished on day 3 after LPS instillation. Transcript and protein expression for these enzymes were not significantly different from control at earlier time points after LPS. B: summary figure showing the kinetics of chemotactic factor expression after intratracheal LPS administration. Data are expressed as means ± SE of n = 6 animals in each group, *P < 0.05, **P < 0.01, and ***P < 0.001 vs. control mice within group.
Neutrophil chemokine production and extracellular ATP accumulation follows a similar biphasic pattern after intratracheal bleomycin instillation.
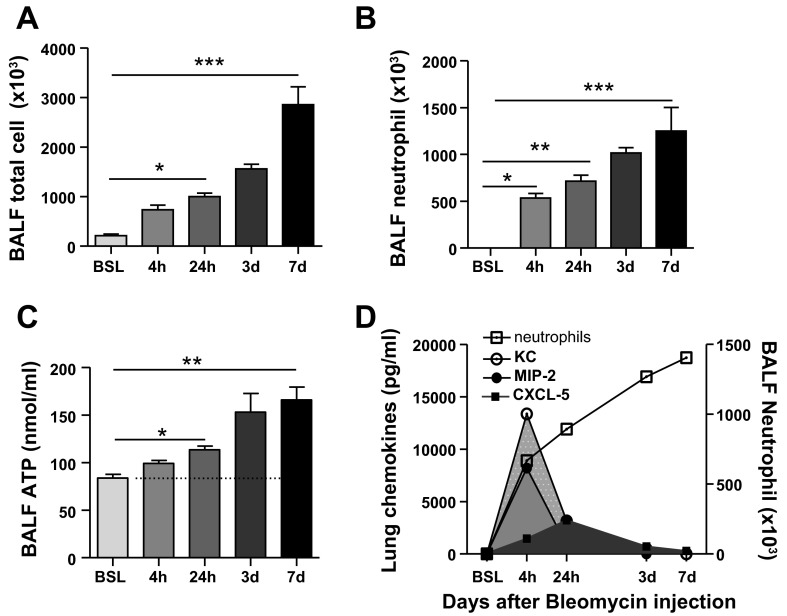
To evaluate whether findings after LPS were generalizable to other lung injury models, we examined the temporal relationship of neutrophil chemotactic factor expression to neutrophil influx in the lung after a one-time intratracheal instillation of bleomycin. Similar to findings observed with LPS, we identified a biphasic pattern of chemotactic factor expression after bleomycin injury. As shown in Fig. 5, transcript and protein levels for the chemokines KC, MIP-2, and CXCL5 peaked early after injury and declined within 24 h of bleomycin instillation. Importantly, this decline in chemokine concentrations occurred while neutrophils were continuing to accumulate in the lung (Fig. 6). Moreover, chemokine levels were undetectable at day 7 after bleomycin injury when neutrophils were maximally recruited to the lung and eATP concentration was markedly increased. Collectively, these findings strongly support the concept that neutrophil influx to the lung after injury is regulated by different early (e.g., chemokines) and late (e.g., eATP) chemotactic factors.
Fig. 5.
Time course of chemokine expression in lung after intratracheal bleomycin. Transcript and protein expression for KC (A and B), MIP-2 (C and D), and CXCL5 (E and F) at 0 h, 4 h, 24 h, and 3 and 7 days after intratracheal bleomycin instillation. Transcript and protein levels peaked at 4 h for KC and MIP and at 24 h for CXCL5. Protein expression for all 3 chemokines was minimally detectable 3 days after intratracheal bleomycin administration. Data are expressed as means ± SE of n = 6 animals in each group, *P < 0.05, **P < 0.01, and ***P < 0.001.
Fig. 6.
Time-dependent recruitment of neutrophils to the lung after intratracheal bleomycin instillation. A and B: total immune cells and neutrophils in BAL fluid of control and bleomycin-injured lung. C: extracellular ATP increases within 4 h after intratracheal bleomycin administration, and levels remained elevated at day 7 after injury. D: graph depicting temporal relationship between neutrophil chemokine expression and neutrophil influx into BALF. Data are expressed as means ± SE of n = 6 animals in each group, *P < 0.05, **P < 0.01, and ***P < 0.001.
Treatment with exogenous ecto-5′-nucleotidase (CD73) decreases the late phase of neutrophil recruitment into lung after LPS.
Because levels of ATP ecto-nucleotidases were decreased in the lung after LPS injury, we tested whether enzyme replacement therapy could reduce neutrophil recruitment to the lung at late time points. The soluble ecto-5′-nucelotidase was administered by intraperitoneal injection twice daily beginning at 24 h after LPS injection. The decision to initiate therapy at this time point was based on the observation that levels of these enzymes were unchanged at 24 h (data not shown). As expected, results demonstrated that treatment with ecto-5′-nucleotidase effectively decreased eATP accumulation in BAL fluid at day 3 (Fig. 7A). This was associated with reductions in neutrophil influx (Fig. 7C), MPO activity (Fig. 7D), and vascular leakage (Fig. 7E). Together, these findings indicate that enhancing ATP catabolism can attenuate injury and block late neutrophil recruitment to the lung during ALI.
Fig. 7.
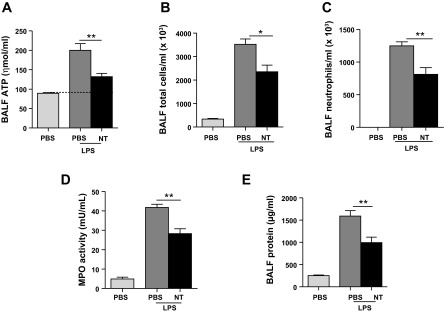
Treatment with ecto-5′-nucleotidase (CD73) attenuated neutrophil recruitment and vascular leak on day 3 after intratracheal LPS. Mice were treated with intraperitoneal injections of soluble ecto-5′-nucleotidase (10 U) twice daily beginning at 24 h and continuing until day 3 after intratracheal LPS. Ecto-5′-nucleotidase (NT) treatment was found to significantly decrease extracellular ATP concentration in BAL fluid (A), total immune cell and neutrophil influx in BAL fluid (B and C), MPO activity in lung (D), and BAL fluid protein concentration in BAL fluid (E) after intratracheal LPS. Data are expressed as means ± SE of n = 6 animals in each group, *P < 0.05 and **P < 0.01 when comparing LPS-NT to mice treated with LPS alone.
Blocking ATP production with 2-DG attenuates the late phase of neutrophil infiltration to the lung after LPS.
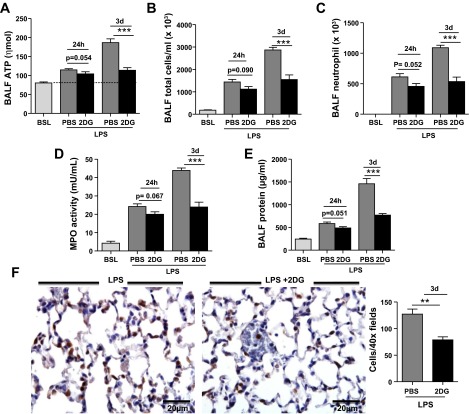
Finally, to support the concept that eATP plays a pathogenic role in late stages of ALI, we administered 2-DG, a therapeutic inhibitor of ATP synthesis, to mice beginning immediately after LPS instillation. 2-DG did not significantly alter eATP, MPO activity, neutrophil influx, or BAL fluid protein concentration at 24 h (Fig. 8). However, significant decreases in each of these parameters were observed on day 3 after LPS administration (Fig. 8). Importantly, treatment with 2-DG did not significantly alter levels of KC, MIP-2, and CXCL5 in the lung after injury, indicating that its protective effects were independent of blocking the first wave of neutrophil chemotactic factor expression in the lung (data not shown). Together, these findings validate that eATP plays a pathogenic role in ALI and strongly suggest that therapeutic approaches that limit eATP accumulation will decrease neutrophil emigration and may attenuate the late manifestations of this condition.
Fig. 8.
2-Deoxy-d-glucose (2-DG) effectively blocks the late stages of neutrophil recruitment to the lung after intratracheal LPS. Treatment with 2-DG did not significantly reduce total cell count, neutrophil cell count, or BAL fluid protein concentration in the lung at 24 h after LPS (A). However, treatment with 2-DG effectively reduced total and neutrophil cell count in BAL fluid (B and C), MPO activity in lung homogenate (D), BAL fluid protein concentration (E), and neutrophil cell count in tissue sections (F) by day 3 after intratracheal LPS. Data are expressed as means ± SE of n = 6 animals in each group, **P < 0.01 and ***P < 0.001 when comparing LPS-2DG to mice treated with LPS alone.
DISCUSSION
To date, the study of neutrophil trafficking to the murine lung has largely focused on the role of chemokines KC, MIP-2, and CXCL5 (2, 14, 23, 31). Their importance in neutrophil mobilization is well documented, but the predominant focus on early time points after injury has provided little insight into the mechanisms involved in sustained mobilization of cells to the lung (8, 10).
In this study, we examined the temporal relationship between neutrophil influx and chemotactic factor expression. Our findings confirmed previous reports demonstrating the transient nature of chemokine expression in the lung (12, 16, 31). For example, we found that chemokines KC and MIP-2 were rapidly induced, peaking within 4 h of LPS and bleomycin administration. This rapid, but coordinated, response likely illustrates functional redundancy in the system and probably is important for ensuring early mobilization to the site of injury (26, 32). Interestingly, CXCL5 expression was also rapidly induced but peaked at slightly later time points than KC and MIP-2. We speculate that delays in CXCL5 expression are necessary for providing a second wave of chemotactic signals that further sustains neutrophil mobilization to the lung (20).
Because of the transient nature of chemokine expression, we also focused our attention on eATP. The role for eATP in neutrophil mobilization has been illustrated in virtually all tissues including the lung, but its relationship and relative importance to chemokines have not been closely investigated (6, 18). In this study, we found that eATP only modestly increases at early time points, whereas eATP levels peaked in BAL fluid at 3 days after LPS and at 7 days after bleomycin. Interestingly, this peak corresponds to the point when neutrophils are maximally recruited to the lung in these models. Furthermore, we showed that, by either enhancing ATP catabolism or by blocking ATP production, we could block neutrophil mobilization and limit vascular leakage at late time points after LPS administration. Together, our findings illustrate the importance of eATP and help to explain the mechanisms mediating sustained mobilization of neutrophils to the lung.
One other finding from our study that requires further discussion is the observation that 2-DG did not significantly inhibit neutrophil influx to the lung at 24 h. This is in contrast to previous reports demonstrating that accumulation of neutrophils was enhanced by LPS at this time point in CD39−/− or CD73−/− mice (27). Importantly, we do not believe our findings contradict those from these other studies. In fact, we found that eATP levels were increased, albeit minimally, in the lung as early as 4 h after LPS administration. Moreover, our treatment with 2-DG was partially effective at reducing eATP levels in BAL fluid at 24 h after LPS, suggesting that higher doses may have demonstrated a more meaningful response. However, the marginal increases in eATP as well as the high levels of neutrophil chemokine expression at 4 and 24 h, at the very least, indicate that eATP is not the dominant chemotactic agent mediating early neutrophil influx to the lung.
Notably, the cellular source for eATP was not determined in our study. However, it is likely that multiple cell types contributed to its accumulation. Previous studies have demonstrated that both structural (type I pneumocytes, type II pneumocytes) and nonstructural (alveolar macrophages) cells release eATP when injured (5, 21, 28, 34). However, we anticipate that identifying the specific source of eATP will ultimately be important for developing targeted strategies. Although we postulate that increases in either production or release contribute to eATP accumulation, the observation that levels of ecto-nucleotidases were decreased in the lung on day 3 after LPS administration strongly suggests that impaired catabolism may also contribute to its accumulation (11, 18, 29).
To date, there are no effective pharmacological strategies for treating patients with established lung injury. However, we showed that intraperitoneal administration of ecto-5′-nucleotidase beginning 24 h after induction of injury was effective in decreasing neutrophil influx and attenuating vascular leakage after LPS. These findings suggest a possible role for ecto-5′-nucleotidase in treating patients with already established lung injury. Importantly, it is also possible that treatment with ecto-5′-nucleotidase may exacerbate lung injury, as the metabolism of eATP to adenosine has been shown to augment the speed of neutrophil chemotaxis into tissue. Based on this understanding, we postulate whether the mode of ecto-5′-nucleotidase delivery might be important in treating patients with ALI, as one could imagine that intratracheal rather than systemic delivery of this enzyme could enhance neutrophil migration to the alveolar space. This hypothesis will need to be tested in future preclinical studies.
Finally, we also believe that the ability of 2-DG to significantly attenuate neutrophil influx and to minimize vascular leakage at late time points after injury has important therapeutic implications. Currently, there are no effective treatments for patients with established ALI except protective lung ventilation (24, 25). Our findings suggest that inhibiting the actions of eATP may be effective in reducing injury induced by prolonged inflammatory states. We recognize that 2-DG treatment only partially reduced the late effects of LPS administration; however, we speculate that this may relate to the dose, timing, and the penetration of 2-DG in the lung, and future studies addressing these issues will be important (40).
In conclusion, this study provides new insight into the complex mechanisms mediating neutrophil recruitment to the murine lung after LPS. Moreover, our findings suggest that therapeutic approaches to modulating neutrophil influx to the lung will require complex interventions that target both early and late chemotactic factor expression.
GRANTS
This work was supported by the National Institutes of Health (NIH) Grant R01HL105490.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.S., F.R., and R.S.S. conception and design of research; D.S., W.S., and M.D. performed experiments; D.S. and R.S.S. analyzed data; D.S. and R.S.S. interpreted results of experiments; D.S. prepared figures; D.S. drafted manuscript; D.S., F.R., W.S., M.D., and R.S.S. approved final version of manuscript; F.R. and R.S.S. edited and revised manuscript.
REFERENCES
- 1.Balamayooran G, Batra S, Cai S, Mei J, Worthen GS, Penn AL, Jeyaseelan S. Role of CXCL5 in leukocyte recruitment to the lungs during secondhand smoke exposure. Am J Respir Cell Mol Biol 47: 104–111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia M, Zemans RL, Jeyaseelan S. Role of chemokines in the pathogenesis of acute lung injury. Am J Respir Cell Mol Biol 46: 566–572, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF. Neutrophils in development of multiple organ failure in sepsis. Lancet 368: 157–169, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Chen SC, Mehrad B, Deng JC, Vassileva G, Manfra DJ, Cook DN, Wiekowski MT, Zlotnik A, Standiford TJ, Lira SA. Impaired pulmonary host defense in mice lacking expression of the CXC chemokine lungkine. J Immunol 166: 3362–3368, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314: 1792–1795, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Cicko S, Lucattelli M, Muller T, Lommatzsch M, De Cunto G, Cardini S, Sundas W, Grimm M, Zeiser R, Durk T, Zissel G, Boeynaems JM, Sorichter S, Ferrari D, Di Virgilio F, Virchow JC, Lungarella G, Idzko M. Purinergic receptor inhibition prevents the development of smoke-induced lung injury and emphysema. J Immunol 185: 688–697, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Cohen AB, Rossi M, Geczy D, Knight L. Neutrophil turnover in normal rabbit lungs. J Clin Invest 69: 794–798, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhaliwal K, Scholefield E, Ferenbach D, Gibbons M, Duffin R, Dorward DA, Morris AC, Humphries D, MacKinnon A, Wilkinson TS, Wallace WA, van Rooijen N, Mack M, Rossi AG, Davidson DJ, Hirani N, Hughes J, Haslett C, Simpson AJ. Monocytes control second-phase neutrophil emigration in established lipopolysaccharide-induced murine lung injury. Am J Respir Crit Care Med 186: 514–524, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frevert CW, Huang S, Danaee H, Paulauskis JD, Kobzik L. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J Immunol 154: 335–344, 1995 [PubMed] [Google Scholar]
- 10.Frevert CW, Wong VA, Goodman RB, Goodwin R, Martin TR. Rapid fluorescence-based measurement of neutrophil migration in vitro. J Immunol Methods 213: 41–52, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, Virchow JC, Jr, Lambrecht BN. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med 13: 913–919, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Jeyaseelan S, Chu HW, Young SK, Worthen GS. Transcriptional profiling of lipopolysaccharide-induced acute lung injury. Infect Immun 72: 7247–7256, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13: 159–175, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Kolosova IA, Mirzapoiazova T, Moreno-Vinasco L, Sammani S, Garcia JG, Verin AD. Protective effect of purinergic agonist ATPγS against acute lung injury. Am J Physiol Lung Cell Mol Physiol 294: L319–L324, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Konter JM, Parker JL, Baez E, Li SZ, Ranscht B, Denzel M, Little FF, Nakamura K, Ouchi N, Fine A, Walsh K, Summer RS. Adiponectin attenuates lipopolysaccharide-induced acute lung injury through suppression of endothelial cell activation. J Immunol 188: 854–863, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin M, Carlson E, Diaconu E, Pearlman E. CXCL1/KC and CXCL5/LIX are selectively produced by corneal fibroblasts and mediate neutrophil infiltration to the corneal stroma in LPS keratitis. J Leukoc Biol 81: 786–792, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linden J. Cell biology. Purinergic chemotaxis. Science 314: 1689–1690, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Lommatzsch M, Cicko S, Muller T, Lucattelli M, Bratke K, Stoll P, Grimm M, Durk T, Zissel G, Ferrari D, Di Virgilio F, Sorichter S, Lungarella G, Virchow JC, Idzko M. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 181: 928–934, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Martin TR. Neutrophils and lung injury: getting it right. J Clin Invest 110: 1603–1605, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mei J, Liu Y, Dai N, Favara M, Greene T, Jeyaseelan S, Poncz M, Lee JS, Worthen GS. CXCL5 regulates chemokine scavenging and pulmonary host defense to bacterial infection. Immunity 33: 106–117, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra A. New insights of P2X7 receptor signaling pathway in alveolar functions. J Biomed Sci 20: 26, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moraes TJ, Zurawska JH, Downey GP. Neutrophil granule contents in the pathogenesis of lung injury. Curr Opin Hematol 13: 21–27, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Mortaz E, Braber S, Nazary M, Givi ME, Nijkamp FP, Folkerts G. ATP in the pathogenesis of lung emphysema. Eur J Pharmacol 619: 92–96, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev 2: CD003844, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 282: 54–61, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Reutershan J, Basit A, Galkina EV, Ley K. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 289: L807–L815, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Reutershan J, Vollmer I, Stark S, Wagner R, Ngamsri KC, Eltzschig HK. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J 23: 473–482, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, Kanellopoulos J, Quesniaux VF, Marchand-Adam S, Crestani B, Ryffel B, Couillin I. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med 182: 774–783, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Robson SC, Kaczmarek E, Siegel JB, Candinas D, Koziak K, Millan M, Hancock WW, Bach FH. Loss of ATP diphosphohydrolase activity with endothelial cell activation. J Exp Med 185: 153–163, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi DL, Hurst SD, Xu Y, Wang W, Menon S, Coffman RL, Zlotnik A. Lungkine, a novel CXC chemokine, specifically expressed by lung bronchoepithelial cells. J Immunol 162: 5490–5497, 1999 [PubMed] [Google Scholar]
- 31.Rovai LE, Herschman HR, Smith JB. The murine neutrophil-chemoattractant chemokines LIX, KC, and MIP-2 have distinct induction kinetics, tissue distributions, and tissue-specific sensitivities to glucocorticoid regulation in endotoxemia. J Leukoc Biol 64: 494–502, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol 32: 452–460, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh NR, Johnson A, Peters AM, Babar J, Chilvers ER, Summers C. Acute lung injury results from failure of neutrophil de-priming: a new hypothesis. Eur J Clin Invest 42: 1342–1349, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Sperlagh B, Hasko G, Nemeth Z, Vizi ES. ATP released by LPS increases nitric oxide production in raw 264.7 macrophage cell line via P2Z/P2X7 receptors. Neurochem Int 33: 209–215, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Suratt BT, Young SK, Lieber J, Nick JA, Henson PM, Worthen GS. Neutrophil maturation and activation determine anatomic site of clearance from circulation. Am J Physiol Lung Cell Mol Physiol 281: L913–L921, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Thomas CJ, Schroder K. Pattern recognition receptor function in neutrophils. Trends Immunol 34: 317–328, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Vitiello L, Gorini S, Rosano G, la Sala A. Immunoregulation through extracellular nucleotides. Blood 120: 511–518, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci USA 86: 612–616, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wuyts A, Haelens A, Proost P, Lenaerts JP, Conings R, Opdenakker G, Van Damme J. Identification of mouse granulocyte chemotactic protein-2 from fibroblasts and epithelial cells. Functional comparison with natural KC and macrophage inflammatory protein-2. J Immunol 157: 1736–1743, 1996 [PubMed] [Google Scholar]
- 40.Yamaguchi R, Janssen E, Perkins G, Ellisman M, Kitada S, Reed JC. Efficient elimination of cancer cells by deoxyglucose-ABT-263/737 combination therapy. PLoS One 6: e24102, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]