Abstract
The lung is an important reservoir of human immunodeficiency virus (HIV). Individuals infected with HIV are more prone to pulmonary infections and chronic lung disorders. We hypothesized that comprehensively profiling the proteomic landscape of bronchoalveolar lavage fluid (BALF) in patients with HIV would provide insights into how this virus alters the lung milieu and contributes to pathogenesis of HIV-related lung diseases. BALF was obtained from five HIV-negative (HIV−) and six asymptomatic HIV-positive (HIV+) subjects not on antiretroviral therapy. Each sample underwent shotgun proteomic analysis based on HPLC-tandem mass spectrometry. Differentially expressed proteins between the groups were identified using statistical methods based on spectral counting. Mechanisms of disease were explored using functional annotation to identify overlapping and distinct pathways enriched between the BALF proteome of HIV+ and HIV− subjects. We identified a total of 318 unique proteins in BALF of HIV− and HIV+ subjects. Of these, 87 were differentially up- or downregulated between the two groups. Many of these differentially expressed proteins are known to interact with key HIV proteins. Functional analysis of differentially regulated proteins implicated downregulation of immune responses in lungs of HIV+ patients. Combining shotgun proteomic analysis with computational methods demonstrated that the BALF proteome is significantly altered during HIV infection. We found that immunity-related pathways are underrepresented in HIV+ patients. These findings implicate mechanisms whereby HIV invokes local immunosuppression in the lung and increases the susceptibility of HIV+ patients to develop a wide range of infectious and noninfectious pulmonary diseases.
Keywords: shotgun proteomics, pulmonary, immunity, mass spectrometry
the lung is an important reservoir of HIV and a site for HIV replication (44). HIV is uniformly detected in alveolar macrophages of infected pediatric patients and in the majority of adult patients (30). Opportunistic lung infections were the leading causes of morbidity and mortality among HIV-positive (HIV+) patients in the precombination antiretroviral therapy (ART) era (26, 27, 33, 42, 43). Since the advent of ART, infectious complications have declined, but other pulmonary disorders such as emphysema, pulmonary hypertension, and lung cancer are becoming increasingly prevalent, with chronic obstructive pulmonary disease (COPD) assuming the leading chronic lung disease in this population (6, 15, 25). Indeed, the incidence of infectious and noninfectious pulmonary disorders is significantly increased in patients with HIV compared with uninfected individuals (5). There is strong experimental evidence that HIV itself evokes a local immune response that facilitates lymphocytic migration and infiltration in the lung. HIV infection appears to confer an independent risk for COPD, even without prior acquired immunodeficiency syndrome-related pulmonary complications (7, 8). Why HIV+ patients are more susceptible to chronic pulmonary diseases is not well understood, but the cause may stem from interactions between HIV and the lung's unique environment that is enriched in specialized immune cells such as alveolar macrophages.
We hypothesized that comprehensively profiling the proteomic landscape of bronchoalveolar lavage fluid (BALF) in asymptomatic HIV+ patients would identify protein signatures that are distinct from uninfected subjects. To this end, we integrated HPLC-tandem mass spectrometry-based proteomics with statistical and computational methods to identify differentially expressed proteins between HIV+ and HIV-negative (HIV−) subjects. We focused on these differentially regulated proteins to assess how HIV alters the functional characteristics of the airspace milieu and thereby promotes susceptibility to lung disease.
METHODS
Subject recruitment and BALF collection.
HIV+ subjects were recruited from acquired immune deficiency syndrome specialty clinics through the University of Washington by screening at routine visits and were asymptomatic, did not have an active pulmonary infection, and were not on ART at screening visit or time of bronchoscopy. Subject characteristics are shown in Table 1. The protocol for collecting human BALF was approved by the Institutional Review board at the University of Washington. Written informed consent was obtained from all subjects. Bronchoalveolar lavage was performed on five HIV− and six HIV+ adults as previously described (14, 22, 38, 40). Individuals with acute respiratory symptoms, including cough, shortness of breath, increased sputum production or pleuritic chest pain, known lung diseases, chronic or active cardiac disease, inhaled illicit drug use, or recent cigarette smoking (within 90 days), were excluded. Briefly, five separate 30-ml aliquots of 0.89% sterile saline were instilled in the right middle lobe or lingula. Immediately on collection the lavage fluid was centrifuged at 200 g for 15 min to pellet the cells, and cell-free supernatants were separated into aliquots and stored at −80°C. Total protein measurements were determined on aliquots of supernatants using the bicinchoninic acid protein assay.
Table 1.
Characteristics of study subjects at time of bronchoscopy
| HIV Status | Age, yr | Gender | Race | Smoker* | Duration of Infection, yr | CD4, cells/μl | HIV-1 RNA, copies/ml |
|---|---|---|---|---|---|---|---|
| Pos | 34 | M | Caucasian | N | 5 | 445 | 14,642 |
| Pos | 34 | M | Caucasian | N | 4 | 405 | <50 |
| Pos | 36 | M | Caucasian | Y | 10 | 426 | 76,691 |
| Pos | 43 | M | African American | Y | 20 | 253 | 40,304 |
| Pos | 37 | M | Asian American | N | 1.5 | 171 | 8,001 |
| Pos | 46 | M | African American | Y | 11 | 503 | 299 |
| Neg | 20 | M | Caucasian | N | NA | ||
| Neg | 30 | M | Caucasian | N | NA | ||
| Neg | 32 | F | Caucasian | N | NA | ||
| Neg | 23 | F | African American | N | NA | ||
| Neg | 45 | M | Caucasian | N | NA |
Pos, positive; Neg, negative; M, male; F, female; N, no; Y, yes; NA, not applicable.
All smokers were required to desist from smoking at least 90 days before bronchoscopy.
Shotgun proteomic analysis.
Tryptic digests of each BALF sample were analyzed by HPLC-tandem mass spectrometry using a NanoAquity HPLC system (Milford, MA) via electrospray ionization on-line to a hybrid LTQ-Velos mass spectrometer (Thermo Fisher, San Jose, CA). Each subject's BALF was analyzed separately, and no samples were pooled. The experiment was repeated in triplicate using gas phase fractionation (GPF) (37) over the following three mass-to-charge ranges: 400–559, 559–846, and 846–2,000. The tandem mass spectra were then matched to peptide sequences in the IPI Human 3.53 database (http://www.ebi.ac.uk/IPI) using SEQUEST (20). Criteria for matching a peptide tandem mass spectrum to a peptide sequence were as follows: cross-correlation (Xcorr) >1.9 with charge state 1+, Xcorr >2.22 with charge state 2+, or Xcorr >3.75 with charge state 3+, as well as ΔCn >0.1. Peptide tandem mass spectra passing these criteria were used for protein identifications. A protein was considered to be identified only if ProteinProphet (28) probability was >0.8 and if more than one unique peptide was found for each protein. To avoid issues of protein grouping with redundant proteins, protein identifications passing these criteria were then subjected to basic local alignment search tool (BLAST; http://blast.ncbi.nlm.nih.gov/Blast.cgi) analysis. Additionally, proteins that had 99% fast alignment sequence similarity were removed to avoid redundant protein matches. Peptide spectral counts were calculated based on the number of times a matched peptide sequence was selected for collision-induced dissociation, including all repeated selections of the sample peptide (34). A protein's spectral count value was calculated by summing the average spectral counts for each GPF segment. Homologous peptides that mapped to multiple proteins were discarded. To improve confidence in our findings, we limited further analysis to proteins detected in the BALF of at least half of the subjects in one group (i.e., three or more HIV− or HIV+ individuals). The specified statistical thresholds to determine protein identification comply with the “minimum information about a proteomics experiment” guidelines (41).
Principal component analysis.
Multidimensional scaling using principal components was performed based on the variability in overall protein expression, as assessed by spectral counts, across HIV− and HIV+ subjects.
Differential protein expression.
To determine differences in relative BALF protein abundance between the two subject populations (HIV+, HIV−), individual protein spectral counts were normalized using the spectral index (SI) metric as we have previously described (12, 13). Briefly, SI compares relative protein abundance between two groups by normalizing their spectral counts to a range of [−1, +1]. An SI close to +1 implies increased abundance of a given protein in one group (e.g., HIV+), whereas an SI close to −1 indicates increased abundance of this protein in the other group (e.g., HIV−). A value close to zero means that the protein is about equally abundant between the subjects of both groups. Statistical significance is determined through a random permutation analysis (n = 1,000 permutations). We chose a 95% confidence threshold to identify significant differential expression of a given BALF protein, which corresponded to SI ≥0.595 or SI less than or equal to −0.595. We performed unsupervised hierarchical clustering of these differentially expressed proteins using Pearson's correlation distance metric to segregate the subjects using the Multi-Experiment Viewer analysis tool (35).
Interaction of BALF proteins with HIV-1 proteome.
We interrogated the “HIV-1, Human Protein Interaction Database” (11) to identify experimentally validated interactions between differentially expressed BALF proteins and HIV-1 proteins (i.e., env, gag, nef, pol, rev, tat, vif, vpr, and vpu). We estimated the enrichment probability of interaction between differentially expressed BALF proteins and HIV-1 proteins using the cumulative hypergeometric distribution:
where k is the number of differentially regulated BALF proteins interacting with HIV-1 proteins (k = 26), n is the total number of differentially expressed BALF proteins (n = 87), K is the number of human proteins known to interact with HIV-1 according to the HIV-1, Human Protein Interaction Database (K = 1,430), and N is number of protein-coding human genes (N = 20,500) (4). This analysis is based on the null hypothesis that the same proportion of differentially expressed BALF proteins interact with HIV-1 proteins (k = 26 out of n = 87) as is observed for the entire human protein-encoding genes (K = 1,430 out of N = 20,500). The underlying assumption is that the human BALF proteome is not selectively overrepresented in proteins that interact with HIV-1. Based on these values, the cumulative probability of observing 26 BALF proteins interacting with HIV-1 proteome is 1.1 × 10−10.
Functional enrichment analysis.
Functional categorization of differentially expressed BALF proteins between HIV− and HIV+ subjects was conducted using WebGestalt software (48) using Gene Ontology annotations (http://www.genontology.org) (1). This analysis was performed separately for differentially upregulated proteins in each of the two groups. Significance of enrichment relative to the entire human proteome was determined based on the hypergeometric test. Enrichment P values were corrected for multiple hypothesis testing using the Benjamini-Hochberg method, with significance threshold set at an adjusted P value <0.05. Sensitivity analyses were performed using a similar statistical approach comparing enrichment of differentially expressed proteins relative to the BALF proteome.
Measurement of cytokines.
We measured the BALF levels of the following cytokines using Luminex Human Cytokine kits (R&D Systems, Minneapolis, MN): IL-1β, IL-1Ra, IL-6, IL-8, IL-10, TNF-α, and MCP-1. The plates were read by the Luminex 100 analyzer (Bio-Rad, Hercules, CA). All samples were run in duplicate. The concentrations of cytokines were extrapolated from a standard curve for each protein.
RESULTS
BALF proteome is altered in HIV disease.
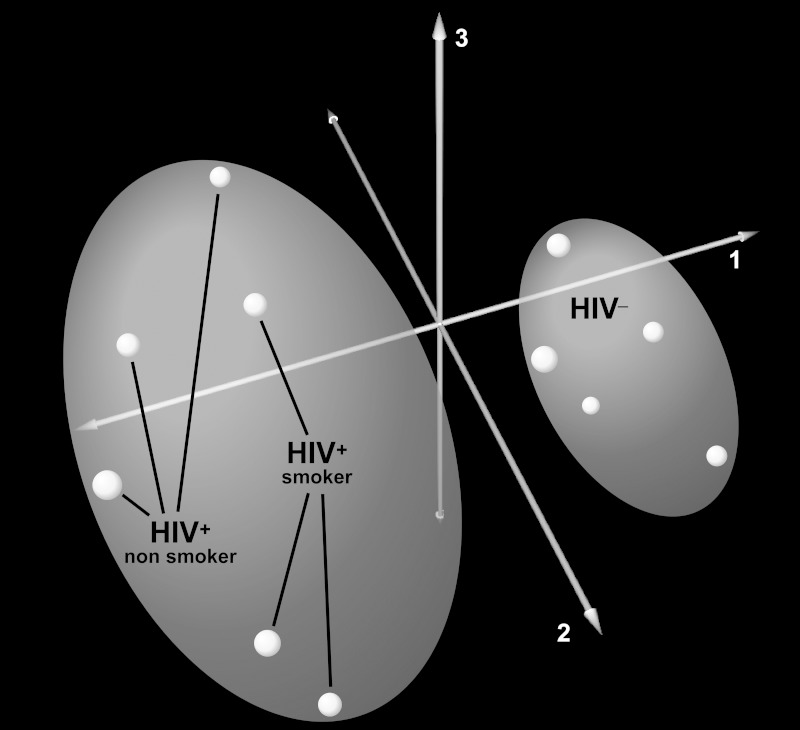
We detected a total of 765 BALF proteins across all 11 subjects. Because many of these proteins were detected in only a few individuals, we focused our analysis to those unique proteins identified in at least half of one group (i.e., at least 3 HIV+ or HIV− subjects). We identified 251 unique proteins in the BALF of HIV− and 223 unique proteins in BALF of HIV+ individuals, for a total of 318 unique proteins across all subjects. The most abundant proteins based on raw spectral counts across all BALF samples were those known to be enriched in normal lung BALF such as albumin and complement C3 (2). Supplemental Table 1 contains a list of the 318 detected proteins in HIV+ and HIV− subjects (Supplemental data for this article may be found on the American Journal of Physiology: Lung Molecular and Cellular Physiology website.). Principal component analysis based on spectral counts of all identified BALF proteins separated HIV+ and HIV− subjects, demonstrating that HIV infection alters global protein expression in the airspaces of asymptomatic HIV+ patients (Fig. 1).
Fig. 1.
Principal component analysis (PCA) of bronchoalveolar lavage fluid (BALF) proteome in human immunodeficiency virus (HIV)-positive (HIV+, magenta, red) HIV-negative (HIV−, cyan) subjects. PCA segregated HIV+ and HIV− subjects based on the variability across all 318 unique proteins detected in BALF, implying that HIV infection induces global changes in airspace protein expression. The first 3 components capturing the largest variance in protein expression (76.3%) are shown.
A distinct signature of differentially expressed proteins discriminates HIV+ patients from HIV− subjects.
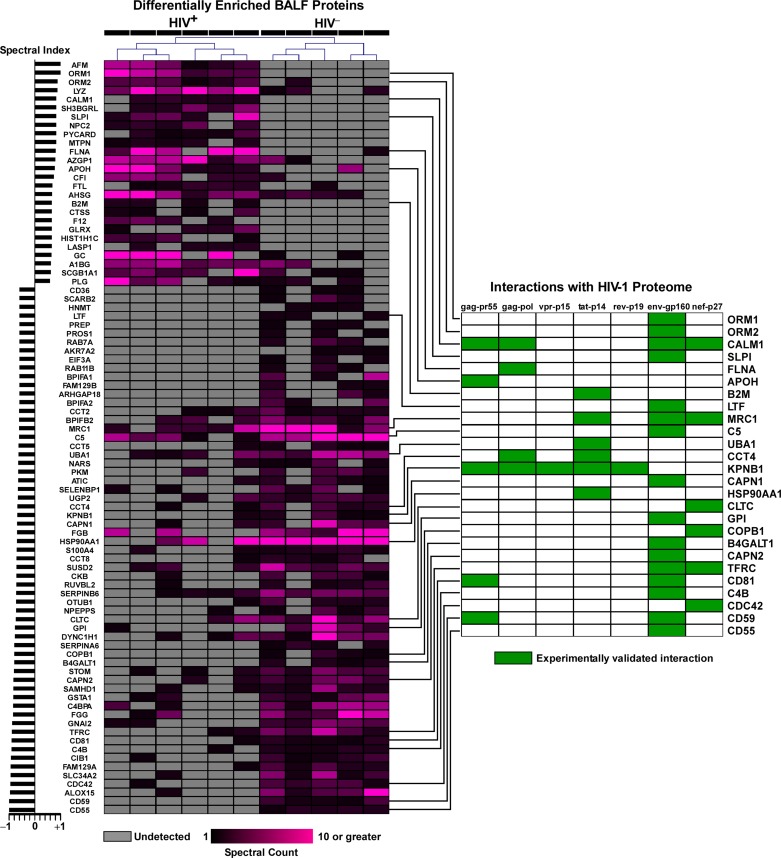
We applied a statistical test based on protein spectral counts known as the SI to identify 87 differentially expressed proteins between HIV+ and HIV− subjects. Of these, 61 proteins were more abundant in BALF of HIV− subjects, whereas 26 were more abundant in the BALF of HIV+ subjects (Supplemental Table 1). Unsupervised hierarchical clustering of differentially expressed BALF proteins demonstrated that this proteomic signature robustly discriminated the two groups (Fig. 2). Furthermore, an unexpectedly large number of these BALF proteins (n = 26) have known interactions with key HIV proteins (enrichment P value 1.1 × 10−10), highlighting putative functional consequences of altered BALF proteome in modulating HIV infection (Fig. 2).
Fig. 2.
Heatmap of 87 differentially expressed proteins in BALF of HIV+ and HIV− individuals. Hierarchical clustering was based Pearson's correlation distance metric of the protein spectral counts. Each protein's spectral index is shown to the left of the heatmap. Twenty six of these differentially regulated proteins are known to interact with one or more HIV-1 proteins, as depicted in the membership profile chart to the right of the heatmap.
HIV infection perturbs the functional repertoire of BALF proteome.
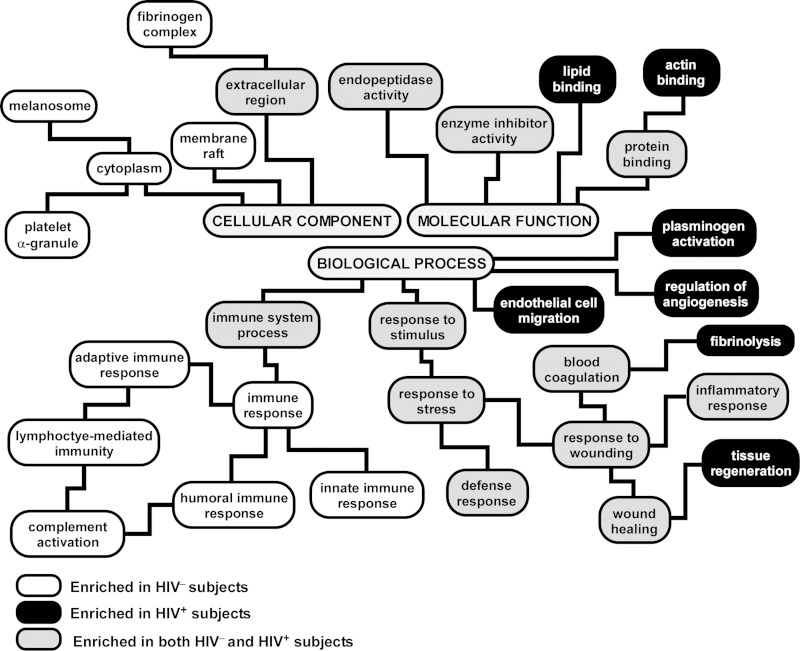
To elucidate the pathways and mechanisms activated or suppressed in the lung during HIV disease, we performed functional enrichment analysis on the differentially expressed BALF proteins (Fig. 3, a complete list is provided in Supplemental Tables 2 and 3). As shown in Fig. 3, several functional categories were commonly enriched in both HIV+ and HIV− subjects, including “response to stress,” “defense response,” “wound healing,” “inflammatory response,” and “endopeptidase activity.” Even though “immune system process” was enriched in both groups, upregulated proteins in BALF of HIV− subjects mapped to a much larger repertoire of immunity-associated pathways such as humoral immune response, innate immune response, adaptive immune response, and complement activation, none of which was enriched in the BALF proteome of HIV+ patients. This finding implies that HIV infection is associated with relative depletion of immune-related mediators and processes in the lung. In contrast, upregulated proteins in BALF of HIV+ subjects were enriched in processes involved in plasminogen activation, angiogenesis, and endothelial cell migration.
Fig. 3.
Functional categorization of differentially expressed proteins in BALF of HIV+ vs. HIV− subjects based the Gene Ontology (GO) annotations. The hierarchical structure of GO is displayed, with branches showing representative enriched functional pathways (adjusted P value <0.05). A complete list is provided in Supplemental Tables 2 and 3. Note that many processes were common to HIV+ and HIV− individuals (gray boxes), consistent with previous reports that BALF is enriched proteins mapping to distinct functional groups such as endopeptidase activity, defense response, and inflammatory response. However, several immune-related pathways were overrepresented only in HIV− subjects (white boxes), implying depletion of their protein members in HIV+ patients, whereas some processes, such as endothelial cell migration and angiogenesis, were selectively enriched in HIV+ individuals (black boxes).
HIV infection does not alter the cytokine profile of BALF.
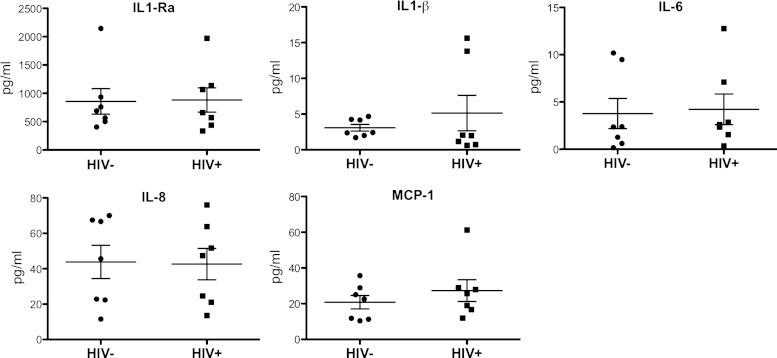
Because proinflammatory cytokines are often not detected by shotgun proteomics because of their small size, we measured the BALF levels of several well-known cytokines using Luminex technology. As shown in Fig. 4, we did not observe any differential expression in IL-1β, IL-1Ra, IL-6, IL-8, or MCP-1 between HIV+ and HIV− subjects. IL-10 and TNF-α were below detection limits in all samples (data not shown).
Fig. 4.
Cytokine levels in BALF of HIV+ and HIV− subjects as measured by Luminex technology. No differences between groups were observed, implying that HIV infection is not associated with altered profiles of key inflammatory molecules in airspaces. Differences were statistically assessed using the nonparametric Mann-Whitney test (Prism; GraphPad Software, La Jolla, CA).
DISCUSSION
The present work is the first systematic investigation of the airspace proteome in asymptomatic HIV+ patients not being treated with ART. HIV infects distinct compartments in the lung, including lymphocytes and alveolar macrophages. While HIV replication rate in the lung can exceed serum during active pulmonary infections, the virus is not uniformly detected in BALF of asymptomatic patients (16). However, a key finding of our study is that the proteomic repertoire of the alveolar lining fluid is significantly altered in these asymptomatic HIV+ subjects, implying that, even in the absence of active pulmonary disease, HIV infection has a profound impact on the constituents of lung compartments. Understanding the functional consequences of this disruption can help delineate how interactions between the lung environment and HIV infection contribute to the increased susceptibility to pulmonary disorders seen in HIV+ individuals.
We identified a signature of differentially expressed BALF proteins that discriminated HIV+ patients from uninfected subjects. Many of these proteins have been previously shown to directly interact with HIV-1 proteins, suggesting putative relationships that affect HIV infectivity and modulate immunity in the lung (Fig. 2). One such protein, CD81, a membrane glycoprotein known to interact with HIV-1 env and gag proteins, was severely depleted in BALF of HIV+ patients. HIV infection can acutely suppress CD81 expression in lymphocytes and knockdown of CD81 using a lentiviral short-hairpin RNA system demonstrated to enhance cell-to-cell viral transmission (17). In contrast, beta-2 microglobin (B2M) was significantly upregulated in HIV+ BALF compared with uninfected individuals. Elevated plasma levels of this major histocompatibility complex class I-associated protein have been linked with HIV disease progression (3, 29), and in vitro models suggest that B2M suppresses immune responsiveness of dendritic cells (45). Secretory leukocyte protease inhibitor (SLPI) was another differentially upregulated BALF protein in HIV+ subjects that interacts with HIV-1 env protein. SLPI has been shown to inhibit HIV infection in vitro (10, 23) and may play a protective role in reducing transmission via its presence in saliva and cervical fluid (9). However, this pleomorphic molecule also has potent antiprotease, anti-inflammatory, and immune-modulatory properties that may mitigate tissue injury in various pulmonary diseases, including COPD, asthma, and acute lung injury (18, 19, 21, 36, 47). Elevated SLPI levels in BALF of asymptomatic HIV+ patients may indicate subclinical activation of proinflammatory processes that if coupled with additional triggers can lead to overt lung disease in this at-risk population.
To obtain a more comprehensive overview of HIV-induced perturbations in BALF proteome, we performed functional enrichment analysis of the differentially expressed proteins (Fig. 3). As expected, many Gene Ontology categories were overrepresented in both HIV+ and HIV− subjects; these reflect common pathways enriched in BALF relative to the entire human proteome (2). However, several processes involved in immune response, including innate, adaptive, and humoral immunity, and complement activity were overrepresented in HIV− individuals but not in HIV+ patients. This finding suggests that HIV infection selectively suppresses the expression of airspace proteins mapping to key immune pathways. A number of complement-associated factors such as C5, C4B, C4BPA, and CD55 were among these downregulated proteins, several of which directly interact with HIV proteins. The complement system is a critical component of innate immunity and a main effector of the host immune response during HIV infection (46). Interestingly, CD59, another regulator of complement activation, is being investigated as a novel therapeutic target against HIV (46). Taken together, these results provide evidence that HIV disease, beyond its well-characterized systemic immunosuppression, is also associated with lung-specific impairments in immunity. This compartmentalized, immune-deficient state may explain the increased susceptibility of HIV+ patients to infectious and noninfectious pulmonary diseases.
Our functional analysis also revealed several processes that were selectively enriched in HIV+ patients, notably those mapping to regulation of angiogenesis, endothelial cell migration, and plasminogen activity. Whereas the clinical implications of these findings in BALF require further study, HIV infection is strongly linked to increased risk of developing pulmonary hypertension (regardless of ART status) (39) and venous thromboembolism (32).
Our study has a number of limitations. Because the number of subjects was small, our analysis should be considered exploratory and requires confirmation in larger cohorts. In addition, we were not able to control for additional clinical variables, such as history of intravenous drug use, prior pneumonias, or smoke exposure. However, we have previously demonstrated that, despite differences in individual constituents of BALF between subjects, enriched pathways are remarkably persevered across subjects (2). We chose to compare enrichment relative to the entire human proteome because a universal BALF proteome reference has not been established. When we performed sensitivity analyses using all detected BALF proteins (n = 765) instead of the entire human proteome, similar enrichment patterns were observed albeit at substantially reduced statistical significance (Supplemental Tables 4 and 5). Although none of the HIV+ subjects was on ART, they had a wide range of CD4 counts and viral load levels. We did not measure viral loads in the BALF. In addition, three of the HIV+ patients were smokers, potentially confounding some of our results. Although all subjects stated that they did not smoke within 3 mo of bronchoscopy, we did not verify smoking status with cotinine levels. BALF protein composition can be altered in smokers compared with nonsmokers (24, 31). In this study, we focused on proteomic profiles in cell-free lavage fluid; the detected proteins likely have multiple cellular sources. Furthermore, the BALF proteome is overrepresented with high-molecular-weight plasma proteins that bias the intensity-based ion-triggered tandem MS spectra for protein identifications and limit the proteome coverage. While immunodepletion is one solution for improving proteomic coverage and extending detectable dynamic range, this methodology was not used since it may also selectively deplete constituents of the native BALF proteome. We did measure BALF levels of several small-molecular-weight proinflammatory cytokines using Luminex and did not observe any significant differences between HIV− individuals and HIV+ patients. Nevertheless, despite the heterogeneity of the clinical population, we were able to define statistically robust differences between the BALF proteomic profiles of the two groups. Finally, because none of the HIV+ patients was receiving ART, we could not assess the effects of therapy in restoring the “normal” BALF proteomic repertoire. Future studies are needed to investigate this important issue.
In conclusion, we have outlined a systematic approach to integrate shotgun proteomics and computational methods to investigate the impact of HIV on BALF proteome. Our findings suggest that HIV infection promotes localized immune suppression in the lung, and we identify candidate proteins, many of which interact with HIV proteome, that potentially regulate this process. A better understanding of HIV's pathogenic role in the lung will provide mechanistic insights into why HIV+ patients have increased susceptibility to pulmonary diseases and may aid in developing targeted therapies for mitigating these HIV-related complications.
GRANTS
This work was supported by National Institutes of Health (NIH) UL1-RR-025014 Institute of Translational Health Sciences award (D. R. Goodlett), an American Heart Association grant-in-aid (L. M. Schnapp), NIH Grants HL-083481 (L. M. Schnapp) and K24-HL-068796 (L. M. Schnapp), Grant 2496490 from the Washington State Life Sciences Discovery Fund to the Center for Intracellular Drug Delivery (L. M. Schnapp), and NIH Grants 1U54-447-AI-57141 (D. R. Goodlett) and 1S10-RR-449017262 (D. R. Goodlett). This research was funded in part by a 2012 developmental grant from the University of Washington Center for AIDS Research, an NIH-funded program under award number P30-AI-027757, which is supported by the following NIH Institutes and Centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA).
DISCLOSURES
None of the authors (EVN, SAG, KC, YHC, DRP, DRG, LMS) have any financial, intellectual or other conflicts of interest pertaining to this work.
AUTHOR CONTRIBUTIONS
Author contributions: E.V.N., Y.-H.C., and D.R.P. performed experiments; E.V.N., S.A.G., and Y.-H.C. analyzed data; E.V.N. and L.M.S. interpreted results of experiments; E.V.N. prepared figures; E.V.N. and S.A.G. drafted manuscript; E.V.N., S.A.G., K.C., D.R.G., and L.M.S. approved final version of manuscript; S.A.G., D.R.G., and L.M.S. conception and design of research; K.C., D.R.G., and L.M.S. edited and revised manuscript.
Supplementary Material
ACKNOWLEDGMENTS
Current affiliation for D. R. Goodlett: Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy.
Glossary
- ART
Antiretroviral therapy
- BALF
Bronchoalveolar lavage fluid
- BLAST
Basic local alignment search tool
- COPD
Chronic obstructive pulmonary disease
- FASTA
Fast alignment
- GO
Gene ontology
- GPF
Gas phase fractionation
- HIV
Human immunodeficiency virus
- HPLC
High-performance liquid chromatography
- m/z
Mass-to-charge ratio
- SI
Spectral index
- Xcorr
Cross-correlation
REFERENCES
- 1.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25: 25–29, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Ryu S, Gharib SA, Goodlett DR, Schnapp LM. Exploration of the normal human bronchoalveolar lavage fluid proteome. Proteomics Clin Appl 2: 585–595, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chitra P, Bakthavatsalam B, Palvannan T. Beta-2 microglobulin as an immunological marker to assess the progression of human immunodeficiency virus infected patients on highly active antiretroviral therapy. Clin Chim Acta 412: 1151–1154, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Clamp M, Fry B, Kamal M, Xie X, Cuff J, Lin MF, Kellis M, Lindblad-Toh K, Lander ES. Distinguishing protein-coding and noncoding genes in the human genome. Proc Natl Acad Sci USA 104: 19428–19433, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crothers K, Huang L, Goulet JL, Goetz MB, Brown ST, Rodriguez-Barradas MC, Oursler KK, Rimland D, Gibert CL, Butt AA, Justice AC. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med 183: 388–395, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crothers K, Justice AC, Rimland D, Gibert CL, Rodriguez-Barradas M, Brown S, Goetz M, Butt AA, Huang L. Increased infectious and non-infectious pulmonary diseases among HIV positive compared to HIV negative veterans (Abstract). Proc Am Thoracic Soc 3: A477, 2006. [Google Scholar]
- 7.Diaz PT, Clanton TL, Pacht ER. Emphysema-like pulmonary disease associated with human immunodeficiency virus infection. Ann Intern Med 116: 124–128, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Diaz PT, King MA, Pacht ER, Wewers MD, Gadek JE, Nagaraja HN, Drake J, Clanton TL. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med 132: 369–372, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Drannik AG, Henrick BM, Rosenthal KL. War and peace between WAP and HIV: role of SLPI, trappin-2, elafin and ps20 in susceptibility to HIV infection. Biochem Soc Trans 39: 1427–1432, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher JM, Kramer LD. An in-practice training scheme for cardiopulmonary resuscitation (CPR). Br Dent J 172: 252–253, 1992. [DOI] [PubMed] [Google Scholar]
- 11.Fu W, Sanders-Beer BE, Katz KS, Maglott DR, Pruitt KD, Ptak RG. Human immunodeficiency virus type 1, human protein interaction database at NCBI. Nucleic Acids Res 37: D417–D422, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu X, Gharib SA, Green PS, Aitken ML, Frazer DA, Park DR, Vaisar T, Heinecke JW. Spectral index for assessment of differential protein expression in shotgun proteomics. J Proteome Res 7: 845–854, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Gharib SA, Vaisar T, Aitken ML, Park DR, Heinecke JW, Fu X. Mapping the lung proteome in cystic fibrosis. J Proteome Res 8: 3020–3028, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Greene KE, Wright JR, Steinberg KP, Ruzinski JT, Caldwell E, Wong WB, Hull W, Whitsett JA, Akino T, Kuroki Y, Nagae H, Hudson LD, Martin TR. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med 160: 1843–1850, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Hull MW, Phillips P, Montaner JS. Changing global epidemiology of pulmonary manifestations of HIV/AIDS. Chest 134: 1287–1298, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Koziel H, Kim S, Reardon C, Li X, Garland R, Pinkston P, Kornfeld H. Enhanced in vivo human immunodeficiency virus-1 replication in the lungs of human immunodeficiency virus-infected persons with Pneumocystis carinii pneumonia. Am J Respir Crit Care Med 160: 2048–2055, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Krementsov DN, Weng J, Lambelé M, Roy NH, Thali M. Tetraspanins regulate cell-to-cell transmission of HIV-1 (Abstract). Retrovirology 6: 64, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lapperre TS, Willems LN, Timens W, Rabe KF, Hiemstra PS, Postma DS, Sterk PJ. Small airways dysfunction and neutrophilic inflammation in bronchial biopsies and BAL in COPD. Chest 131: 53–59, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Lentsch AB, Jordan JA, Czermak BJ, Diehl KM, Younkin EM, Sarma V, Ward PA. Inhibition of NF-kappaB activation and augmentation of IkappaBbeta by secretory leukocyte protease inhibitor during lung inflammation. Am J Pathol 154: 239–247, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Link AJ, Eng J, Schieltz DM, Carmack E, Mize GJ, Morris DR, Garvik BM, Yates JR., 3rd Direct analysis of protein complexes using mass spectrometry. Nat Biotechnol 17: 676–682, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Marino R, Thuraisingam T, Camateros P, Kanagaratham C, Xu YZ, Henri J, Yang J, He G, Ding A, Radzioch D. Secretory leukocyte protease inhibitor plays an important role in the regulation of allergic asthma in mice. J Immunol 186: 4433–4442, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matute-Bello G, Liles WC, Radella F, Steinberg KP, 2nd, Ruzinski JT, Jonas M, Chi EY, Hudson LD, Martin TR. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med 156: 1969–1977, 1997. [DOI] [PubMed] [Google Scholar]
- 23.McNeely TB, Dealy M, Dripps DJ, Orenstein JM, Eisenberg SP, Wahl SM. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Invest 96: 456–464, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merkel D, Rist W, Seither P, Weith A, Lenter MC. Proteomic study of human bronchoalveolar lavage fluids from smokers with chronic obstructive pulmonary disease by combining surface-enhanced laser desorption/ionization-mass spectrometry profiling with mass spectrometric protein identification. Proteomics 5: 2972–2980, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Morris A, Crothers K, Beck JM, Huang L, Disease ATSCoHP. An official ATS workshop report: Emerging issues and current controversies in HIV-associated pulmonary diseases. Proc Am Thorac Soc 8: 17–26, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray JF, Mills J. Pulmonary infectious complications of human immunodeficiency virus infection. Part I. Am Rev Respir Dis 141: 1356–1372, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Murray JF, Mills J. Pulmonary infectious complications of human immunodeficiency virus infection. Part II. Am Rev Respir Dis 141: 1582–1598, 1990. [DOI] [PubMed] [Google Scholar]
- 28.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Jul 75: 4646–4658, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Nyamweya S, Townend J, Zaman A, Steele SJ, Jeffries D, Rowland-Jones S, Whittle H, Flanagan KL, Jaye A. Are plasma biomarkers of immune activation predictive of HIV progression: a longitudinal comparison and analyses in HIV-1 and HIV-2 infections? PLoS One 7: e44411, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearce TE, Nowakowski M, Eden E, Huang ZB, Steiner P, Shahabuddin M, Potash MJ, Volsky DJ. Uniform detection of HIV-1 in alveolar macrophages of pediatric but not adult AIDS patients. Jun 53: 722–726, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Plymoth A, Lofdahl CG, Ekberg-Jansson A, Dahlback M, Lindberg H, Fehniger TE, Marko-Varga G. Human bronchoalveolar lavage: biofluid analysis with special emphasis on sample preparation. Proteomics 3: 962–972, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen LD, Dybdal M, Gerstoft J, Kronborg G, Larsen CS, Pedersen C, Pedersen G, Jensen J, Pedersen L, Sørensen HT, Obel N. HIV and risk of venous thromboembolism: a Danish nationwide population-based cohort study. HIV Med 12: 202–210, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Rosen MJ, Clayton K, Schneider RF, Fulkerson W, Rao AV, Stansell J, Kvale PA, Glassroth J, Reichman LB, Wallace JM, Hopewell PC. Intensive care of patients with HIV infection: utilization, critical illnesses, and outcomes. Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med 155: 67–71, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Ryu S, Gallis B, Goo YA, Shaffer SA, Radulovic D, Goodlett DR. Comparison of a label-free quantitative proteomic method based on peptide ion current area to the isotope coded affinity tag method. Cancer Inform 6: 243–255, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34: 374–378, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Sallenave JM. Secretory leukocyte protease inhibitor and elafin/trappin-2: versatile mucosal antimicrobials and regulators of immunity. Am J Respir Cell Mol Biol 42: 635–643, 2010. [DOI] [PubMed] [Google Scholar]
- 37.Scherl A, Shaffer SA, Taylor GK, Kulasekara HD, Miller SI, Goodlett DR. Genome-specific gas-phase fractionation strategy for improved shotgun proteomic profiling of proteotypic peptides. Anal Chem 80: 1182–1191, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Schnapp LM, Donohoe S, Chen J, Sunde DA, Kelly PM, Ruzinski J, Martin T, Goodlett DR. Mining the acute respiratory distress syndrome proteome: identification of the insulin-like growth factor (IGF)/IGF-binding protein-3 pathway in acute lung injury. Am J Pathol 169: 86–95, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, Gressin V, Clerson P, Sereni D, Simonneau G. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med 177: 108–113, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Steinberg KP, Milberg JA, Martin TR, Maunder RJ, Cockrill BA, Hudson LD. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am J Respir Crit Care Med 150: 113–122, 1994. [DOI] [PubMed] [Google Scholar]
- 41.Taylor CF, Paton NW, Lilley KS, Binz PA, Julian RK, Jr, Jones AR, Zhu W, Apweiler R, Aebersold R, Deutsch EW, Dunn MJ, Heck AJ, Leitner A, Macht M, Mann M, Martens L, Neubert TA, Patterson SD, Ping P, Seymour SL, Souda P, Tsugita A, Vandekerckhove J, Vondriska TM, Whitelegge JP, Wilkins MR, Xenarios I, Yates JR, 3rd, Hermjakob H. The minimum information about a proteomics experiment (MIAPE). Nat Biotechnol 25: 887–893, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Wallace JM, Hansen NI, Lavange L, Glassroth J, Browdy BL, Rosen MJ, Kvale PA, Mangura BT, Reichman LB, Hopewell PC. Respiratory disease trends in the pulmonary complications of HIV Infection Study cohort. Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med 155: 72–80, 1997. [DOI] [PubMed] [Google Scholar]
- 43.White DA, Matthay RA. Noninfectious pulmonary complications of infection with the human immunodeficiency virus. Am Rev Respir Dis 140: 1763–1787, 1989. [DOI] [PubMed] [Google Scholar]
- 44.White NC, Agostini C, Israel-Biet D, Semenzato G, Clarke JR. The growth and the control of human immunodeficiency virus in the lung: implications for highly active antiretroviral therapy. 29: 964–972, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Xie J, Wang Y, Freeman ME, Barlogie B, Yi Q. Beta 2-microglobulin as a negative regulator of the immune system: high concentrations of the protein inhibit in vitro generation of functional dendritic cells. Blood 101: 4005–4012, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Yu Q, Yu R, Qin X. The good and evil of complement activation in HIV-1 infection. Cell Mol Immunol 7: 334–340, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zani ML, Tanga A, Saidi A, Serrano H, Dallet-Choisy S, Baranger K, Moreau T. SLPI and trappin-2 as therapeutic agents to target airway serine proteases in inflammatory lung diseases: current and future directions. Biochem Soc Trans 39: 1441–1446, 2011. [DOI] [PubMed] [Google Scholar]
- 48.Zhang B, Kirov S, Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res 33: W741–W748, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.