Abstract
Acute lung injury is marked by profound influx of activated neutrophils, which have delayed apoptosis, along with fluid accumulation that impairs lung function and causes high mortality. Inflammatory and antimicrobial molecules, such as reactive oxygen species from activated neutrophils with prolonged lifespan, cause tissue damage and contribute to lung dysfunction. Angiostatin, an endogenous antiangiogenic molecule, is expressed in the lavage fluid of patients with acute respiratory distress syndrome and modifies neutrophil infiltration in a mouse model of peritonitis. Our aim was to investigate the therapeutic role of angiostatin in acute lung injury. We analyzed bronchoalveolar lavage and lung tissues from C57BL/6 mouse model of Escherichia coli LPS-induced acute lung injury to assess the effects of angiostatin treatment. Subcutaneous angiostatin administered at 5 h after LPS treatment reduces histological signs of inflammation, protein accumulation, lung Gr1+ neutrophils, myeloperoxidase activity, and expression of phosphorylated p38 MAPK in lung tissues and peripheral blood neutrophils, while increasing the number of apoptotic cells in the lungs without affecting the levels of macrophage inflammatory protein-1 α, IL-1β, keratinocyte chemoattractant, and monocyte chemoattractant protein-1 in lavage and lung homogenates at 9 and 24 h after LPS treatment. In contrast, angiostatin administered intravenously 5 h after LPS treatment did not reduce histological sign of inflammation, BAL cell recruitment, and protein concentration at 9 h of LPS treatment. We conclude that angiostatin administered subcutaneously after LPS challenge inhibits acute lung inflammation up to 24 h after LPS treatment.
Keywords: neutrophils, vascular permeability, inflammation, apoptosis
inflammation is the host response to microbial or chemical stimuli and typically involves migration of inflammatory cells, such as neutrophils into the affected organ (25). Acute lung injury (ALI) imposes a heavy burden on public health, with more than a 50% in-hospital mortality rate (32). ALI is characterized by the migration of activated neutrophils into lung vasculature and alveoli and can arise from local pulmonary or systemic inflammation (32). The activated neutrophils live longer and cause significant tissue damage through inflammatory products such as proteinases and reactive oxygen species (13, 32). The paradox of neutrophil biology is highlighted by the fact that although activated neutrophils are essential for clearing bacteria, the tissue damage caused by them contributes to organ failure, morbidity, and mortality (1, 32). Hence, there is a need to find ways to regulate neutrophil migration, activation, and their life span in ALI.
Angiostatin is a potent endogenous antiangiogenic molecule that is a Kringle 1–4 or Kringle 1–3 product generated from plasminogen-derived plasmin through actions of phosphoglycerate kinase, serine proteinase, and matrix metalloprotease (19, 28, 43). Proteolysis of membrane-bound plasmin generates angiostatic molecules in vitro and in vivo (11). Angiostatin and plasminogen appear as early as day 1 and day 3 after generation of the wounds, peaks during the last phase of wound healing, and almost disappears after healing (6). Angiostatin released from neutrophils mediates the initial angiogenic switch in the mouse model of multistage carcinoma (4, 26, 30). Platelets are also well known to produce angiostatin from urokinase-type plasminogen activator (uPA) (17).
Angiostatin binds to several cell surface proteins (38, 43), such as ATP synthase F1F0, heat shock proteins 27 and 70, annexin II (33, 36), and angiomotin (40) to produce anoikis, apoptosis, and cell migration. Angiostatin binds to adhesion proteins, such as integrin αvβ3 expressed on neutrophils and the apical and basal surfaces of endothelial and epithelial cells (34, 39). Angiostatin inhibits recruitment of peripheral blood leukocytes and angiogenesis in a mouse model of peritonitis (8) by inducing the expression of adhesion molecules (ICAM-1, E-selectin) (9, 22), interacting with Mac-1 (αMβ2) integrin and inhibiting NF-κB. Increased levels of angiostatin in bronchoalveolar lavage (BAL) from patients with acute respiratory distress syndrome are cytotoxic to endothelial cells in vitro (12, 21). We have reported increased immunohistological expression of angiostatin and its receptor integrin αvβ3 in septic lungs compared with normal human lungs (35). However, there are no data on the effects of angiostatin on the pathogenesis of ALI.
There have not been any major breakthroughs in the development of new therapeutics for acute respiratory distress syndrome and ALI (32). One of the therapeutic targets in ALI is modulation of the exuberant migration of activated neutrophils into inflamed lungs to mitigate lung injury. Because angiostatin has been shown to inhibit neutrophil migration in a mouse model of peritonitis (8) and because there are no data on its roles in ALI, we tested a hypothesis that angiostatin will inhibit endotoxin-induced lung inflammation and injury in a mouse model.
MATERIALS AND METHODS
All of the animal experiments were conducted in accordance with the institutional guidelines and following approval from the University of Saskatchewan Animal Ethics Board. Six- to eight-week-old male C57BL/6 mice were purchased from Animal Resource Center of the University of Saskatchewan. Escherichia coli 0127:B8 LPS, myeloperoxidase (MPO) from human leukocytes (Sigma Chemicals, St. Louis, MO), angiostatin K1–4 (Haematologic Technologies, Essex Junction, VT), mouse Ly-6G Ly-6C (Gr1) antibody (BD Biosciences, San Jose, CA), human vWF, polyclonal secondary anti-rat and anti-rabbit HRP (horseradish peroxidase)-conjugated antibody (Dako, Glostrup, Denmark), Vector VIP peroxidase substrate kit (Vector Laboratories, Burlingame, CA), Roche in situ cell death detection kit AP (Roche Diagnostics Canada, Laval, QC, Canada) were purchased commercially.
Intranasal E. coli LPS-induced Mouse Model of Acute Lung Injury
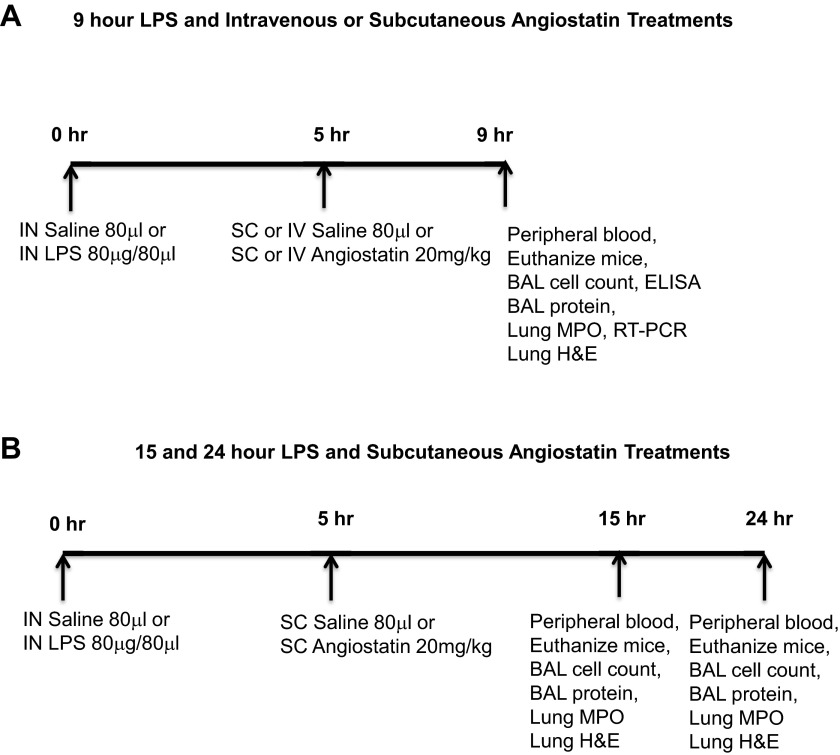
We used an intra-nasal LPS instillation mouse model of ALI (37) and the 9-h treatment groups are shown in Fig. 1A. Peripheral blood was collected through cardiac puncture from anesthetized animals for differential, as well as total peripheral leukocyte counts, and lungs were lavaged with a total of 1.5 ml of ice-cold Hank's balanced salt solution. The right lung bronchus was ligated while the left lung was fixed in situ through intra-tracheal instillation of 4% paraformaldehyde for 1 h, after which the right lung was snap-frozen. In another set of experiments, angiostatin (20 mg/kg) was subcutaneously administered 5 h following LPS instillation, and mice were euthanized at 15 or 24 h after LPS treatment (Fig. 1B). We also conducted experiments in which angiostatin was either coadministered at 10 mg/kg intravenously with intranasal LPS or injected intravenously 5 h (20 mg/kg) after treatment with LPS. The mice were euthanized at 12 h after LPS treatment.
Fig. 1.
Study design for 9 h LPS (A) as well as 15 and 24 h (B) post-LPS treatment are shown. Sample collection and analyses are described in materials and methods.
Peripheral blood leukocyte counts.
Total cell counts and differential leukocyte counts were done after red blood cell hemolysis with 2% acetic acid. Blood smears were stained with Diffquick.
Bronchoalveolar lavage.
BAL was centrifuged at 700 g, and supernatant was stored at −80°C for protein estimation and cytokine assay. Cells were resuspended at 106/ml to make cytospin for differential cell count.
Protein assay.
Bradford's reagent was used to assess total lung as well as BAL protein.
Hematoxylin-and-eosin staining.
The Tissue-Tek O.C.T. (Optimal Cutting Temperature) Compound-embedded left lung was cut into 4-μm sections and stained with hematoxylin and eosin to assess degree of lung inflammation.
Anti-Gr1 and phosphorylated p38 MAPK immunohistochemistry.
Briefly, cryosections were subjected to endogeneous peroxide removal followed by antibody blocking, as well as treatments with primary rat anti-mouse Gr1 or anti-phosphorylated p38 MAPK (anti-pp38 MAPK) and secondary anti-rat HRP-conjugated antibody. Negative controls and positive controls comprising omission of primary antibody and von Willebrand factor (vWF) stain, respectively. Isotype-matched controls, namely mouse IgG2b and rabbit IgG antibodies, were also run to check the specificity of the Gr1 and pp38 MAPK antibodies, respectively. We also performed phosphorylated p38 MAPK (pp38 MAPK) immunostaining on peripheral blood neutrophils isolated from the 24 h in vivo subcutaneous angiostatin treatment experiments (27). Briefly, the neutrophil cytospin slides were fixed with 4% paraformaldehyde for 15 min, washed with PBS, permeabilized with 0.5% Triton-X, and processed for standard pp38 MAPK immunostaining.
MPO assay.
Lung homogenates were prepared in 50 mM HEPES (pH 8.0) and centrifuged at 900 g (3,000 rpm) for 20 min at 4°C. The pellet was resuspended in 0.5% CTAC (cetyl trimethylammonium chloride) solution and rehomogenized. MPO colorimetric assay was performed (31) using 3,3′,5,5′-tetramethylbenzidine as substrate for H2O2 under low pH conditions. MPO from human leukocytes was used as a standard. Results were expressed as MPO units per milligram of lung tissue.
Terminal deoxynucleotidyl transferase biotin-dUTP nick-end labeling assay of lung.
Terminal deoxynucleotidyl transferase biotin-dUTP nick-end labeling (TUNEL)/staining (Roche in situ cell death detection kit) was used to assay cellular apoptosis, as recommended by the manufacturer. Ten fields per section from the regions with cell apoptosis were examined at a magnification of ×100.
Cytokine assay.
Sandwich ELISA was performed on lung homogenates for monocyte chemoattractant protein-1 (MCP-1), keratinocyte chemoattractant (KC), interleukin-1β (IL-1β), macrophage inflammatory protein-1 alpha (MIP-1α) using ELISA kits (R&D Systems, Minneapolis, MN).
Lung cytokine/chemokine analysis.
RNA was isolated from lung samples and purified using the RNase mini kit followed by RNase-free DNase treatment (Qiagen, Mississauga, ON, Canada). RNA integrity was confirmed by agarose gel electrophoresis and quantified with Nanodrop spectrophotometry (Thermo Fisher Scientific, Ottawa, ON, Canada). The mRNA was reverse transcribed using the QuantiTect reverse transcription kit (Qiagen) with a mixture of universal oligo dT and random primers and followed by reverse-transcriptase PCR analysis using QuantiFast SYBR Green PCR kit (Qiagen) using the primer pairs: 5-ATGGCAACTGTTCCTGAACTC-3′ and 5′-CTGCCTGAAGCTCTTGTTGAT-3′ for interleukin-1β (GenBank accession no. BC011437), 5′-ATGAAGGTCTCCACCACTG-3′ and 5′-GCAAAGGCTGCTGGTTTCA-3′ for MIP-1 (GenBank accession no. NM_011337), 5′-ATGCAGGTCCCTGTCATGC-3′ and 5′-GCTGCTGGTGATCCTCTTGTA-3′ for MCP-1 (GenBank accession no. NM_011333), 5′-AGTGCCTGCAGACCATGG-3′ and 5′-CTTGCCTTGACCCTGAAGC-3′ for KC-1 (GenBank accession no. NM_008176), and 5′-TGCATCCTGCACCACCAACTG-3′ and 5′-GGGCCATCCACAGTCTTCTGG-3′ for GAPDH (GenBank accession no. BC096042). The negative control consisted of all the components of the reaction mixture except RNA. The reaction analysis was performed using the MX3005P Light-Cycler (Stratagene, La Jolla, CA), as per the manufacturer's instructions. The cDNA was denatured for 5 min at 95°C, followed by amplification of target DNA through 45 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 60°C for 45 s. Relative expression levels were calculated after the correction for expression of GAPDH using MxPro software.
Western blots.
Briefly, lung homogenates extracted with equal volume of 2× Laemmli sample buffer concentrate (Sigma) were electrophoresed in 12% SDS-PAGE at 180 V followed by electro-transfer to nitrocellulose membranes at 100 V for 60 min. The resulting membranes were blocked (5% skim milk in 0.1% PBS-Tween for 60 min), incubated with rat monoclonal plasminogen antibody (ab61387) overnight at 4°C, washed three times with PBS-Tween for 5 min each, and exposed to HRP-conjugated anti-rat IgG antibody for 60 min. The membrane was treated with ECL (enhanced chemiluminescence system) solutions (Western blotting detection reagents; GE Amersham, Little Chalfont, Buckinghamshire, UK) and developed with special ECL-sensitive films (GE Amersham). The probed membranes were restored with blot-restore solution (Millipore, Temecula, CA) to be reprobed with β-actin. Angiostatin expression was normalized to actin through densitometric quantification with Adobe Photoshop.
Statistical analysis.
Results were analyzed by utilizing GraphPad Software (San Diego, CA). One-way ANOVA was applied followed by Bonferroni's post hoc test for all group comparison to assess differences between two groups. The treatment significance was set at P < 0.05.
RESULTS
Subcutaneous Treatment with Angiostatin Inhibits Early Signs of LPS-Induced Lung Inflammation
Peripheral blood leukocyte counts.
While total cell counts in the peripheral blood did not differ among treatment groups, monocytes were decreased and neutrophils were significantly increased in all the LPS-challenged animals compared with the saline controls (data not shown).
BAL protein.
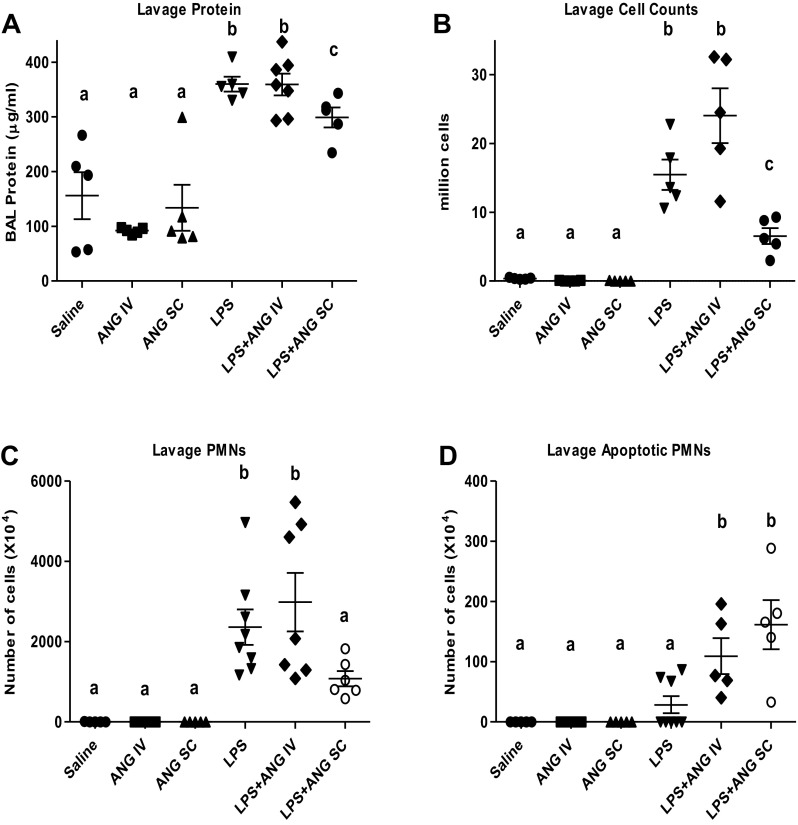
BAL protein concentration (Fig. 2A), an indicator of lung vascular permeability, was significantly increased in LPS-treated mice (343.7 ± 11.3 μg/ml) compared with the saline-treated mice (156.0 ± 42.8 μg/ml). All of the LPS groups with or without angiostatin treatment had significantly higher BAL protein concentration compared with the control groups (saline only, intravenous angiostatin only, and subcutaneous angiostatin only). The subcutaneous but not intravenous angiostatin treatment of LPS-instilled animals caused a significant reduction in protein concentration (303.4 ± 15.7 μg/ml) compared with the LPS-treated control mice at 9 h (Fig. 2A) post-LPS instillation.
Fig. 2.
Bronchoalveolar lavage (BAL) protein content (A) total leukocyte counts (B), polymorphonuclear neutrophils (PMNs) (C), and apoptotic cells (D). Different letters indicate statistical differences (P < 0.05) between the groups while letters that match each other indicate no statistical difference.
BAL cell counts.
LPS caused a significant increase in BAL total cell counts, as well as neutrophil counts, compared with the controls (Figs. 2, B and C) at 9 h after LPS treatment. The subcutaneous, but not intravenous, angiostatin treatment of LPS-treated mice significantly decreased the BAL leukocytes compared with the LPS-only group at the 9-h time point (Fig. 2, B and C). Intravenous, as well as subcutaneous, angiostatin treatment of LPS-challenged mice, caused a significant increase in apoptotic neutrophils in the BAL (Fig. 2D) compared with the saline, intravenous angiostatin-only, subcutaneous angiostatin-only, and LPS-alone groups. BAL from mice treated with angiostatin alone, irrespective of the route, primarily showed alveolar macrophages and lacked neutrophil recruitment.
Lung histology.
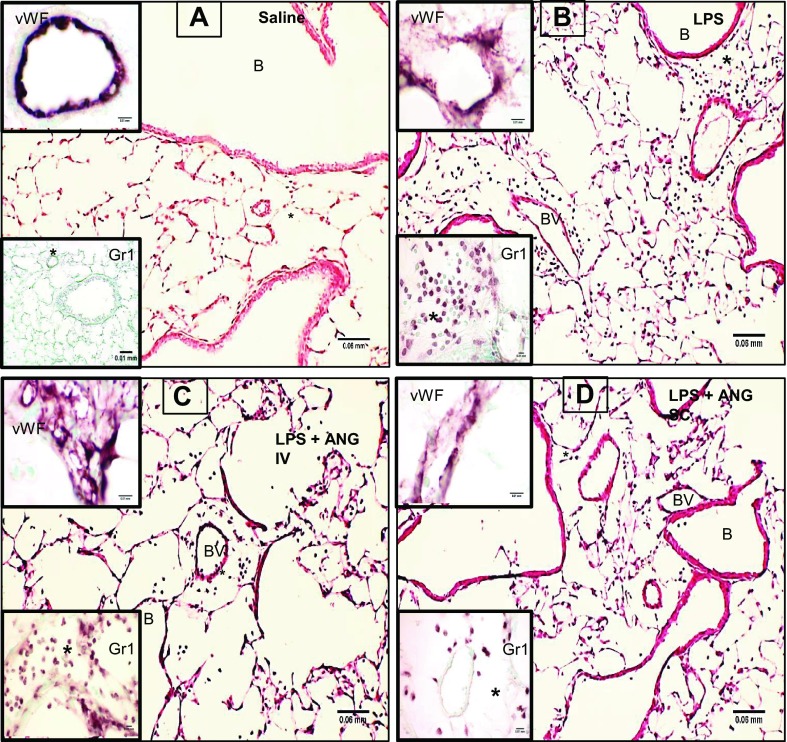
Lung sections from the saline group showed normal alveolar septa (Fig. 3A), while those from the LPS-treated mice had septal congestion, neutrophil accumulation in the septa, and the perivascular and peribronchiolar spaces (Fig. 3B). Compared with the lung sections from the LPS+intravenous angiostatin mice (Fig. 3C), those from LPS+subcutaneous angiostatin mice showed markedly reduced histological signs of ALI (Fig. 3D). Although all of the lungs were lavaged, we still observed neutrophils in the alveoli of lung sections from the LPS, as well as LPS+intravenous angiostatin, mice, which suggests more robust migration of neutrophils or their increased adhesion to lung tissues. Mice treated with angiostatin only, either intravenously or subcutaneously, showed normal lung histology (data not shown) similar to the saline-only group.
Fig. 3.
Hematoxylin-and-eosin (H&E)-stained lung sections from saline-treated control mouse show normal histology (A) and signs of inflammation in 9-h LPS-exposed mice (B). The inflammation at 9 h post-LPS treatment is much reduced in LPS-treated mice given subcutaneous angiostatin treatment (C) compared with those given angiostatin intravenously (D). The insets labeled vWF show expression of inflammatory marker von Willebrand Factor (vWF) to be more prominent in LPS+intravenous angiostatin compared with other groups. Gr1 immunohistochemistry insets show lack of neutrophils in perivascular compartment (*) in control mouse (A), while LPS-treated mouse lung showed robust accumulation of neutrophils in the perivascular compartment. The accumulation is nearly absent in lungs from mice given LPS followed by subcutaneous angiostatin (C) but not in the mice treated with angiostatin intravenously (D). BV, blood vessel; B, bronchiole.
Lung vWF immunohistology.
We also used vWF staining (vWF insets in Fig. 3) as an indicator of inflammation because vWF is released from Weibel-Palade bodies in endothelial cells and plays a role in cell adhesion. Although vWF was present in the blood vessels in the saline-treated lungs (Fig. 3A), the expression was increased in LPS-treated lungs (Fig. 3B). The intravenous (Fig. 3C) angiostatin treatment of LPS-treated mice led to intense vWF expression in lung vessels. The subcutaneous treatment with angiostatin (Fig. 3D) resulted in vWF expression similar to the control (Fig. 3A) and angiostatin-only treated animals (data not shown).
Anti-Gr1 immunohistochemistry.
Gr1 antibody was used to evaluate the neutrophils still trapped in the interstitial compartment of the lungs after the lavage. Perivascular spaces in the saline-treated control (inset in Fig. 3A), as well as angiostatin-only treated mice (data not shown) were devoid of neutrophils in contrast to robust staining for neutrophils in the perivascular compartment of LPS-treated mice (Fig. 3B) and those treated intravenously with angiostatin following LPS administration (Fig. 3C). Lungs from LPS-treated mice given angiostatin subcutaneously (Fig. 3D) showed very few neutrophils in the septum and the perivascular compartment.
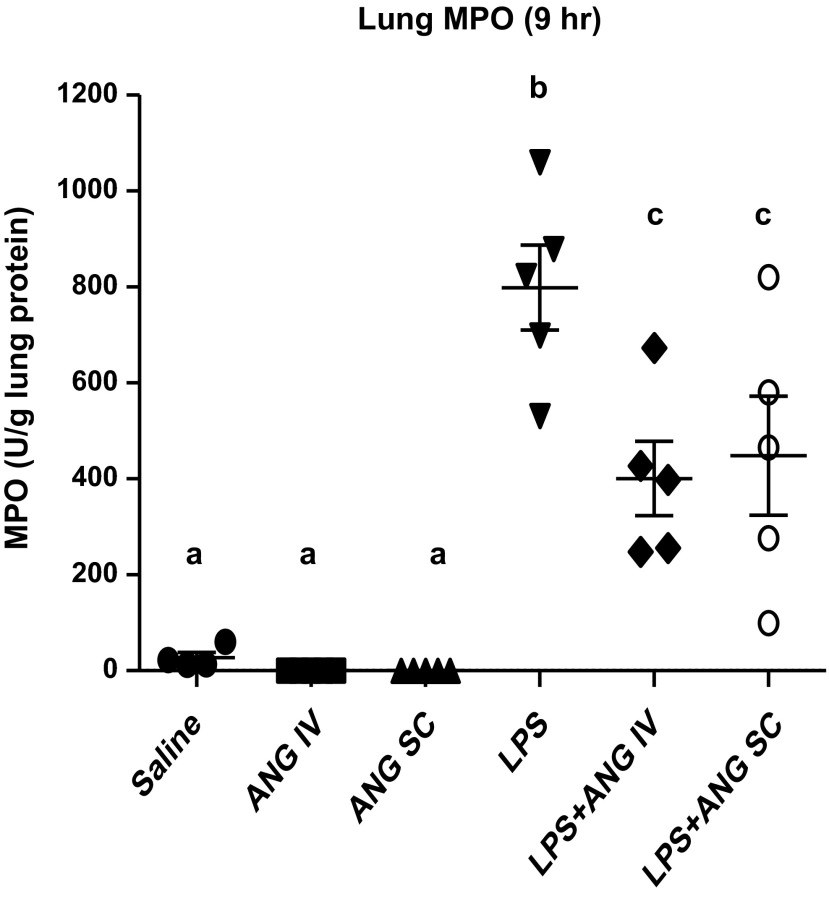
Lung MPO.
MPO, as a surrogate indicator of neutrophils trapped in the lavaged lungs, was significantly lower in the saline, as well as angiostatin intravenous and angiostatin subcutaneous-only groups, compared with the LPS or LPS+intravenous angiostatin treatments at 9 h following LPS treatment (Fig. 4). However, LPS-challenged mice treated with angiostatin, either subcutaneously or intravenously, showed significantly lower MPO compared with the LPS-only animals.
Fig. 4.
Lung myeloperoxidase (MPO) per unit gram of lung protein. Lung homogenates were prepared as described in materials and methods; results were expressed as MPO U/g lung protein. Lungs collected at 9 h after the LPS treatment had significantly higher MPO compared with all other groups, while both routes of angiostatin treatment had significantly lower MPO compared with the LPS-only group. a,b,cDifferent letters indicate statistical differences (P < 0.05) between the groups, while letters that match each other indicate no statistical difference.
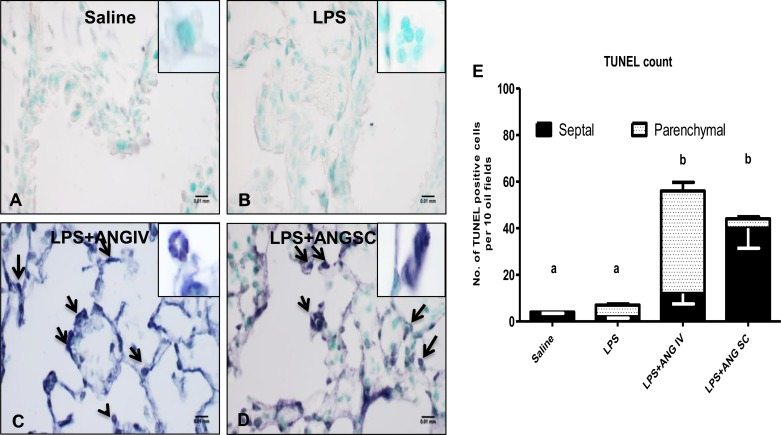
TUNEL assay of the lung.
Because angiostatin induces apoptosis in endothelial cells and we noticed higher numbers of apoptotic neutrophils in the BAL of mice treated with both angiostatin and LPS, we evaluated the extent of apoptosis in lungs from mice exposed to various treatments. While TUNEL-positive cells were absent in saline (Fig. 5A), as well as angiostatin-only treated (data not shown) and LPS-treated (Fig. 5B) mice, there were apparently more apoptotic cells, including neutrophils in alveolar septa and alveoli of LPS+angiostatin subcutaneous mice (Fig. 5, D and E). Numerical counts of TUNEL cells showed significantly more apoptotic cells in lungs from LPS-challenged mice treated with angiostatin either intravenously (Fig. 5C) or subcutaneously, compared with those treated with LPS only (Figs. 5E). Within the limitations of resolution of a light microscope, some endothelial cells especially in LPS+angiostatin intravenous mice (Fig. 5C) appeared to be TUNEL-positive.
Fig. 5.
Lung terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL): Ten fields per section from the regions with cell apoptosis were examined at a magnification of ×100. Lung sections from a saline-treated mouse (A) and LPS-treated (B) mouse did not show TUNEL-positive cells. Intravenous treatment with angiostatin of LPS-treated mouse (C) showed many apoptotic cells (arrows). An LPS-treated mouse given angiostatin subcutaneously (D) showed apoptosis, which appeared to be mostly in septal cells (40 ± 8.6/10 fields), whereas the intravenous angiostatin treatment group showed a significantly high number of TUNEL-positive cells in the parenchyma (bronchioles, septa, and blood vessels) (44 ± 3.7/10 fields) (E). a,bDifferent letters indicate statistical differences (P < 0.05) between the groups, while letters that match each other indicate no statistical difference.
Expression of IL-1β, MIP-1α, MCP-1, and KC in BAL and lung tissues.
To understand mechanisms through which angiostatin may be influencing neutrophil migration into the lung, we assayed IL-1β, MIP-1α, MCP-1, and KC protein in BAL and mRNA lung homogenates because of their well-established roles in ALI. The data showed significant increase of all the cytokines tested in LPS-treated groups compared with those given only saline (data not shown), but no differences among the LPS-treated animals with or without angiostatin, irrespective of the administration route. Also, mRNA levels of these cytokines and chemokines in lung tissue were not different among endotoxin-treated animals (data not shown).
pp38 MAPK immunohistochemistry.
To determine whether angiostatin altered the expression of phosphorylated p38 MAPK, which regulates apoptosis and other cell responses (2), we stained lung sections for pp38 MAPK. Only occasional pp38 MAPK-positive cells were noticed in lungs of saline- (Fig. 6A) and angiostatin-only treated mice (data not shown). The pp38 MAPK staining was intense in neutrophils and alveolar septa in the lungs of LPS-treated mice (Fig. 6B). Many neutrophils expressing pp38 MAPK were adhered to the endothelium (Figs. 6, C and D). Although there were many positively stained neutrophils in LPS+intravenous angiostatin (Fig. 6D), subcutaneous treatment with angiostatin caused a marked decrease in pp38 MAPK expression in leukocytes and near absence in alveolar septa (Fig. 6E).
Fig. 6.
Phosphorylated p38 MAPK expression. The immunohistochemical protocol did not detect any staining for phosphorylated p38 MAPK in a section from control lung (A) but showed intense staining in many leukocytes (arrows) and septum (asterisks) in mice treated with LPS (B) or LPS+ANG IV (D). Higher magnification view (C) from an LPS-treated mouse lung shows leukocytes (double arrows) showing intense staining for phosphorylated p38 MAPK in contact with the endothelium (En). Lung section from a mouse treated with LPS+ANG SC (E) showed a few cells (single arrows) but not alveolar septum (asterisks) positive for phosphorylated p38 MAPK. Scale bar = 10 μm except bar in A = 50 μm.
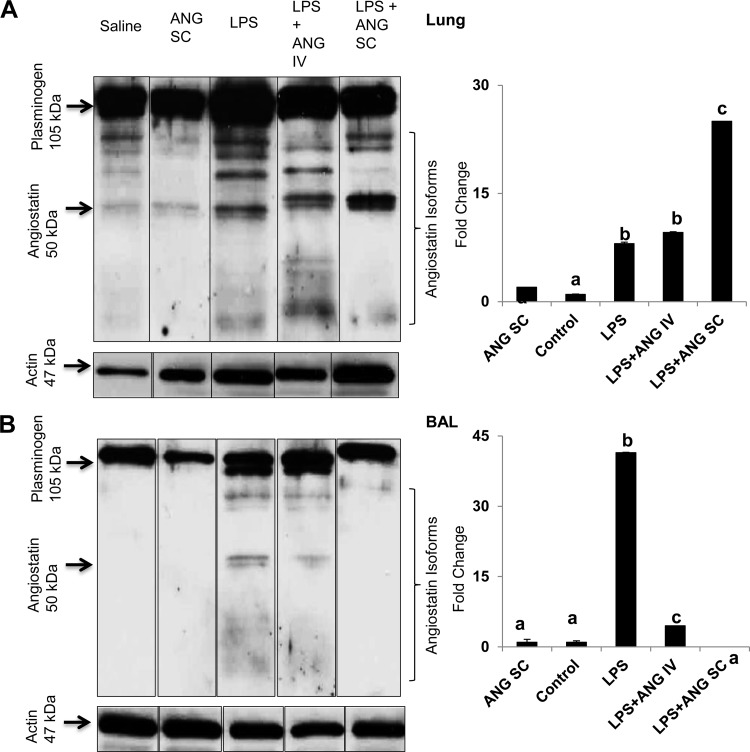
Western blots for angiostatin.
Finally, we determined whether exogenous angiostatin treatment altered amounts of angiostatin in the lungs. We found plasminogen bands at 105 kDa BAL in lung homogenates from mice in all of the groups. Angiostatin forms (50 kDa) were detectable in LPS-treated lung homogenates as well as in BAL (Fig. 7, A and B). Very faint bands for 50-kDa angiostatin were also observed in the lung homogenates from subcutaneous angiostatin-only-treated mice (Fig. 7A). Densitometry for 50-kDa angiostatin bands in relation to actin bands showed a significant increase in angiostatin expression in lung homogenates from all of LPS-treated mice compared with the saline or subcutaneous angiostatin-only group (Fig. 7A). Lung homogenates, as well as the lavage supernatant from intravenous angiostatin-only-treated mice lungs, show no detectable 50-kDa angiostatin bands (data not shown). The lung homogenates from mice administered both LPS and subcutaneous angiostatin showed significantly more angiostatin compared with the LPS only as well as LPS+angiostatin intravenous mice (Fig. 7A).
Fig. 7.
Representative Western blots of plasminogen and angiostatin (ANG) isoforms and densitometric analysis for 50 kDa ANG in lung homogenates (A) and bronchoalveolar lavage (BAL) fluid (B) from a 9-h study. The boxes around the lanes indicate that these were spliced and rearranged gel lanes obtained from gels run on a single day. Also shown is corresponding β-actin expression. y-axis represents fold difference in ANG expression after normalization for actin expression. a,b,cDifferent letters indicate statistical differences (P < 0.05) between the groups, while letters that match each other indicate no statistical difference.
BAL from mice treated with saline only, subcutaneous angiostatin only, and both LPS+subcutaneous angiostatin did not show any bands corresponding to 50 kDa angiostatin (Fig. 7B). The angiostatin expression in BAL from LPS-treated mice was 40-fold higher compared with those treated with saline, as well as all of the angiostatin-treated mice (Fig. 7B). Interestingly, there were no differences in angiostatin levels in BAL from the control, as well as mice treated with LPS and subcutaneous angiostatin.
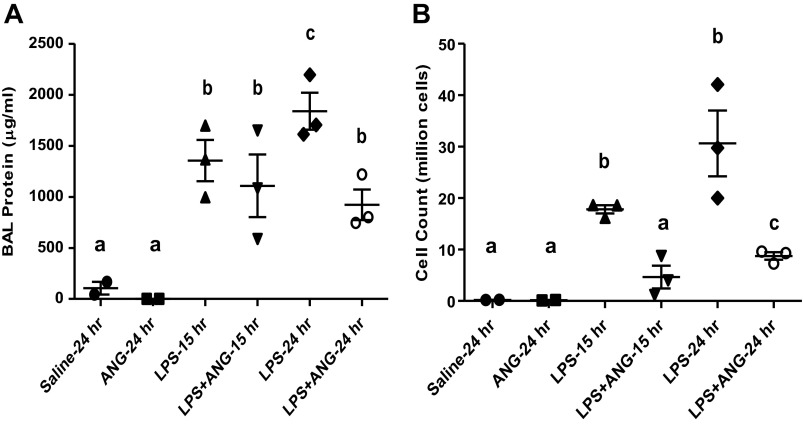
Subcutaneous angiostatin treatment inhibits LPS-induced lung inflammation at 15 and 24 h.
To further extend observations on the beneficial effects of subcutaneous angiostatin on LPS-induced lung inflammation, we performed additional studies to find out whether effects of angiostatin lasted up to 24 h. BAL protein was significantly lower in the saline and subcutaneous angiostatin-only-treated groups at 15 (1,355.4 ± 202.9 μg/ml) and 24 h (1,838.0 ± 181.1 μg/ml) than the LPS-only groups (Fig. 8A). The LPS and subcutaneous angiostatin treatment caused significant reduction in BAL protein compared with the time-matched 24 h LPS-only group (922.3 ± 150.1 μg/ml) (Fig. 8A). The saline (0.2 × 106 ± 0.0 cells) and subcutaneous angiostatin-only (0.1 × 106 ± 0.0 cells)-treated mice show significantly less BAL leukocytes compared with 15 (18.6 × 106 ± 0.01 cells) and 24 h (36.0 × 106 ± 5.0 cells) LPS-only groups (Fig. 8B). We noted a significant reduction in BAL leukocyte counts at 15 (4.7 × 106 ± 2.2 cells), as well as 24 h (8.7 × 106 ± 0.7 cells) in LPS-treated mice given angiostatin subcutaneously compared with the time-matched LPS-only groups (Fig. 8B).
Fig. 8.
Bronchoalveolar lavage (BAL) protein content (μg/ml; A) and total leukocyte counts (B) in mice from various treatment groups as described in Fig. 1B and materials and methods. Significantly reduced BAL protein and cell counts were observed in at 24-h post-LPS treatment mice that were treated subcutaneously with angiostatin at 5 h after LPS treatment. a,b,cDifferent letters indicate statistical differences (P < 0.05) between the groups, while letters that match each other indicate no statistical difference.
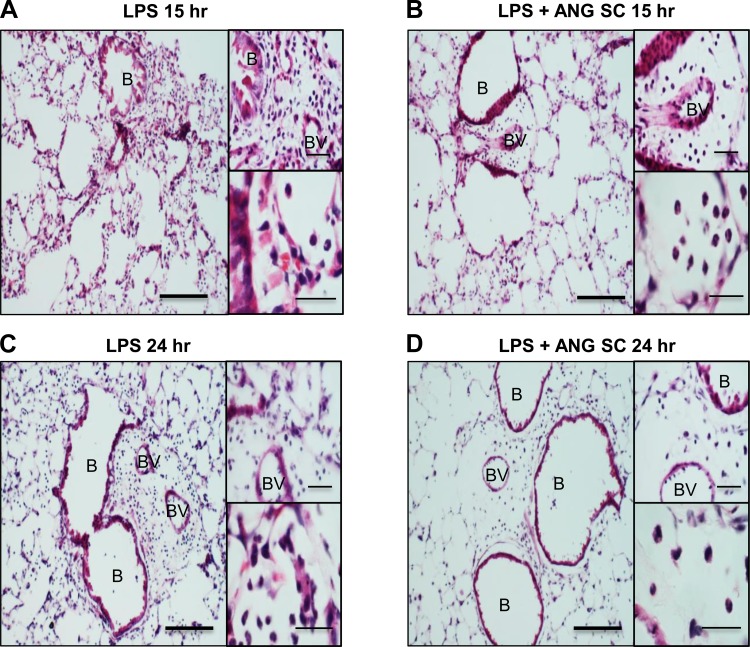
Lungs from mice treated with LPS showed intense inflammation characterized by neutrophil influx in alveolar septa, as well as perivascular and peribronchiolar areas, septal thickening, and edema (Figs. 9, A and C and insets). Mice treated subcutaneously with angiostatin following LPS challenge showed reduced inflammation at 15 h (Fig. 9B and insets) and 24 h (Fig. 9D and insets).
Fig. 9.
Hematoxylin-and-eosin-stained lung sections of mice at 15 h (A and insets) and 24 h (C and insets) show more inflammation compared with time-matched LPS+subcutaneous ANG-treated mice (B and D). The insets show reduced perivascular and alveolar neutrophils in LPS-challenged mice treated with subcutaneous angiostatin. Scale bar = 50 μm for wide fields; 20 μm for insets.
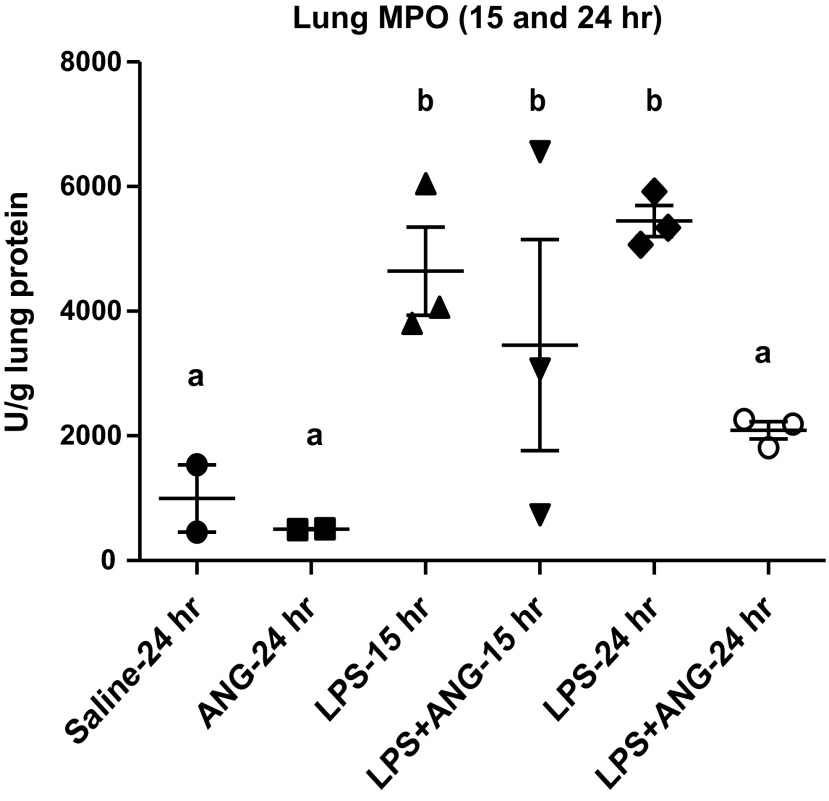
Lungs from both of the groups of mice challenged with LPS alone showed significantly more MPO concentrations compared with the saline control (Fig. 10). Subcutaneous angiostatin administration 5 h after LPS treatment caused significant (P < 0.01) reduction in MPO concentration compared with the LPS group at 24 h but not 15-h time point (Fig. 10).
Fig. 10.
Lung MPO per unit gram of lung protein. Lung homogenates prepared as described in materials and methods show significantly higher MPO in LPS-treated mice compared with saline or angiostatin only treated mice at 15, as well as 24 h post-LPS instillation. Subcutaneous angiostatin treatment 5 h after LPS instillation significantly reduced lung MPO 24 h after LPS instillation. a,bDifferent letters indicate statistical differences (P < 0.05) between the groups, while letters that match each other indicate no statistical difference.
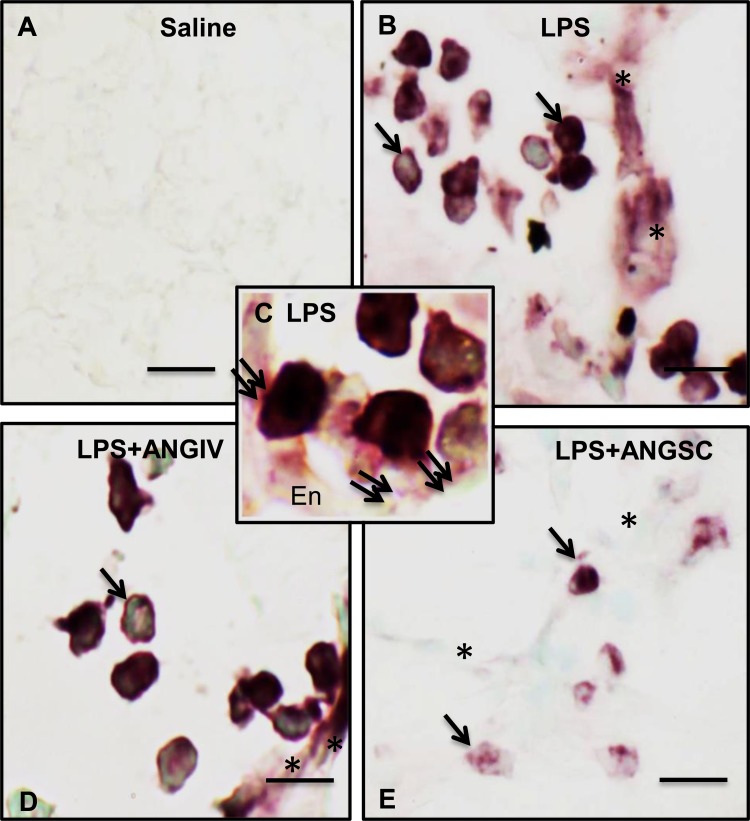
Ex vivo neutrophil pp38 MAPK immunocytochemistry.
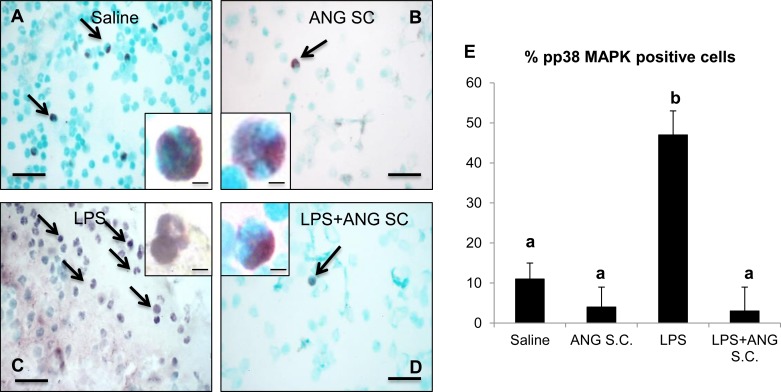
To further clarify whether a single treatment with angiostatin inhibited expression of pp38 MAPK in peripheral blood neutrophils, we stained peripheral blood neutrophils from the 24-h study for pp38 MAPK. Compared with pp38 MAPK staining in occasional neutrophils from both saline (Fig. 11A), angiostatin-only (Fig. 11B), and LPS+angiostatin (Fig. 11D) treatment groups, we observed significantly more pp38 MAPK-positive-stained neutrophils from 24-h LPS-only group (Fig. 11, C and E).
Fig. 11.
Phosphorylated p38 MAP kinase (pp38 MAPK) staining of peripheral blood neutrophil isolated from various mice groups, as described in materials and methods shows intense positive staining (marked by arrows) for LPS (C and inset) compared with saline (A and inset), subcutaneous angiostatin only (B and inset) and LPS+subcutaneous angiostatin (D and inset). Two-hundred peripheral blood neutrophils were counted from each group and plotted to represent the percentage of positive cells (E). a,bDifferent letters indicate statistical differences (P < 0.05) between the groups, while letters that match each other indicate no statistical difference. Scale bar = 10 μm for wide fields; 5 μm for insets.
Intravenous angiostatin treatment and lung inflammation.
To further clarify the effects of intravenously administered angiostatin on LPS-induced ALI, we either coadministered angiostatin with LPS or 5 h after LPS treatment (data not shown). There was a significant increase in protein concentration in BAL from the 12-h LPS-treated mice (366.4 ± 2.7 μg/ml) compared with the time-matched controls (54.7 ± 1.0 μg/ml) (P < 0.02). Intravenous coadministration of angiostatin with LPS resulted in significant reduction in BAL protein (159.7 ± 0.9 μg/ml) compared with the 12-h LPS-only group. However, there was no difference between the LPS-only mice and those receiving angiostatin 5 h after LPS treatment (260.3 ± 4.6 μg/ml) and euthanized 12 h after LPS treatment.
Total cell counts in the BAL were significantly higher in the LPS-only group (6.6 × 106 ± 0.9) compared with the saline control (0.4 × 106 ± 0.1) at 12-h time point. Neither intravenous coadministration (3.3 × 106 ± 0.1) nor administration of angiostatin 5 h after LPS treatment (7.2 × 106 ± 0.3) affected BAL cell counts or MPO concentrations in lung tissues compared with the LPS-only group (data not shown).
DISCUSSION
We report important data showing inhibition of both early and late signs of acute lung inflammation by single subcutaneous treatment with angiostatin 5 h after a single intranasal challenge with LPS. These data are significant because of the ongoing lack of major therapeutic breakthroughs against acute lung injury. We used a well-established mouse model of lung inflammation where intranasal E. coli LPS induces neutrophil migration, increased microvascular permeability, and monocyte/macrophage recruitment, and all these changes are observed within 24 h of LPS administration (5, 16). The subcutaneous angiostatin treatment caused a significant reduction in neutrophil numbers in the BAL at 9, 15, as well as 24 h after LPS instillation and reduced MPO concentrations in lung homogenates at 9 as well as 24 h after LPS instillation. Furthermore, there was reduced septal congestion and near absence of inflammatory cells in the perivascular, peribronchiolar, and septal compartments of LPS-treated mice that were administered angiostatin subcutaneously. The subcutaneous treatment with angiostatin also inhibited LPS-induced edema formation, as indicated by reduction in BAL protein concentration, in the lungs at 9 and 24 h after LPS instillation. These data show that subcutaneous administration of angiostatin inhibits two major characteristics, neutrophil accumulation and vascular permeability, of LPS-induced ALI in mice.
Neutrophil migration is a hallmark of ALI and is induced through a complex interplay of adhesion molecules, cytokines, and chemokines (20). To explore potential mechanisms of actions of angiostatin, we examined the expression of chemokines such as KC, MCP-1, and MIP-1α, which are known mediators of neutrophil migration in the lung (43) and IL-1β, which is a major player in activation of endothelium in the LPS-induced lung inflammation. The subcutaneous angiostatin treatment did not alter the LPS-induced increases in expression of MCP-1, KC, MIP-1α, and IL-1β protein and mRNA in BAL and lung homogenates, respectively. Angiostatin, however, reduced immunohistologic expression of vWF, an inflammatory adhesive protein, in lung vessels, and pp38 MAPK in neutrophils in lung tissues, to suggest potential mechanisms underlying the reduced recruitment of neutrophils into inflamed lungs. The data showing reduced immunocytological staining of pp38 MAPK in neutrophils isolated from peripheral blood of mice treated with LPS and subcutaneous angiostatin may be important because p38 MAPK critically regulates neutrophil chemotaxis, survival, and production of reactive oxygen species (18, 29). These in vivo data are supported by our recent in vitro observations that angiostatin shuts down phosphorylation of p38 MAPK, alters cytoskeleton of activated neutrophils, and inhibits chemotaxis (3). In addition to the suppression of pp38 MAPK in lung tissues and peripheral blood neutrophils by angiostatin, it is possible that angiostatin may also manifest its actions through interactions with one of its receptors such as integrin αvβ3, which is expressed by neutrophils and endothelial cells in the normal and the inflamed lungs (15, 34). The αvβ3 integrin plays a role in neutrophil migration, and its ligation by multimeric vitronectin increases lung capillary permeability (14, 41). Interestingly, we recently showed increased immunohistologic expression of angiostatin, vitronectin, and integrin αvβ3 in alveolar septa and inflammatory cells in lungs from human sepsis patients (35). The exogenous as well as endogenous angiostatin may interfere with the functioning of integrin αvβ3 to reduce migration of neutrophils and also maintain lung barrier function to suppress lung edema formation in LPS-treated mice. We have also found with confocal microscopy and coimmunoprecipitation that angiostatin interacts with integrin αvβ3 (3). Nevertheless, subcutaneous treatment with angiostatin inhibited phosphorylated p38 MAPK expression without altering expression of the inflammatory mediators examined in this study.
Apoptosis is a noninflammatory way of eliminating activated neutrophils, inhibiting release of reactive oxygen species and lysosomal enzymes, and initiating processes, such as IL-10 production and macrophage recruitment to restore physiological homeostasis (23, 24). Intravenous, as well as subcutaneous angiostatin injection in LPS-treated mice, induced significant apoptosis in BAL neutrophils compared with mice treated with LPS or angiostatin only. The integrin αvβ3 also plays a role in increased survival and proliferation of endothelial cells (6, 7, 10, 41). Angiostatin binds to and inhibits F1F0 ATP synthase under conditions of low pH to cause ATP deficiency and consequently caspase-mediated apoptosis (42, 44). In vitro data from our laboratory show that angiostatin binds to F1F0 ATP synthase, induces activated caspase-3, and causes apoptosis in LPS-treated neutrophils (3). Although both intravenous and subcutaneous treatments with angiostatin increased apoptotic cells in the LPS-treated lungs, only subcutaneous angiostatin had clear anti-inflammatory effects.
In contrast to therapeutic effects of subcutaneous angiostatin in LPS-treated mice, the intravenously administered angiostatin had intriguing effects on lung inflammation. Intravenous treatment of LPS-challenged mice with angiostatin shows significant lung inflammation, as indicated by migration of neutrophils and vascular expression of vWF, and it had no effect on BAL protein and lung MPO compared with LPS-treated mice, even at longer time points, such as 12 h (data not shown). It is important to note that angiostatin alone administered via either of the routes did not cause lung inflammation in normal mice. The specific mechanisms underlying the inflammatory actions of intravenous angiostatin compared with the subcutaneous angiostatin in LPS-treated mice could not be ascertained from this study. The bolus nature of angiostatin concentrations in the vascular compartment leading to endothelial activation and potential induction of apoptosis via integrin αvβ3 may lead to increased hydraulic conductivity and inflammation (41). On the basis of these data, it appears that intravenous in contrast to subcutaneous angiostatin may not yield beneficial effects in endotoxin-induced ALI.
The angiostatin levels in BAL and lung tissues following subcutaneous or intravenous treatments were also different. Higher levels of angiostatin in BAL in LPS-treated mice most likely were contributed by alveolar macrophages, newly migrated neutrophils, as well as leakage of angiostatin from lung vasculature, and these levels were reversed by anti-inflammatory actions of subcutaneous angiostatin. The subcutaneous angiostatin, however, led to higher levels of angiostatin in lung tissues in LPS-treated mice compared with only LPS treatment, most likely through retention of angiostatin in lung vasculature through binding to endothelial integrin αvβ3, strengthening of vascular barrier to prevent leakage into alveoli, and reduced production in the alveoli because of inhibition of alveolar recruitment of activated neutrophils that can produce angiostatin. While BAL levels of angiostatin were lower in LPS-treated mice administered intravenous angiostatin compared with the LPS-only mice, the lung homogenate levels were not different between the two groups. Although the reasons for these differences are not clear from our study, it seems that an intravenous bolus of angiostatin is not absorbed at high levels by the lung tissues compared with subcutaneous treatment because of potentially quick metabolism and excretion from the body. Therefore, the sustained, but not sharp, bolus availability of angiostatin in vascular compartment compared with BAL levels seems to be critical for its therapeutic effects. Additional experiments are required to delineate pharmacokinetics of angiostatin administered via different routes in normal and LPS-challenged animals.
On the basis of these data, we conclude that subcutaneous treatment with angiostatin ameliorates LPS-induced acute lung inflammation through reduction of neutrophil migration, vascular permeability, and phosphorylated p38 MAPK expression in the lungs. The angiostatin treatment also caused apoptosis in neutrophils and lung parenchymal cells. While further studies are needed on the effects of angiostatin on lung inflammation in a bacterial infection model of pneumonia, it appears that angiostatin treatment fine-tunes neutrophil migration without completely shutting it down and abrogating the innate inflammatory response.
GRANTS
The work was supported by a Discovery Grant to Baljit Singh from Natural Sciences and Engineering Research Council of Canada. Dr. Aulakh was supported by a Dean's Scholarship from College of Graduate Studies and Research of the University of Saskatchewan.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: G.K.A. and B.S. conception and design of research; G.K.A. and S.S.S. performed experiments; G.K.A. and B.S. analyzed data; G.K.A. and B.S. interpreted results of experiments; G.K.A. prepared figures; G.K.A. and B.S. drafted manuscript; G.K.A. and B.S. edited and revised manuscript; G.K.A., S.S.S., and B.S. approved final version of manuscript.
REFERENCES
- 1.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: From mechanisms to disease. Annu Rev Immunol 30: 459–489, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Aoshiba K, Yasui S, Hayashi M, Tamaoki J, Nagai A. Role of p38-mitogen-activated protein kinase in spontaneous apoptosis of human neutrophils. J Immunol 162: 1692–1700, 1999 [PubMed] [Google Scholar]
- 3.Aulakh GK, Balachandran Y, Liu L, Singh B. Angiostatin inhibits activation and migration of neutrophils. Cell Tissue Res In press doi:10.1007/s00441-013-1753-0 [DOI] [PubMed] [Google Scholar]
- 4.Benelli R, Morini M, Carrozzino F, Ferrari N, Minghelli S, Santi L, Cassatella M, Noonan DM, Albini A. Neutrophils as a key cellular target for angiostatin: implications for regulation of angiogenesis and inflammation 1. FASEB J 16: 267–269, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Brigham KL, Meyrick B. Endotoxin and lung injury. Am Rev Respir Dis 133: 913–927, 1986 [PubMed] [Google Scholar]
- 6.Brooks PC, Clark RAF, Cheresh DA. Requirement of vascular integrin α(v)β3 for angiogenesis. Science 264: 569–571, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Brooks PC, Montgomery AMP, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin α(v)β 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 79: 1157–1164, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Chavakis T, Athanasopoulos A, Rhee JS, Orlova V, Schmidt-Woll T, Bierhaus A, May AE, Celik I, Nawroth PP, Preissner KT. Angiostatin is a novel anti-inflammatory factor by inhibiting leukocyte recruitment. Blood 105: 1036–1043, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Chen YH, Huang YH, Wu HL, Wu MP, Chang WT, Kuo YZ, Lu KC, Wu LW. Angiostatin K1–3 induces E-selectin via AP1 and Ets1: a mediator for anti-angiogenic action of K1–3. J Thromb Haemost 6: 1953–1961, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Drake CJ, Cheresh DA, Little CD. An antagonist of integrin α(v)β3 prevents maturation of blood vessels during embryonic neovascularization. J Cell Sci 108: 2655–2661, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Falcone DJ, Khan KMF, Layne T, Fernandes L. Macrophage formation of angiostatin during inflammation. A byproduct of the activation of plasminogen. J Biol Chem 273: 31480–31485, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Hamacher J, Lucas R, Lijnen HR, Buschke S, Dunant Y, Wendel A, Grau GE, Suter PM, Ricou B. Tumor necrosis factor-α and angiostatin are mediators of endothelial cytotoxicity in bronchoalveolar lavages of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 166: 651, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am J Respir Crit Care Med 160: 5, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Hendey B, Lawson M, Marcantonio EE, Maxfield FR. Intracellular calcium and calcineurin regulate neutrophil motility on vitronectin through a receptor identified by antibodies to integrins αv and β3. Blood 87: 2038–2048, 1996 [PubMed] [Google Scholar]
- 15.Janardhan KS, Appleyard GD, Singh B. Expression of integrin subunits αv and β3 in acute lung inflammation. Histochem Cell Biol 121: 383–390, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Janardhan KS, Sandhu SK, Singh B. Neutrophil depletion inhibits early and late monocyte/macrophage increase in lung inflammation. Front Biosci 11: 1569–1576, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Jurasz P, Santos-Martinez MJ, Radomska A, Radomski MW. Generation of platelet angiostatin mediated by urokinase plasminogen activator: effects on angiogenesis. J Thromb Haemost 4: 1095–1106, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Kutsuna H, Suzuki K, Kamata N, Kato T, Hato F, Mizuno K, Kobayashi H, Ishii M, Kitagawa S. Actin reorganization and morphological changes in human neutrophils stimulated by TNF, GM-CSF, and G-CSF: The role of MAP kinases. Am J Physiol Cell Physiol 286: C55–C64, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Lay AJ, Jiang XM, Kisker O, Flynn E, Underwood A, Condron R, Hogg PJ. Phosphoglycerate kinase acts in tumour angiogenesis as a disulphide reductase. Nature 408: 869–873, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Rev Immunol 7: 678–689, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Lucas R, Lijnen HR, Suffredini AF, Pepper MS, Steinberg KP, Martin TR, Pugin J. Increased angiostatin levels in bronchoalveolar lavage fluids from ARDS patients and from human volunteers after lung instillation of endotoxin. Thromb Haemost 87: 966–971, 2002 [PubMed] [Google Scholar]
- 22.Luo J, Lin J, Paranya G, Bischoff J. Angiostatin upregulates E-selectin in proliferating endothelial cells. Biochem Biophys Res Commun 245: 906–911, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Matute-Bello G, Martin T. Apoptosis in acute lung injury. Crit Care 7: 355–358, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mecklenburgh K, Murray J, Brazil T, Ward C, Rossi AG, Chilvers ER. Role of neutrophil apoptosis in the resolution of pulmonary inflammation. Mon Arch Chest Dis 54: 345–349, 1999 [PubMed] [Google Scholar]
- 25.Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med 358: 716–727, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA 103: 12493–12498, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nuzzi PA, Lokuta MA, Huttenlocher A. Analysis of neutrophil chemotaxis. Methods Mol Biol 370: 23–36, 2007 [DOI] [PubMed] [Google Scholar]
- 28.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79: 315–328, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Sabroe I, Whyte MK. Toll-like receptor (TLR)-based networks regulate neutrophilic inflammation in respiratory disease. Biochem Soc Trans 35: 1492–1495, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Scapini P, Nesi L, Morini M, Tanghetti E, Belleri M, Noonan D, Presta M, Albini A, Cassatella MA. Generation of biologically active angiostatin Kringle 1–3 by activated human neutrophils 1. J Immunol 168: 5798–5804, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Schierwagen C, Bylund-Fellenius AC, Lundberg C. Improved method for quantification of tissue PMN accumulation measured by myeloperoxidase activity. J Pharmacol Methods 23: 179–186, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Seeley EJ, Matthay MA, Wolters PJ. Inflection points in sepsis biology: from local defense to systemic organ injury. Am J Physiol Lung Cell Mol Physiol 303: L355–L363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma MR, Rothman V, Tuszynski GP, Sharma MC. Antibody-directed targeting of angiostatin's receptor annexin II inhibits Lewis lung carcinoma tumor growth via blocking of plasminogen activation: possible biochemical mechanism of angiostatin's action. Exp Mol Pathol 81: 136–145, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Singh B, Fu C, Bhattacharya J. Vascular expression of the αvβ3-integrin in lung and other organs. Am J Physiol Lung Cell Mol Physiol 278: L217–L226, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Singh B, Janardhan KS, Kanthan R. Expression of angiostatin, integrin αvβ3, and vitronectin in human lungs in sepsis. Exp Lung Res 31: 771–782, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Syed SP, Martin AM, Haupt HM, Arenas-Elliot CP, Brooks JJ. Angiostatin receptor annexin II in vascular tumors including angiosarcoma. Hum Pathol 38: 508–513, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Szarka RJ, Wang N, Gordon L, Nation PN, Smith RH. A murine model of pulmonary damage induced by lipopolysaccharide via intranasal instillation. J Immunol Methods 202: 49–57, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Tabruyn SP, Griffioen AW. Molecular pathways of angiogenesis inhibition. Biochem Biophys Res Commun 355: 1–5, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Tarui T, Miles LA, Takada Y. Specific Interaction of Angiostatin with Integrin αvβ3 in endothelial cells. J Biol Chem 276: 39562–39568, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Troyanovsky B, Levchenko T, Mansson G, Matvijenko O, Holmgren L. Angiomotin an angiostatin binding protein that regulates endothelial cell migration and tube formation. J Cell Biol 152: 1247–1254, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsukada H, Ying X, Fu C, Ishikawa S, McKeown-Longo P, Albelda S, Bhattacharya S, Bray BA, Bhattacharya J. Ligation of endothelial alpha v beta 3 integrin increases capillary hydraulic conductivity of rat lung. Circ Res 77: 651–659, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Veitonmäki N, Cao R, Wu LH, Moser TL, Li B, Pizzo SV, Zhivotovsky B, Cao Y. Endothelial cell surface ATP synthase-triggered caspase-apoptotic pathway is essential for k1–5-induced antiangiogenesis. Cancer Res 64: 3679–3686, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Wagner JG, Roth RA. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev 52: 349–374, 2000 [PubMed] [Google Scholar]
- 44.Wahl ML, Kenan DJ, Gonzalez-Gronow M, Pizzo SV. Angiostatin's molecular mechanism: aspects of specificity and regulation elucidated. J Cell Biochem 96: 242–261, 2005 [DOI] [PubMed] [Google Scholar]