Abstract
Cystic fibrosis-related diabetes (CFRD) is the most common comorbidity associated with cystic fibrosis (CF), impacting more than half of patients over age 30. CFRD is clinically significant, portending accelerated decline in lung function, more frequent pulmonary exacerbations, and increased mortality. Despite the profound morbidity associated with CFRD, little is known about the underlying CFRD-related pulmonary pathology. Our aim was to develop a murine model of CFRD to explore the hypothesis that elevated glucose in CFRD is associated with reduced lung bacterial clearance. A diabetic phenotype was induced in gut-corrected CF transmembrane conductance regulator (CFTR) knockout mice (CFKO) and their CFTR-expressing wild-type littermates (WT) utilizing streptozotocin. Mice were subsequently challenged with an intratracheal inoculation of Pseudomonas aeruginosa (PAO1) (75 μl of 1–5 × 106 cfu/ml) for 18 h. Bronchoalveolar lavage fluid was collected for glucose concentration and cell counts. A portion of the lung was homogenized and cultured as a measure of the remaining viable PAO1 inoculum. Diabetic mice had increased airway glucose compared with nondiabetic mice. The ability to clear bacteria from the lung was significantly reduced in diabetic WT mice and control CFKO mice. Critically, bacterial clearance by diabetic CFKO mice was significantly more diminished compared with nondiabetic CFKO mice, despite an even more robust recruitment of neutrophils to the airways. This finding that CFRD mice boast an exaggerated, but less effective, inflammatory cell response to intratracheal PAO1 challenge presents a novel and useful murine model to help identify therapeutic strategies that promote bacterial clearance in CFRD.
Keywords: cystic fibrosis-related diabetes, murine model, Pseudomonas, pneumonia, cystic fibrosis
cystic fibrosis (CF) is the most common inheritable lethal disorder among Caucasians (25). Although often primarily characterized by a progressive pulmonary decline due to frequent pulmonary exacerbations, the disease carries risk for numerous other comorbidities (27). More than 75% of adults with CF demonstrate some aberrancy in glucose regulation, for many of which constitutes a comorbidity termed cystic fibrosis-related diabetes (CFRD) (4, 12, 25). CFRD is now recognized as the most common comorbidity associated with CF, affecting more than 50% of patients over the age of 30 with increasing prevalence rates rising with increasing age (4, 5, 25). CFRD is punctuated by delayed insulin secretion, glucose dysregulation, and hyperglycemia (22–24, 34). Consequences of prolonged hyperglycemia can include significant microvascular complications similar to what is observed in other types of diabetes (36, 40, 44). However, by far the most egregious insult to a patient with CFRD is the rapid decline in pulmonary function and increase in pulmonary exacerbations associated with onset of the disease (19). The inception of CFRD at any age accelerates pulmonary decline, as noted in multiple epidemiological studies documenting both deteriorating pulmonary function (forced expiratory volume in 1 s) and increased frequency of pulmonary exacerbations (17, 19). Even in patients with CF without overt CFRD, insulin sensitivity can be erratic, especially in the setting of infection with heightened inflammation (35). There is evidence to suggest that patients with CF even with glucose intolerance have a significant reduction in their pulmonary function tests 2–4 yr before meeting clinical criteria for diagnosis of CFRD (7, 18, 21). Although 85% of all patients with CF ultimately die with severe advanced lung disease, the ensuing exponential deficit in lung function associated with the onset of CFRD is detrimental, as patients with CFRD experience a sixfold higher mortality rate compared with their non-CFRD cohorts (8, 26). Although the pathogenesis of CFRD remains largely unknown, epidemiological studies have shown that patients with CF with either CFTR class I or II mutations, which account for over 50% of the CF patient population, are more likely to develop CFRD regardless of other risk factors, including pancreatic insufficiency (1, 4). As such, it is imperative to understand the pulmonary mechanisms by which the onset of hyperglycemia promotes a more rapid functional decline in patients with CF. However, despite the significant morbidity and mortality associated with the onset of CFRD, exploring the mechanisms by which CFRD contributes to poor clinical outcomes has been difficult. This gap in knowledge of various mechanisms underlying CFRD may in part be due to the lack of a well-established animal model of CFRD. In the present study, we used an animal model of CFRD to assess the bacterial clearance of the most common CF-related pulmonary pathogen, Pseudomonas aeruginosa (P. aeruginosa). These approaches will help establish this CFRD animal model to study other aspects of CFRD-related pathologies.
MATERIALS AND METHODS
The care and use of mouse strains.
Experiments utilized the gut-corrected CF transmembrane conductance regulator (CFTR) knockout mouse strain Cftrtm1Unc Tg(FABPCFTR)-1Jaw/J, which has been described previously (referred to as CFKO) (48). These mice demonstrate human CFTR expression regulated by expression of the fatty acid-binding protein promoter. As such, expression is limited to the small and large intestine with subsequent improved viability and no dietary restrictions or special dietary supplementation. These mice were developed on a mixed background of C57BL/6 and FVB/Nj; therefore, CFTR-expressing wild-type littermates (WT) were utilized as controls for experimental conditions. All animals were housed in specific pathogen-free housing with 12-h:12-h light/dark cycles at the Emory University Division of Animal Resources in accordance with NIH guidelines. Animals had allowances of food and water ad libitum with the exception of predetermined fasting states for streptozotocin (STZ) injections or intraperitoneal glucose tolerance tests. All experimental procedures were carried out with the approval from the Institutional Animal Care and Use Committee (IACUC) at Emory University (Atlanta, GA).
STZ induction of diabetes.
Male and female mice (8–12 wk of age) underwent induction of hyperglycemia with serial low-dose STZ (Sigma, St. Louis, MO) intraperitoneal injections as previously described (14). Following a 4-h fast, CFKO mice and their WT littermates were administered an intraperitoneal injection of STZ (50 mg/kg) serially every 24 h for 5 days. Weight measurements were recorded on the first day of STZ administration (day 1) and then subsequently on days 14 and 30. Four-hour fasting blood glucose levels were measured by tail vein sampling using a commercially available glucometer (Freestyle Lite; Abbott, Alameda, CA) on days 1, 14, and 30. Mice that did not demonstrate fasting blood glucose values ≥230 mg/dl on day 14 were removed from further analysis.
Intraperitoneal glucose tolerance test.
Thirty days following STZ induction of hyperglycemia, hyperglycemic CFKO and WT mice and their normoglycemic controls underwent an intraperitoneal glucose tolerance test (n = 3–5 per group). Mice were fasted for 16 h with free access to water. They then received an intraperitoneal injection of glucose (1 g/kg). Subsequent blood glucose levels were measured as described above at 0, 15, 30, 60, 90, 120, 150, and 180 min.
Bronchoalveolar lavage glucose measures.
Bronchoalveolar lavage fluid (BALF) was collected from mice 30 days following STZ-mediated induction of hyperglycemia. Mice were killed by intraperitoneal overdose of pentobarbital (150 mg/kg, Euthasol; Virbac, Fort Worth, TX). The anterior cervical region was sterilized with 70% ethanol. An anterior cervical midline incision was then made to expose the trachea. A small tracheal incision was made, and an 18-gauge × ½ inch blunt tip needle tubing adaptor (Becton Dickinson, Roswell, GA) was inserted into the trachea and secured with a 4.0 silk suture tied around the trachea. BALF was then collected with two 750-μl aliquots of sterile PBS. Cells were removed from the BALF through centrifugation at 3,000 g for 5 min. The supernatant was then stored at −20°C until glucose measures were taken. BALF supernatant glucose was measured with a commercially available colorimetric glucose assay kit (Cayman Chemical, Ann Arbor, MI) in duplicate.
PAO1 inoculum preparation.
Intratracheal bacterial challenges were performed with a non-mucoid strain of Pseudomonas aeruginosa expressing green fluorescence protein (PAO1). PAO1 was grown on LB agar plates for 24 h at 37°C with atmospheric CO2. Several colony-forming units (CFU) were removed from the plate with a sterile cotton tip applicator and resuspended in 1 ml of sterile PBS. The PAO1 solution was then washed twice with sterile PBS, and OD600 measurements were taken of the solution during growth at 37°C. Based on a previously determined OD600 growth curve for PAO1, the solution was diluted to a target of 5 × 106 CFU/ml in sterile PBS. This concentration was confirmed by culturing serial dilutions of the inoculums on LB agar plates at 37°C with atmospheric CO2 with colony counts measured at 24 h of growth.
In vivo bacterial pneumonia challenge.
Mice were placed under general anesthesia with a combination of intraperitoneal injection of ketamine/xylazine (100 mg/kg and 10 mg/kg, respectively). Once appropriately anesthetized, as determined by a lack of reflexive response to toe pinch, the anterior cervical region was sterilized with 70% ethanol. An anterior cervical midline incision was then made to expose the trachea. Using a 28-gauge insulin syringe (Becton Dickinson), the proximal trachea was punctured between cartilaginous rings, and 75 μl of 1–5 × 106 CFU/ml of PAO1 was instilled intratracheally. Direct tracheal instillation was confirmed by visualized fluid movement within the trachea, a change in respiratory status of the animal, and coughing following the instillation. The surgical wound was closed and secured with surgical staples. Mice were placed on their right side down, under a warming lamp and allowed to awake from general anesthesia. Eighteen hours following the intratracheal instillation of PAO1, the mice were killed by intraperitoneal overdose of pentobarbital (150 mg/kg, Euthasol) for specimen collection.
BAL cell counts.
Following death, BALF was collected as described above. A 50-μl aliquot of BALF was diluted 1:4 with 150 μl of sterile PBS for cytospin preparation. The 200-μl suspension was spun at 500 g for 5 min. The slides were then stained with a modified Giemsa stain (Diff Quick; ThermoScientific, Kalamazoo, MI) and allowed to dry for 24 h. Slides were then read with an Olympus upright microscope (model Bx51TF) with Olympus DP71 (U-TVO.63xC) digital camera (settings: ISO400, White Balance “on”, image size = 1360 × 1024, shift times = 1, Exposure = Auto, Spot = 30%). Differential counts were made counting leukocytes (neutrophils and macrophages) on 10 sequential high-powered (×40) fields. Relative absolute counts were multiplied by 4 to account for dilution factor.
Lung histology.
Sternotomy was performed to expose the lungs in the anterior chest. The pulmonary artery was identified and cannulated with a 30-gauge soft tip catheter. A small incision was made in the left atrium to ease fluid flow through the pulmonary vessels. Five milliliters of sterile PBS were used to flush the stagnant blood from the pulmonary vasculature. The left lobe was then resected and fixed in 4% paraformaldehyde followed by paraffin embedding and subsequent hematoxylin & eosin staining. Microscopic images were obtained with an Olympus upright microscope (model Bx51TF) as described previously.
Lung culture.
Following pulmonary arterial flush, the remainder of the lung was resected. Lobes utilized for culture were then weighed and homogenized in sterile PBS at a dilution of 1:10 (i.e., 1 g of lung wet weight: 10 ml of PBS) to standardize dilution between samples based on lung mass. Homogenates were then cultured with serial dilutions (25 μl of undiluted homogenate and 100 μl of 1:10, 1:100, and 1:1,000 dilutions) on LB agar plates fortified with ampicillin (50 μl/ml) to select for PAO1. Culture plates were incubated at 37°C and atmospheric CO2 with colony counts measured at 24 h.
Statistical analyses.
Data are expressed as means ± SE. Statistical analyses were performed with SigmaPlot 12.0 software (Jandel Scientific, San Jose, CA). Area under the curve was determined by the trapezoidal method. Analyses for all comparisons were performed by ANOVA followed by a Tukey's post hoc test, except fasting blood glucose values where differences were determined through a Student's two-tailed unequally-paired t-test. Animal numbers are noted in figure legends.
RESULTS
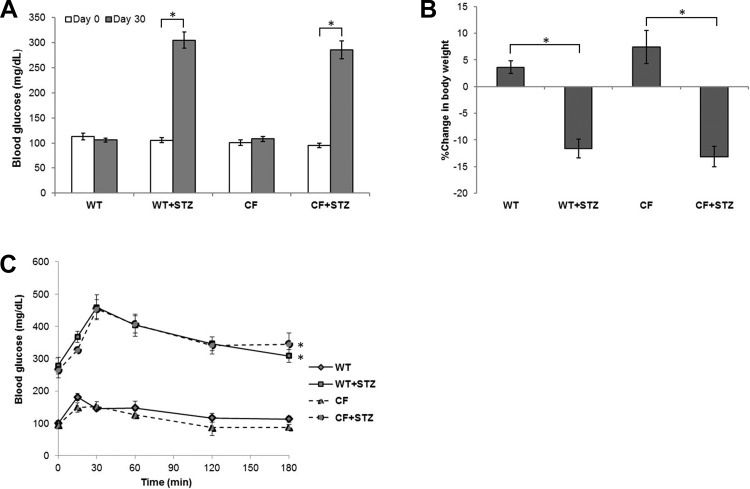
Induction of hyperglycemia in CFTR knockout (CFKO) mice was performed utilizing a previously well-established model of STZ administration (14). Before STZ administration, fasting blood glucose values in CFKO and WT mice were similar. STZ-treated mice developed fasting hyperglycemia (Fig. 1A) by 2 wk with no difference between WT and CFKO mice. Consistent with the catabolic state demonstrated in a diabetic phenotype, both WT and CFKO STZ-treated mice lost more body mass than controls over a 30-day period post-STZ injection (Fig. 1B).
Fig. 1.
Diabetic phenotypes in wild-type (WT) and cystic fibrosis (CF) transmembrane conductance regulator (CFTR) knockout (CFKO) mice. A: Increase in blood glucose levels in streptozotocin (STZ)-treated mice (n = 11–19). Under basal conditions, no significant differences between WT and CFKO mice were observed. 30 days after STZ treatments, blood glucose levels were significantly elevated in both WT and CFKO mice but were not significantly different between genotypes. B: changes in mouse weight 30 days after STZ treatment. STZ-treated animals showed a greater loss of body weight than untreated controls regardless of genotype (*P < 0.05; n = 22–31). No differences between non-STZ-treated WT and CFKO mice were observed. C: intraperitoneal glucose tolerance test. STZ-treated animals showed an exaggerated increase in blood glucose levels following intraperitoneal glucose administration where differences from baseline to peak glucose concentrations were greater (*P < 0.05; n = 3–5). Based on area-under-the-curve analyses, CFKO and WT mice treated with STZ were not statistically different, as both STZ-treated CFKO and WT mice produced similar glycemic profiles. No differences between non-STZ-treated WT and CFKO mice were observed.
Thirty days following STZ administration, an intraperitoneal glucose tolerance test was performed (Fig. 1C). STZ-treated mice had significantly higher levels of fasting blood glucose even following a 16-h fast. Additionally, both STZ-treated CFKO and STZ-treated WT mice demonstrated greater peak blood glucose levels (>200 mg/dl above baseline) compared with their respective group controls (∼100 mg/dl above baseline) consistent with the diabetic phenotype seen in CFRD (24). The lack of functional CFTR did not diminish or augment the effect of STZ-induced glycemic profiles.
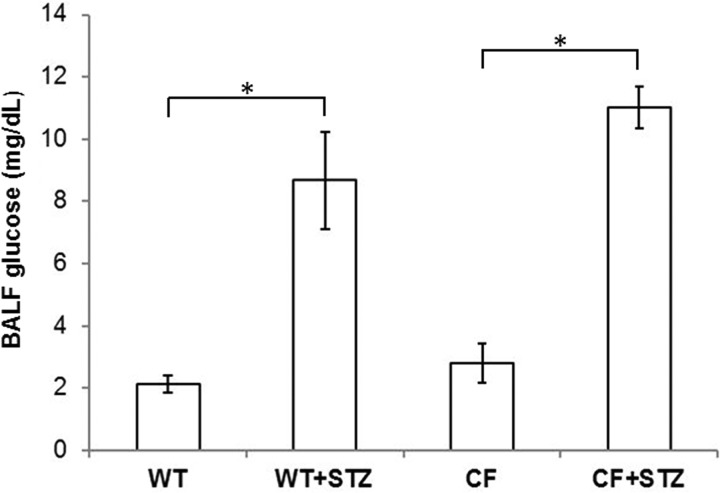
Airway glucose is normally 12.5-fold lower then blood levels and is tightly regulated but has been shown to be elevated in settings of airway inflammation or systemic hyperglycemia (2, 30). To measure baseline airway glucose, we performed tracheal cannulation with subsequent BALF collection. Consistent with their blood values, both STZ-treated WT and STZ-treated CFKO mice demonstrated significantly higher levels of BALF glucose at baseline (Fig. 2).
Fig. 2.
Glucose concentrations in the bronchoalveolar lavage fluid (BALF). Uninfected WT and CFKO mice treated with STZ displayed significantly higher levels of glucose in BALF compared with nondiabetic, genotype-matched controls (*P < 0.05; n = 4–6). No differences between STZ-treated wild-type and CFKO mice were observed.
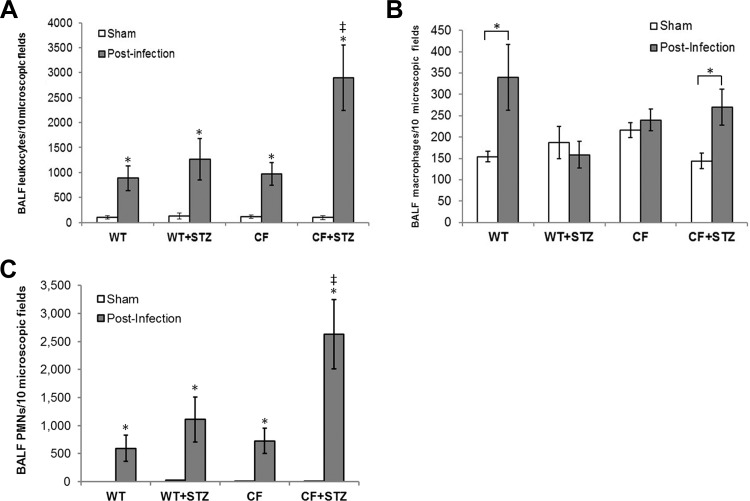
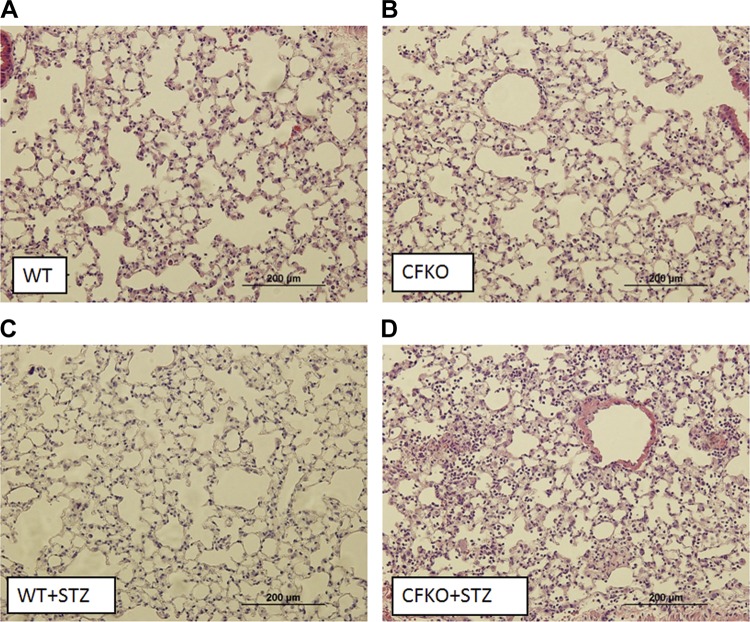
Thirty days following STZ treatments, mice underwent a bacterial pneumonia challenge with a non-mucoid strain of P. aeruginosa (PAO1). Eighteen hours after PAO1 challenge, BALF was obtained for cell counts and differential staining. All groups had elevated BALF leukocyte counts compared with sham-operated controls without bacterial installation (Fig. 3A). The increase in the airway leukocytic infiltrate in response to infection in the diabetic CFKO mice was significantly higher than in both diabetic and nondiabetic WT mice and in normoglycemic CFKO controls. Additionally, the increase in leukocytic infiltration was primarily due to neutrophilic infiltration (Fig. 3, B and C). Sham-treated, diabetic WT and CFKO mice displayed no significant differences in leukocytic infiltrate compared with nondiabetic WT controls. To confirm the enhanced cellularity in the infected diabetic CFKO mouse lung, lung histological sections were stained with hematoxylin and eosin and were found to exhibit a much higher level of cellular infiltration in the diabetic CFKO lung (Fig. 4).
Fig. 3.
Pulmonary leukocytic infiltrate levels following Pseudomonas aeruginosa (PAO1) instillation. Data are presented as the means ± SE of positively identified leukocytes in 10 high-powered fields following differential staining. A: relative leukocytic infiltrates in BALF from sham and infected mice. PAO1 infection caused a significant increase in leukocyte infiltration compared with basal levels of leukocytes in sham-operated controls of both genotypes (*P < 0.05; n = 8–12). PAO1-infected diabetic CFKO mice showed statistically higher levels of leukocytic infiltration compared with infected WT, diabetic WT, and CFKO mice (*P < 0.05). B: pulmonary macrophage levels in the BALF. BALF macrophage levels were increased by ∼2-fold following infection with PAO1 in WT mice (*P < 0.05) but not in diabetic WT mice. Macrophages in CFKO mice were not higher than in WT mice but were higher in diabetic CFKO mice (*P < 0.05). C: polymorphonuclear cells (PMNs, neutrophils) in the BALF. PMNs increased in each group with infection (*P < 0.05). The relative increase in total leukocytic infiltration is primarily a consequence of an increase of PMNs in the BALF, constituting upwards of 90% of the total infiltrate. *Statistical difference (P < 0.05) between sham and postinfection mice within each group. ‡Statistical difference (P < 0.05) between diabetic CFKO and other groups.
Fig. 4.
Lung histology following PAO1 infection. Left lobes were processed and stained with hematoxylin and eosin for morphological analyses. Findings support relative levels of leukocytes in the BALF determined through differential staining, where PAO1-infected diabetic CFKO mice displayed higher levels of leukocytic infiltration.
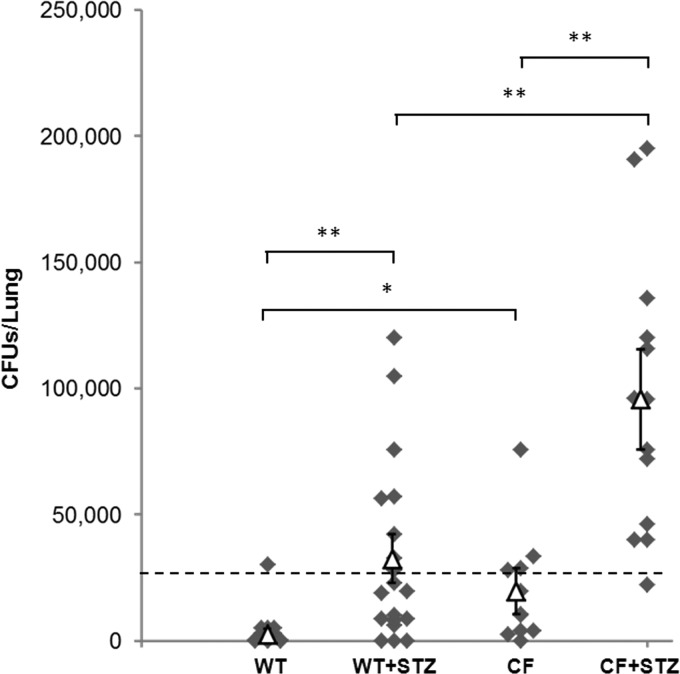
Patients with CFRD have more hospitalizations for pulmonary exacerbations, suggesting that, in CFRD, the ability to properly handle bacterial infections is even further complicated (43). Following exposure to intratracheal PAO1, a portion of the lung was resected, homogenized, and cultured for CFUs as a measure of the remaining viable PAO1 inoculum. Nondiabetic CFKO lungs contained significantly more viable PAO1 compared with nondiabetic WT mice, where most WT mice showed complete clearance of the infection (Fig. 5). Both diabetic WT and diabetic CFKO mice harbored significantly more live PAO1 compared with nondiabetic controls, suggesting that airway hyperglycemia levels significantly reduced the capacity to clear the bacterial pneumonia. Importantly, diabetic CFKO mice showed an even greater inability to clear pulmonary bacteria compared with both nondiabetic CFKO and diabetic WT mice.
Fig. 5.
Pulmonary bacterial clearance after 18 h of PAO1 infection. Data are presented as a scatterplot of lung homogenate colony-forming units (CFUs) from individual mice (⧫; n = 8–16 per group) 18 h after PAO1 infection (△ = average CFUs/lung ± SE). The dashed line represents an approximate clearance threshold, based on 3 times the observed standard deviation above the mean value in nondiabetic WT mice for this dose of bacteria. Lung homogenates that displayed less than 25,000 CFUs/lung were considered to be cleared of the instilled PAO1 bacteria. Most WT mice were cleared 18 h postinfection. Counts in nondiabetic WT mice were significantly lower than in nondiabetic CFKO mice. Cultures from diabetic WT mice had substantially increased CFUs/lung compared with nondiabetic WT mice. CFUs/lung from diabetic CFKO mice were also substantially increased compared with nondiabetic CFKO mice. Interestingly, lungs from diabetic CFKO mice had significantly increased numbers of PAO1 CFUs compared with lungs from diabetic WT mice, suggesting that diabetic CFKO mice have a diminished ability to properly clear PAO1 infections. *P < 0.05, **P < 0.01.
DISCUSSION
CFRD is a relative insulin deficiency, leading initially to ineffective postprandial glucose control followed by sustained fasting hyperglycemia (24, 47). The onset of CFRD is associated with dysglycemia and increased pulmonary exacerbations with a decline in overall health (7, 19). Despite this significance, the underlying mechanisms of pulmonary decline in CFRD are not well understood. The present results show that hyperglycemia in mice lacking functional CFTR disrupts airway glucose control, exaggerates pulmonary neutrophilic infiltration in response to a bacterial challenge, and reduces lung bacterial clearance over time. To our knowledge, this is the first murine model of CFRD that has been utilized to study lung bacterial clearance and establishes an easily manipulated and useful animal model for further exploration of this significant disease.
Following administration of STZ, CFKO mice developed significant fasting hyperglycemia and weight loss compared with both nondiabetic WT and CFKO controls. Unlike previous reports where CFTR-deficient mice showed an exacerbation of STZ-induced hyperglycemia, our findings showed no difference between STZ-treated CFKO and WT mice (38). The rationale for these differences is likely a result in the variations of STZ treatment regimens. Stalvey et al. (37, 38) used a single low dose of STZ (100 mg/kg). This produced glycemic effects in the CFKO mice, suggestive that the pancreata from these mice may be more sensitive to a single STZ treatment. Our approach did not focus on the susceptibility of the pancreas to STZ but rather to make the CFKO and WT mice diabetic. Thus we used serial doses of STZ (50 mg/kg) over 5 days, which resulted in all mice, both CFKO and WT, becoming hyperglycemic.
In humans, it is well recognized that the onset of CFRD correlates with a drop in BMI, which is observed even before a clinical diagnosis of hyperglycemia is made (10, 21). Related to hyperglycemia, insulin deficiency can lead to episodes of heightened protein catabolism, particularly in times of acute physiological stress, such as that associated with pulmonary exacerbations (17). Historical data show that patients with CF that have low BMIs generally exhibit a more rapid decline in pulmonary function (39). Regardless of the overall effects, treatment of mice with STZ caused weight loss, which helps to further establish this model of CFRD as being relevant to the human CFRD condition.
Diabetic CFKO mice demonstrated a robust yet ineffective inflammatory response to bacterial pneumonia. Stalvey and coworkers (37, 38) previously demonstrated that splenocytes from CFKO mice treated with STZ demonstrated greater differences in cytokine production compared with controls when stimulated in vitro. These data suggest that hyperglycemia primes immunoreactivity and inflammation with CFTR deficiency (37). The current study differs in that the effects were assessed in the lung, being the primary target organ in CFRD. Findings here show that pulmonary immunocytic infiltrate, primarily neutrophils, is exacerbated in diabetic CFKO mice compared with other mouse groups following tracheal bacterial instillation in vivo. Overabundance of neutrophilic infiltrate in the CF lung can alter the pulmonary environment through release of elastase, overproduction of reactive oxygen species, and excessive cytokine production (6). These features were not measured in this study, but excessively high levels of infiltrate in response to bacterial pneumonia may serve as a means to suggest the potentiation of a more rapid decline in lung function in patients with CFRD.
Another observation made in patients with CFRD is elevated glucose concentrations in the airway. Recent work in humans showed that exhaled breath condensate glucose levels, as well as breath-to-blood glucose ratios in patients with CFRD, were significantly higher than airway glucose levels in patients with CF without CFRD (2). In our study, loss of glucose control in the airway was determined in the BALF of diabetic WT and CFKO mice. Uncontrolled airway glucose may have a myriad of implications in CF. First, the onset of hyperglycemia may aggravate structural changes to the lung tissue itself. Pathology samples from diabetic lung tissue have demonstrated thickened alveolar epithelial basal lamina compared with age-matched controls (46). Similarly, changes in Type II alveolar cells and lung endothelial cells and extracellular matrix hyperplasia have been seen in animal models of diabetes (31, 32, 42). These changes may complicate the already distorted lung parenchyma and small airway changes associated with CF (5a, 33, 41).
With regard to lung infection, dysregulation of airway glucose control may also promote an environment that supports bacterial survival and growth. In the setting of high airway glucose concentrations, excessive glucose may serve as a steady source of both carbon and energy for proliferating infectious bacteria, creating an environment within the lung conducive to bacterial growth and endurance (11). Recent in vivo animal data have demonstrated that airway glucose control is essential to maintaining airway sterility (29). In particular, PAO1 growth is positively correlated to glucose concentrations in vitro, suggesting that even relatively small errors in airway glucose control may contribute to heightened bacterial growth in CFRD (3).
Other CF animal models have strengths and weaknesses related to studying CFRD. Unlike CF mouse models, both the CF pig and ferret develop spontaneous pulmonary disease and demonstrate various levels of hepatic, gastrointestinal, and pancreatic dysfunction similar to various stages of pathology seen in patients with CF (16). Although these models provide exciting tools with which to further study CFRD, like all models, they also have various limitations. CF pigs are born with severe pancreatic exocrine inflammation and fibrosis mimicking the most advanced pancreatic disease in patients with CF (20). CF ferrets are born with substantially less pancreatic fibrosis; however, they demonstrate significantly reduced first-phase insulin secretion and abnormal glucose tolerance shortly after birth (28). Although these models will hopefully yield insights into the pancreatic decline in CF, they may be limited in respect to studying the addition of hyperglycemia on a CF background, as they demonstrate only a narrow window in which they do not display significant pancreatic dysfunction, unlike patients with CF who rarely develop CFRD before the age of 10 (25). Critically, compared with the CF pig and ferret models, the mouse model offers the advantage that the onset of diabetes can be controlled experimentally, which allows for an easily obtainable cohort of nondiabetic CF mice for controls.
Although murine models for CF do not exhibit all of the hallmarks of human CF lung pathology, they demonstrate exuberant inflammation and reduced lung bacterial clearance in response to pathogen exposure (13). This model utilized the gut-corrected CFTR-knockout mouse that expresses the human CFTR in cells with the fatty acid-binding protein 1 motif (48). Benefits of this model include ease of pup weaning and no dietary limitations, but molecularly this mouse model does not fully recapitulate CFTR dysfunction seen in the majority of patients with CF, who express mutant CFTR as opposed to being CFTR deficient. Patients with CF typically have a genetic mutation leading to lack of functional, although not absent, CFTR protein, the most common of which is the ΔF508 mutation (4). It previously has been demonstrated that presence of ΔF508 triggers endoplasmic reticulum overload and may affect gene expression (15, 45). Utilizing this model, the onset of diabetes can also be controlled by STZ treatment in mice expressing ΔF508 CFTR or other mutations. Future studies will need to make use of other CF mouse models to better mimic CFRD as it is expressed in humans. Our data suggest that the lack of CFTR alone is sufficient to exacerbate the consequences of CFRD. However, it is likely that the added expression of mutant CFTR (such as the common ΔF508 or G551D mutants) may further enhance CFRD-related pulmonary pathophysiology.
In this study, diabetic CFKO mice recapitulated phenotypic characteristics of the CF population with CFRD. Namely, they developed dysregulated systemic and airway glucose control and significant weight loss. In response to bacterial infection, we observed that pulmonary neutrophilic infiltration was substantially increased in diabetic CFKO mice, but, perhaps most importantly, bacterial clearance was significantly attenuated in diabetic CFKO mice. Together, these observations may capture two salient features of CFRD in humans, namely a more rapid decline in lung function as a result of excessive neutrophilic infiltration and more frequent, and possibly persistent, pulmonary exacerbations. This model can be further developed to understand other aspects of CFRD to address potential mechanisms in related pathologies.
GRANTS
This work was supported by NIH DK056481-08 (N. McCarty) NIH HL116958 (M. Koval), and NHLBI T32 training grant HL076118-08 (W. Hunt).
DISCLOSURES
W. Hunt was partially supported by a T32 training grant from NHLBI (HL076118-08) during portions of this study. M. Koval reports grants from NHLBI (HL116958) and from Emory University Research Committee during the conduct of the study and grants from NIAAA, grants from NHLBI, and grants from Kimberly Clark outside the submitted work. This work was supported by NIH DK056481-08 (N. McCarty). No funding source had any role in the experimental design, data collection and analysis, or the preparation of the manuscript. All other authors have no conflicts of interests, financial or otherwise, to declare.
AUTHOR CONTRIBUTIONS
Author contributions: W.R.H., M.K., N.A.M., and J.M.H. conception and design of research; W.R.H., S.M.Z., D.E.G., M.A.S., and J.M.H. performed experiments; W.R.H., S.M.Z., D.E.G., M.A.S., M.K., N.A.M., and J.M.H. analyzed data; W.R.H., S.M.Z., D.E.G., M.A.S., M.K., N.A.M., and J.M.H. interpreted results of experiments; W.R.H. and J.M.H. prepared figures; W.R.H. and J.M.H. drafted manuscript; W.R.H., S.M.Z., D.E.G., M.A.S., M.K., N.A.M., and J.M.H. edited and revised manuscript; W.R.H., S.M.Z., D.E.G., M.A.S., M.K., N.A.M., and J.M.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Frank Harris, Dr. LouAnn Brown, and other members of the Brown laboratory at Emory University for assistance and technical expertise. We also thank the Emory+Children's Center for Cystic Fibrosis Research CF Mouse Core for supplying the mice used in this project. We also thank Drs. Bruce Stanton and George O'Toole (Dartmouth University) for the generous donation of the PAO1.
REFERENCES
- 1.Adler AI, Shine BS, Chamnan P, Haworth CS, Bilton D. Genetic determinants and epidemiology of cystic fibrosis-related diabetes: results from a British cohort of children and adults. Diabetes Care 31: 1789–1794, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker EH, Clark N, Brennan AL, Fisher DA, Gyi KM, Hodson ME, Philips BJ, Baines DL, Wood DM. Hyperglycemia and cystic fibrosis alter respiratory fluid glucose concentrations estimated by breath condensate analysis. J Appl Physiol 102: 1969–1975, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Brennan AL, Gyi KM, Wood DM, Johnson J, Holliman R, Baines DL, Philips BJ, Geddes DM, Hodson ME, Baker EH. Airway glucose concentrations and effect on growth of respiratory pathogens in cystic fibrosis. J Cyst Fibros 6: 101–109, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Cystic Fibrosis Foundation Cystic Fibrosis Foundation Patient Registry Annual Data Report. Bethesda, MD: CF Foundation, 2010 [Google Scholar]
- 5.Cystic Fibrosis Foundation Cystic Fibrosis Foundation Patient Registry Annual Data Report. Bethesda, MD: CF Foundation, 2011 [Google Scholar]
- 5a.Durieu I, Peyrol S, Gindre D, Bellon G, Durand DV, Pacheco Y. Subepithelial fibrosis and degradation of the bronchial extracellular matrix in cystic fibrosis. Am J Respir Crit Care Med 158: 580–588, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Elborn JS, Shale DJ. Cystic fibrosis. 2. Lung injury in cystic fibrosis. Thorax 45: 970–973, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkelstein SM, Wielinski CL, Elliott GR, Warwick WJ, Barbosa J, Wu SC, Klein DJ. Diabetes mellitus associated with cystic fibrosis. J Pediatr 112: 373–377, 1988 [DOI] [PubMed] [Google Scholar]
- 8.Flume PA, Mogayzel PJ, Jr, Robinson KA, Rosenblatt RL, Quittell L, Marshall BC. Cystic fibrosis pulmonary guidelines: pulmonary complications: hemoptysis and pneumothorax. Am J Respir Crit Care Med 182: 298–306, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Hameed S, Morton JR, Jaffe A, Field PI, Belessis Y, Yoong T, Katz T, Verge CF. Early glucose abnormalities in cystic fibrosis are preceded by poor weight gain. Diabetes Care 33: 221–226, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han SO, Inui M, Yukawa H. Effect of carbon source availability and growth phase on expression of Corynebacterium glutamicum genes involved in the tricarboxylic acid cycle and glyoxylate bypass. Microbiology 154: 3073–3083, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Hardin DS, Moran A. Diabetes mellitus in cystic fibrosis. Endocrinol Metab Clin North Am 28: 787–800; ix, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Heeckeren A, Walenga R, Konstan MW, Bonfield T, Davis PB, Ferkol T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Invest 100: 2810–2815, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito M, Kondo Y, Nakatani A, Hayashi K, Naruse A. Characterization of low dose streptozotocin-induced progressive diabetes in mice. Environ Toxicol Pharmacol 9: 71–78, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol 143: 1883–1898, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keiser NW, Engelhardt JF. New animal models of cystic fibrosis: what are they teaching us? Curr Opin Pulm Med 17: 478–483, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch C, Rainisio M, Madessani U, Harms HK, Hodson ME, Mastella G, McKenzie SG, Navarro J, Strandvik B. Presence of cystic fibrosis-related diabetes mellitus is tightly linked to poor lung function in patients with cystic fibrosis: data from the European Epidemiologic Registry of Cystic Fibrosis. Pediatr Pulmonol 32: 343–350, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Lanng S, Hansen A, Thorsteinsson B, Nerup J, Koch C. Glucose tolerance in patients with cystic fibrosis: five year prospective study. BMJ 311: 655–659, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall BC, Butler SM, Stoddard M, Moran AM, Liou TG, Morgan WJ. Epidemiology of cystic fibrosis-related diabetes. J Pediatr 146: 681–687, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Meyerholz DK, Stoltz DA, Pezzulo AA, Welsh MJ. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am J Pathol 176: 1377–1389, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med 162: 891–895, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Mohan K, Miller H, Dyce P, Grainger R, Hughes R, Vora J, Ledson M, Walshaw M. Mechanisms of glucose intolerance in cystic fibrosis. Diabet Med 26: 582–588, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Moran A, Brunzell C, Cohen RC, Katz M, Marshall BC, Onady G, Robinson KA, Sabadosa KA, Stecenko A, Slovis B. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 33: 2697–2708, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moran A, Doherty L, Wang X, Thomas W. Abnormal glucose metabolism in cystic fibrosis. J Pediatr 133: 10–17, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care 32: 1626–1631, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moran A, Hardin D, Rodman D, Allen HF, Beall RJ, Borowitz D, Brunzell C, Campbell PW, 3rd, Chesrown Duchow SE, Fink C, Fitzsimmons RJ, Hamilton SC, Hirsch N, Howenstine I, Klein MS, Madhun DJ, Pencharz Z, Quittner PB, Robbins AL, Schindler MK, Schissel T, Schwarzenberg K, Stallings SJ, Zipf VA, et al. Diagnosis, screening and management of cystic fibrosis related diabetes mellitus: a consensus conference report. Diabetes Res Clin Pract 45: 61–73, 1999 [DOI] [PubMed] [Google Scholar]
- 27.O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet 373: 1891–1904, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Olivier AK, Yi Y, Sun X, Sui H, Liang B, Hu S, Xie W, Fisher JT, Keiser NW, Lei D, Zhou W, Yan Z, Li G, Evans TI, Meyerholz DK, Wang K, Stewart ZA, Norris AW, Engelhardt JF. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest 122: 3755–3768, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pezzulo AA, Gutierrez J, Duschner KS, McConnell KS, Taft PJ, Ernst SE, Yahr TL, Rahmouni K, Klesney-Tait J, Stoltz DA, Zabner J. Glucose depletion in the airway surface liquid is essential for sterility of the airways. PLoS One 6: e16166, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Philips BJ, Meguer JX, Redman J, Baker EH. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med 29: 2204–2210, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Plopper CG, Morishige WK. Alterations in granular (type II) pneumocyte ultrastructure by streptozotocin-induced diabetes in the rat. Lab Invest 38: 143–148, 1978 [PubMed] [Google Scholar]
- 32.Popov D, Simionescu M. Alterations of lung structure in experimental diabetes, and diabetes associated with hyperlipidaemia in hamsters. Eur Respir J 10: 1850–1858, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Ratjen F, Doring G. Cystic fibrosis. Lancet 361: 681–689, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Ripa P, Robertson I, Cowley D, Harris M, Masters IB, Cotterill AM. The relationship between insulin secretion, the insulin-like growth factor axis and growth in children with cystic fibrosis. Clin Endocrinol (Oxf) 56: 383–389, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Sc NN, Shoseyov D, Kerem E, Zangen DH. Patients with cystic fibrosis and normoglycemia exhibit diabetic glucose tolerance during pulmonary exacerbation. J Cyst Fibros 9: 199–204, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Schwarzenberg SJ, Thomas W, Olsen TW, Grover T, Walk D, Milla C, Moran A. Microvascular complications in cystic fibrosis-related diabetes. Diabetes Care 30: 1056–1061, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Stalvey MS, Brusko TM, Mueller C, Wasserfall CH, Schatz DA, Atkinson MA, Flotte TR. CFTR mutations impart elevated immune reactivity in a murine model of cystic fibrosis related diabetes. Cytokine 44: 154–159, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Stalvey MS, Muller C, Schatz DA, Wasserfall CH, Campbell-Thompson ML, Theriaque DW, Flotte TR, Atkinson MA. Cystic fibrosis transmembrane conductance regulator deficiency exacerbates islet cell dysfunction after beta-cell injury. Diabetes 55: 1939–1945, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Steinkamp G, Wiedemann B. Relationship between nutritional status and lung function in cystic fibrosis: cross sectional and longitudinal analyses from the German CF quality assurance (CFQA) project. Thorax 57: 596–601, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan MM, Denning CR. Diabetic microangiopathy in patients with cystic fibrosis. Pediatrics 84: 642–647, 1989 [PubMed] [Google Scholar]
- 41.Tomashefski JF, Jr, Konstan MW, Bruce MC, Abramowsky CR. The pathologic characteristics of interstitial pneumonia cystic fibrosis. A retrospective autopsy study. Am J Clin Pathol 91: 522–530, 1989 [DOI] [PubMed] [Google Scholar]
- 42.Uyy E, Antohe F, Ivan L, Haraba R, Radu DL, Simionescu M. Upregulation of caveolin-1 expression is associated with structural modifications of endothelial cells in diabetic lung. Microvasc Res 79: 154–159, 2010 [DOI] [PubMed] [Google Scholar]
- 43.van den Berg JM, Kouwenberg JM, Heijerman HG. Demographics of glucose metabolism in cystic fibrosis. J Cyst Fibros 8: 276–279, 2009 [DOI] [PubMed] [Google Scholar]
- 44.van den Berg JM, Morton AM, Kok SW, Pijl H, Conway SP, Heijerman HG. Microvascular complications in patients with cystic fibrosis-related diabetes (CFRD). J Cyst Fibros 7: 515–519, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Venkatakrishnan A, Stecenko AA, King G, Blackwell TR, Brigham KL, Christman JW, Blackwell TS. Exaggerated activation of nuclear factor-kappaB and altered IkappaB-beta processing in cystic fibrosis bronchial epithelial cells. Am J Respir Cell Mol Biol 23: 396–403, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Vracko R, Thorning D, Huang TW. Basal lamina of alveolar epithelium and capillaries: quantitative changes with aging and in diabetes mellitus. Am Rev Respir Dis 120: 973–983, 1979 [DOI] [PubMed] [Google Scholar]
- 47.Yung B, Noormohamed FH, Kemp M, Hooper J, Lant AF, Hodson ME. Cystic fibrosis-related diabetes: the role of peripheral insulin resistance and beta-cell dysfunction. Diabet Med 19: 221–226, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Zhou L, Dey CR, Wert SE, DuVall MD, Frizzell RA, Whitsett JA. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science 266: 1705–1708, 1994 [DOI] [PubMed] [Google Scholar]