Abstract
Secretoglobin (SCGB) 3A2 is a member of the SCGB gene superfamily of small secreted proteins, predominantly expressed in lung airways. We hypothesize that human SCGB3A2 may exhibit anti-inflammatory, growth factor, and antifibrotic activities and be of clinical utility. Recombinant human SCGB3A2 was expressed, purified, and biochemically characterized as a first step to its development as a therapeutic agent in clinical settings. Human SCGB3A2, as well as mouse SCGB3A2, readily formed a dimer in solution and exhibited novel phospholipase A2 inhibitory activity. This is the first demonstration of any quantitative biochemical measurement for the evaluation of SCGB3A2 protein. In the mouse as an experimental animal, human SCGB3A2 exhibited growth factor activity by promoting embryonic lung development in both ex vivo and in vivo systems and antifibrotic activity in the bleomycin-induced lung fibrosis model. The results suggested that human SCGB3A2 can function as a growth factor and an antifibrotic agent in humans. When SCGB3A2 was administered to pregnant female mice through the tail vein, the protein was detected in the dam's serum and lung, as well as the placenta, amniotic fluids, and embryonic lungs at 10 min postadministration, suggesting that SCGB3A2 readily crosses the placenta. The results warrant further development of recombinant SCGB3A2 as a therapeutic agent in treating patients suffering from lung diseases or preterm infants with respiratory distress.
Keywords: SCGB, embryonic lung ex vivo organ culture, bleomycin-induced pulmonary fibrosis, phospholipase A2 inhibitory activity, drug candidate treating lung diseases
secretoglobin (SCGB) 3A2 belongs to the SCGB gene superfamily, consisting of cytokine-like secreted proteins of small molecular weight (∼10 kDa) (12). The members of the SCGB gene superfamily are known to form a homo- or heterodimer and are exclusively found in mammals. Their concentrations are high in secretions of the lung, salivary gland, lachrymal gland, prostate, and uterus (11). Although their biological functions remain elusive, SCGBs are thought to play a role in the modulation of inflammation, tissue repair, and tumorigenesis.
SCGB1A1, the founding member of the SCGB gene superfamily, also called club (Clara) cell secretory protein or club (Clara) cell 10-kDa protein is the most well-characterized secretoglobin (21). SCGB1A1 has two cysteine residues at the NH2- and COOH-terminal regions of polypeptide, which assemble in antiparallel orientation to form a homodimer (3, 21, 25). SCGB1A1 is predominantly expressed in airway club cells, as the conventional name indicates. A number of studies demonstrated that SCGB1A1 possesses anti-inflammatory, antifibrotic, and immunomodulatory functions (10, 15, 18, 21). The anti-inflammatory and immunomodulatory activities are partly due to the ability of SCGB1A1 to inhibit phospholipase A2 (PLA2) activity (8, 20). SCGB1A1 also exhibits tumor suppressor activity (21). Thus, when SCGB1A1 was added to human lung adenocarcinoma-derived A549 cells in vitro, cells showed decreased invasiveness (17). Scgb1a1-null mice demonstrated increased incidence of airway epithelial hyperplasia and lung adenomas in a tobacco carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced carcinogenesis model (33), and enhanced colonization of B16F10 melanoma cells was found in their lungs (27).
SCGB3A2 is predominantly expressed in airway epithelial club cells (22). In fact, SCGB1A1, SCGB3A2, and another member of the SCGB3A subfamily, SCGB3A1, are all expressed in club cells (21, 22, 26). The temporal and spatial expression pattern is unique to each gene/protein, yet their expression appears to be interrelated and to balance each other. For instance, Scgb3a2 expression was increased in Scgb1a1-null lungs (31). Furthermore, Scgb1a1 was increased by the addition of oncostatin M in in vitro culture of transformed murine Clara cell-derived (mtCC) cells, whereas Scgb3a2 expression decreased (29). How the expression of these three genes/proteins is related, which affects function of club cells (in turn affecting the homeostasis and physiology of lung and etiology of lung diseases) is not known.
Mouse SCGB3A2 was previously shown to exhibit anti-inflammatory activity in ovalbumin-induced airway inflammation model mouse (5), growth factor activity by promoting lung development ex vivo and in vivo (14), and antifibrotic activity by using a bleomycin (BLM)-induced mouse pulmonary fibrosis model (13). The antifibrotic activity was due to SCGB3A2-induced increase of STAT1 phosphorylation and increased expression of inhibitory SMAD7, which led to the inhibition of the TGF-β signaling, resulting in reduced expression of various collagen genes and development of fibrotic tissues (13). The results altogether suggested the potential for use of SCGB3A2 to modify these processes in patients suffering from various lung diseases.
In this article, as the first step to provide the evidence for the feasibility of SCGB3A2 to be used as a drug in humans, recombinant human (rh) SCGB3A2 was prepared, characterized, and tested for its various activities in the mouse system. The rhSCGB3A2 exhibited both antifibrotic and growth factor activities comparable to those of recombinant mouse (rm) SCGB3A2. The results suggested that human SCGB3A2 protein may have potential to be used in future to inhibit fibrosis in patients with pulmonary fibrosis and promote lung development in premature infants.
MATERIALS AND METHODS
Purification of recombinant human SCGB3A2.
The human SCGB3A2 protein was expressed in Escherichia coli bacteria (strain HMS174/DE3). The amino acid sequence for rhSCGB3A2 was obtained from GenBank entries NP_473364, AAL26215, and Q96PL1, which were all in agreement, specifically by using predicted residues A19–V93 in the expressed protein, which corresponded to one of two possible predicted NH2 termini for the mature native protein. Another predicted NH2 terminus of the mature protein corresponded to F22. A synthetic gene for rhSCGB3A2 was constructed by codon usage optimized for E. coli K-12, and the gene was cloned into expression vector pTXB1 (New England Biolabs, Ipswich, MA). The rhSCGB3A2 was expressed as a COOH-terminal fusion with a synthetic derivative of ubiquitin, or ubiquitin-like protein (UBL). The UBL component contained a histidine tag enabling purification of the fusion protein using nickel-IMAC (immobilized metal ion chromatography) and anion exchange chromatography. The rhSCGB3A2 was then separated from the UBL in vitro by using a UBL protease and purified via cation exchange chromatography. Endotoxin content was assessed by using the LAL gel clot assay (Associates of Cape Cod, Falmouth, MA) with control standard endotoxin from E. coli O113:H10. The endotoxin content of the rhSCGB3A2 preparation was between 25 and 125 EU/mg, which was suitable for animal studies.
Materials.
Preparation of rmSCGB3A2, rhSCGB1A1, and rmSCGB1A1 with endotoxin levels suited for animal studies and of anti-mouse SCGB3A2 antibody was as previously described (4, 14, 22). Anti-human SCGB3A2 antibody was raised in rabbit against purified rhSCGB3A2. The protein concentration was determined by using Pierce BCA protein assay kit with bovine serum albumin as a standard. Chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
Tracheal aspirate fluids from premature infants were obtained in a previous clinical trial. The patients were enrolled at four participating hospitals: the University of Maryland School of Medicine (Baltimore, MD); Mercy Medical Center (Baltimore, MD); Winthrop-University Hospital, SUNY Stony Brook School of Medicine (Mineola, NY); and Christiana HealthCare Systems (Wilmington, DE). The Institutional Review Boards at each site approved the study protocol and patient informed consent forms (16). Tracheal aspirate fluid (TAF) samples were normalized by total protein amounts measured by BCA assay as previously described (16). Adult human serum and urine samples were obtained from healthy volunteers with informed consent.
Preparation of mutant variant of mouse SCGB3A2 (rmSCGB3A2-C47S).
The plasmid, encoding mutated variant of recombinant mouse SCGB3A2 (C47S of the mature protein, number of which is on the basis of L22 as its NH2 terminus), was generated from the original SCGB3A2 expression plasmid (pDEST-544-His6-NusA-TEV-SCGB3A2) (14) by using the QuickChange Site-Directed mutagenesis kit (Stratagene, San Diego, CA) with the following primers: 5′-AGGACTGAAGAAGTCTGTGGACGAGCTGGG-3′ and 5′-AGCTCGTCCACAGACTTCTTCAGTCCTGTC-3′, where the underlined nucleotides code for Ser. The DNA sequence of the mutant rhSCGB3A2-C47S coding sequence in the His6-NusA-TEV-SCGB3A2-C47S plasmid was confirmed. Expression of SCGB3A2-C47S protein was carried out in E. coli strain BL21(DE3)RIPL (Invitrogen/Life Technologies, Grand Island, NY) or Rosetta DE3 (Novagen, San Diego, CA). The expressed protein was purified through Ni-NTA column (Qiagen, Valencia, CA), which was then digested by His6-TEV protease, followed by ion-exchange chromatography with the Q Sepharose HP column (GE Healthcare Life Sciences, Piscataway, NJ) in the inverse mode, i.e., with collection of the flow through and gel-filtration chromatography using the Superdex 75 HR column (GE Healthcare Life Sciences).
PLA2 inhibition assay.
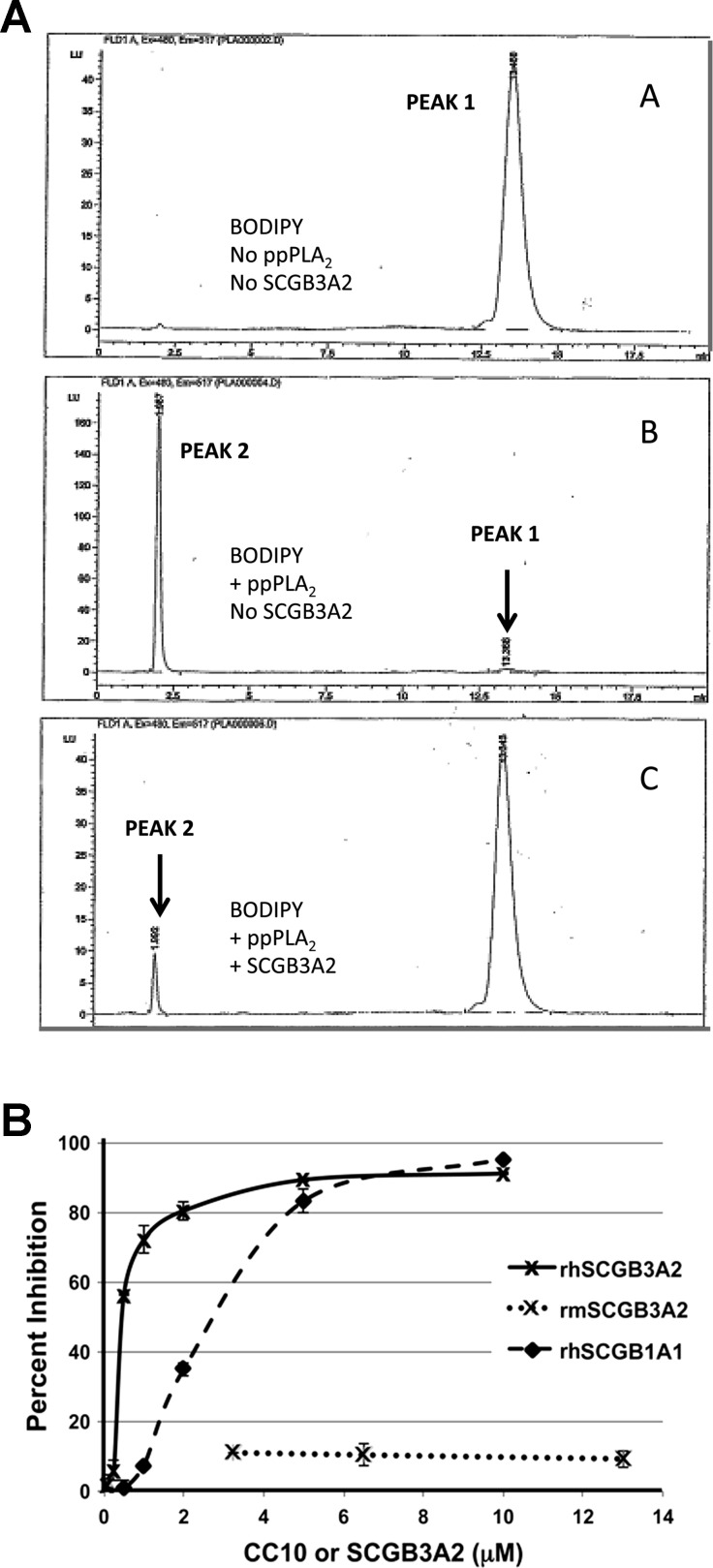
The rhSCGB1A1, rhSCGB3A2, or rmSCGB3A2 (5.5 μg each) was mixed with porcine pancreatic secretory PLA2 (ppPLA2) Type 1B (0.1 μg, Sigma-Aldrich) in Hanks' buffered saline solution (5.36 mM KCl, 0.44 mM KH2PO4, 140 mM NaCl, 4.19 mM NaHCO3, 3.49 mM Na2HPO4, 1 mM CaCl2, pH 7.4) and incubated at 37°C. The reaction was started through the addition of the fluorescent phospholipid analog 2-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl)-1-hexadecanoyl-sn-glycero-3-phosphocholine (BODIPY, 100 ng, Molecular Probes/Life Technologies), as previously described (16). After 15 min the reaction was terminated through the addition of 2-propanol/n-hexane. The cleavage product was separated from the substrate on a Spherisorb silica column (Waters, Milford, MA) run on an Agilent 1100 series HPLC (Santa Clara, CA). The cleavage product was quantified by use of a G1321A fluorescence detector (see Fig. 2A). The positive control was PLA2 alone, which cleaved the substrate into two HPLC peaks in which the product was directly quantified. The inhibitory activity was expressed as a percent inhibition as follows: [1−(average of the cleaved area with SCGB3A2/average of the cleaved area without SCGB3A2)] × 100.
Fig. 2.
Phospholipase A2 (PLA2) inhibitory activity of SCGB3A2. A: representative HPLC separation patterns of assay for PLA2 hydrolysis of specific fluorescent substrate (BODIPY). Top: BODIPY substrate with no porcine pancreatic secretory PLA2 (ppPLA2), no rhSCGB3A2. Peak 1 shows the intact BODIPY phospholipid substrate. Middle: BODIPY substrate plus ppPLA2, no rhSCGB3A2. This was used as positive control. Peak 2 represents the product after cleavage of the BODIPY substrate by ppPLA2. Bottom: BODIPY substrate plus ppPLA2, plus rhSCGB3A2; peak 2 is reduced demonstrating inhibition of PLA2 activity. Each reaction set was run in duplicate. B: inhibition of ppPLA2 activity by rhSCGB1A1, rhSCGB3A2, and rmSCGB3A2. Each curve is composed of average data points of 2 (rh and rmSCGB3A2) or 3 (rhSCGB1A1) measurements. Error bar is SD.
SDS-PAGE and Western blotting.
Purified recombinant protein or tracheal aspirate fluids were mixed with sodium dodecyl sulfate (2% SDS) in standard protein sample loading buffer (Invitrogen) in the presence or absence of 1 mM 2-mercaptoethanol, followed by electrophoresis on SDS-polyacrylamide gels (10–20%-tricine, Invitrogen) in 1 × running buffer (Invitrogen). Western blotting was carried out by electroblotting proteins to a PVDF membrane (Millipore, Billerica, MA). Membranes were blocked with TBS-Tween (20 mM Tris·HCl, pH 7.5, 150 mM NaCl, and 0.1% Tween 20) and 5% skim milk and were incubated with purified anti-human SCGB3A2 antibody raised against rhSCGB3A2 (diluted 1:2,500) in TBS-Tween. Membranes were washed with TBS-Tween and then incubated with horseradish peroxidase-conjugated secondary antibody (Pierce/Thermo Scientific, Rockford, IL). Protein bands were detected at ∼6 and 12 kDa in Coomassie-stained SDS-PAGE gels, representing reduced and nonreduced rhSCGB3A2 monomer and homodimer, respectively.
For SDS-polyacrylamide gels of rmSCGB3A2, samples were prepared using LDS (lithium dodecyl sulfate) sample buffer (Invitrogen/Life Technologies) containing 2% LDS in the presence or absence of 25 mM TCEP [Tris (2-carboxyethyl) phosphine] reducing agent (Bio-Rad, Hercules, CA). Samples were applied to Criterion Tris-glycine 10–20% precast gels (Bio-Rad) after heat treatment. Electrophoresis was performed in 1 × running buffer (25 mM Tris, pH 8.6, 0.192 mM glycine, 0.1% SDS). Proteins were visualized by use of 0.005% Coomassie Brilliant Blue R-250 in 0.3% vol/vol acetic acid.
Animal studies.
Mouse embryonic lungs collected from C57BL/6 pregnant females at embryonic day (E) 10.5 were subjected to ex vivo organ culture. Noon of the day on which a vaginal plug was found was considered as E0.5. For in vivo analysis of growth factor activity of SCGB3A2, pregnant mice were intravenously injected daily with 0.5 mg/kg rhSCGB3A2 from E12.5 through E16.5, and the pups were removed on E17.5, followed by breathing score assessment. For the BLM-induced pulmonary fibrosis model, ∼8-wk-old mice were intratracheally intubated and dosed with BLM (1.2 U/kg, Sigma-Aldrich, B8416–15UN) at day 0 and were intravenously injected at the same time each day with rhSCGB3A2, rmSCGB3A2, rhSCGB1A1, rmSCGB1A1 (all 0.25 mg/kg each), or PBS as control, for 5 consecutive days starting at day 7. The last injection was on day 11. Mice were euthanized on day 21, and bronchoalveolar lavage (BAL) fluids were obtained by lavaging lungs with 1 ml PBS. The collected BAL fluids were used for counting inflammatory cell numbers with a hemocytometer and cell differentiation by Cytospin 4 (Thermo Scientific). All animal studies were carried out after approval by the NCI Animal Care and Use Committee.
Embryonic lung ex vivo organ culture.
Embryonic lungs were cultured in DMEM/F12 on a 0.8-μm-pore membrane, which was placed on the top of steel wire mesh in an organ culture dish (BD Biosciences, Franklin Lakes, NJ). Branching scores were determined by counting the number of terminal branches visible around the periphery of each cultured lung with transillumination to visualize the structures and photomicrography to record permanent images (30).
Breathing score assessment.
Breathing scores were determined by observing pups placed on moistened filter paper at 37°C for 2 min immediately after removal from the mother (14). Scoring assignment was performed according to the criteria described by Ozdemir et al. (23): 0, no breathing; 1, gasping; 2, gasping/labored breathing; 3, labored breathing; 4, labored breathing/unlabored breathing; 5, unlabored breathing.
Histological analysis of lung sections.
Lungs were inflated and fixed in 10% buffered formalin under 20 cmH2O pressure, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin (H&E). The degree of embryonic lung development was determined by using radial alveolar count (RAC) as described by Cooney et al. (6). The assessment was repeated for 10 terminal respiratory units in one random tissue section per mouse. For the BLM study, the sections were subjected to Masson trichrome staining that detects collagen fibers. Fibrosis was quantified by the Ashcroft scoring system (2) for the entire lung. The degree of fibrosis was graded from 0 (normal lung) to 8 (severe distortion of structure, large fibrous areas, and honeycomb lesions). The mean score from all fields (magnification ×200, average 30 fields/animal) was taken as the fibrosis score.
Quantitation of hydroxyproline content in lung.
Hydroxyproline content was measured by using a hydroxyproline assay kit from Biovision (Milpitas, CA) according to the manufacturer's instructions with slight modification. In brief, whole lungs were homogenized in deionized H2O, using 100 μl H2O for every 10 mg of tissue. To a 100 μl of tissue homogenate, 200 μl concentrated HCl (6 N) was added in a pressure-tight, Teflon-capped vial. The mixture was hydrolyzed at 120°C for 3 h, followed by filtration through a 45-μm syringe filter (Millipore, Bedford, MA), and 10 μl of hydrolyzed sample was transferred to a 96-well plate and evaporated to dryness under vacuum, then 100 μl chloramine-T reagent was added to each well. After incubation at room temperature for 5 min, 100 μl p-dimethylaminobenzaldehyde reagent was added to each well and further incubated for 90 min at 60°C. Absorbance was measured at 560 nm in a microplate reader (SpectroMax Plus384, Molecular Devices, Sunnyvale, CA).
qRT-PCR analysis.
The whole left lobe of each lung was used for total RNA isolation with TRIzol, digested with DNase I, and reverse transcribed with Superscript II reverse transcriptase. Quantitative RT-PCR (qRT-PCR) was performed with an ABI Prism 7900 Sequence Detection System (Applied Biosystems, Foster City, CA) by using SYBR Green master mixture. The ΔΔCt method was used with β-actin or 18S as normalization control. PCR conditions used were 50°C, 2 min; 95°C, 10 min; followed by 95°C, 15 s; 60°C, 40 s for 40 cycles with the following primers: Col1a1 (forward) 5′-TAGGCCATTGTGTATGCAGC-3′, (reverse) 5′-ACATGTTCAGCTTTGTGGACC-3′, Col3a1 (forward) 5′-TAGGACTGACCAAGGTGGCT-3′, (reverse) 5′-GGAACCTGGTTTCTTCTCACC-3′, Col4a1 (forward) 5′-CACATTTTCCACAGCCAGAG-3′, (reverse) 5′-GTCTGGCTTCTGCTGCTCTT-3′, Col5a2 (forward) 5′-CATGGAGAAGGTTTCCAAATG-3′, (reverse) 5′-AAAGCCCAGGAACAAGAGAA-3′, Col12a1 (forward) 5′-TGAGGTCTGGGTAAAGGCAA-3′, (reverse) 5′-GTATGAGGTCACCGTCCAGG-3′, β-actin (forward) 5′-ATGGAGGGGAATACAGCCC-3′, (reverse) 5′-TTCTTTGCAGCTCCTTCGTT-3′, and 18S (forward) 5′-CGCGGTTCTATTTTGTTGGT-3′, (reverse) 5′-AGTCGGCATCGTTTATGGTC-3′.
Bioavailability of human SCGB3A2 by competitive ELISA.
E17.5 pregnant females were euthanized at 10 min after intravenous administration of 0.5 mg/kg rhSCGB3A2 or saline as control through the tail vein. Blood for serum preparation and lung were taken from the dam, and placenta, amniotic fluid, and embryonic lung were collected, the tissues being snapped frozen in liquid N2. Frozen tissues were weighed, put into a buffer at a ratio of 100 mg tissue per ml at 4°C, containing a protease-inhibitor cocktail of 1 mmol/l phenylmethylsulfonylfluoride, 1 μg/ml pepstatin A, 1 μg/ml aprotinin, and 1 μg/ml leupeptin in phosphate-buffered saline, pH 7.2 and were homogenized with Precellys 24 tissue homogenizer/grinder (Bertin Technologies, Saint Quentin en Yvelines, France). Homogenates were then centrifuged at 10,000 g for 10 min, and the supernatants were used for competitive ELISA assay.
The competitive ELISA for rhSCGB3A2 was developed in conditions similar to those developed for rhCC10 (16) as follows: 96-well plates were coated with 200 ng of purified anti-human SCGB3A2 IgG (1 mg/ml stock, diluted to 2 μg/ml in 0.1 M carbonate/bicarbonate buffer, pH 9.5) per well. Plates were blocked in 5% sucrose, 2.5% BSA in PBS, then dried and stored at 4°C. A conjugate of horseradish peroxidase (HRP) and rhSCGB3A2 was made by using the EZ-Link maleimide-activated HRP kit (Thermo-Fisher-Pierce Biochemical) basically according to the manufacturer's protocol, and the conjugate was used in the assay at a dilution of 1:130,000 in 1% BSA in PBS, pH 7.2. ELISA optimization was carried out by coating with a range of antibody (100–300 ng/well), followed by testing a standard curve with various combinations of different dilutions of conjugate and coatings until the best sigmoidal curve was obtained. Samples and conjugate (50 μl each per well) were mixed and then added to the ELISA plate prepared as above in duplicate wells. The plate was incubated at room temperature for 1 h with gentle rocking, followed by three washes. The plate was incubated at room temperature for 10–15 min with 3,3′,5,5′-tetramethylbenzidine (TMB, Sigma) and was read at 450 nm after stopping the reaction by adding 1 N HCl. The limit of detection of the assay was 5 ng/ml.
ELISA for detection of mouse SCGB3A2.
Samples diluted with a coating solution (500 mM bicarbonate buffer, pH 9.6) were applied onto each well of 96-well plates and the plates were incubated at 4°C overnight. Calibration curves were constructed with 12 points by serially diluting a solution of rmSCGB3A2 (1 μg/ml). The plates were washed four times with washing solution (PBS 1 mM, pH 7.4 containing 0.5% of Tween), followed by addition of blocking buffer (PBS 1 mM, pH 7.4 containing 1% of BSA) to each well. After incubation for 2 h at 37°C, the plates were washed four times with the washing solution. Purified anti-mouse SCGB3A2 IgG (dilution 4,000×, concentration 4.27 mg/ml) was applied to each well and the plates were incubated for 4 h at 37°C or 4°C overnight. The plates were washed seven times with the washing buffer, 100 μl of ECL anti-rabbit IgG horseradish peroxidase-linked F(ab′) fragment (from donkey) was added to each well, and the plates were placed at 37°C for 2 h. After further washing, the amount of SCGB3A2 was determined by addition of TMB as described above.
Statistical analysis.
Statistical analysis was carried out by using the one-way ANOVA with Bonferroni correction (α = 0.05) except for the analysis of weight loss and bioavailability of rhSCGB3A2, in which Student's t-test was used. P < 0.05 was considered statistically significant.
RESULTS
Characterization of human SCGB3A2.
This is the first report describing the in vitro and in vivo characterization of a novel version of rhSCGB3A2 that includes residues 19–93 of the predicted full-length amino acid sequence and may be suitable for use as a therapeutic agent. The rhSCGB3A2 was produced using an E. coli expression system as described in materials and methods. The purified rhSCGB3A2 was a 8.1-kDa monomer that readily dimerized in vitro. SCGB3A2 migrated lower on SDS-PAGE (∼6 and 12-kDa bands for monomer and dimer, respectively) than its predicted molecular weight. The final preparation of rhSCGB3A2 was >97% homogeneous by SDS-PAGE, composed of ∼95% dimer and 5% monomer, as determined by densitometric analysis of the digitally imaged gel from a single determination (Fig. 1A). Similar dimerization was also observed with the rmSCGB3A2; the dimer was maintained even in 2% LDS loading buffer and was more predominant than the monomer form (Fig. 1B). These two bands were isolated and subjected to MALDI-TOF analysis, which confirmed that both bands were identical, indeed derived from mouse SCGB3A2. A mutant rmSCGB3A2 having a serine residue instead of a cysteine (C47S of the predicted consensus of the mature protein) did not form a dimer in nonreducing condition of SDS gel (Fig. 1C), suggesting that the C47 is involved in dimerization. Thus the tendency to dimerize in vitro seems to be a common characteristic for both human and mouse SCGB3A2.
Fig. 1.
Analysis of secretoglobin (SCGB)3A2 protein. A–C: SDS-PAGE analysis of purified recombinant human (rh)SCGB3A2 under nonreducing (NR) and reducing (R) conditions (A), purified recombinant mouse (rm)SCGB3A2 under reducing conditions (B), and purified rmSCGB3A2 (left) and its mutant rmSCGB3A2-C47S (right) under nonreducing conditions (C). MW, molecular weight. D: ELISA titration curve to determine mouse SCGB3A2 protein levels. E: competitive ELISA titration curve to determine human SCGB3A2 protein levels. F: human SCGB3A2 titration using competitive ELISA in the presence of piglet bronchoalveolar lavage (BAL) (13.5 μg), 1% BSA, and PBS as control. Addition of piglet BAL or 1% BSA had similar SCGB3A2 readouts as PBS control. G: tracheal aspirate fluids from 6 patients (lanes 2–7) were subjected to SDS-PAGE under nonreducing conditions, followed by Western blotting with anti-rhSCGB3A2 antibody. Lanes 1 and 8 are 5 and 1 ng, respectively, of rhSCGB3A2 as control. H: competitive ELISA assay to determine the amount of human SCGB3A2 found in tracheal aspirate fluids used in F. Tracheal aspirate fluid (TAF) samples were diluted so the readings fell in between 10 and 100 ng/ml.
The specificity of anti-mouse and anti-human SCGB3A2 antibodies was measured by ELISA (Fig. 1, D and E). Mouse SCGB3A2 antibody cross-reacted with rhSCGB3A2 in the ELISA format used at concentrations above ∼0.4 μg/ml rhSCGB3A2 (Fig. 1D). On the other hand, human SCGB3A2 antibody did not cross-react with rmSCGB3A2 in the competitive ELISA format used (Fig. 1E). Furthermore, the human SCGB3A2 antibody did not recognize any proteins contained in piglet BAL fluids (Fig. 1F) or human cancer cells (data not shown), suggesting that the specificity of human SCGB3A2 antibody is likely to be high. With this antibody, Western blotting was performed under nonreducing conditions by using TAF from premature infants (Fig. 1G). The Western blot results showed that both monomer and dimer forms of SCGB3A2, as well as other immunoreactive bands <15 kDa, were present in TAF. Interestingly, the dimer was predominant in certain patients (lanes 3, 6, and 7), whereas monomer was predominant in others (lanes 2 and 5). The SCGB3A2 in patients of lanes 3 and 7 showed degradation products (“smears”) toward the bottom of the gel, whereas these degradation products were not present in other patient samples. The levels of SCGB3A2 in these TAFs as determined by competitive ELISA were quite high in most lungs and generally agreed with the Western blot band intensities (Fig. 1H). By comparison, SCGB3A2 was also measured in three human adult serum samples, reporting values of 0, 29, and 32 ng/ml. SCGB3A2 could not be detected in unconcentrated or 10× concentrated human urine (data not shown).
PLA2 inhibitory activity of SCGB3A2.
SCGB3A2 shares similarities to SCGB1A1 (10, 13–15, 18, 21, 22). Since SCGB1A1 is known to exhibit PLA2 inhibitory activity (8, 20), SCGB3A2 was tested to see whether it has PLA2 inhibitory activity (Fig. 2). The curve showing percent inhibition as a function of SCGB concentration was very steep for both recombinant human proteins (rhSCGB1A1 and rhSCGB3A2), with rhSCGB3A2 being apparently a stronger inhibitor than rhSCGB1A1 (Fig. 2B). At 10 μM of SCGB concentration, rhSCGB3A2 and rhSCGB1A1 had 91 and 95% PLA2 inhibitory activity, respectively. The inhibition of rhSCGB1A1 was consistent with the previous observations (8, 19). In contrast, rmSCGB3A2 exhibited only 11% of inhibitory activity at concentrations used (3–13 μM).
Antifibrotic activity of human SCGB3A2.
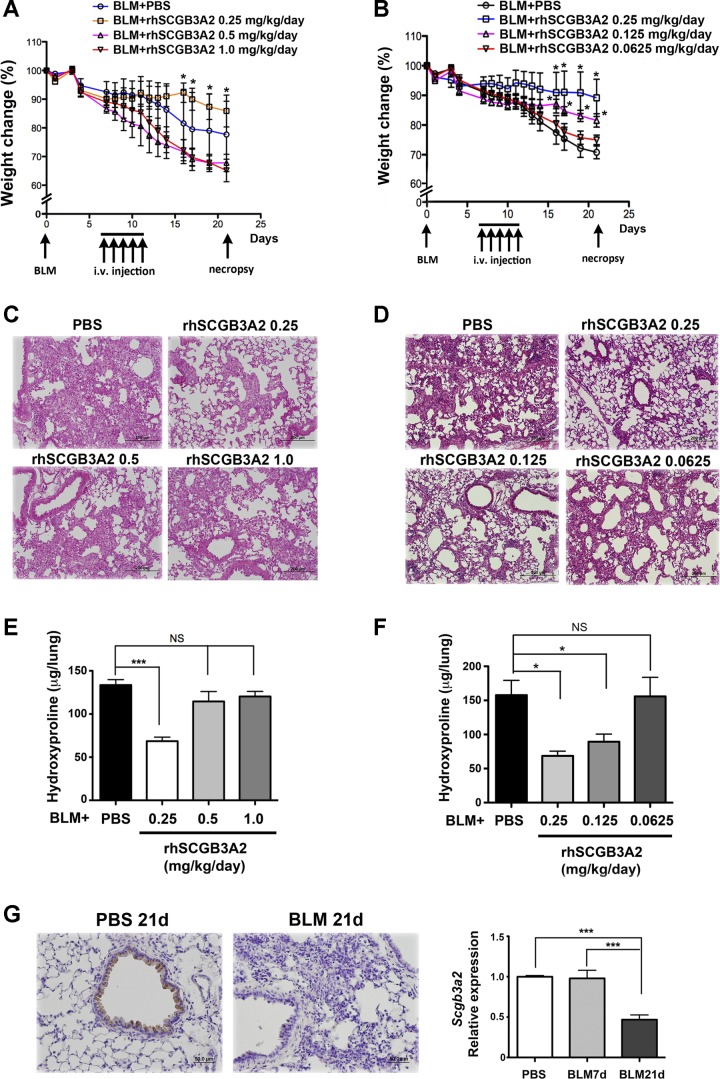
To examine whether human SCGB3A2 exhibits antifibrotic activity, rhSCGB3A2 was evaluated in a BLM-induced pulmonary fibrosis mouse model, in which rhSCGB3A2 was administered daily to BLM-treated mice from day 7 through day 11, and their lungs were examined for the degree of fibrosis on day 21 (Figs. 3 and 4). BLM-treated mice gradually lost weight during the entire experimental period of 21 days, which could be used as an indicator for lung injury and consequent development of pulmonary fibrosis. Mice were treated with 0.25, 0.5, and 1.0 (Fig. 3A), or 0.25, 0.125, and 0.0625 (Fig. 3B) mg·kg−1·day−1 for 5 consecutive days for a total of 1.25, 2.5, and 5.0, or 1.25, 0.625, and 0.3125 mg/kg of rhSCGB3A2, respectively. The results demonstrated that the administration of a total of 1.25 mg/kg rhSCGB3A2, given over 5 days, exhibited the least weight loss with statistical significance compared with the other groups of mice. Groups given 0.5 and 1.0 mg·kg−1·day−1 rhSCGB3A2 lost weight more rapidly than PBS control (Fig. 3A), whereas those with 0.0625 and 0.125 mg·kg−1·day−1 exhibited a similar and lesser weight loss, respectively, compared with control (Fig. 3B). These results suggested that the lungs treated with 0.25 mg·kg−1·day−1 for 5 days may have developed least fibrosis, and this was indeed the case as judged by tissue histology and hydroxyproline content (Fig. 3, C–F). Note that even though mice with 0.5 and 1.0 mg·kg−1·day−1 rhSCGB3A2 treatment lost weight more rapidly than control, the degree of fibrosis did not appear to be more severe than PBS control (Fig. 3, C and E).
Fig. 3.
Dose effect of rhSCGB3A2 on the amelioration of bleomycin (BLM)-induced pulmonary fibrosis. A and B: body weight curves of mice intubated and treated with BLM on day 0, then treated with various doses of rhSCGB3A2 proteins from day 7 through day 11 [total of 1.25, 2.5, and 5.0 mg/kg (A) or 1.25, 0.625, and 0.3125 (B) over 5 days], followed by euthanasia and necropsy on day 21. Body weights are shown as the percentage of day 0 weight set as 100%. A: *P < 0.05 for BLM+rhSCGB3A2 0.25 mg·kg−1·day−1 treated group vs. BLM+rhSCGB3A2 0.5 and 1.0 mg·kg−1·day−1 treated groups. No significant difference between BLM+rhSCGB3A2 0.25 mg·kg−1·day−1 treated group and PBS control group. N = 3–4 per group. B: *P < 0.05 for BLM+rhSCGB3A2 0.25 and 0.125 mg·kg−1·day−1 treated groups vs. PBS control groups or BLM+rhSCGB3A2 0.0625 mg·kg−1·day−1 treated groups. No significant difference between BLM+rhSCGB3A2 0.0625 mg·kg−1·day−1 treated group and PBS control group. N = 6–8 per group. C and D: hematoxylin and eosin (H&E) staining of day 21 lungs from mice treated with various doses of rhSCGB3A2 or PBS as control. E and F: hydroxyproline content of various groups of lungs. NS, not significant. G: SCGB3A2 immunohistochemistry using lungs of 21-day (21d) post-BLM or PBS as control (left). Quantitative RT-PCR (qRT-PCR) analysis of Scgb3a2 mRNA levels of PBS, 7-day (7d), and 21-day post-BLM lungs (right). N = 6–8 per group, ***P < 0.005. Error bars in all graphs are SD.
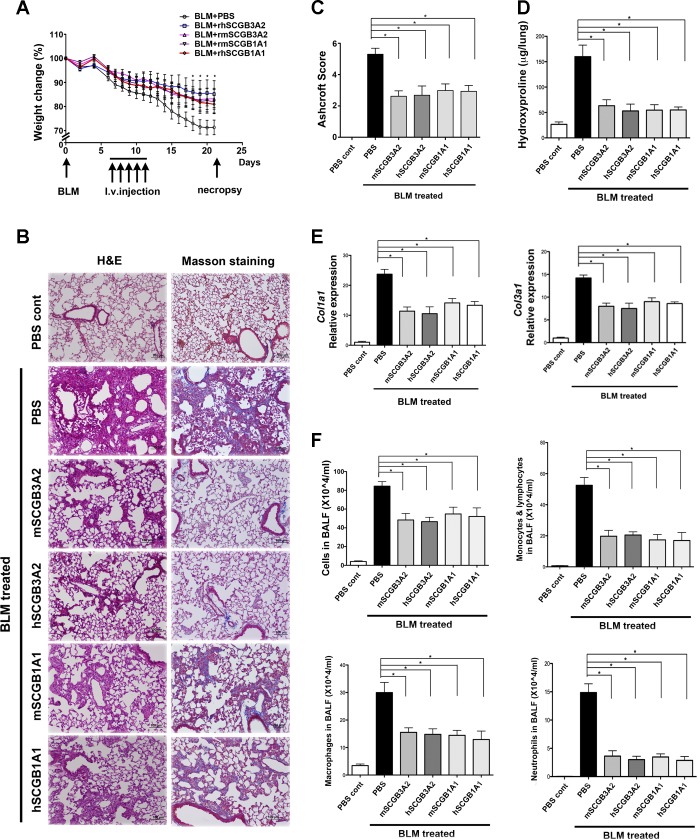
Fig. 4.
Effect of various recombinant SCGBs on the amelioration of BLM-induced pulmonary fibrosis. A: effect of rhSCGB3A2, rmSCGB3A2, rhSCGB1A1, and rmSCGB1A1 on weight loss of mice treated with BLM on day 0. SCGB proteins (total of 1.25 mg/kg over 5 days) were injected intravenously on day 7 through day 11, followed by necropsy on day 21. *P < 0.05 for all SCGB-treated groups vs. corresponding control group. No statistical difference among SCGB-treated groups. B: histological analysis of day 21 lungs from various groups. H&E (left) and Masson trichrome staining (right), in which blue color indicates collagen expression. BLM+PBS group of mice exhibited extensive fibrosis in their lungs, which were largely suppressed by administration of various SCGB proteins. C: BLM-induced damaged areas in lungs of various groups of mice were determined by the Ashcroft scoring method. D: hydroxyproline content of various groups of lungs. E: qRT-PCR analysis of collagen 1a1 and 3a1. F: number of total inflammatory cells, macrophages, monocytes/lymphocytes, and neutrophils in bronchoalveolar lavage fluid (BALF) in various groups of mice. N = 7–10 per group in all experiments. C–F: *P < 0.05 for all SCGB-treated groups vs. PBS control. Error bars in all graphs are SD.
The status of endogenous SCGB3A2 expression was examined by immunohistochemistry and qRT-PCR for SCGB3A2 protein and mRNA levels in lung, respectively (Fig. 3G). Levels of mRNA did not change in 7-day post-BLM mouse lungs compared with PBS control, which reduced by ∼50% by day 21. Immunohistochemical analysis showed no clear positive staining in 21-day BLM lungs, in sharp contrast to 21-day control PBS lungs, in which SCGB3A2 was clearly expressed in epithelial cells of bronchioles (22, 28).
Using the dose of 0.25 mg·kg−1·day−1, we then compared the effect of rhSCGB3A2 on the weight loss of BLM-treated mice with the same amount of rmSCGB3A2, rhSCGB1A1, and rmSCGB1A1 (Fig. 4). BLM-treated control mice injected with PBS on days 7 through 11 gradually lost more than 20% of their original weight. All other groups of mice treated with various SCGBs lost weight at similar rate compared with each other and to PBS controls during the first ∼1 wk after BLM administration. After the first week, their weight loss slowed and began differentiating from that of PBS-treated controls. On day 21, the SCGB-treated groups had lost only about half of the weight lost by the PBS control group, which was statistically significant (Fig. 4A).
H&E and Masson trichrome staining of lung sections showed that the lungs of BLM+PBS-treated control group developed extensive fibrosis, characterized by the presence of collagen fibers detected by Masson staining with the loss of alveolar structures (Fig. 4B). The extent of fibrous damage of lung histological sections was calculated by use of the Ashcroft scoring system (Fig. 4C). All groups of BLM+SCGB-treated mice had significantly reduced Ashcroft scores compared with BLM+PBS control group. Furthermore, the extensive fibrosis of the control group was confirmed by high levels of hydroxyproline content, which was clearly reduced by the treatment with SCGBs (Fig. 4D). When collagen expression was determined by qRT-PCR, the levels of collagen 1a1, 3a1, 4a2, 5a2, and 12a1 expression in SCGB-treated mouse lungs were about the half of those of BLM+PBS mice (Fig. 4E and data of 4a2, 5a2, and 12a1 not shown). Finally, the increased inflammatory cell numbers including macrophages, monocytes/lymphocytes and neutrophils in BAL fluids were similarly reduced to about or less than a half in all four groups of various SCGB-treated mice compared with BLM+PBS group of mice (Fig. 4F). These results demonstrated that rhSCGB3A2 inhibited BLM-induced pulmonary fibrosis in mice, and the degree of inhibition was similar to those of rmSCGB3A2, rhSCGB1A1, and rmSCGB1A1. This was in good agreement with the weight loss curves, suggesting that weight loss following BLM intubation can be used as an indicator for the lung injury and consequent development of pulmonary fibrosis in this mouse model.
Growth factor activity of human SCGB3A2.
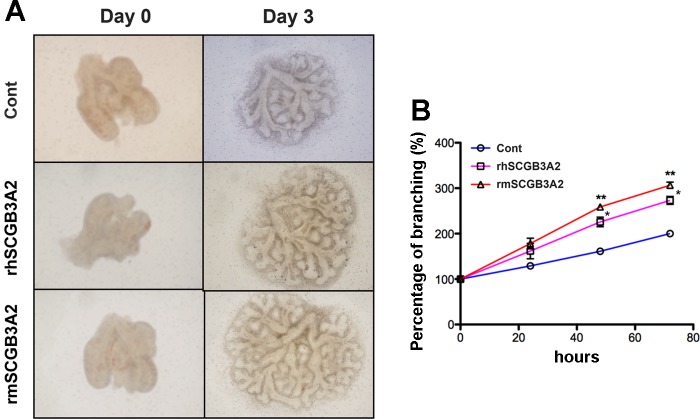
To determine whether human SCGB3A2 exhibits growth factor activity, rhSCGB3A2 was added to the medium for mouse embryonic lung ex vivo organ culture (Fig. 5A). By comparison, the same amount of rmSCGB3A2 was added to the medium. Branching degrees were counted and compared. The rhSCGB3A2 significantly increased branching of ex vivo cultured embryonic lung compared with control, to a comparable extent as those treated with rmSCGB3A2 (Fig. 5B). These results indicated that human SCGB3A2 was functional in mouse, promoting mouse embryonic lung branching morphogenesis comparable to mouse SCGB3A2 as evaluated by ex vivo mouse embryonic lung organ culture.
Fig. 5.
Promoted branching morphogenesis of embryonic lungs by recombinant SCGBs in ex vivo organ culture. A: representative photos of ex vivo cultured embryonic lungs of embryonic day (E) 10.5, taken right after plating (day 0) and 3 days after culture (day 3) in the absence [control (Cont)] or presence of rhSCGB3A2 or rmSCGB3A2 (1 μg/ml each). B: the degree of branching was calculated and plotted with day 0 as 100%. At least 6 lungs per group. Error bars are SD. *P < 0.05, **P < 0.01 vs. corresponding control group.
The in vivo effect of SCGB3A2 on embryonic lung development was next examined by administrating rhSCGB3A2 to pregnant female mice of E12.5 and on consecutive days until E16.5, followed by examination of embryos at E17.5. All body length, body weight, and lung weight of E17.5 embryos from the dam treated with rhSCGB3A2 were larger than E17.5 PBS control, but smaller than E18.5 mature controls with statistical significance (Fig. 6A). The E17.5 rhSCGB3A2-treated lungs had red color similar to E18.5 mature controls, indicative of the presence of aerated blood circulation, in contrast to a pale color of E17.5 PBS control lungs (Fig. 6B). The breathing score of E17.5 rhSCGB3A2-treated pups was similar to mature control and was significantly larger than E17.5 immature control (Fig. 6C). Embryonic lungs were further examined histologically. H&E-stained lung sections showed that rhSCGB3A2-treated E17.5 mouse lungs had a histological appearance between those of E17.5 and E18.5 controls (Fig. 6D). The RACs were obtained to determine the degree of development of embryonic lungs (Fig. 6E). RAC value of the lungs of rhSCGB3A2-treated E17.5 embryos was ∼3, which was significantly higher than 2.7 obtained with E17.5 controls that did not receive rhSCGB3A2 but was significantly lower than 3.5 of the E18.5 controls that did not receive rhSCGB3A2.
Fig. 6.
In vivo analysis of rhSCGB3A2 promoting mouse fetal lung development. Mice were intravenously injected with 0.5 mg/kg of rhSCGB3A2 from E12.5 through E16.5 daily, and the pups were taken out on E17.5. A: body length, body weight, and lung weight of E17.5 pups from mothers treated with either PBS or rhSCGB3A2, and E18.5 pups as control. B: representative gross picture of embryonic lungs in different treatment groups as indicated. C: breathing scores of embryos in different treatment groups as indicated. D: representative histological sections of embryonic lungs in different treatment groups as indicated. Magnification: left, ×40, and right, ×100. E: radial alveolar counts (RAC) of embryonic lungs calculated using histological sections as shown in D. At least 7 mice per group were used in all experiments. Error bars in all graphs are SD. *P < 0.05, **P < 0.01, ***P < 0.005.
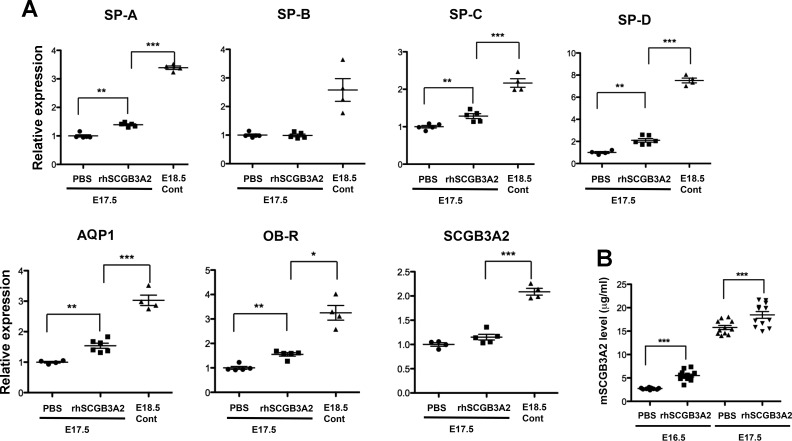
Furthermore, the expression of genes known to increase toward the end of gestation, including surfactant protein (SP)-A, B, C, and D, aquaporin 1, leptin receptor, and Scgb3a2, were analyzed by using lung tissue RNAs by qRT-PCR (Fig. 7A). Expression of all genes but SP-B was significantly higher (P < 0.005) in the rhSCGB3A2-treated group of embryonic lungs than the control group at E17.5. The expression of all genes was drastically increased in E18.5 mature controls. Finally, mouse SCGB3A2 levels in amniotic fluids were determined by ELISA using E16.5 and 17.5 PBS-treated control embryos as well as those treated with rhSCGB3A2 (Fig. 7B). The level of endogenous mouse SCGB3A2 was significantly higher at E17.5 than E16.5 regardless of rhSCGB3A2 treatment, and at each time point rhSCGB3A2-treated embryos had significantly higher mouse SCGB3A2 than PBS control (P < 0.005). These results altogether suggested that rhSCGB3A2 promoted lung development in mice in vivo.
Fig. 7.
Enhancement of the expression of genes related to fetal mouse maturation by rhSCGB3A2 treatment. A: qRT-PCR analysis of various genes as indicated by using E17.5 pups from mothers treated with either PBS or rhSCGB3A2 (0.5 mg/kg daily from E12.5 through E16.5), and E18.5 pups as control. SP, surfactant protein; AQP, aquaporin; OB-R, leptin receptor. Relative expression is shown on the basis of that of E17.5 PBS control as 1. B: mouse SCGB3A2 levels in amniotic fluids determined by ELISA. Amniotic fluids were collected from E16.5 and E17.5 embryos whose dams were treated with and without rhSCGB3A2 from E11.5 to E15.5 and from E12.5 to E16.5, respectively, and euthanized 1 day later. N > 5 with SD. *P < 0.05, **P < 0.01, ***P < 0.005.
Bioavailability of human SCGB3A2.
To understand the bioavailability of intravenously administered rhSCGB3A2, particularly whether the protein passed through the placenta, the levels of rhSCGB3A2 were determined by collecting lung and blood for serum preparation from the dam and placenta, amniotic fluid, and lung from the embryos at 10 min following administration of rhSCGB3A2 or saline as control. Protein extracts were made from the tissues and the extracts and fluids were analyzed by competitive ELISA (Fig. 8). Higher levels of human SCGB3A2 were detected in all the samples analyzed that received rhSCGB3A2 compared with saline control with statistical significance. The possible cross-reactivity of mouse SCGB3A2 with human SCGB3A2 antibody in this ELISA system was negligible because even 500 ng of rmSCGB3A2 did not show any positive reactivity above the background (see Fig. 1E). These results suggested that rhSCGB3A2 crossed the placenta and reached the embryo lungs within a very short time of 10 min after administration.
Fig. 8.
Bioavailability of rhSCGB3A2. E17.5 pregnant females were euthanized at 10 min after intravenous administration of 0.5 mg/kg rhSCGB3A2 or saline as control (n = 5 for each group). Blood for serum preparation and lung from the dam, and placenta, amniotic fluids, and embryonic lung were collected for measurement of rhSCGB3A2 levels by competitive ELISA. Placenta and embryonic lungs were collected from 2 embryos per dam. Amniotic fluids from all embryos were pooled (n = 7–9) per each dam to obtain sufficient amount for ELISA analysis. The levels of rhSCGB3A2 are shown as μg/ml for fluids and ng/ml of total protein for tissue extracts. Error bars are SD. *P < 0.05, ***P < 0.005.
DISCUSSION
We described, for the first time to our knowledge, the biochemical characterization of rhSCGB3A2 and rmSCGB3A2. We also demonstrated, using mouse as a model animal, that intravenously administered rhSCGB3A2 exhibits growth factor and antifibrotic activities in the lungs. It is most remarkable that the activity of rhSCGB3A2 was so similar to rmSCGB3A2, despite that the proteins are only 79% identical and have different NH2 termini. By analogy, we hypothesize that our rhSCGB3A2 may stimulate lung development and repair and inhibit fibrosis in humans. Thus rhSCGB3A2 may have potential to be used as a targeted molecule in pulmonary regenerative medicine, and/or treatment of premature babies with underdeveloped lungs, and/or patients with pulmonary fibrosis in humans.
This study demonstrated that the rhSCGB3A2 and rmSCGB3A2 appear to readily dimerize in vitro and the lack of dimerization of the rmSCGB3A2-C47S mutant showed that the sole cysteine is required for efficient dimer formation and/or stability. When TAF from premature infants were examined by nonreducing SDS-PAGE, some samples showed primarily dimer, whereas others showed primarily monomer. There were also other minor bands observed in between the monomers and dimers, which may represent alternatively folded isomers of intact SCGB3A2 or specific proteolysis products. Multiple bands on SDS-PAGE and Western blot are likely to be a characteristic of SCGBs as reported for SCGB1A1 (1, 19). Both the dimeric and monomeric forms of SCGB1A1 can appear as two to three distinct bands that are thought to represent different conformations that migrate through the gel. The multiple hSCGB3A2 bands in the infant TAF appear to correspond with dimer (∼12 kDa) and monomer (∼6 kDa), with bands corresponding to alternate conformations of each that migrate close by. Furthermore, some samples showed low-molecular-weight smears, suggesting that some nonspecific proteolytic degradation may have occurred in the samples. Although a possibility remains that the multiple bands are cross-reacting proteins with anti-human SCGB3A2 antibody, this appears unlikely on the basis of the competitive ELISA results with piglet BAL fluid spiked with rhSCGB3A2 and Western blot results using several colon and lung cancer cells. The anti-human SCGB3A2 antibody seems to be very specific. In our previous study, when mouse SCGB3A2 was expressed in COS1 cells by transfecting the expression plasmid, followed by nonreducing gel analysis, mouse SCGB3A2 was observed predominantly in its dimer form (22). Thus both human and mouse SCGB3A2 may predominantly exist as a dimer in vivo. Whether there is any correlation between levels of SCGB3A2 in TAF and condition of the lungs in premature infants awaits further studies.
SCGB1A1 is known to form a homodimer of globin structure in antiparallel fashion through two cysteine residues located in the NH2- and COOH-terminal regions of the polypeptide (3, 21, 25). In contrast, there is only one cysteine residue present in the middle of the SCGB3A2 polypeptide for both human and mouse (11, 22). The area of sequence containing this cysteine residue in SCGB3A2 overlaps the antiflammin peptide of SCGB1A1, which is the putative active site of this protein thought to be responsible for its anti-inflammatory activity, partly because of its ability to inhibit PLA2 activity (8, 20–22). The present results showed that both rhSCGB3A2 and rmSCGB3A2 possess PLA2 inhibitory activity. SCGB3A2 showed anti-inflammatory activity in a mouse model of ovalbumin-induced allergic inflammation (5). Whether PLA2 inhibitory activity is a genuine in vivo activity of SCGB3A2 that could be at least partially responsible for its anti-inflammatory effects awaits further studies. Nevertheless, to our knowledge, this is the first demonstration of any in vitro biochemical measurement for the evaluation of SCGB3A2 protein. It is curious that rmSCGB3A2 showed much lower activity in the PLA2 inhibition assay compared with rhSCGB3A2. Since both rmSCGB3A2 and rhSCGB3A2 demonstrate similar activities and potencies in in vivo experiments we performed, these results suggest that PLA2 inhibition is not required for the antifibrotic and growth factor properties of SCGB3A2 in vivo.
The present study demonstrated that rh and rm SCGB1A1 and SCGB3A2 proteins all share similar levels of antifibrotic activity. The best suppression/inhibition of the development of pulmonary fibrosis was obtained when a total of 1.25 mg/kg of rhSCGB3A2 was administered to mouse among five different doses tested (total of 0.3125, 0.625, 1.25, 2.5, and 5.0 mg/kg over 5 days). The accelerated weight loss was found with both higher and lower doses of rhSCGB3A2 than 1.25 mg/kg, whereas the extent of pulmonary fibrosis with these doses was at most similar to the control, but not worse. We do not know the exact reasons why higher dose rhSCGB3A2-treated BLM mice lost weights as quickly as the BLM controls. It is generally known that any drug has the effective range of concentration, and a high dose of drug causes opposite effects (7, 24). In relation to this, administration of an excess of rhSCGB3A2 to adult lungs in the BLM model could have caused decreased expression of endogenous SCGB3A2 and other SCGBs in the lungs and other organs, which have affected the homeostasis of the body. It was reported that intratracheal instillation of rhSCGB1A1 transiently inhibited expression of the endogenous SCGB1A1 in the lungs (16, 32). Expression feedback loops are common mechanisms that regulate protein expression. Notably, however, rhSCGB3A2 administered intravenously to pregnant dams did not reduce expression of native mouse SCGB3A2 in fetal lungs at E16.5 or 17.5, as shown in this study. Furthermore, in the case of BLM model mouse without SCGB3A2 treatment, SCGB2A2 mRNA and protein abundance appeared to have decreased between 7 and 21 days post-BLM administration, suggesting that there might be a loss of responsive epithelial cells that secrete SCGB3A2 and that the feedback loop may not be operable in the BLM model mouse. It is known that the expression patterns of SCGB3A2 and SCGB1A1 in the mouse are unique to each protein; SCGB3A2 is expressed mainly in bronchus and bronchioles whereas SCGB1A1 is mainly expressed in larger airways such as trachea and bronchus (22, 26). Furthermore, SCGB3A2 expression can be detected earlier during lung development than SCGB1A1 (9). Different degrees and patterns of combinatorial expression of SCGB3A2 and SCGB1A1 may direct their overall antifibrotic activity in the lung.
Our bioavailability study showed that rhSCGB3A2 was very quick to cross the placenta and reach the embryos. Since the ELISA for human SCGB3A2 does not cross-react significantly with mouse SCGB3A2, the levels of SCGB3A2 detected in the tissue extracts and fluids of embryos are most likely derived from exogenously administered rhSCGB3A2. In our preliminary study, the peak rhSCGB3A2 levels were found at 1–10 min postadministration in dam's lung, liver, and kidney, which almost returned to the basal level at 30 min (data not shown), suggesting that exogenously administered rhSCGB3A2 is very rapidly cleared. Further studies are required to address the details of this phenomenon. However, because of this rapid elimination, we believe that the mouse SCGB3A2 levels detected in amniotic fluids 1 day after the last rhSCGB3A2 injection to pregnant females (Fig. 7B) represent mouse SCGB3A2 levels. In fact, human SCGB3A2 was not detected in any of the amniotic fluids examined 1 day after administration of rhSCGB3A2.
We demonstrated in this study that mouse and human SCGB3A2 appear to share the same activities, responsible for lung development and as antifibrotic agent. As for the latter, we previously demonstrated that SCGB3A2 activates STAT1 phosphorylation, increases expression of SMAD7, thus decreases phosphorylation of SMAD2/3, resulting in the suppression of TGF-β signaling pathway, one of the major pathways responsible for fibrotic process (13). On the other hand, we do not know the signaling pathway for growth factor activity of SCGB3A2, nor whether the same receptor activates pathways for STAT1 signaling and growth factor activity, assuming that SCGB3A2 signal starts from its binding to a specific cell surface receptor. Further studies are required to address these questions.
In conclusion, SCGB3A2 may potentially be used as a targeted molecule in lung regenerative medicine and/or treatment of various lung diseases, especially those of premature babies and patients with pulmonary fibrosis.
GRANTS
This study was carried out under the CRADA (Corporate Research and Development Agreement) between National Cancer Institute and Clarassance, Inc. and funded in part by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research (ZIABC010449).
DISCLOSURES
ALP is an employee of Clarassance and has a >5% interest in Clarassance, Inc., which has an interest in commercializing the rhSCGB3A2. No conflicts of interest, financial or otherwise, are declared by the other authors.
AUTHOR CONTRIBUTIONS
Y.C., A.L.P., and S.K. conception and design of research; Y.C., M.E.W., J.K.Z., W.K.G., and J.T.L. performed experiments; Y.C., M.E.W., J.K.Z., W.K.G., and S.K. analyzed data; Y.C., M.E.W., J.K.Z., W.K.G., A.L.P., and S.K. interpreted results of experiments; Y.C., M.E.W., J.K.Z., W.K.G., A.L.P., and S.K. prepared figures; Y.C., A.L.P., and S.K. drafted manuscript; Y.C., A.L.P., and S.K. edited and revised manuscript; Y.C., M.E.W., J.K.Z., W.K.G., J.T.L., A.L.P., and S.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Timothy Veenstra (SAIC, NCI-Frederick) for MALDI-TOF analysis of rmSCGB3A2 protein.
REFERENCES
- 1.Antico G, Lingen MW, Sassano A, Melby J, Welch RW, Fiore S, Pilon AL, Miele L. Recombinant human uteroglobin/CC10 inhibits the adhesion and migration of primary human endothelial cells via specific and saturable binding to fibronectin. J Cell Physiol 207: 553–561, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 41: 467–470, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callebaut I, Poupon A, Bally R, Demaret JP, Housset D, Delettre J, Hossenlopp P, Mornon JP. The uteroglobin fold. Ann NY Acad Sci 923: 90–112, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Chandra S, Davis JM, Drexler S, Kowalewska J, Chester D, Koo HC, Pollack S, Welch R, Pilon A, Levine CR. Safety and efficacy of intratracheal recombinant human Clara cell protein in a newborn piglet model of acute lung injury. Pediatr Res 54: 509–515, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Chiba Y, Kurotani R, Kusakabe T, Miura T, Link BW, Misawa M, Kimura S. Uteroglobin-related protein 1 expression suppresses allergic airway inflammation in mice. Am J Respir Crit Care Med 173: 958–964, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 2—intrauterine and early postnatal lung growth. Thorax 37: 580–583, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costandi J, Melone M, Zhao A, Rashid S. Human resistin stimulates hepatic overproduction of atherogenic ApoB-containing lipoprotein particles by enhancing ApoB stability and impairing intracellular insulin signaling. Circ Res 108: 727–742, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Facchiano A, Cordella-Miele E, Miele L, Mukherjee AB. Inhibition of pancreatic phospholipase A2 activity by uteroglobin and antiflammin peptides: possible mechanism of action. Life Sci 48: 453–464, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Guha A, Vasconcelos M, Cai Y, Yoneda M, Hinds A, Qian J, Li G, Dickel L, Johnson JE, Kimura S, Guo J, McMahon J, McMahon AP, Cardoso WV. Neuroepithelial body microenvironment is a niche for a distinct subset of Clara-like precursors in the developing airways. Proc Natl Acad Sci USA 109: 12592–12597, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrod KS, Mounday AD, Stripp BR, Whitsett JA. Clara cell secretory protein decreases lung inflammation after acute virus infection. Am J Physiol Lung Cell Mol Physiol 275: L924–L930, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Jackson BC, Thompson DC, Wright MW, McAndrews M, Bernard A, Nebert DW, Vasiliou V. Update of the human secretoglobin (SCGB) gene superfamily and an example of ‘evolutionary bloom’ of androgen-binding protein genes within the mouse Scgb gene superfamily. Hum Genomics 5: 691–702, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klug J, Beier HM, Bernard A, Chilton BS, Fleming TP, Lehrer RI, Miele L, Pattabiraman N, Singh G. Uteroglobin/Clara cell 10-kDa family of proteins: nomenclature committee report. Ann NY Acad Sci 923: 348–354, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Kurotani R, Okumura S, Matsubara T, Yokoyama U, Buckley JR, Tomita T, Kezuka K, Nagano T, Esposito D, Taylor TE, Gillette WK, Ishikawa Y, Abe H, Ward JM, Kimura S. Secretoglobin 3A2 suppresses bleomycin-induced pulmonary fibrosis by transforming growth factor beta signaling down-regulation. J Biol Chem 286: 19682–19692, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurotani R, Tomita T, Yang Q, Carlson BA, Chen C, Kimura S. Role of secretoglobin 3A2 in lung development. Am J Respir Crit Care Med 178: 389–398, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YC, Zhang Z, Mukherjee AB. Mice lacking uteroglobin are highly susceptible to developing pulmonary fibrosis. FEBS Lett 580: 4515–4520, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Levine CR, Gewolb IH, Allen K, Welch RW, Melby JM, Pollack S, Shaffer T, Pilon AL, Davis JM. The safety, pharmacokinetics, and anti-inflammatory effects of intratracheal recombinant human Clara cell protein in premature infants with respiratory distress syndrome. Pediatr Res 58: 15–21, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Linnoila RI, Szabo E, DeMayo F, Witschi H, Sabourin C, Malkinson A. The role of CC10 in pulmonary carcinogenesis: from a marker to tumor suppression. Ann NY Acad Sci 923: 249–267, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Mandal AK, Zhang Z, Ray R, Choi MS, Chowdhury B, Pattabiraman N, Mukherjee AB. Uteroglobin represses allergen-induced inflammatory response by blocking PGD2 receptor-mediated functions. J Exp Med 199: 1317–1330, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantile G, Miele L, Cordella-Miele E, Singh G, Katyal SL, Mukherjee AB. Human Clara cell 10-kDa protein is the counterpart of rabbit uteroglobin. J Biol Chem 268: 20343–20351, 1993 [PubMed] [Google Scholar]
- 20.Miele L, Cordella-Miele E, Facchiano A, Mukherjee AB. Inhibition of phospholipase A2 by uteroglobin and antiflammin peptides. Adv Exp Med Biol 279: 137–160, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee AB, Zhang Z, Chilton BS. Uteroglobin: a steroid-inducible immunomodulatory protein that founded the secretoglobin superfamily. Endocr Rev 28: 707–725, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Niimi T, Keck-Waggoner CL, Popescu NC, Zhou Y, Levitt RC, Kimura S. UGRP1, a uteroglobin/Clara cell secretory protein-related protein, is a novel lung-enriched downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor. Mol Endocrinol 15: 2021–2036, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Ozdemir H, Guvenal T, Cetin M, Kaya T, Cetin A. A placebo-controlled comparison of effects of repetitive doses of betamethasone and dexamethasone on lung maturation and lung, liver, and body weights of mouse pups. Pediatr Res 53: 98–103, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Pang X, Cheng J, Krausz KW, Guo DA, Gonzalez FJ. Pregnane X receptor-mediated induction of CYP3A by black cohosh. Xenobiotica 41: 112–123, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pattabiraman N, Matthews JH, Ward KB, Mantile-Selvaggi G, Miele L, Mukherjee AB. Crystal structure analysis of recombinant human uteroglobin and molecular modeling of ligand binding. Ann NY Acad Sci 923: 113–127, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Reynolds SD, Reynolds PR, Pryhuber GS, Finder JD, Stripp BR. Secretoglobins SCGB3A1 and SCGB3A2 define secretory cell subsets in mouse and human airways. Am J Respir Crit Care Med 166: 1498–1509, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Saha A, Lee YC, Zhang Z, Chandra G, Su SB, Mukherjee AB. Lack of an endogenous anti-inflammatory protein in mice enhances colonization of B16F10 melanoma cells in the lungs. J Biol Chem 285: 10822–10831, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomita T, Kido T, Kurotani R, Iemura S, Sterneck E, Natsume T, Vinson C, Kimura S. CAATT/enhancer-binding proteins alpha and delta interact with NKX2–1 to synergistically activate mouse secretoglobin 3A2 gene expression. J Biol Chem 283: 25617–25627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomita T, Yamada A, Miyakoshi M, Kido T, Sheikh F, Srisodsai A, Miyajima A, Donnelly RP, Kimura S. Oncostatin M regulates secretoglobin 3A1 and 3A2 expression in a bidirectional manner. Am J Respir Cell Mol Biol 40: 620–630, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warburton D, Seth R, Shum L, Horcher PG, Hall FL, Werb Z, Slavkin HC. Epigenetic role of epidermal growth factor expression and signalling in embryonic mouse lung morphogenesis. Dev Biol 149: 123–133, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Watson TM, Reynolds SD, Mango GW, Boe IM, Lund J, Stripp BR. Altered lung gene expression in Ccsp-null mice suggests immunoregulatory roles for Clara cells. Am J Physiol Lung Cell Mol Physiol 281: L1523–L1530, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Wolfson MR, Funanage VL, Kirwin SM, Pilon AL, Shashikant BN, Miller TL, Shaffer TH. Recombinant human Clara cell secretory protein treatment increases lung mRNA expression of surfactant proteins and vascular endothelial growth factor in a premature lamb model of respiratory distress syndrome. Am J Perinatol 25: 637–645, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Zhang Z, Mukherjee AB, Linnoila RI. Increased susceptibility of mice lacking Clara cell 10-kDa protein to lung tumorigenesis by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a potent carcinogen in cigarette smoke. J Biol Chem 279: 29336–29340, 2004 [DOI] [PubMed] [Google Scholar]