Abstract
The monoamine serotonin (5-HT) has been previously implicated in pulmonary arterial remodeling and is considered a potential therapeutic target for the disease pulmonary arterial hypertension (PAH). More recently, it has been recognized that the enzyme tissue transglutaminase (TG2) mediates cross-linking of proteins with 5-HT, a posttranslational process of monoaminylation known as “serotonylation.” TG2 activity and serotonylation of protein participate in both smooth muscle proliferation and contraction produced by 5-HT. Indeed, markedly increased TG2 activity has now been identified in lung tissue of an experimental rodent model of pulmonary hypertension, and elevated serotonylation of fibronectin and the signaling molecule Rho, downstream products of transglutamidation, have been found in blood of patients with PAH. The basic mechanism by which TG2 is activated and the potential role(s) of serotonylated proteins in pulmonary hypertension remain a mystery. In the present review we have tried to address the current understanding of 5-HT metabolism in pulmonary hypertension and relate it to what is currently known about the evolving cellular process of serotonylation.
Keywords: serotonin, transglutaminase 2, transglutamidation, serotonylation, pulmonary hypertension
serotonin (5-hydroxytryptamine/5-HT) is a monoamine synthesized from the essential amino acid tryptophan by a two-step reaction that requires both hydroxylation and decarboxylation (Fig. 1). Tryptophan hydroxylase (Tph) is rate limiting in its synthesis, and at least some locations of peripheral synthesis presently identified in mammals include the gut and endothelium of the vasculature (15, 84). There are likely to be other peripheral locations as yet unidentified. The peripheral synthesis is under regulation of the Tph1 gene. 5-HT is also synthesized centrally in the nervous system under regulation of the Tph2 gene and does not cross the blood-brain barrier, so peripheral and central 5-HT are thought to be compartmentalized (84). In its degradation 5-HT is oxidized sequentially by monoamine oxidase and aldehyde dehydrogenase, and its metabolite, 5-hydroxyindolacetic acid (5-HIAA), is excreted in the urine. 5-HT was first identified as a vasoconstricting substance in blood and it is this action from which it gets its name, i.e., sero (serum) and tonin (tone) (66).
Fig. 1.
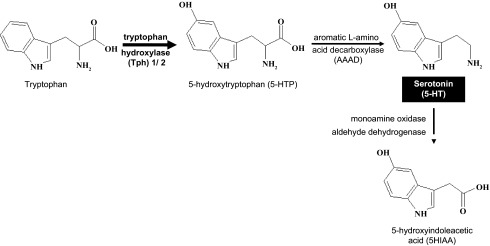
Scheme of 5-HT pathway showing hydroxylation of tryptophan by the rate-limiting enzyme tryptophan hydroxylase (Tph) to 5-hydroxytryptophan (5-HTP). 5-HTP is then decarboxylated to 5-HT by the enzyme aromatic amino acid decarboxylase (AAAD). 5-HT is metabolized to its inactive metabolite 5-hydroxyindoleacetic acid (5HIAA) by a 2-step enzymatic process involving monoamine oxidase and aldehyde dehydrogenase.
Serotonin that is released into the blood from tissue is taken up and stored by platelets that serve as a source for its participation in such actions as containment of bleeding by clot formation and local vasoconstriction. 5-HT is best known for its participation in a variety of neuroregulatory functions in humans, including neurotransmission, behavior regulation, and mood alterations (5), but it also takes part in such activities as contraction of the gut (20) and vasculature (89) and bone formation (88). In addition to its presence in humans and animals, 5-HT exists in various species of the plant kingdom and has been identified in such diverse organisms as fruit flies, locusts, and fungi, where its functions are largely unknown. Thus 5-HT is a simple molecule with considerable participation in biological systems and of current general interest.
Serotonin-Related Cell Signaling Pathways
Serotonin basically interacts with cells at their surfaces in two ways, one through a receptor and the other through a transporter. Increasing numbers of different 5-HT receptors have been recognized; there are now ∼15–16 of them that have been individually identified (28, 61). The reason for their large number and possible redundancy in function remains unknown. They participate in multiple signaling pathways to produce a cellular effect. To further complicate matters there is also a 5-HT transporter (only one is known presently) (SERT) that acts somewhat like a receptor and internalizes 5-HT through signaling pathways by a sodium-driven active transport process (8). Both the transporter and receptors are known to exist on a large variety of cells throughout the body so they have the potential to interact in numerous biological events. Among these are pulmonary artery smooth muscle cell (PASMC) proliferation and contraction, hallmarks of pulmonary arterial hypertension (PAH) (19, 52).
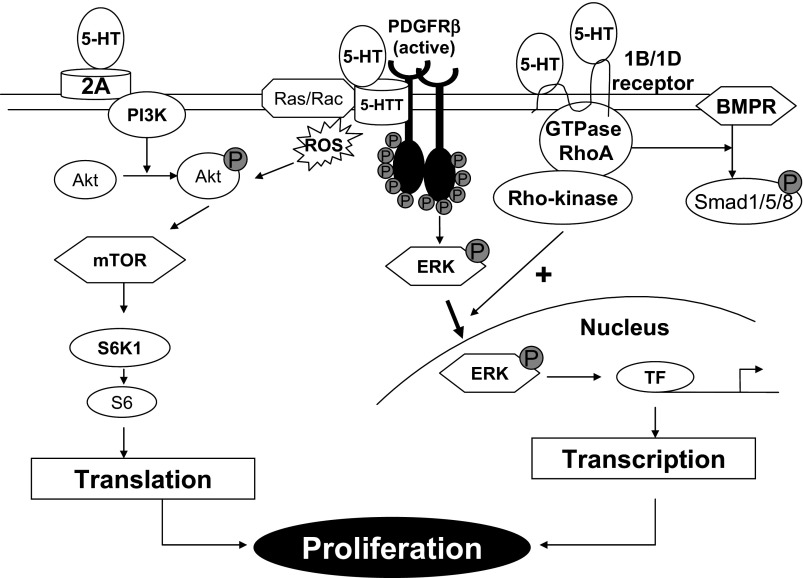
Cell signaling in PASMCs produced by 5-HT occurs through multiple pathways that include MAPK, Rho/ROCK, and Akt (Fig. 2). There is interaction between these pathways and this has been referred to as “combinatorial” cell signaling (47). Additionally, SERT is known to bind and interact with other receptors such as the platelet-derived growth factor receptor (PDGFR) (46, 68). There may be similar interactions with other cell receptors including that for bone morphogenetic protein (BMP) type 2 (49). Generation of reactive oxygen species (ROS) participates in the signaling events produced by 5-HT (41, 43, 80). The physiological events to which these signals lead include PASMC proliferation and contraction (18, 54).
Fig. 2.
Summary for the hypothesis of combinatorial 5-HT-related cell signaling pathways. 2A, 5-HT2A receptor; 5HTT, serotonin transporter; BMPR, bone morphogenetic protein receptor; ERK, extracellular signal-related kinase; PDGFR, plaetlet-derived growth factor receptor; PI3K, phosphatidylinositide 3-kinase; Rho, Ras homolog gene; TF, transcription factor; ROS, reactive oxygen species; mTOR, mammalian target of rapamycin.
Serotonin and Pulmonary Hypertension
There have been multiple animal studies that relate 5-HT biology to pulmonary hypertension (PH). The fawn hooded rat, known to have a defect in 5-HT transport, was found many years ago to develop spontaneous PH (74). Eddahibi et al. (16) first reported that SERT knockout mice failed to develop PH when exposed to hypoxia and Guignabert et al. (22) later showed that mice with a SM22-activated promoter overexpressing vascular SERT spontaneously develop PH. Additional studies have demonstrated a role for 5-HT receptors in PH (9, 36, 40, 52). These receptors have been of variable types and there is no indication of their specificity for the pulmonary vasculature. More recent studies utilizing pharmacological and genetic approaches have emphasized the importance of Tph1 (and, thus, 5-HT synthesis) in experimental PH in mice and have suggested that this is a possible target of therapy for PAH (2, 56, 57, 70). Presently, both Tph1 and SERT knockout mice are available for study, and although these animals show no overt phenotype they both fail to respond to an experimental stimulus that produces PH.
Perhaps the first observation in humans that there may be an association between 5-HT and PH was that made by Herve et al. (25, 26), who found elevation in plasma 5-HT and reduced platelet 5-HT in patients with combined familial platelet storage disease and PAH. Shortly after this, it became widely recognized that ingestion of derivatives of fenfluramine, an appetite suppressant and agonist of SERT (42), is associated with development of PAH (1, 51, 69). Eddahibi et al. (17) found increased expression of SERT in PASMCs derived from patients with PAH and chronic obstructive pulmonary disease associated with PH (14), compared with control subjects. They also reported that SERT gene polymorphism, LL genotype rather than LS and SS genotypes, is associated with increased SERT expression and activity in these patients. Elevated plasma levels of 5-HT have been found in humans with pulmonary hypertensive disease, thereby further supporting a likelihood of association of PAH with alterations in 5-HT metabolism (37). Thus there is a growing recognition of an association between 5-HT and PH both in animal and human studies. However, the specific related mechanism of this association has not been identified.
Transglutaminase 2-Mediated Posttranslational Protein Modification by 5-HT
The recent recognition of posttranslational modification of proteins by the enzyme transglutaminase may offer new insight into an end-product chemical function for 5-HT. First identified in the food industry, where it changes the consistency of meats the enzyme transglutaminase produces a cross-linking between glutamine and lysine moieties of proteins, peptides, and small amine-containing molecules. This transglutamidation reaction has been identified to occur for the monoamine 5-HT and the term for its chemical reaction has been coined “serotonylation” (85) (Fig. 3). Furthermore, multiple other monoamines have been found to produce similar posttranslational modifications of proteins by transglutaminase and the process is broadly referred to as “monoaminylation” (30, 85, 86). Recent advances in studying these posttranslational protein modifications have identified novel regulatory pathways that may be helpful in providing insights into poorly understood pathogenesis of diseases such as PAH and hypoxia-induced PH that occurs in chronic obstructive pulmonary disease and high-altitude exposure.
Fig. 3.
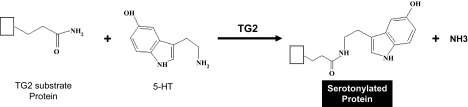
Schematic illustration of serotonylation of transglutaminase (TG2) substrate protein by 5-HT. This posttranslational modification is catalyzed by the TG2 enzyme resulting in formation of serotonylated protein and ammonia.
Transglutaminase 2 Expression and Activation
Initial studies on transglutaminases were focused on Factor XIII of the coagulation cascade. Factor XIII was shown to be required for cross-linking of fibrin and in turn for smooth muscle cell migration during tissue repair (60). A variety of transglutaminases have been identified; the cellular one that is ubiquitously present in many mammalian organs has been referred to as tissue transglutaminase type 2 or TG2 (50, 77). TG2 is present in many cell types including endothelial, smooth muscle, and immunologically related cells (72). Intracellular TG2 is constitutively localized in the cytosol, and it has also been shown to translocate and is found in the plasma membrane, mitochondria, and nucleus (63). It has been shown to be involved in cell adhesion, migration, survival, proliferation, apoptosis, and regulation of gene expression (35, 39, 50, 53, 59, 63). In addition, TG2 is also secreted into the extracellular matrix via a constitutive nonclassical pathway and has been well studied for its role in matrix stabilization and wound healing (7, 11). Nitric oxide (NO) has been shown to induce S-nitrosylation of TG2, thereby inhibiting TG2 secretion into the extracellular space (73), whereas an increase in intracellular Ca2+ induces TG2 secretion and activation (98). However, the specific mechanisms involved in TG2 secretion are not well understood. Several intra- and extracellular proteins including the ones that are important for vascular contraction have been reported to be TG2 substrates (21). TG2 has high affinity for the fibronectin NH2-terminal domain (27) and binds strongly to fibronectin (4, 55, 82).
Recently Oh et al. (62) reported that TG2 is required for differentiation of a subset of T-helper cells producing interleukin 17 and the fibrogenic effects of TGF-β in a bleomycin-induced pulmonary fibrosis model. Activation of TG2 has been observed to be mediated through ROS generation in response to TGF-β (44) and arachidonic acid (95) treatment in a fibroblast cell line. Studies in mice have shown that inhibition of TG2 activity reduces inflammation associated with acute lung injury (78) and bronchospasm produced by ovalbumin challenge (38). Jang et al. (31) showed that TG2 expression and intracellular activity are increased through a hypoxia-inducible factor-1 (HIF1)-dependent pathway in hypoxic tumor cells. We have observed that TG2 expression and activity are increased in bovine distal PASMCs exposed to hypoxia and this is related to cellular proliferation (unpublished data).
Pinkas et al. (65) demonstrated that activated TG2 undergoes major conformational change, simultaneously allowing access of protein substrates to its active site. The constitutively dormant TG2 was observed to be activated during cellular stress and tissue injury (76). Interestingly, extracellular TG2 is activated by both a disulfide-reducing agent, thioredoxin (Trx) (32), and a ligand for Toll-like receptor 3, polyinosinic-polycytidylic acid (76).
Regulation of Transglutaminase 2 by Intracellular Calcium
Achyuthan and Greenberg (3) first observed that GTP is a specific substrate for TG2 and is required for regulation of TG2 function through inhibition of transglutamidating activity. In addition, these investigators showed that GTP protects TG2 against proteolytic degradation and reported that Ca2+ reversed the effects of GTP on TG2 activity and proteolysis. Later Zhang et al. (100) showed for the first time that in situ TG2 activity is directly stimulated by muscarinic cholinergic receptor-mediated Ca2+ mobilization through production of inositol 1,4,5-trisphosphate (IP3) and that inhibition of GTP increases TG2 proteolytic degradation. Liu et al. (48) observed that 5-HT-induced posttranslational modification of TG2 substrate protein was markedly decreased in the presence of the Ca2+-chelating agent EGTA. Ca2+-dependent TG2-mediated posttranslational modification of proteins at selected glutamine residues is beginning to demonstrate a potentially important presence in multiple diseases including PAH (72, 75, 86).
The importance of calcium channels and receptors in PH is complex and not completely understood. Clinical investigations have suggested that calcium channel blockers are beneficial in some patients with PAH, whereas other patients do not respond (81). Intracellular Ca2+ in PASMCs is mobilized from both intra- and extracellular sources through a variety of channels including voltage-dependent, receptor-operated, and store-operated calcium channels (94). Recently, the extracellular Ca2+-sensing receptor was reported to be an important regulator of Ca2+ mobilization and has been implicated in increased PASMC proliferation in PAH (94). In addition, Xia et al. (92) have reported that the nonselective Ca2+-permeating transient receptor potential (TRP) vanilloid 4 channel is required for 5-HT-dependent contraction of PASMCs in a hypoxia-induced mouse model of PH. Interestingly, 5-HT was found to induce IP3 and Ca2+ mobilization through a 5-HT2 receptor in rat aortic smooth muscle cells (SMCs) (13). Later Saini et al. (71) showed that inhibitors of the Ca2+ channel, sarcoplasmic reticular Ca2+ pumps, and 5-HT2A-specific receptor attenuated the 5-HT induced intracellular Ca2+ levels in these cells.
Nitric oxide has been found to inhibit 5-HT-induced Ca2+ mobilization and thereby to produce vasodilation in PASMCs (97). In addition to 5-HT, dexfenfluramine has been reported to increase intracellular Ca2+ flux from both sarcoplasmic reticulum and extracellular sources in rat PASMCs (67). In a similar experimental model, BMP2 decreased TRP canonical (TRPC) channel expression and attenuated intracellular Ca2+ levels, cell proliferation, and migration; furthermore, these cellular effects were reversed with BMP2 knockdown (101). Yu et al. (96) observed that PDGF-induced PASMC proliferation is mediated by intracellular Ca2+ influx through a TRPC6 channel. Given the direct links between 5-HT and intracellular Ca2+ levels, we may speculate that Ca2+-dependent TG2-mediated protein modification such as that seen with serotonylation is the defining step in regulating 5-HT-induced cellular effects. However, further studies are needed to evaluate this potential mechanism.
Serotonylation as a Function of Transglutaminase 2
Studies have demonstrated that serotonylation of protein may participate in such diverse processes as insulin secretion (64), vascular muscle contractility (90), and neurotransmission (29). Of considerable interest is the recognition that in addition to that of 5-HT similar transglutamidation reactions may occur in the presence of other primary amines, such as histamine (83), dopamine, and noradrenaline (30).
Studies concerning serotonylation of protein or other intermediates in disease states are currently very limited. Walther et al. (85) first published about this phenomenon when they showed that serotonylation of small GTPases triggered platelet α-granule release. Guilluy et al. (24) noted the occurrence of transglutaminase-dependent RhoA activation and depletion by 5-HT in vascular smooth muscle cells and related this to SERT activity. This group later showed enhancement of serotonylation of RhoA in platelets of patients with PAH (23), an observation indirectly suggesting that TG2 activity that participates in the activation of RhoA may be elevated in these patients. Other cell-signaling molecules may be activated by serotonylation as recently demonstrated by Lin et al. (45) in the case of Ras.
More recently, TG2 was shown to interact with fibronectin and participate in PDGF- and PDGFR-mediated activation of downstream signaling events in vascular SMCs (99). In addition, these investigators also showed that TG2 activation induces fibronectin expression and promotes PDGF-dependent proliferation and migration of SMCs. Curiously, transglutamidation was reported to be associated with fibronectin in several of the early reports (29, 55, 58, 79).
Liu et al. (48) pursued further investigations of TG2 activity and specifically of serotonylation of protein such as fibronectin in experimental studies with PASMCs in culture. These initial studies showed that treatment of PASMCs in culture with 5-HT resulted in serotonylation of multiple proteins of these cells (48). One predominant protein that was serotonylated was fibronectin. This is of interest as alterations in fibronectin have been associated with experimental and clinical PH (33, 93). Inhibition of SERT or TG2 activity resulted in blockade of the serotonylation that occurred in the PASMCs (48). Furthermore, inhibition of TG2 activity blocked cellular proliferation and migration, hallmarks of PH, that were stimulated by 5-HT.
With the cellular findings in mind, these investigators sought evidence of enhanced TG2 activity in experimental animal models of PH and in patients with PAH (91). Mouse and rat models of PH produced both by exposure to hypoxia and injection of monocrotaline showed increased serotonylation of fibronectin in lungs and blood, suggesting enhanced transglutaminase activity in these lungs and possible release of serotonylated fibronectin into the blood (91). This observation suggested that the enhanced serotonylation of fibronectin might reflect an elevation of TG2 activity. Further collaborative studies by these investigators with Khosla and associates (12), utilizing a TG2 substrate, 5-(biotinamido)pentylamine, whose product can be identified by fluorescent microscopy, strongly confirmed an in vivo elevation of TG2 activity in mouse lungs after 16 days of exposure of animals to hypoxia. Interestingly, Wang et al. (87) recently showed that serotonylation of Rho in Rho/ROCK signaling is important in monocrotaline-induced PH in rats and that this action is blocked by inhibition of the SERT by fluoxetine.
The aforementioned observations signal a strong importance of enhanced TG2 activity in the experimental model of PH and identify an elevation of products of TG2 activation in blood of humans with PAH. It is now needed to define possible mechanisms by which the elevated TG2 activity might translate to the physiological effects of the disease. Ball et al. (6) observed that HIF1α conditional knockout combined with tamoxifen-inducible smooth muscle-specific Cre recombinase expression attenuated PH in hypoxia-exposed mice. Interestingly, Jang et al. (31) showed that HIF1 is a transcriptional regulator of TG2, which is required for evading apoptosis in tumor cells exposed to hypoxia. Recently, Chen et al. (10) showed that Trx1 expression and activity are increased in mouse lungs of a chronic hypoxia PH model. In addition, they showed that siRNA knockdown or pharmacological inhibition of Trx1 reduced PASMC proliferation by blocking HIF1 and consequent PI3K-Akt activation. In human aortic endothelial cells, NO has been shown to inhibit cytoplasmic TG2 secretion into the extracellular matrix (73). These investigators further reported that extracellular TG2 secretion, TG2 cross-linking activity, and vascular stiffness are significantly increased in endothelial NO synthase (eNOS) knockout mice compared with the wild-type controls (34).
5-HT, calcium channels, HIF1, Toll receptors, and Trx may all interact with TG2 and participate in the process. The nature of these interactions needs to be addressed in future experiments. Several potential pathways that may produce a cellular effect related to TG2 need to be evaluated. These include 1) activation of central cell signaling molecules such as Rho kinase in the development of PH; 2) alteration by serotonylation of matrix proteins such as fibronectin that promote vascular remodeling; 3) activation of vascular contractile proteins by serotonylation such as has been demonstrated for the systemic circulation (82); 4) direct physiological alteration of interstitial proteins by TG2 that result in vascular wall “stiffness”; 5) transcription of cell proliferation-related genes; and 6) a combination of these effects.
Conclusions
From these observations it may be hypothesized that PAH is associated with a heightened state of activation of TG2 that is nurtured by changes in synthesis and fluxes of 5-HT. These alterations also may be activated by cellular Ca2+ fluxes that are modified by 5-HT and known to stimulate TG2. Whether or not posttranslational modification of protein by serotonylation or direct actions of TG2 on cross-linking of vascular proteins participates in the pathogenesis of clinical PAH that can be altered by inhibition of TG2 remains to be determined. At the very least these protein modifications may provide new insights into features of PAH and offer novel biomarkers for the disease.
GRANTS
This study was supported by NIH Research Grant RO1 HL 107713 (B. L. Fanburg).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.C.P. and B.L.F. prepared figures; K.C.P. and B.L.F. drafted manuscript; K.C.P. and B.L.F. edited and revised manuscript; K.C.P. and B.L.F. approved final version of manuscript.
REFERENCES
- 1.Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, Higenbottam T, Oakley C, Wouters E, Aubier M, Simonneau G, Begaud B. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. N Engl J Med 335: 609–616, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Abid S, Houssaini A, Chevarin C, Marcos E, Tissot CM, Gary-Bobo G, Wan F, Mouraret N, Amsellem V, Dubois-Rande JL, Hamon M, Adnot S. Inhibition of gut- and lung-derived serotonin attenuates pulmonary hypertension in mice. Am J Physiol Lung Cell Mol Physiol 303: L500–L508, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Achyuthan KE, Greenberg CS. Identification of a guanosine triphosphate-binding site on guinea pig liver transglutaminase. Role of GTP and calcium ions in modulating activity. J Biol Chem 262: 1901–1906, 1987 [PubMed] [Google Scholar]
- 4.Akimov SS, Krylov D, Fleischman LF, Belkin AM. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol 148: 825–838, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arroll B, Macgillivray S, Ogston S, Reid I, Sullivan F, Williams B, Crombie I. Efficacy and tolerability of tricyclic antidepressants and SSRIs compared with placebo for treatment of depression in primary care: a meta-analysis. Ann Fam Med 3: 449–456, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ball MK, Waypa GB, Mungai PT, Nielsen JM, Czech L, Dudley VJ, Beussink L, Dettman RW, Berkelhamer SK, Steinhorn RH, Shah SJ, Schumacker PT. Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle HIF-1alpha. Am J Respir Crit Care Med 2013. November 19. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belkin AM. Extracellular TG2: emerging functions and regulation. FEBS J 278: 4704–4716, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blakely RD, Berson HE, Fremeau RT, Jr, Caron MG, Peek MM, Prince HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat brain. Nature 354: 66–70, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Callebert J, Esteve JM, Herve P, Peoc'h K, Tournois C, Drouet L, Launay JM, Maroteaux L. Evidence for a control of plasma serotonin levels by 5-hydroxytryptamine2B receptors in mice. J Pharmacol Exp Ther 317: 724–731, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Chen B, Nelin VE, Locy ML, Jin Y, Tipple TE. Thioredoxin-1 mediates hypoxia-induced pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 305: L389–L395, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collighan RJ, Griffin M. Transglutaminase 2 cross-linking of matrix proteins: biological significance and medical applications. Amino Acids 36: 659–670, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Diraimondo TR, Klock C, Warburton R, Herrera Z, Penumatsa K, Toksoz D, Hill N, Khosla C, Fanburg B. Elevated transglutaminase 2 activity is associated with hypoxia-induced experimental pulmonary hypertension in mice. ACS Chem Biol 2013. November 5 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doyle VM, Creba JA, Ruegg UT, Hoyer D. Serotonin increases the production of inositol phosphates and mobilises calcium via the 5-HT2 receptor in A7r5 smooth muscle cells. Naunyn Schmiedebergs Arch Pharmacol 333: 98–103, 1986 [DOI] [PubMed] [Google Scholar]
- 14.Eddahibi S, Chaouat A, Morrell N, Fadel E, Fuhrman C, Bugnet AS, Dartevelle P, Housset B, Hamon M, Weitzenblum E, Adnot S. Polymorphism of the serotonin transporter gene and pulmonary hypertension in chronic obstructive pulmonary disease. Circulation 108: 1839–1844, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Eddahibi S, Guignabert C, Barlier-Mur AM, Dewachter L, Fadel E, Dartevelle P, Humbert M, Simonneau G, Hanoun N, Saurini F, Hamon M, Adnot S. Cross talk between endothelial and smooth muscle cells in pulmonary hypertension: critical role for serotonin-induced smooth muscle hyperplasia. Circulation 113: 1857–1864, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Eddahibi S, Hanoun N, Lanfumey L, Lesch KP, Raffestin B, Hamon M, Adnot S. Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene. J Clin Invest 105: 1555–1562, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F, Simonneau G, Dartevelle P, Hamon M, Adnot S. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest 108: 1141–1150, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fanburg BL, Lee SL. A new role for an old molecule: serotonin as a mitogen. Am J Physiol Lung Cell Mol Physiol 272: L795–L806, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Fanburg BL, Lee SL. A role for the serotonin transporter in hypoxia-induced pulmonary hypertension. J Clin Invest 105: 1521–1523, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132: 397–414, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature's biological glues. Biochem J 368: 377–396, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guignabert C, Izikki M, Tu LI, Li Z, Zadigue P, Barlier-Mur AM, Hanoun N, Rodman D, Hamon M, Adnot S, Eddahibi S. Transgenic mice overexpressing the 5-hydroxytryptamine transporter gene in smooth muscle develop pulmonary hypertension. Circ Res 98: 1323–1330, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Guilluy C, Eddahibi S, Agard C, Guignabert C, Izikki M, Tu L, Savale L, Humbert M, Fadel E, Adnot S, Loirand G, Pacaud P. RhoA and Rho kinase activation in human pulmonary hypertension: role of 5-HT signaling. Am J Respir Crit Care Med 179: 1151–1158, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Guilluy C, Rolli-Derkinderen M, Tharaux PL, Melino G, Pacaud P, Loirand G. Transglutaminase-dependent RhoA activation and depletion by serotonin in vascular smooth muscle cells. J Biol Chem 282: 2918–2928, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Herve P, Drouet L, Dosquet C, Launay JM, Rain B, Simonneau G, Caen J, Duroux P. Primary pulmonary hypertension in a patient with a familial platelet storage pool disease: role of serotonin. Am J Med 89: 117–120, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Herve P, Launay JM, Scrobohaci ML, Brenot F, Simonneau G, Petitpretz P, Poubeau P, Cerrina J, Duroux P, Drouet L. Increased plasma serotonin in primary pulmonary hypertension. Am J Med 99: 249–254, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann BR, Annis DS, Mosher DF. Reactivity of the N-terminal region of fibronectin protein to transglutaminase 2 and factor XIIIA. J Biol Chem 286: 32220–32230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev 46: 157–203, 1994 [PubMed] [Google Scholar]
- 29.Hummerich R, Schloss P. Serotonin—more than a neurotransmitter: transglutaminase-mediated serotonylation of C6 glioma cells and fibronectin. Neurochem Int 57: 67–75, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Hummerich R, Thumfart JO, Findeisen P, Bartsch D, Schloss P. Transglutaminase-mediated transamidation of serotonin, dopamine and noradrenaline to fibronectin: evidence for a general mechanism of monoaminylation. FEBS Lett 586: 3421–3428, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Jang GY, Jeon JH, Cho SY, Shin DM, Kim CW, Jeong EM, Bae HC, Kim TW, Lee SH, Choi Y, Lee DS, Park SC, Kim IG. Transglutaminase 2 suppresses apoptosis by modulating caspase 3 and NF-kappaB activity in hypoxic tumor cells. Oncogene 29: 356–367, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Jin X, Stamnaes J, Klock C, DiRaimondo TR, Sollid LM, Khosla C. Activation of extracellular transglutaminase 2 by thioredoxin. J Biol Chem 286: 37866–37873, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones PL, Cowan KN, Rabinovitch M. Tenascin-C, proliferation and subendothelial fibronectin in progressive pulmonary vascular disease. Am J Pathol 150: 1349–1360, 1997 [PMC free article] [PubMed] [Google Scholar]
- 34.Jung SM, Jandu S, Steppan J, Belkin A, An SS, Pak A, Choi EY, Nyhan D, Butlin M, Viegas K, Avolio A, Berkowitz DE, Santhanam L. Increased tissue transglutaminase activity contributes to central vascular stiffness in eNOS knockout mice. Am J Physiol Heart Circ Physiol 305: H803–H810, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang SK, Yi KS, Kwon NS, Park KH, Kim UH, Baek KJ, Im MJ. Alpha1B-adrenoceptor signaling and cell motility: GTPase function of Gh/transglutaminase 2 inhibits cell migration through interaction with cytoplasmic tail of integrin alpha subunits. J Biol Chem 279: 36593–36600, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Keegan A, Morecroft I, Smillie D, Hicks MN, MacLean MR. Contribution of the 5-HT1B receptor to hypoxia-induced pulmonary hypertension: converging evidence using 5-HT1B-receptor knockout mice and the 5-HT1B/1D-receptor antagonist GR127935. Circ Res 89: 1231–1239, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Kereveur A, Callebert J, Humbert M, Herve P, Simonneau G, Launay JM, Drouet L. High plasma serotonin levels in primary pulmonary hypertension. Effect of long-term epoprostenol (prostacyclin) therapy. Arterioscler Thromb Vasc Biol 20: 2233–2239, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Kim DY, Park BS, Hong GU, Lee BJ, Park JW, Kim SY, Ro JY. Anti-inflammatory effects of the R2 peptide, an inhibitor of transglutaminase 2, in a mouse model of allergic asthma, induced by ovalbumin. Br J Pharmacol 162: 210–225, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuo TF, Tatsukawa H, Kojima S. New insights into the functions and localization of nuclear transglutaminase 2. FEBS J 278: 4756–4767, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Launay JM, Herve P, Peoc'h K, Tournois C, Callebert J, Nebigil CG, Etienne N, Drouet L, Humbert M, Simonneau G, Maroteaux L. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med 8: 1129–1135, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Lee SL, Simon AR, Wang WW, Fanburg BL. H2O2 signals 5-HT-induced ERK MAP kinase activation and mitogenesis of smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 281: L646–L652, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Lee SL, Wang WW, Fanburg BL. Dexfenfluramine as a mitogen signal via the formation of superoxide anion. FASEB J 15: 1324–1325, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Lee SL, Wang WW, Finlay GA, Fanburg BL. Serotonin stimulates mitogen-activated protein kinase activity through the formation of superoxide anion. Am J Physiol Lung Cell Mol Physiol 277: L282–L291, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Lee ZW, Kwon SM, Kim SW, Yi SJ, Kim YM, Ha KS. Activation of in situ tissue transglutaminase by intracellular reactive oxygen species. Biochem Biophys Res Commun 305: 633–640, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Lin JC, Chou CC, Gao S, Wu SC, Khoo KH, Lin CH. An in vivo tagging method reveals that Ras undergoes sustained activation upon transglutaminase-mediated protein serotonylation. Chembiochem 14: 813–817, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Li M, Warburton RR, Hill NS, Fanburg BL. The 5-HT transporter transactivates the PDGFβ receptor in pulmonary artery smooth muscle cells. FASEB J 21: 2725–2734, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Suzuki YJ, Day RM, Fanburg BL. Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ Res 95: 579–586, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Wei L, Laskin DL, Fanburg BL. Role of protein transamidation in serotonin-induced proliferation and migration of pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol 44: 548–555, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Long L, MacLean MR, Jeffery TK, Morecroft I, Yang X, Rudarakanchana N, Southwood M, James V, Trembath RC, Morrell NW. Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circ Res 98: 818–827, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 4: 140–156, 2003 [DOI] [PubMed] [Google Scholar]
- 51.MacLean MR. Pulmonary hypertension, anorexigens and 5-HT: pharmacological synergism in action? Trends Pharmacol Sci 20: 490–495, 1999 [DOI] [PubMed] [Google Scholar]
- 52.MacLean MR, Herve P, Eddahibi S, Adnot S. 5-Hydroxytryptamine and the pulmonary circulation: receptors, transporters and relevance to pulmonary arterial hypertension. Br J Pharmacol 131: 161–168, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malorni W, Farrace MG, Matarrese P, Tinari A, Ciarlo L, Mousavi-Shafaei P, D'Eletto M, Di Giacomo G, Melino G, Palmieri L, Rodolfo C, Piacentini M. The adenine nucleotide translocator 1 acts as a type 2 transglutaminase substrate: implications for mitochondrial-dependent apoptosis. Cell Death Differ 16: 1480–1492, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Marcos E, Fadel E, Sanchez O, Humbert M, Dartevelle P, Simonneau G, Hamon M, Adnot S, Eddahibi S. Serotonin-induced smooth muscle hyperplasia in various forms of human pulmonary hypertension. Circ Res 94: 1263–1270, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Martinez J, Chalupowicz DG, Roush RK, Sheth A, Barsigian C. Transglutaminase-mediated processing of fibronectin by endothelial cell monolayers. Biochemistry 33: 2538–2545, 1994 [DOI] [PubMed] [Google Scholar]
- 56.Morecroft I, Dempsie Y, Bader M, Walther DJ, Kotnik K, Loughlin L, Nilsen M, MacLean MR. Effect of tryptophan hydroxylase 1 deficiency on the development of hypoxia-induced pulmonary hypertension. Hypertension 49: 232–236, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Morecroft I, White K, Caruso P, Nilsen M, Loughlin L, Alba R, Reynolds PN, Danilov SM, Baker AH, Maclean MR. Gene therapy by targeted adenovirus-mediated knockdown of pulmonary endothelial Tph1 attenuates hypoxia-induced pulmonary hypertension. Mol Ther 20: 1516–1528, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mosher DF. Cross-linking of plasma and cellular fibronectin by plasma transglutaminase. Ann NY Acad Sci 312: 38–42, 1978 [DOI] [PubMed] [Google Scholar]
- 59.Nadalutti C, Viiri KM, Kaukinen K, Maki M, Lindfors K. Extracellular transglutaminase 2 has a role in cell adhesion, whereas intracellular transglutaminase 2 is involved in regulation of endothelial cell proliferation and apoptosis. Cell Prolif 44: 49–58, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naito M, Nomura H, Iguchi A, Thompson WD, Smith EB. Effect of crosslinking by factor XIIIa on the migration of vascular smooth muscle cells into fibrin gels. Thromb Res 90: 111–116, 1998 [DOI] [PubMed] [Google Scholar]
- 61.Nichols DE, Nichols CD. Serotonin receptors. Chem Rev 108: 1614–1641, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Oh K, Park HB, Byoun OJ, Shin DM, Jeong EM, Kim YW, Kim YS, Melino G, Kim IG, Lee DS. Epithelial transglutaminase 2 is needed for T cell interleukin-17 production and subsequent pulmonary inflammation and fibrosis in bleomycin-treated mice. J Exp Med 208: 1707–1719, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park D, Choi SS, Ha KS. Transglutaminase 2: a multi-functional protein in multiple subcellular compartments. Amino Acids 39: 619–631, 2010 [DOI] [PubMed] [Google Scholar]
- 64.Paulmann N, Grohmann M, Voigt JP, Bert B, Vowinckel J, Bader M, Skelin M, Jevsek M, Fink H, Rupnik M, Walther DJ. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol 7: e1000229, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pinkas DM, Strop P, Brunger AT, Khosla C. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol 5: e327, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rapport MM, Green AA, Page IH. Serum vasoconstrictor, serotonin; isolation and characterization. J Biol Chem 176: 1243–1251, 1948 [PubMed] [Google Scholar]
- 67.Reeve HL, Archer SL, Soper M, Weir EK. Dexfenfluramine increases pulmonary artery smooth muscle intracellular Ca2+, independent of membrane potential. Am J Physiol Lung Cell Mol Physiol 277: L662–L666, 1999 [DOI] [PubMed] [Google Scholar]
- 68.Ren W, Watts SW, Fanburg BL. Serotonin transporter interacts with the PDGFβ receptor in PDGF-BB-induced signaling and mitogenesis in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 300: L486–L497, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rothman RB, Ayestas MA, Dersch CM, Baumann MH. Aminorex, fenfluramine, and chlorphentermine are serotonin transporter substrates. Implications for primary pulmonary hypertension. Circulation 100: 869–875, 1999 [DOI] [PubMed] [Google Scholar]
- 70.Rothman RB, Cadet JL, Dersch CM, McCoy MT, Lehrmann E, Becker KG, Bader M, Alenina N, Baumann MH. Altered gene expression in pulmonary tissue of tryptophan hydroxylase-1 knockout mice: implications for pulmonary arterial hypertension. PLoS One 6: e17735, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saini HK, Sharma SK, Zahradka P, Kumamoto H, Takeda N, Dhalla NS. Attenuation of the serotonin-induced increase in intracellular calcium in rat aortic smooth muscle cells by sarpogrelate. Can J Physiol Pharmacol 81: 1056–1063, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Sane DC, Kontos JL, Greenberg CS. Roles of transglutaminases in cardiac and vascular diseases. Front Biosci 12: 2530–2545, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santhanam L, Berkowitz DE, Belkin AM. Nitric oxide regulates non-classical secretion of tissue transglutaminase. Commun Integr Biol 4: 584–586, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sato K, Webb S, Tucker A, Rabinovitch M, O'Brien RF, McMurtry IF, Stelzner TJ. Factors influencing the idiopathic development of pulmonary hypertension in the fawn hooded rat. Am Rev Respir Dis 145: 793–797, 1992 [DOI] [PubMed] [Google Scholar]
- 75.Siegel M, Khosla C. Transglutaminase 2 inhibitors and their therapeutic role in disease states. Pharmacol Ther 115: 232–245, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Siegel M, Strnad P, Watts RE, Choi K, Jabri B, Omary MB, Khosla C. Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PLoS One 3: e1861, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smethurst PA, Griffin M. Measurement of tissue transglutaminase activity in a permeabilized cell system: its regulation by Ca2+ and nucleotides. Biochem J 313: 803–808, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suh GY, Ham HS, Lee SH, Choi JC, Koh WJ, Kim SY, Lee J, Han J, Kim HP, Choi AM, Kwon OJ. A peptide with anti-transglutaminase activity decreases lipopolysaccharide-induced lung inflammation in mice. Exp Lung Res 32: 43–53, 2006 [DOI] [PubMed] [Google Scholar]
- 79.Szasz R, Dale GL. Thrombospondin and fibrinogen bind serotonin-derivatized proteins on COAT-platelets. Blood 100: 2827–2831, 2002 [DOI] [PubMed] [Google Scholar]
- 80.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028, 2000 [DOI] [PubMed] [Google Scholar]
- 81.Tonelli AR, Alnuaimat H, Mubarak K. Pulmonary vasodilator testing and use of calcium channel blockers in pulmonary arterial hypertension. Respir Med 104: 481–496, 2010 [DOI] [PubMed] [Google Scholar]
- 82.Turner PM, Lorand L. Complexation of fibronectin with tissue transglutaminase. Biochemistry 28: 628–635, 1989 [DOI] [PubMed] [Google Scholar]
- 83.Vowinckel J, Stahlberg S, Paulmann N, Bluemlein K, Grohmann M, Ralser M, Walther DJ. Histaminylation of glutamine residues is a novel posttranslational modification implicated in G-protein signaling. FEBS Lett 586: 3819–3824, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol 66: 1673–1680, 2003 [DOI] [PubMed] [Google Scholar]
- 85.Walther DJ, Peter JU, Winter S, Holtje M, Paulmann N, Grohmann M, Vowinckel J, Alamo-Bethencourt V, Wilhelm CS, Ahnert-Hilger G, Bader M. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell 115: 851–862, 2003 [DOI] [PubMed] [Google Scholar]
- 86.Walther DJ, Stahlberg S, Vowinckel J. Novel roles for biogenic monoamines: from monoamines in transglutaminase-mediated post-translational protein modification to monoaminylation deregulation diseases. FEBS J 278: 4740–4755, 2011 [DOI] [PubMed] [Google Scholar]
- 87.Wang HM, Wang Y, Liu M, Bai Y, Zhang XH, Sun YX, Wang HL. Fluoxetine inhibits monocrotaline-induced pulmonary arterial remodeling involved in inhibition of RhoA-Rho kinase and Akt signalling pathways in rats. Can J Physiol Pharmacol 90: 1506–1515, 2012 [DOI] [PubMed] [Google Scholar]
- 88.Warden SJ, Bliziotes MM, Wiren KM, Eshleman AJ, Turner CH. Neural regulation of bone and the skeletal effects of serotonin (5-hydroxytryptamine). Mol Cell Endocrinol 242: 1–9, 2005 [DOI] [PubMed] [Google Scholar]
- 89.Watts SW, Morrison SF, Davis RP, Barman SM. Serotonin and blood pressure regulation. Pharmacol Rev 64: 359–388, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Watts SW, Priestley JR, Thompson JM. Serotonylation of vascular proteins important to contraction. PLoS One 4: e5682, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wei L, Warburton RR, Preston IR, Roberts KE, Comhair SA, Erzurum SC, Hill NS, Fanburg BL. Serotonylated fibronectin is elevated in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 302: L1273–L1279, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xia Y, Fu Z, Hu J, Huang C, Paudel O, Cai S, Liedtke W, Sham JS. TRPV4 channel contributes to serotonin-induced pulmonary vasoconstriction and the enhanced vascular reactivity in chronic hypoxic pulmonary hypertension. Am J Physiol Cell Physiol 305: C704–C715, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu Y, Shiraishi K, Mori M, Motomiya M. Changes of fibronectin in the right and left ventricles of rats exposed to chronic normobaric hypoxia. Tohoku J Exp Med 168: 573–582, 1992 [DOI] [PubMed] [Google Scholar]
- 94.Yamamura A, Guo Q, Yamamura H, Zimnicka AM, Pohl NM, Smith KA, Fernandez RA, Zeifman A, Makino A, Dong H, Yuan JX. Enhanced Ca2+-sensing receptor function in idiopathic pulmonary arterial hypertension. Circ Res 111: 469–481, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yi SJ, Choi HJ, Yoo JO, Yuk JS, Jung HI, Lee SH, Han JA, Kim YM, Ha KS. Arachidonic acid activates tissue transglutaminase and stress fiber formation via intracellular reactive oxygen species. Biochem Biophys Res Commun 325: 819–826, 2004 [DOI] [PubMed] [Google Scholar]
- 96.Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol 284: C316–C330, 2003 [DOI] [PubMed] [Google Scholar]
- 97.Yuan XJ, Bright RT, Aldinger AM, Rubin LJ. Nitric oxide inhibits serotonin-induced calcium release in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 272: L44–L50, 1997 [DOI] [PubMed] [Google Scholar]
- 98.Zemskov EA, Mikhailenko I, Hsia RC, Zaritskaya L, Belkin AM. Unconventional secretion of tissue transglutaminase involves phospholipid-dependent delivery into recycling endosomes. PLoS One 6: e19414, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zemskov EA, Mikhailenko I, Smith EP, Belkin AM. Tissue transglutaminase promotes PDGF/PDGFR-mediated signaling and responses in vascular smooth muscle cells. J Cell Physiol 227: 2089–2096, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang J, Lesort M, Guttmann RP, Johnson GV. Modulation of the in situ activity of tissue transglutaminase by calcium and GTP. J Biol Chem 273: 2288–2295, 1998 [DOI] [PubMed] [Google Scholar]
- 101.Zhang Y, Lu W, Yang K, Xu L, Lai N, Tian L, Jiang Q, Duan X, Chen M, Wang J. Bone morphogenetic protein 2 decreases TRPC expression, store-operated Ca2+ entry, and basal [Ca2+]i in rat distal pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 304: C833–C843, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]