Abstract
Pre-existing diabetes increases the risk of maternal and fetal complications during pregnancy, which may be due to underlying maternal vascular dysfunction and impaired blood supply to the uteroplacental unit. Endothelial dysfunction and reduced vascular smooth muscle responsiveness to nitric oxide (NO) are common vascular impairments in type 2 diabetes (T2D). We hypothesized that uterine arteries from diabetic rats would have reduced vascular smooth muscle sensitivity to NO compared with nondiabetic rats due to impairment in the NO/soluble guanylate cyclase (sGC)/cGMP signaling pathway. Uterine arteries from pregnant Goto-Kakizaki (GK; model of T2D) and Wistar (nondiabetic) rats were studied in a wire myograph. GK nonpregnant uterine arteries had reduced responses to ACh and sodium nitroprusside (SNP) but increased responses to propylamine propylamine NONOate and greater sensitivity to sildenafil compared with Wistar nonpregnant arteries. In late pregnancy, Wistar rats had reduced uterine vascular smooth muscle responsiveness to SNP, but GK rats failed to show this adaptation and had reduced expression of sGC compared with the nonpregnant state. GK rats had a smaller litter size (13.9 ± 0.48 vs. 9.8 ± 0.75; P < 0.05) and a greater number of resorptions compared with Wistar controls (0.8 ± 0.76% vs. 19.9 ± 6.06%; P < 0.05). These results suggest that uterine arteries from rats with T2D show reduced sensitivity of uterine vascular smooth muscle sGC to NO. During pregnancy, the GK uterine vascular smooth muscle fails to show relaxation responses similar to those of arteries from nondiabetic rats.
Keywords: uterine artery, soluble guanylate cyclase, vascular smooth muscle, vasorelaxation, gestation
pregestational diabetes carries a high risk of mortality and morbidity for the mother and the fetus and comprises a hostile environment for implantation, and embryonic and fetal development (6, 25). Macrosomia, intrauterine growth restriction, shoulder dystocia, congenital abnormalities, and stillbirth are only a few of the complications seen in pregnancies with maternal diabetes (3, 24). The majority of diabetic pregnancies are complicated by gestational diabetes, but in recent years, the prevalence of pregnancies associated with type 2 diabetes (T2D) has increased steadily as it parallels the increasing rates of obesity and is influenced by the occurrence of childbearing in older ages (2, 7).
During healthy pregnancy, uterine blood flow increases to facilitate adequate nutrition and oxygen supply to the fetus. To accommodate this increase in blood flow, the uterine vasculature undergoes structural and functional changes including hypertrophy and hyperplasia of vascular smooth muscle cells (4, 31), increase in vasodilatory capacity (5, 49), and refractoriness to potent vasoconstrictors (46), respectively. Previous studies have reported that women with pregestational diabetes have increased uterine artery impedance (33). It was proposed that uterine endothelial dysfunction due to hyperglycemia may contribute to increased uterine resistance (41). Animal studies have primarily focused on uterine artery function in models of experimental diabetes that resemble characteristics of type 1 (10, 32, 40) or gestational diabetes (41, 42). These studies showed that a hyperglycemic environment during pregnancy promotes vascular dysfunction and adverse pregnancy outcomes. To the best of our knowledge, no data are currently available on uterine artery dilatory function in animal models of T2D, since the occurrence of T2D during pregnancy is a relatively recent phenomenon.
Endothelial dysfunction is well documented in T2D (13, 23, 38). Emerging clinical and experimental data, however, demonstrate that vascular smooth muscle cells may also be functionally impaired and thus contributing to vascular dysfunction in T2D (29, 35). Endothelium-derived factors [i.e., nitric oxide (NO)] or exogenous nitrates can induce vascular smooth muscle relaxation via activation of the soluble guanylate cyclase (sGC)/cGMP pathway. In humans, relaxation responses of the umbilical vascular smooth muscle change throughout pregnancy (18, 19), showing an increase in early pregnancy and a reduction in late pregnancy back to levels seen in the nonpregnant state (20). Humans and rodents with T2D show attenuated responsiveness of vascular smooth muscle to exogenous NO (30, 34, 47) and persistent reduction in vascular NO-sensitive sGC (48). One could speculate, therefore, that women with T2D enter pregnancy with preexisting deficiency of the NO/sGC/cGMP pathway; yet whether such impairment is evident in uterine vascular smooth muscle and if it compromises uterine artery adaptations to pregnancy is unknown.
Due to methodological and ethical considerations, studies in pregnant women with T2D are limited to measurements of uterine blood flow and uterine artery resistance using ultrasound techniques. Thus appropriate rodent models are important tools for the characterization of signaling mechanisms associated with uterine artery function. The Goto-Kakizaki (GK) rat model of T2D was produced by selective inbreeding of nondiabetic Wistar rats that had glucose intolerance (12). GK rats have glucose intolerance, reduced glucose-stimulated insulin release, mild hyperglycemia, decreased functional β-cell mass, and changes in islet microarchitecture (36). Most importantly, the GK rat is a nonobese model of T2D, allowing the investigation of the effect of diabetes in the absence of the confounding influences of obesity. In this study, we used the GK model to examine the effects of pregestational diabetes on uterine artery dilatory adaptations to pregnancy. We hypothesized that uterine arteries from GK rats would have reduced vascular smooth muscle sensitivity to NO compared with those from nondiabetic rats due to an impairment in NO/sGC/cGMP signaling pathway.
MATERIALS AND METHODS
Reagents.
Phenylephrine (PE), ACh, sodium nitroprusside (SNP), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), sildenafil citrate salt, Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME), 8-bromoguanosine 3′,5′-cyclic monophosphate sodium salt (8-Br-cGMP), and antibody against β-actin were obtained from Sigma Chemical (St. Louis, MO). Propylamine propylamine NONOate (PAPA NONOate) and antibodies against sGCα1 and sGCβ1 were purchased from Cayman Chemical (Ann Arbor, MI). Antibody against phosphodiesterase 5A (PDE5A) was obtained from Abcam (Cambridge, MA). Stock solutions were prepared in deionized water or dimethyl sulfoxide (DMSO).
Animals.
Female, 20-wk-old, virgin GK rats (inhouse bred, derived from the Tampa colony) and age-matched Wistar rats (Charles River Laboratories International) were used in this study. The animals were housed in a temperature- and humidity-controlled environment under 12-h:12-h light/dark cycles and had free access to tap water and standard laboratory rodent chow. Half of the female rats were paired with a fertile male (Wistar, 12–16 wk old) and the morning on which spermatozoa were found in vaginal smears was considered day 1 of pregnancy. In all experiments, rats were anesthetized with isoflurane via a nose cone for surgical procedures (initially with 5% and then maintained at 2.5% in 100% oxygen) and euthanized with isoflurane overdose followed by cutting their diaphragm on gestational days 19 and 20 (term = 21 to 22 days). Fetuses were euthanized immediately following removal from the dam via decapitation. All procedures were performed in accordance with the Guiding Principles in the Care and Use of Animals and approved by the Georgia Regents University Committee on the Use of Animals in Research and Education.
Metabolic parameters.
Tail blood samples were used for measurements of nonfasted whole blood glucose (FreeStyle Lite, Alemeda, CA) before vascular reactivity studies. Blood was also collected from the inferior vena cava for measurement of serum insulin (Rat Insulin ELISA; ALPCO, Salem, NH).
In vitro assessment of uterine artery reactivity.
Uterine artery reactivity was measured using a wire myograph (Danish Myo Technology A/S, Aarhus, Denmark). After euthanization, the uterus with attached vasculature was excised and placed in ice-cold physiological solution (PSS) of the following composition (in mM): 130 NaCl, 4.7 KCl, 14.9 NaHCO3, 5.5 dextrose, 1.18 KH2PO4, 1.17 MgSO4, 1.6 CaCl2, and 0.026 EDTA. The main uterine arteries were carefully isolated by dissection of fat and connective tissue. One of the main uterine arteries was frozen immediately in liquid nitrogen and stored at −80°C for subsequent Western blot experiments. The midpoints of the contralateral uterine artery (2 mm in length) were mounted in an isometric wire myograph system using two 40-μm wires and allowed to equilibrate for 30–45 min before resting tension was applied. Optimum resting tension was determined via a length-tension curve. Arterial rings were allowed to equilibrate for 45 min in a tissue bath filled with 5 ml PSS, continuously gassed with 95% O2-5% CO2 at 37°C. Vascular integrity was assessed by contracting uterine arterial segments with a depolarizing concentration of potassium chloride (KCl, 120 mM). Vascular endothelium viability was examined by assessing relaxation responses to ACh (3 × 10−6 M) in uterine arteries preconstricted with PE (3 × 10−6 M). Endothelium-dependent relaxation was assessed by concentration-response curves to ACh (10−9 to 10−6 M) in the presence or absence of a NO synthase (NOS) inhibitor (l-NAME; 10−4 M, 30 min incubation). Endothelium-independent relaxation was assessed by concentration-response curves to two NO donors—SNP (10−10 - 3 × 10−6 M) and PAPA NONOate (10−9 - 3 × 10−4 M) in the presence and absence of a specific inhibitor of sGC (ODQ, 10−6 M, 30 min incubation)—and a cGMP analog (8-Br-cGMP, 10−9 - 3 × 10−4 M). Concentration-response curves to a PDE5 inhibitor (sildenafil; 10−10 to 10−6 M) were also performed. All concentration-response curves to various reagents were performed in endothelium-intact arteries preconstricted with PE in a concentration that elicited isometric force corresponding to 80% of maximum response to KCl.
Western blot analysis.
Uterine arteries were homogenized in ice-cold lysis buffer containing T-Per tissue protein extraction solution (Thermo Scientific, Rockford, IL), 100 mM sodium orthovanadate (Na3VO4), 100 mM PMSF, and 1% proteinase inhibitor cocktail (Sigma). Homogenates were centrifuged at 10,000 g for 15 min at 4°C, the supernatant was collected, and the proteins were solubilized in Laemmli's buffer containing mercaptoethanol. Protein concentration in the supernatant was measured by bicinchoninic acid assay (Thermo Scientific). Samples (10 μg protein/lane) were resolved by electrophoresis on 10% SDS-PAGE gels and then transferred to nitrocellulose membranes. Membranes were blocked in blocking solution (Tris-buffered saline-Tween 20 with 5% skim dry milk or 5% bovine serum albumin) and subsequently incubated with primary antibodies overnight at 4°C. The immunostaining was detected using horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) (GE Healthcare, Buckinghamshire, UK) or anti-mouse IgG (GE Healthcare) for 1 h at room temperature. Results were normalized by β-actin expression. Primary antibodies were as follows: rabbit anti-sGCα1 (77–82 kDa; 1:1,000), rabbit anti-sGCβ1 (70 kDa; 1:1,000), rabbit anti-PDE5A (105 kDa; 1:500), mouse anti-β-actin (42 kDa; 1:15,000). Immunoreactive bands were visualized with an enhanced chemiluminescence detection system and quantified using UN-SCAN-IT gel analysis software (v. 6.1; Silk Scientific, Orem, UT).
Data analysis.
Sigmoidal curve fitting was performed on wire myography concentration-response curve data using GraphPad Prism software (v. 5.0; GraphPad Software, San Diego, CA). Two pharmacological parameters were obtained from these curves: the maximal effect generated by the agonist (Emax) and the EC50 (molar concentration of agonist producing 50% of the maximum response). EC50 was calculated only for those responses that showed a sigmoidal curve. Maximal vasodilatory responses were calculated and expressed relative to the maximal changes from the contraction produced by PE in each segment, which was determined as 0% relaxation. The baseline tension before addition of PE was considered as 100% relaxation.
In addition to Emax and EC50 calculations, the area under the curve (AUC) of the concentration-response curves to SNP, in the presence and absence of ODQ, was calculated (GraphPad Prism) to determine total relaxation. The contribution of sGC-dependent mechanisms to SNP- and PAPA NONOate-induced responses was calculated as the difference (%Δ) between AUC corresponding to the agonist alone and AUC corresponding to the agonist in the presence of ODQ.
Statistical analysis.
Values are presented as means ± SEM, and n represents the number of animals used in the experiments. Before statistical analysis, all data sets were tested for normality using the Kolmogorov-Smirnov test. Statistical differences were calculated by Student's t-test (fetal physical parameters), two-way ANOVA followed by Bonferroni post hoc test (body weight, blood glucose, insulin, Emax, EC50, AUC) and two-way ANOVA with repeated measures (concentration-response curves). Group differences in protein levels were determined using Kruskal-Wallis one-way ANOVA. All statistical tests were performed with GraphPad Prism (v. 5.0; GraphPad Software). The significance level of all tests was set at α = 0.05.
RESULTS
Body weight and metabolic parameters.
Body weight was lower in pregnant and nonpregnant GK rats compared with pregnant and nonpregnant Wistar rats, respectively (P < 0.05; Table 1). GK nonpregnant rats had increased blood glucose levels compared with Wistar controls, and pregnancy reduced blood glucose levels only in GK rats (P < 0.05; Table 1). Furthermore, GK animals had reduced serum insulin levels compared with Wistar controls and this difference was unchanged at the late pregnancy stage (P < 0.05; Table 1).
Table 1.
Body weight, serum glucose, and insulin levels
| Wistar |
GK |
|||
|---|---|---|---|---|
| Nonpregnant | Pregnant | Nonpregnant | Pregnant | |
| Body weight, g*$ | 269.8 ± 3.77a | 362.0 ± 8.58b | 228.2 ± 3.56 | 269.1 ± 5.21c,d |
| Nonfasting glucose, mg/dL*$ | 89.5 ± 6.33a | 87.2 ± 6.95 | 210.9 ± 10.14 | 116.8 ± 7.03c,d |
| Insulin, ng/ml* | 1.92 ± 0.388a | 3.14 ± 0.632 | 0.35 ± 0.053 | 0.41 ± 0.076d |
Values are means ± SE; n = 7–11 for Wistar nonpregnant, n = 6–10 for Wistar pregnant, n = 7–10 for Goto-Kakizaki (GK) nonpregnant, and n = 5–10 for GK pregnant.
P < 0.05: main effect, Wistar vs. GK;
P < 0.05: main effect, nonpregnant vs. pregnant;
P < 0.05: Wistar nonpregnant vs. GK nonpregnant;
P < 0.05: Wistar nonpregnant vs. Wistar pregnant;
P < 0.05: GK pregnant vs. GK nonpregnant;
P < 0.05: GK pregnant vs. Wistar pregnant.
Maternal and fetal parameters.
There were no significant differences in either fetal weights (Wistar, n = 7: 1.68 ± 0.142 g vs. GK, n = 5: 1.96 ± 0.184 g; P > 0.05) or placental weights (Wistar, n = 7: 0.43 ± 0.001 g vs. GK, n = 5: 0.45 ± 0.001 g; P > 0.05). GK rats (n = 11) had a smaller litter size (9.8 ± 0.75 vs. 13.9 ± 0.48; P < 0.05) and a greater number of resorptions compared with Wistar controls (n = 10) (19.9 ± 6.06% vs. 0.8 ± 0.76%; P < 0.05).
Endothelium-dependent relaxation in rat uterine arteries.
In the nonpregnant state, GK uterine arteries had reduced responses to ACh at submaximal concentrations (i.e., 3 × 10−7 M, 10−7 M, and 3 × 10−6 M) compared with Wistar arteries (P < 0.05; Fig. 1A). Uterine arteries from Wistar nonpregnant rats had increased sensitivity to ACh compared with those from Wistar pregnant rats (−logEC50, Wistar nonpregnant: 7.43 ± 0.127 vs. Wistar pregnant: 6.83 ± 0.134; P < 0.05), but there were no differences between GK pregnant and nonpregnant uterine arteries (GK nonpregnant: 6.71 ± 0.176 vs. GK pregnant: 6.67 ± 0.204; P > 0.05). In late pregnancy, there were no differences in uterine artery sensitivity to ACh between GK and Wistar rats (P > 0.05; Fig. 1B). The presence of the NOS inhibitor l-NAME abolished uterine artery responses to ACh in all groups (Fig. 1, A and B).
Fig. 1.
Concentration-response curves to ACh in the presence and absence of a nitric oxide synthase inhibitor (Nω-nitro-l-arginine methyl ester hydrochloride, or l-NAME; 10−4 M) in uterine arteries from nonpregnant (A) and pregnant (B) control (Wistar) and diabetic (Goto-Kakizaki, GK) rats. ACh-induced uterine relaxation was reduced in GK nonpregnant (n = 8) and Wistar pregnant (n = 8) rats compared with Wistar nonpregnant rats (n = 8), but there were no differences between GK nonpregnant and GK pregnant (n = 6) rats. Values are means ± SE. *P < 0.05, Wistar vs. GK ACh alone; #P < 0.05, ACh alone vs. ACh plus l-NAME for each group. PE, phenylephrine.
Endothelium-independent uterine artery relaxations.
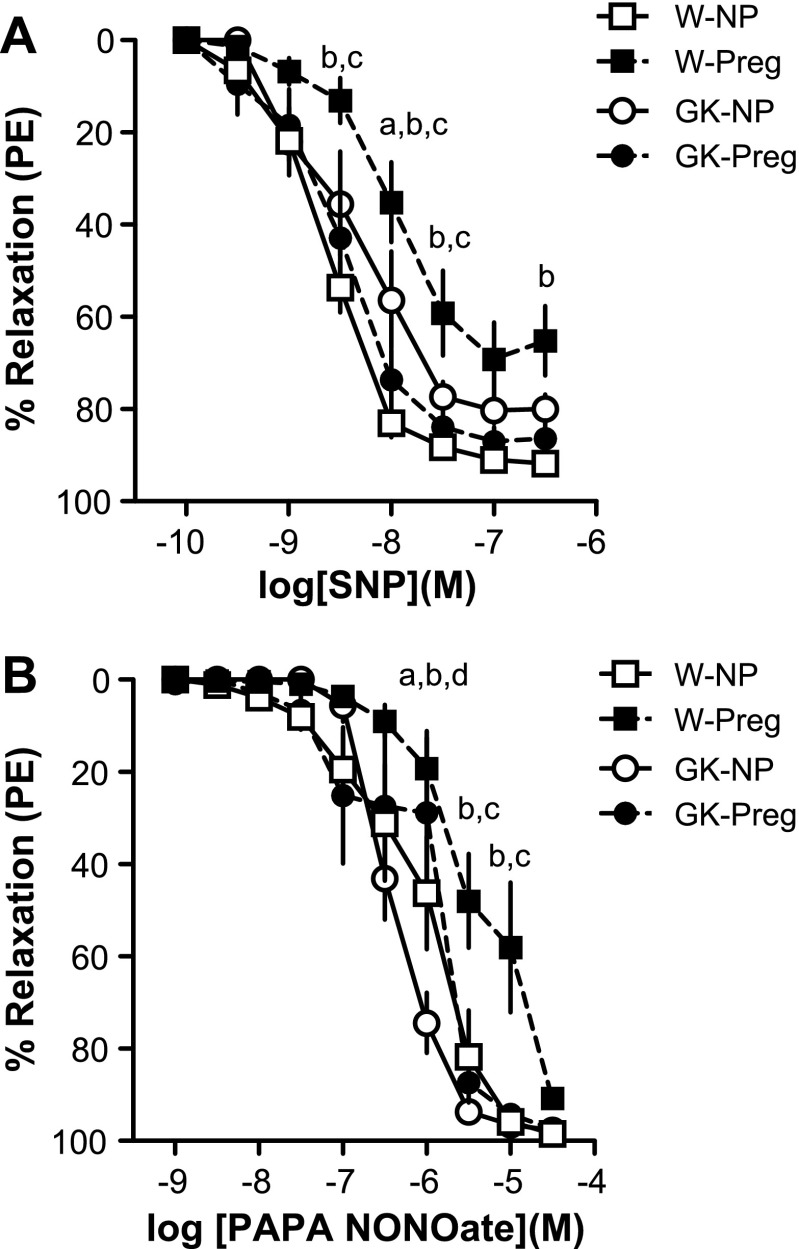
Uterine arteries from GK nonpregnant rats had diminished total relaxation responses to SNP compared with those from Wistar nonpregnant rats (AUC; Wistar nonpregnant: 195.0 ± 6.06 vs. GK nonpregnant: 144.6 ± 20.04; P < 0.05; Fig. 2A). Late pregnancy caused a rightward shift in the uterine SNP concentration-response curve in Wistar (−logEC50, nonpregnant: 8.67 ± 0.07 vs. pregnant: 7.99 ± 0.09; P < 0.05; Fig. 2A) but not in GK rats (−logEC50, nonpregnant: 8.48 ± 0.19 vs. pregnant: 8.65 ± 0.18; P > 0.05; Fig. 2A). In addition, Wistar pregnant uterine arteries had reduced maximum (Table 2 and Fig. 2A) and total relaxation responses to SNP (AUC) compared with Wistar nonpregnant arteries (AUC; Wistar nonpregnant: 195.0 ± 6.06 vs. Wistar pregnant: 108.8 ± 17.18; P < 0.05; Fig. 2A), and there were no differences in responses to SNP between GK nonpregnant uterine arteries and pregnant arteries (AUC; GK nonpregnant: 144.6 ± 20.04 vs. GK pregnant: 179.3 ± 18.10; P > 0.05; Fig. 2A and Table 2). Wistar pregnant rats had reduced responses (maximum and total relaxation) to SNP compared with GK pregnant rats (P < 0.05; Fig. 2A and Table 2).
Fig. 2.
Concentration-response curves to sodium nitroprusside (SNP; A) and propylamine propylamine NONOate (PAPA NONOate; B) in uterine arteries from nonpregnant and pregnant control [Wistar (W)] and diabetic (GK) rats. SNP-induced relaxation was reduced in GK-nonpregnant (NP; n = 9) rats compared with W-NP (n = 9) rats and in W-NP and GK-pregnant (Preg; n = 9) compared with W-Preg (n = 7). PAPA NONOate-induced relaxation was increased in GK-NP (n = 8) compared with W-NP (n = 9) and GK-Preg (n = 9) rats and in GK-Preg compared with W-Preg (n = 7) rats, and was reduced in W-Preg compared with W-NP rats. Values are means ± SE. aP < 0.05, GK-NP vs. W-NP; bP < 0.05, W-NP vs. W-Preg; cP < 0.05, GK-Preg vs. W-Preg; dP < 0.05, GK-NP vs. GK-Preg.
Table 2.
Maximum uterine artery relaxation responses (percent relaxation from submaximal responses to phenylephrine) to various agonists in Wistar and GK nonpregnant and pregnant rats
| Wistar |
GK |
|||
|---|---|---|---|---|
| Nonpregnant | Pregnant | Nonpregnant | Pregnant | |
| ACh | 87.2 ± 3.56 | 80.2 ± 4.71 | 74.7 ± 6.24 | 84.3 ± 4.10 |
| Sodium nitroprusside | 92.3 ± 1.31 | 69.7 ± 7.82a,b | 81.8 ± 2.61 | 88.3 ± 2.81 |
| NONOate | 97.5 ± 1.27 | 90.3 ± 2.73a | 97.4 ± 0.76 | 96.1 ± 1.67 |
| 8-Bromoguanosine 3′,5′-cyclic monophosphate sodium salt | 66.6 ± 6.07 | 66.5 ± 7.92 | 65.9 ± 11.73 | 71.9 ± 3.02 |
| Sildenafil | 85.1 ± 5.91c | 46.7 ± 4.18a | 53.1 ± 6.05 | 44.8 ± 5.63 |
Values are means ± SE; n = 8–10 for Wistar nonpregnant, n = 4–9 for Wistar pregnant, n = 4–10 for GK nonpregnant, and n = 4–9 for GK pregnant.
P < 0.05: Wistar nonpregnant vs. Wistar pregnant;
P < 0.05: Wistar pregnant vs. GK pregnant;
P < 0.05: Wistar nonpregnant vs. GK nonpregnant.
In contrast with SNP responses, GK nonpregnant uterine arteries had greater relaxation responses to PAPA NONOate compared with Wistar nonpregnant arteries (−logEC50, GK nonpregnant: 6.36 ± 0.061 vs. Wistar nonpregnant: 6.00 ± 0.119; P < 0.05; Fig. 2B). Pregnant GK and Wistar rats had reduced uterine artery sensitivity to PAPA NONOate compared with their nonpregnant counterparts (−logEC50, GK nonpregnant: 6.46 ± 0.08 vs. GK pregnant: 5.71 ± 0.11; Wistar nonpregnant: 6.10 ± 0.21 vs. Wistar pregnant: 5.84 ± 0.15; P < 0.05; Fig. 2B).
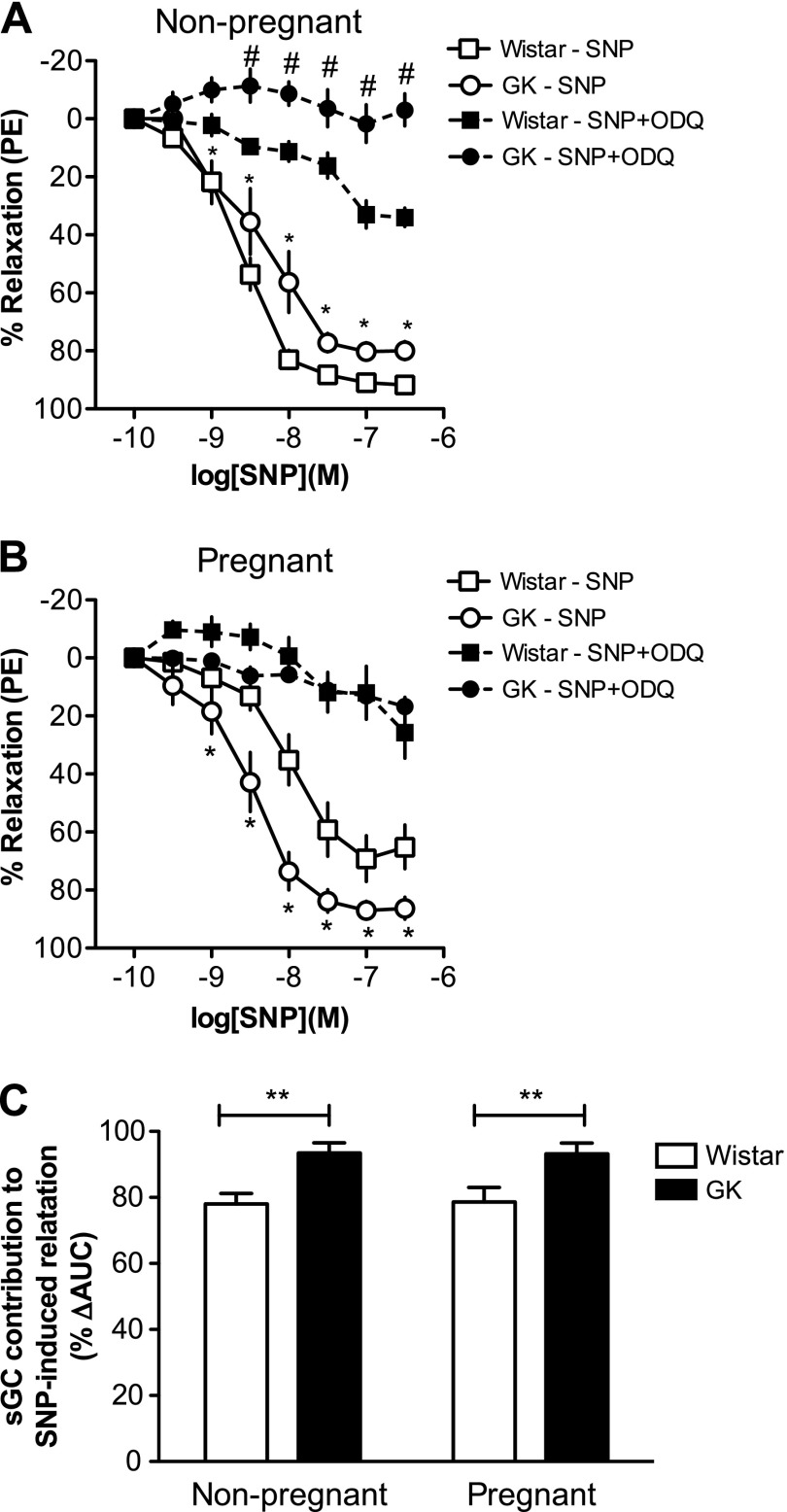
Inhibition of sGC by ODQ significantly reduced SNP-induced uterine artery relaxation in all groups (P < 0.05; Fig. 3, A and B). ODQ abolished the group differences in SNP-induced responses in pregnant but not in nonpregnant uterine arteries (Fig. 3, A and B). The contribution of sGC-dependent mechanisms to SNP-induced responses was greater in uterine arteries from GK rats compared with arteries from Wistar rats regardless of pregnancy status (main effect of diabetes, P < 0.05; Fig. 3C).
Fig. 3.
Concentration-response curves to SNP in the presence and absence of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one [ODQ; soluble guanylate cyclase (sGC) inhibitor, 10−6 M] in uterine arteries from nonpregnant (A) and pregnant (B) control (Wistar) and diabetic (GK) rats, and sGC contribution to SNP-induced relaxation (C). ODQ reduced SNP-induced uterine artery relaxations in GK nonpregnant (n = 9), Wistar nonpregnant (n = 9), GK pregnant (n = 9), and Wistar pregnant (n = 7) rats. GK nonpregnant and pregnant rats had increased contribution of sGC to SNP-induced relaxation compared with Wistar nonpregnant and pregnant rats, respectively. Values are means ± SE. *P < 0.05, Wistar vs. GK SNP alone; #P < 0.05, SNP alone vs. SNP plus ODQ for each group; **P < 0.05 vs. Wistar. AUC, area under the curve.
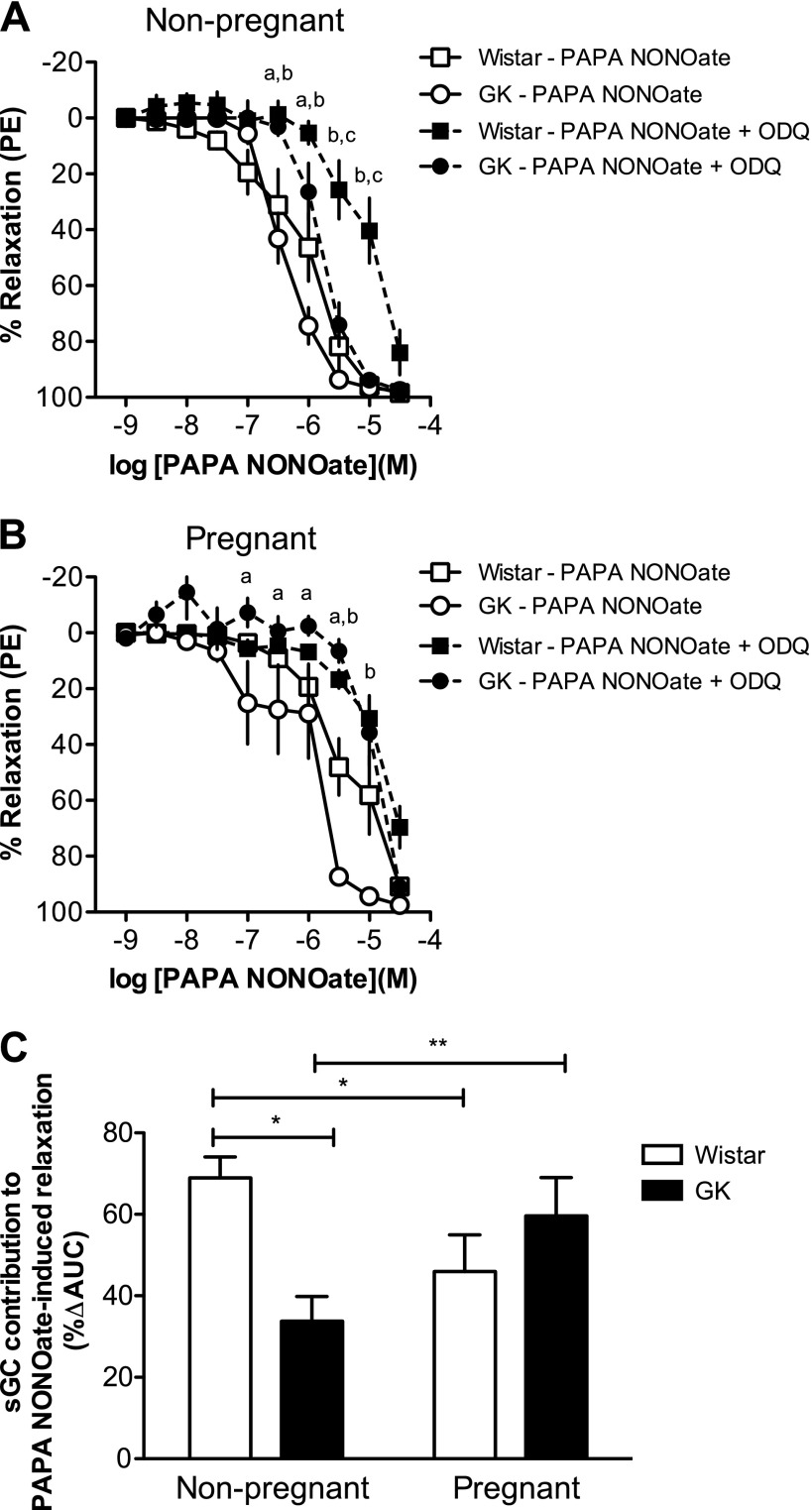
Inhibition of sGC caused a rightward shift in the PAPA NONOate concentration-response curves in all groups but did not affect maximum responses to PAPA NONOate (Fig. 4, A and B). ODQ abolished the group differences in pregnant uterine arteries (Fig. 4B). The contribution of sGC to PAPA NONOate-induced relaxation (%ΔAUC; Fig. 4C) was smaller in GK nonpregnant uterine arteries compared with Wistar nonpregnant arteries (P < 0.05; Fig. 4C). Pregnancy reduced this contribution in uterine arteries from Wistar rats (%ΔAUC, Wistar nonpregnant: 69.0 ± 5.15 vs. Wistar pregnant: 46.0 ± 9.03; P < 0.05), whereas the opposite was found for the GK uterine arteries (%ΔAUC, GK nonpregnant: 33.8 ± 6.04 vs. GK pregnant: 59.6 ± 9.44; P < 0.05).
Fig. 4.
Concentration-response curves to PAPA NONOate in the presence and absence of ODQ (sGC inhibitor, 10−6 M) in uterine arteries from nonpregnant (A) and pregnant (B) control (Wistar) and diabetic (GK) rats, and sGC contribution to PAPA NONOate-induced relaxation (C). ODQ decreased sensitivity to PAPA NONOate in uterine arteries from GK nonpregnant (n = 8), Wistar nonpregnant (n = 9), GK pregnant (n = 9), and Wistar pregnant (n = 7) rats. Values are means ± SE. aP < 0.05, PAPA NONOate alone vs. PAPA NONOate plus ODQ for GK rats; bP < 0.05, PAPA NONOate alone vs. PAPA NONOate plus ODQ for Wistar rats; cP < 0.05, Wistar nonpregnant rats PAPA NONOate plus ODQ vs. GK nonpregnant PAPA NONOate plus ODQ; *P < 0.05 vs. Wistar nonpregnant; **P < 0.05 vs. GK nonpregnant.
Neither diabetes nor pregnancy had any effects on 8-Br-cGMP-induced uterine artery relaxation (concentration-response curves not shown; Emax at Table 2).
Effects of PDE5 inhibition on rat uterine artery function.
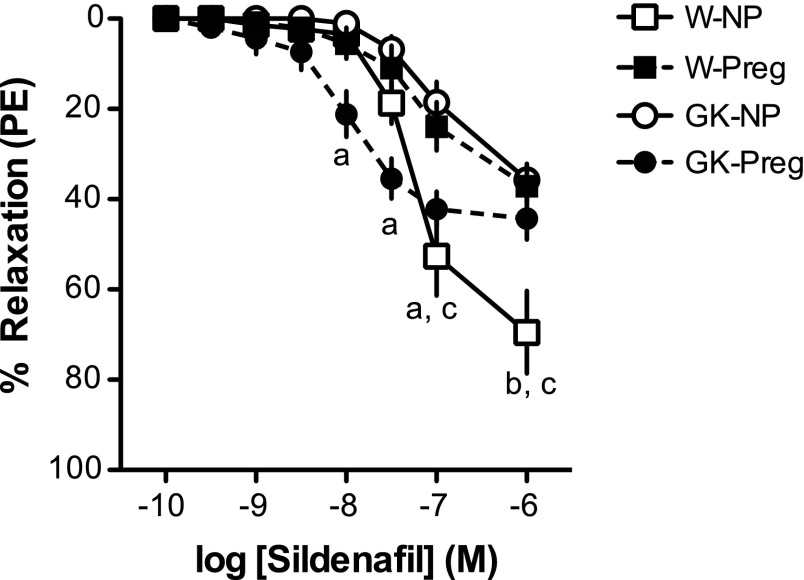
Sildenafil induced greater maximum relaxation responses in uterine arteries from Wistar compared with GK nonpregnant rats (P < 0.05; Fig. 5 and Table 2). However, GK nonpregnant uterine arteries had greater sensitivity to sildenafil compared with Wistar controls (−logEC50, Wistar: 7.10 ± 0.052 vs. GK: 7.96 ± 0.282; P < 0.05; Fig. 5). Pregnancy diminished the maximum responses to sildenafil in uterine arteries from Wistar rats (P < 0.05; Fig. 5 and Table 4) and reduced sensitivity (−logEC50, GK nonpregnant: 7.94 ± 0.143 vs. GK pregnant: 6.90 ± 0.118; P < 0.05; Fig. 5) but did not alter maximum responses to PDE5 inhibition in arteries from GK rats (P > 0.05; Fig. 5 and Table 2). There were no differences in uterine artery responses to sildenafil between pregnant uterine arteries from Wistar and GK rats (P > 0.05; Fig. 5).
Fig. 5.
Concentration-response curves to sildenafil [phosphodiesterase 5A (PDE5) inhibitor] in uterine arteries from nonpregnant (NP) and pregnant (Preg) control (Wistar, or W) and diabetic (GK) rats. GK-NP rats (n = 8) had increased uterine artery responses to sildenafil compared with W-NP rats (n = 10). W-Preg (n = 7) and GK-Preg (n = 7) rats had reduced responses to sildenafil compared with W-NP and GK-NP rats, and there were no differences between W-Preg and GK-Preg rats. Values are means ± SE. aP < 0.05, GK-NP vs. GK-Preg; bP < 0.05, GK-NP vs. W-NP; cP < 0.05, W-NP vs. W-Preg.
Protein expression of components of the sGC/cGMP pathway.
No differences existed between GK and Wistar nonpregnant uterine arteries in basal protein expression levels of the sGC isoforms α1 and β1 (P > 0.05; Fig. 6) and PDE5 (P > 0.05; Fig. 7). GK pregnant uterine arteries had reduced protein levels of sGC (both isoforms) compared with nonpregnant GK arteries (P < 0.05), but pregnancy had no effect on these proteins in Wistar uterine arteries (Fig. 6). Pregnancy did not alter the expression of PDE5 in uterine arteries from any of the groups.
Fig. 6.
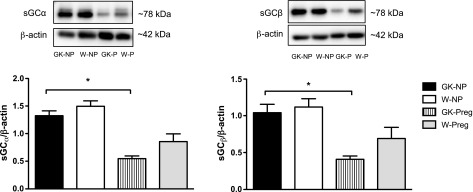
Protein expression levels of sGCα1 and sGCβ1 in uterine arteries from control (Wistar) and diabetic (GK) nonpregnant (NP) and pregnant (Preg) rats. GK-Preg rats had reduced expression levels of sGCα1 and sGCβ1 compared with GK-NP rats. Values are means ± SE; n = 7 for GK-NP, W-NP, and GK-Preg and n = 6 for W-Preg. *P < 0.05, vs. GK-NP.
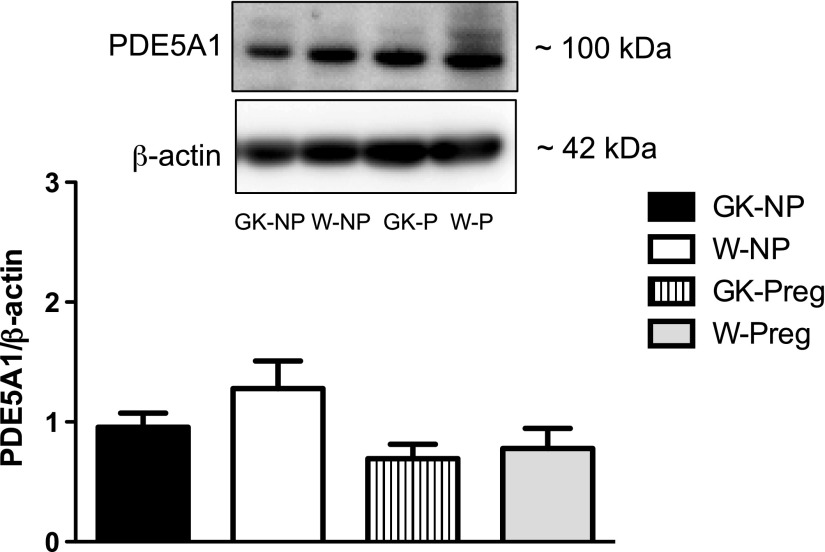
Fig. 7.
Protein expression levels of PDE5 in uterine arteries from control (Wistar) and diabetic (GK) nonpregnant and pregnant rats. There were no differences in PDE5 levels between groups. Values are means ± SE; n = 6 for GK-NP and GK-Preg, n = 5 for W-Preg, and n = 4 for W-NP. P > 0.05.
DISCUSSION
The main finding of this study is that the uterine vascular smooth muscle from rats with T2D has attenuated responsiveness to NO due to impairment in sGC-dependent mechanisms. These results suggest that rodents with T2D enter pregnancy with preexisting dysfunction of the uterine vasculature. In late pregnancy, uterine arteries from nondiabetic pregnant rats show attenuated vascular smooth muscle relaxation, but uterine arteries from GK rats fail to display this adaptation and exhibit greater vascular smooth muscle sensitivity to NO, despite a substantial reduction in protein levels of sGC.
Our studies showed that endothelium-dependent relaxation was reduced in uterine arteries from nonpregnant rats with T2D, and this impairment was concomitant with a decrease in vascular smooth muscle responsiveness to SNP. These data indicate that in addition to endothelial dysfunction, GK rats exhibit uterine vascular smooth muscle dysfunction. Currently, less is known about the function of the vascular smooth muscle cells in T2D compared with vascular endothelial cells (35). To the best of our knowledge, our study is the first to demonstrate dysfunction of the uterine vascular smooth muscle in the nonpregnant and pregnant states in rats with T2D. Importantly, GK rats have only mild hyperglycemia, which suggests that chronically small elevations in blood glucose may be adequate to induce impairment in smooth muscle function of the reproductive vascular bed.
In contrast with a reduction in SNP-induced relaxation, uterine arteries from GK rats had increased sensitivity to another NO donor, PAPA NONOate. These differences may be explained by dissimilarities in the metabolism and actions of these two drugs. The vasodilator effects of NO are primarily mediated by activation of sGC to induce the generation of the second messenger, cGMP. Therefore, it was speculated that NO donors act via the sGC/cGMP pathway. Previous studies, however, identified a NO-related sGC-independent component of vasodilation in response to high concentrations of NO (8, 16, 26). It was suggested that this sGC-independent action of NO was exclusively related to agents that generate NO extracellularly.
PAPA NONOate is a large molecule that does not enter vascular smooth muscle cells. It spontaneously dissociates to generate NO, and this process is not catalyzed by either thiols or biological tissue (26). On the other hand, SNP requires intracellular metabolism to generate NO. Using a specific inhibitor of sGC, we demonstrate for first time that in rat uterine arteries SNP-induced relaxation is primarily mediated by sGC-dependent mechanisms, whereas PAPA NONOate has both sGC-dependent and independent actions. Interestingly, the contribution of sGC-associated mechanisms to SNP and PAPA NONOate-induced relaxations differ between Wistar and GK nonpregnant uterine arteries. Furthermore, uterine arteries from GK rats exhibited reduced sGC-dependent vasodilator mechanisms, whereas the sGC-independent component of vasorelaxation was enhanced. Previous work has suggested that the contribution of NO-related sGC-independent pathways may be upregulated in cardiovascular diseases or inflammatory conditions, where inducible NOS (iNOS) is expressed (15). In addition, Miller et al. (26) found that the sGC-independent actions of NONOates were significantly reduced by extracellular NO scavengers. These authors suggested that NONOates release sufficient NO extracellularly to react with molecular oxygen and form nitrosating species (26). S-Nitrosation of thiol-containing enzymes and ion channels, therefore, may be a potential sGC-independent mechanism of relaxation in the uterine arteries of GK rats.
According to our findings, diabetic uterine arteries use sGC-independent mechanisms to compensate for a reduction in sGC-dependent relaxation. Because responses to 8-Br-cGMP did not differ between GK and Wistar uterine arteries, we hypothesize that the signaling defect in GK uterine vascular smooth muscle cells lies upstream of cGMP (e.g., sGC-induced cGMP generation) and may act in concert with reduced basal NO biovailability. Due to the small size of rat uterine arteries, we were unable to measure production of cGMP in response to sGC activation. Previous studies, however, have shown reduced sGC-induced cGMP production in conduit arteries from GK rats (48).
The intracellular levels of cGMP are controlled by the rate of cGMP synthesis (sGC-mediated) and by the rate of cGMP hydrolysis (cyclic nucleotide PDE dependent). PDE5 is a cGMP-binding, cGMP-specific PDE that controls the hydrolysis of cGMP in vascular smooth muscle cells. Basal levels of PDE5 did not differ between GK and Wistar nonpregnant rats but this does not exclude the possibility of group differences in PDE5 activity.
In late pregnancy and in the presence of equal amounts of exogenous NO, GK uterine arteries had increased relaxation compared with Wistar rats; however, endothelium-dependent relaxation did not differ between groups. Inhibition of sGC abolished the group differences in uterine responses to NO donors, indicating that these differences were sGC dependent. Our findings are in agreement with previous reports showing reductions in endothelium-independent relaxation to SNP in late pregnant rats (nondiabetic) (20, 46). Pregnancy increases endothelium-derived NO production (11, 50) and sGC activity (17) in the uterine vasculature; thus further increases in sGC activity in the presence of exogenous NO to induce vascular smooth muscle relaxation may not be feasible (46). In contrast, GK rats showed a reduction in uterine artery sGC expression and no change in SNP-induced relaxation, whereas PAPA NONOate-induced relaxation (largely dependent on a sGC-independent mechanism) was reduced in response to pregnancy. These data show that the expression of sGC (both subunits) and the sGC-dependent relaxation of smooth muscle in uterine arteries are mediated by the combined effect of pregnancy and T2D, whereas T2D or pregnancy alone have no effect on basal sGC expression and induce relaxation responses opposite from those induced by pregnancy and diabetes together.
The adverse pregnancy outcomes and impairment in uterine vascular smooth muscle responsiveness to NO could be attributed to several factors associated with the pathology of T2D, such as oxidative stress and hyperglycemia. It has been reported that the exposure to a diabetes-associated hyperglycemic environment within the first 7 wk of human pregnancy (equivalent to the first 13.5 gestational days of rat pregnancy) are associated with pre-implantation embryo loss and increased resorption rates (28). Our findings in corroboration with other investigations demonstrated dramatically higher rates of resorptions in rats with T2D compared with nondiabetic pregnant rats (21). Stanley et al. showed endothelial dysfunction and increased production of superoxide in uterine arteries from mice with gestational diabetes, which was attributed to NOS uncoupling (41). These investigators, however, did not investigate the effects of gestational diabetes on vascular smooth muscle sensitivity to NO. Defects in sGC-dependent vascular smooth muscle relaxation have been previously reported in aorta from GK rats and attributed to a reduction in the heme content of the enzyme and/or oxidation of the heme iron (48). Hyperglycemia-induced oxidation of the prosthetic heme group of sGC would lead to reduced uterine vascular smooth muscle sensitivity to NO.
Estrogen and progesterone have been shown to affect vascular smooth muscle reactivity and production of endothelium-derived factors (45). Thus different levels of estrogen and progesterone may be responsible for the differential vascular responses in GK and Wistar rats. However, we had previously demonstrated no significant differences in these hormones between GK and Wistar nonpregnant rats, suggesting that the pregestational vascular dysfunction seen in GK rats cannot be attributed to these hormones (1). Nevertheless, the levels of estrogen and progestins significantly change during pregnancy (37). It is possible that the alterations in uterine vascular dilatory mechanisms seen in GK pregnant rats are mediated by different estrogen and progesterone levels between GK and Wistar rats.
Pregnant Wistar and GK uterine arteries showed a reduction in sensitivity to sildenafil (PDE5 inhibitor) compared with the nonpregnant state, whereas GK nonpregnant uterine arteries had increased sensitivity to sildenafil compared with Wistar nonpregnant arteries. This is the first report to document such pregnancy- and diabetes-induced adaptations. Sildenafil has been previously used in pregnant women and animal models to increase uterine dilatory responses and promote an increase in uterine blood flow. Indeed, sildenafil improved endothelial function of isolated myometrial vessels from pregnant women with intrauterine growth restriction and increased uterine blood flow in women with healthy pregnancies (14, 27, 44). In animal models of pregnancy-induced hypertension and preeclampsia, sildenafil also improved endothelial function (9, 43). Moreover, the use of sildenafil has been recommended for pregnancies complicated with pulmonary hypertension because of the dilatory actions of this PDE5 inhibitor in the pulmonary circulation (22). Because sildenafil has the ability to increase uterine blood flow, it could be a reasonable choice of treatment for women with T2D and vascular dysfunction. Nevertheless, high drug concentrations may be necessary in pregnant women and the toxicity of those concentrations should be considered (39). On the contrary, if sildenafil treatment starts before the beginning of pregnancy, where women with T2D may have increased uterine sensitivity to sildenafil, lower concentrations of this drug may be necessary to adequately increase blood flow to the uterine and decidua tissues.
In conclusion, our study demonstrated that uterine arteries from rats with T2D had endothelial dysfunction, which may be partially explained by reduced sensitivity of uterine vascular smooth muscle sGC to NO. During pregnancy, the GK uterine vascular smooth muscle fails to show relaxation responses similar to those of arteries from nondiabetic rats of the same gestational age and GK rats have dramatically greater rates of resorptions compared with Wistar rats. We propose that the diabetic uterine vascular smooth muscle may be a novel pharmacological target to induce vascular improvements. Prenatal combination of PDE5 inhibitor treatment with good glycemic control may improve function of the uterine vasculature in women with T2D, increase blood flow to the uteroplacental unit, and improve pregnancy outcomes.
GRANTS
This study was supported in part by the National Institutes of Health Grants R01 HL-071138, R01 DK-083685, and T32 HL-066993-09); American Heart Association Scientist Development Grant 13SDG17050056; the Naito Foundation Japan; the Heart and Stroke Foundation Canada; and the Society for Women's Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.G., J.L.H., T.M., and R.C.W. conception and design of research; S.G., J.L.H., and T.M. performed experiments; S.G. and T.M. analyzed data; S.G., A.E., and R.C.W. interpreted results of experiments; S.G. prepared figures; S.G. drafted manuscript; S.G., J.L.H., T.M., A.E., and R.C.W. edited and revised manuscript; S.G., J.L.H., T.M., A.E., and R.C.W. approved final version of manuscript.
REFERENCES
- 1.Allahdadi KJ, Hannan JL, Ergul A, Tostes RC, Webb RC. Internal pudendal artery from type 2 diabetic female rats demonstrate elevated endothelin-1-mediated constriction. J Sex Med 8: 2472–2483, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brydon P, Smith T, Proffitt M, Gee H, Holder R, Dunne F. Pregnancy outcome in women with type 2 diabetes mellitus needs to be addressed. Int J Clin Pract 54: 418–419, 2000 [PubMed] [Google Scholar]
- 3.Cheng YW, Chung JH, Kurbisch-Block I, Inturrisi M, Shafer S, Caughey AB. Gestational weight gain and gestational diabetes mellitus: perinatal outcomes. Obstet Gynecol 112: 1015–1022, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Cipolla M, Osol G. Hypertrophic and hyperplastic effects of pregnancy on the rat uterine arterial wall. Am J Obstet Gynecol 171: 805–811, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Cooke CL, Davidge ST. Pregnancy-induced alterations of vascular function in mouse mesenteric and uterine arteries. Biol Reprod 68: 1072–1077, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Evers IM, de Valk HW, Visser GH. Risk of complications of pregnancy in women with type 1 diabetes: nationwide prospective study in the Netherlands. BMJ 328: 915, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feig DS, Palda VA. Type 2 diabetes in pregnancy: a growing concern. Lancet 359: 1690–1692, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol 48: 184–188, 1995 [PubMed] [Google Scholar]
- 9.George EM, Palei AC, Dent EA, Granger JP. Sildenafil attenuates placental ischemia induced hypertension. Am J Physiol Regul Integr Comp Physiol 305: R397–R403, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gokina NI, Bonev AD, Gokin AP, Goloman G. Role of impaired endothelial cell Ca2+ signaling in uteroplacental vascular dysfunction during diabetic rat pregnancy. Am J Physiol Heart Circ Physiol 304: H935–H945, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gokina NI, Mandala M, Osol G. Induction of localized differences in rat uterine radial artery behavior and structure during gestation. Am J Obstet Gynecol 189: 1489–1493, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Goto Y, Kakizaki M, Masaki N. Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med 119: 85–90, 1976 [DOI] [PubMed] [Google Scholar]
- 13.Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag 3: 853–876, 2007 [PMC free article] [PubMed] [Google Scholar]
- 14.Hale SA, Jones CW, Osol G, Schonberg A, Badger GJ, Bernstein IM. Sildenafil increases uterine blood flow in nonpregnant nulliparous women. Reprod Sci 17: 358–365, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobbs AJ, Higgs A, Moncada S. Inhibition of nitric oxide synthase as a potential therapeutic target. Annu Rev Pharmacol Toxicol 39: 191–220, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Homer KL, Fiore SA, Wanstall JC. Inhibition by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) of responses to nitric oxide-donors in rat pulmonary artery: influence of the mechanism of nitric oxide generation. J Pharm Pharmacol 51: 135–139, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Itoh H, Bird IM, Nakao K, Magness RR. Pregnancy increases soluble and particulate guanylate cyclases and decreases the clearance receptor of natriuretic peptides in ovine uterine, but not systemic, arteries. Endocrinology 139: 3329–3341, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Izumi H, Garfield RE, Makino Y, Shirakawa K, Itoh T. Gestational changes in endothelium-dependent vasorelaxation in human umbilical artery. Am J Obstet Gynecol 170: 236–245, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Izumi H, Makino Y, Shirakawa K, Garfield RE. Role of nitric oxide on vasorelaxation in human umbilical artery. Am J Obstet Gynecol 172: 1477–1484, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Jain V, Vedernikov YP, Saade GR, Chwalisz K, Garfield RE. Effect of gestational age on in-vitro responses of pregnant rat aorta. Hum Reprod 13: 214–219, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Jawerbaum A, Gonzalez E. The role of alterations in arachidonic acid metabolism and nitric oxide homeostasis in rat models of diabetes during early pregnancy. Curr Pharm Des 11: 1327–1342, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Kass DA, Champion HC, Beavo JA. Phosphodiesterase type 5: expanding roles in cardiovascular regulation. Circ Res 101: 1084–1095, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113: 1888–1904, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Kwik M, Seeho SK, Smith C, McElduff A, Morris JM. Outcomes of pregnancies affected by impaired glucose tolerance. Diabetes Res Clin Pract 77: 263–268, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: the consequences of not treating. Am J Obstet Gynecol 192: 989–997, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Miller MR, Okubo K, Roseberry MJ, Webb DJ, Megson IL. Extracellular nitric oxide release mediates soluble guanylate cyclase-independent vasodilator action of spermine NONOate: comparison with other nitric oxide donors in isolated rat femoral arteries. J Cardiovasc Pharmacol 43: 440–451, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Miller SL, Loose JM, Jenkin G, Wallace EM. The effects of sildenafil citrate (Viagra) on uterine blood flow and well being in the intrauterine growth-restricted fetus. Am J Obstet Gynecol 200: 102 e101–e107, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Mills JL, Baker L, Goldman AS. Malformations in infants of diabetic mothers occur before the seventh gestational week. Implications for treatment. Diabetes 28: 292–293, 1979 [DOI] [PubMed] [Google Scholar]
- 29.Montero D, Walther G, Perez-Martin A, Vicente-Salar N, Roche E, Vinet A. Vascular smooth muscle function in type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetologia 56: 2122–2133, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Oniki H, Goto K, Fujii K, Kansui Y, Murakami N, Ohtsubo T, Matsumura K, Kitazono T. Effects of the superoxide dismutase mimetic tempol on impaired endothelium-dependent and endothelium-independent relaxations in type II diabetic rats. Clin Exp Hypertens 35: 112–119, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 24: 58–71, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips JK, Vance AM, Raj RS, Mandala M, Linder EA, Gokina NI. Impact of experimental diabetes on the maternal uterine vascular remodeling during rat pregnancy. Reprod Sci 19: 322–331, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pietryga M, Brazert J, Wender-Ozegowska E, Biczysko R, Dubiel M, Gudmundsson S. Abnormal uterine Doppler is related to vasculopathy in pregestational diabetes mellitus. Circulation 112: 2496–2500, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Pitei DL, Watkins PJ, Edmonds ME. NO-dependent smooth muscle vasodilatation is reduced in NIDDM patients with peripheral sensory neuropathy. Diabet Med 14: 284–290, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Porter KE, Riches K. The vascular smooth muscle cell: a therapeutic target in Type 2 diabetes? Clin Sci (Lond) 125: 167–182, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Portha B, Lacraz G, Kergoat M, Homo-Delarche F, Giroix MH, Bailbe D, Gangnerau MN, Dolz M, Tourrel-Cuzin C, Movassat J. The GK rat beta-cell: a prototype for the diseased human beta-cell in type 2 diabetes? Mol Cell Endocrinol 297: 73–85, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Puri CP, Garfield RE. Changes in hormone levels and gap junctions in the rat uterus during pregnancy and parturition. Biol Reprod 27: 967–975, 1982 [DOI] [PubMed] [Google Scholar]
- 38.Rask-Madsen C, King GL. Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab 3: 46–56, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Sasser JM, Baylis C. Effects of sildenafil on maternal hemodynamics and fetal growth in normal rat pregnancy. Am J Physiol Regul Integr Comp Physiol 298: R433–R438, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanley JL, Ashton N, Taggart MJ, Davidge ST, Baker PN. Uterine artery function in a mouse model of pregnancy complicated by diabetes. Vascul Pharmacol 50: 8–13, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Stanley JL, Cheung CC, Rueda-Clausen CF, Sankaralingam S, Baker PN, Davidge ST. Effect of gestational diabetes on maternal artery function. Reprod Sci 18: 342–352, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Stanley JL, Sankaralingam S, Baker PN, Davidge ST. Previous gestational diabetes impairs long-term endothelial function in a mouse model of complicated pregnancy. Am J Physiol Regul Integr Comp Physiol 299: R862–R870, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Turgut NH, Temiz TK, Bagcivan I, Turgut B, Gulturk S, Karadas B. The effect of sildenafil on the altered thoracic aorta smooth muscle responses in rat pre-eclampsia model. Eur J Pharmacol 589: 180–187, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Wareing M, Myers JE, O′Hara M, Baker PN. Sildenafil citrate (Viagra) enhances vasodilatation in fetal growth restriction. J Clin Endocrinol Metab 90: 2550–2555, 2005 [DOI] [PubMed] [Google Scholar]
- 45.White MM, Zamudio S, Stevens T, Tyler R, Lindenfeld J, Leslie K, Moore LG. Estrogen, progesterone, and vascular reactivity: potential cellular mechanisms. Endocr Rev 16: 739–751, 1995 [DOI] [PubMed] [Google Scholar]
- 46.Wight E, Kung CF, Moreau P, Takase H, Bersinger NA, Luscher TF. Aging, serum estradiol levels, and pregnancy differentially affect vascular reactivity of the rat uterine artery. J Soc Gynecol Investig 7: 106–113, 2000 [PubMed] [Google Scholar]
- 47.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol 27: 567–574, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Witte K, Jacke K, Stahrenberg R, Arlt G, Reitenbach I, Schilling L, Lemmer B. Dysfunction of soluble guanylyl cyclase in aorta and kidney of Goto-Kakizaki rats: influence of age and diabetic state. Nitric Oxide 6: 85–95, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Xiao D, Pearce WJ, Zhang L. Pregnancy enhances endothelium-dependent relaxation of ovine uterine artery: role of NO and intracellular Ca2+. Am J Physiol Heart Circ Physiol 281: H183–H190, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Yi FX, Boeldt DS, Magness RR, Bird IM. [Ca2+]i signaling vs. eNOS expression as determinants of NO output in uterine artery endothelium: relative roles in pregnancy adaptation and reversal by VEGF165. Am J Physiol Heart Circ Physiol 300: H1182–H1193, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]