Abstract
Amyloid-β (Aβ) has long been implicated as a causative protein in Alzheimer's disease. Cellular Aβ accumulation is toxic and causes mitochondrial dysfunction, which precedes clinical symptoms of Alzheimer's disease pathology. In the present study, we explored the possible use of epoxyeicosatrienoic acids (EETs), epoxide metabolites of arachidonic acid, as therapeutic target against Aβ-induced mitochondrial impairment using cultured neonatal hippocampal astrocytes. Inhibition of endogenous EET production by a selective epoxygenase inhibitor, MS-PPOH, caused a greater reduction in mitochondrial membrane potential in the presence of Aβ (1, 10 μM) exposure versus absence of Aβ. MS-PPOH preincubation also aggravated Aβ-induced mitochondrial fragmentation. Preincubation of the cells with either 14,15- or 11,12-EET prevented this mitochondrial depolarization and fragmentation. EET pretreatment also further improved the reduction observed in mitochondrial oxygen consumption in the presence of Aβ. Preincubation of the cells with EETs significantly improved cellular respiration under basal condition and in the presence of the protonophore, carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP). The uncoupling of ATP synthase from the electron transfer chain that occurred in Aβ-treated cells was also prevented by preincubation with EETs. Lastly, cellular reactive oxygen species production, a hallmark of Aβ toxicity, also showed significant reduction in the presence of EETs. We have previously shown that Aβ reduces EET synthesis in rat brain homogenates and cultured hippocampal astrocytes and neurons (Sarkar P, Narayanan J, Harder DR. Differential effect of amyloid beta on the cytochrome P450 epoxygenase activity in rat brain. Neuroscience 194: 241–249, 2011). We conclude that reduction of endogenous EETs may be one of the mechanisms through which Aβ inflicts toxicity and thus supplementing the cells with exogenous EETs improves mitochondrial dynamics and prevents metabolic impairment.
Keywords: EETs, Aβ, mitochondrial membrane potential, mitochondrial fragmentation, ROS, oxygen consumption
alzheimer's disease (AD) is a debilitating, late-onset, progressive, neurodegenerative disease that affects cognition, memory, and behavior and is clinically determined by the formation of neurofibrillary tangles and extracellular deposition of the protein amyloid-β (Aβ) in the form of plaques (47). However, it is the intracellular accumulation of soluble oligomeric Aβ that is believed to play a central role in the pathogenesis of AD (34). A growing body of research suggests that mitochondrial dysfunction is one of the leading causes of neurodegeneration in aging (50) and AD (29). Aβ has been shown to negatively affect mitochondrial function (16, 21) through pathways such as impaired oxidative phosphorylation, loss of mitochondrial membrane potential, altered morphology, and increased oxidative stress [reviewed in (22)]. This deregulation of mitochondrial function generates a vicious loop decreasing the proteolytic activity of mitochondrial presequence peptidases (PreP peptidasome), which metabolizes Aβ (6, 24), leading to further accumulation of Aβ in the mitochondria. Decreased mitochondrial activity leads to cellular dysfunction by deregulating calcium influx (5) and activating apoptotic pathways (42). Mitochondrial accumulation of Aβ and amyloid precursor protein has been reported in AD patients (19, 35) and mouse models of AD (7, 18, 36). Furthermore, both sporadic and familial AD patients show mitochondrial dysfunction, implying that irrespective of the cause of Aβ accumulation, the primary target is mitochondria (10, 27, 30). In fact, mitochondrial damage occurs before clinically detectable pathologies surface and follows the progression of AD damage in human and mouse models, establishing this as an early biomarker of AD pathology (17, 28, 57).
Astrocytes play a crucial role in neurodegeneration seen in AD (1). Aβ causes mitochondrial dysfunction in astrocytes by reducing mitochondrial membrane potential, altering calcium influx, suppressing oxygen consumption, and increasing formation and release of reactive oxygen species (ROS), which leads to cell death in surrounding neurons (1, 2).
We have previously reported that Aβ affects epoxygenase activity in cultured astrocytes (46), resulting in decreased 14,15- and 11,12-epoxyeicosatrienoic acid (EET) production. EET (4 regioisomers: 5,6-, 8,9-, 11,12-, and 14,15-EET) are epoxides of the essential fatty acid arachidonic acid and have well-established cytoprotective properties in cardiovascular and cerebrovascular injury models (43, 49, 59). It has been proposed that the cytoprotective action of EETs is mediated by improving mitochondrial function (31), modulation of the ATP-sensitive K+ channels (12), and preventing the loss of mitochondrial membrane potential (23). EETs have also been reported to inhibit ROS production in ischemia-reperfusion injury (25) and prevent oxidant-induced neuronal damage (51), but the effect of EETs in Aβ-induced mitochondrial dysfunction is not known. Therefore, in this study we have addressed the potential effectiveness of 14,15- and 11,12-EET in preventing soluble oligomeric Aβ-induced mitochondrial damage. We hypothesized that: 1) inhibition of endogenous EET production will contribute to Aβ toxicity and 2) supplementation with exogenous EETs will protect the cells from Aβ injury.
METHODS
Cell culture.
Primary cultures of neonatal hippocampal astrocytes were obtained as previously described (46). All protocols were approved by the Institutional Animal Care and Use Committee at the Medical College of Wisconsin and in accordance with National Institutes of Health guidelines. Briefly, Sprague-Dawley rat pup brains (1 to 2 days) were quickly removed, and hippocampi were dissected in media containing Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Carlsbad, CA), supplemented with 1% penicillin-streptomycin, 0.1% gentamicin, and 0.005% fungizone (Invitrogen). Minced hippocampal tissue was then digested for 45 min at 37°C with gentle shaking in papain (20 U/ml, Worthington Biochemical, Freehold, NJ) and l-cystine (1.5×10−4 g/ml, Sigma-Aldrich, St. Louis, MO) in dissection media. The pellet was washed three times and triturated in growth media consisting of DMEM supplemented with 10% FBS (Invitrogen), 1% penicillin-streptomycin, and 0.1% gentamicin. Cells were plated on 10-cm culture dishes (Sigma Chemicals, St. Louis, MO) at a density of ∼2 × 105 cells per cm2 and incubated at 37°C in an atmosphere of 5% CO2 in room air. Medium was replaced the next day and, thereafter, every third day with gentamicin-free growth media. Cells from passages 1 to 2 were used for experiments.
Oligomeric Aβ and preincubation with EETs and MS-PPOH.
Soluble oligomers of Aβ (Aβ 1–42, Sigma) were prepared as previously described, and the quality of the oligomers was checked with Western blot analysis (46). Briefly, Aβ was dissolved in hexafluoroisopropanol, and the aliquots were dried in a Speed-Vac and stored at −80°C. Before experimentation, the aliquots were dissolved in DMSO and media and allowed to oligomerize for 24 h at 4°C. Serum-starved cells were incubated with Aβ or vehicle (equivalent mixture of DMSO and media) for 24 h. Control experiments were done using reverse Aβ (42–1, Sigma), which was oligomerized following the same protocol for Aβ (1–42), and it had no effect on the mitochondrial membrane potential, morphology, and ROS production. Stock solutions of MS-PPOH (10 mM, Cayman Chemicals, Ann Arbor, MI) and EETs (32.5 mM, kindly donated by Dr. John R. Falck, Department of Biochemistry, University of Texas Southwestern Medical Center) were prepared in ethanol. To block endogenous EET production, the epoxygenase inhibitor MS-PPOH (40 μM) was added to the cells 12 h before Aβ incubation. Different concentrations of EETs were added 30 min after MS-PPOH. Since bioavailability of EETs declines rapidly, at the end of 12 h EETs were added again followed by the addition of Aβ after 30 min (Fig. 1A).
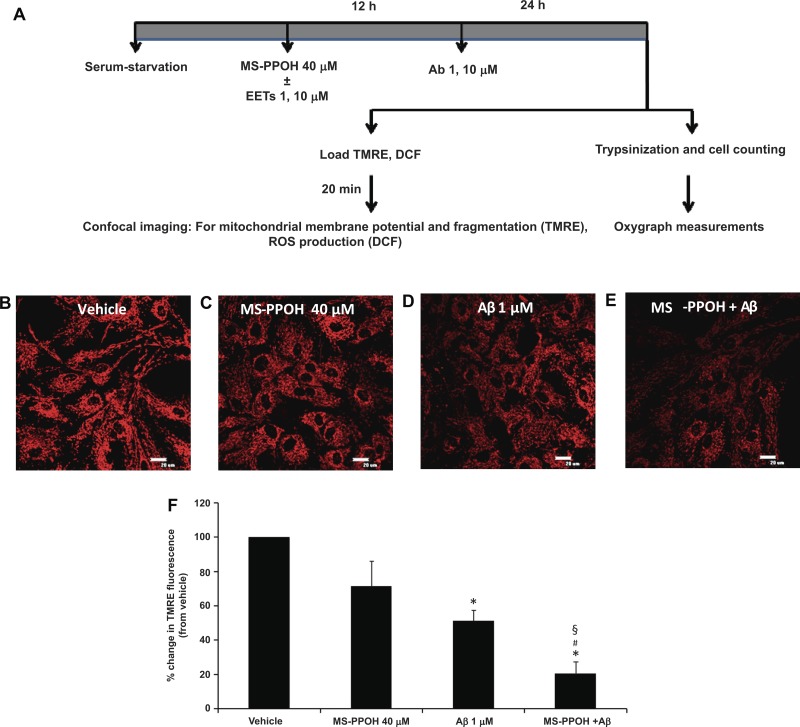
Fig. 1.
Preincubation with MS-PPOH aggravates amyloid-β (Aβ, Ab)-induced mitochondrial membrane depolarization. A schematic diagram depicting experimental procedure (A). EETs, epoxyeicosatrienoic acids; DCF, 5 (and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester; ROS, reactive oxygen species. For mitochondrial membrane potential measurements astrocytes were loaded with tetramethylrhodamine ethyl ester perchlorate (EET; which localizes to the inner mitochondrial membrane) after being treated with vehicle (B), MS-PPOH (C), Aβ (D), and MS-PPOH + Aβ (E) (×60 magnification; scale bar = 20 μm). The relative change in fluorescence intensity is expressed as percent change from vehicle set at 100%. Aβ (1 μM) causes a decrease in mitochondrial membrane potential (seen as a decrease in EET fluorescence) compared with vehicle, which is further decreased by incubation with MS-PPOH (F). *P < 0.001 vs. vehicle; #P < 0.05 vs. Aβ; §P < 0.001 vs. MS-PPOH; n = 5 to 6.
Confocal microscopy.
For measurement of mitochondrial membrane potential and fragmentation, cells were plated on Matrigel-coated (Sigma) glass coverslips at a density of 20,000 cells/cover slip (1 cm diameter, Thermo Fisher, Waltham, MA), grown for 24 h in DMEM with 0.1% bovine serum albumin. Cells were treated with Aβ with or without MS-PPOH and EETs. The coverslips were immersed in phenol-red free DMEM (Invitrogen) containing 30 nM tetramethylrhodamine ethyl ester perchlorate (EET, Invitrogen), a membrane-potential sensitive dye, which accumulates in the inner mitochondrial membrane, for 20 min and washed for 5 min before imaging.
For cellular ROS production measurements, a similar method was followed, except that the coverslips were immersed in phenol-red free media containing 1 μM 5 (and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA or DCF; Molecular Probes, Eugene, OR) instead of EET.
Images of dye-loaded cells were captured using a confocal microscope (Eclipse TE2000-U; Nikon) with a ×60 oil-immersion objective, 1.4 numerical aperature, and a ND4 filter to prevent photobleaching. EET was excited at 543 nm with a helium-neon laser, and emission spectra were recorded through a band-pass 590 to 640-nm filter. An argon laser was used to excite DCF at 488 nm, and emission was recorded through a band-pass filter (515 to 530 nm).
Image analysis.
Images were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD).
Mitochondrial membrane potential and ROS generation.
Ten images from random, nonoverlapping fields were taken per coverslip from cells loaded with EET or DCF. The mean fluorescence intensity/image calculated after background subtraction was averaged over the ten captured images from at least five independent experiments. Relative change in intensity was expressed as percent change from the vehicle control and calculated separately for each individual experiment, with vehicle set at 100%.
Mitochondrial morphology analysis.
Images of 56 μm × 56 μm area were captured that approximately focused on single cells stained with EET. A minimum of five cells were imaged per coverslip. For quantitative analysis of mitochondrial fragmentation, a median filter was applied to the acquired images to equalize fluorescence intensity. After converting to a binary form, the image was subjected to particle analysis that calculated the circularity and aspect ratio (AR = major axis/minor axis of the ellipse equivalent to that object) of each mitochondrial object per image. The form factor [FF = 1/circularity = perimeter2/(4π·area)] was calculated from the circularity values. These values were averaged over four to six images over at least four independent experiments per group to obtain the mean AR and FF values. A perfect circle has an AR of 1, and the value increases as it becomes more elongated. Whereas AR is an indicator of length and shape of the mitochondria, FF denotes length and branching. Hence, low values of AR and FF signify fragmented, unbranched mitochondria, and higher values mean more elongated, branched mitochondria (32, 52).
Mitochondrial respiration in whole cells.
Confluent cells grown in six-well plates (Sigma) were used for measurement of mitochondrial oxygen consumption after incubation with Aβ, EETs, and MS-PPOH, as described above. At the end of 24 h, cells were trypsinized, centrifuged, and resuspended in 300 μl growth media (Fig. 1A). Cell number was calculated using an automated cell counter (TC10, Bio-Rad, Hercules, CA) by trypan blue exclusion method. The cell suspension was then placed with a magnetic stirrer into a closed glass chamber thermostatically maintained at 37°C. Oxygen consumption was followed with an S1Clark type oxygen electrode connected to an Oxygraph (version 1.02) control unit (Hansatech Instruments, Norfolk, England). The slopes of the obtained traces represent the rate of consumption. Three distinct states of respiration were measured: 1) baseline, depicting cellular oxygen consumption at a basal level without the presence of any modulator; 2) oligomycin (2 μM, Sigma), added to block ATP synthase and H+ flux through it; and 3) a protonophore, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP, 2 μM, Sigma), added to uncouple electron transport chain from ATP synthesis, which in turn increases the oxygen consumption, indicating the maximal respiratory state. Non-mitochondrial respiration was obtained by adding 1 μM complex III blocker antimycin A (Sigma) and was subtracted from all recordings (1).
The values were normalized to the total amount of protein present in the cell suspension, which was calculated at the end of each experiment by Bio-Rad protein assay kit.
Statistical analysis.
All data are presented as means ± SE of n ≥ 4 independent experiments. One-way analysis of variance was performed to determine the difference between the mean values of the groups, which was followed by a post hoc Holm-Sidak (all pairwise comparison) test to assess intergroup differences. P < 0.05 was considered to be significant.
RESULTS
Decreasing endogenous EETs aggravates Aβ-induced depolarization of mitochondrial membrane potential.
Exposure to Aβ for 24 h (1 μM) caused a decrease in mitochondrial membrane potential as revealed by a reduction in EET fluorescence (51.3 ± 6% of vehicle control, n = 6, P < 0.001) in astrocytes compared with vehicle (Fig. 1, B–D) as previously reported (2). To block endogenous EET production (44), we used the specific epoxygenase inhibitor MS-PPOH (40 μM). Preincubation of cells with MS-PPOH (12 h) before Aβ treatment (1 μM, Fig. 1E) for 24 h caused a further reduction in mitochondrial membrane potential (20.6 ± 6.8% of vehicle control, n = 5 independent experiments, P < 0.001 vs. vehicle) compared with Aβ alone (P < 0.05). MS-PPOH itself did not cause a significant reduction in membrane potential (71.5 ± 14.6% of vehicle, n = 6), but a decreasing trend was observed that worsened in the presence of Aβ (P < 0.001, Fig. 1F).
Exogenous EETs prevent Aβ-induced depolarization of mitochondrial membrane potential.
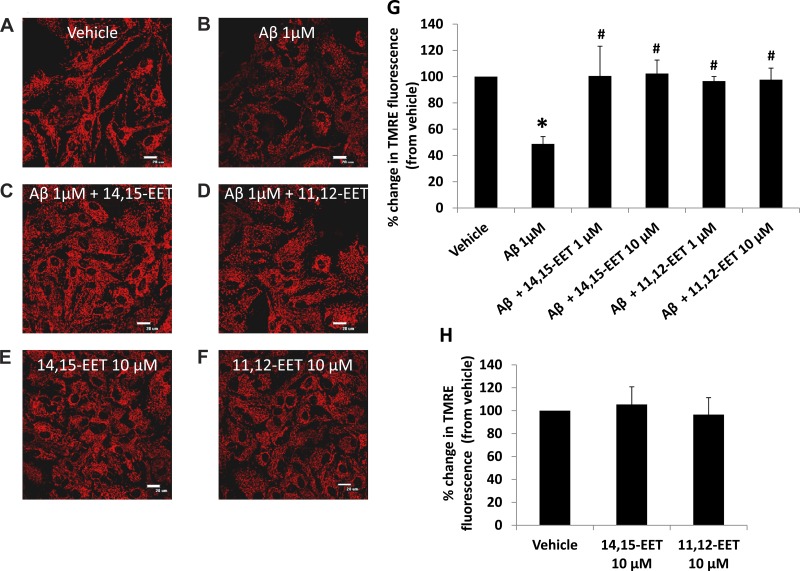
Since blockade of endogenous EET production aggravated Aβ-induced mitochondrial membrane potential depolarization, we investigated whether supplementation of exogenous EETs could reverse this effect of Aβ on mitochondrial membrane potential. Astrocytes were incubated with 1 or 10 μM 11,12- or 14,15-EET 12 h before incubation of the cells with Aβ (1 μM). To ensure that we observed the effect of exogenous EETs only, MS-PPOH (40 μM) was added to block endogenous EET production 30 min before addition of EETs. Preincubation with 10 μM 14,15-EET completely prevented the loss of membrane potential in the presence of Aβ with a relative fluorescence intensity of 102.3 ± 10.2% compared with that of vehicle (n = 5, P < 0.005 vs. Aβ alone). 11,12-EET (10 μM) preincubation (Fig. 2, A–D) showed similar results (97.6 ± 8.8% of the control, n = 6 independent experiments, P < 0.005 vs. Aβ alone), establishing the effectiveness of EETs in sustaining mitochondrial membrane potential by counteracting the effects of Aβ. Lower doses of both EETs (1 μM) elicited similar results as the 10-μM groups (Fig. 2G). EETs alone had no effect on mitochondrial membrane potential (Fig. 2H).
Fig. 2.
Preincubation with EETs prevents Aβ-induced mitochondrial depolarization. Astrocytes were loaded with EET after being treated with vehicle (A), Aβ for 24 h (B), Aβ with 12-h preincubation of 10 μM 14,15-EET (C), 10 μM 11,12-EET (D), or EETs alone (E and F) (×60 magnification, scale bar = 20 μm). The relative change in fluorescence intensity is expressed as percent change from vehicle set at 100% (G and H). Aβ causes a decrease in membrane potential compared with that of vehicle, which is reverted back to control levels after incubation with both EET regioisomers (G). EETs themselves do not affect the membrane potential (H). *P < 0.05 vs. vehicle; #P < 0.01 vs. Aβ; n = 5 to 6.
Aβ causes fragmentation in astrocytic mitochondria, which is exacerbated by preincubation with MS-PPOH.
Aβ-induced mitochondrial fragmentation in neurons is well documented (54), as well as its effect on mitochondrial function. Although mitochondrial depolarization and metabolic failure have been reported after Aβ exposure in astrocytes (1, 2), no definitive effect on mitochondrial morphology is known. We observed for the first time that a 24-h Aβ incubation (1 μM, n = 27 cells) causes significant fragmentation in the mitochondria of astrocytes compared with vehicle (Fig. 3, A and C, n = 37, P < 0.001), Mitochondrial fragmentation was also observed when astrocytes were incubated for a 3-h period with a range of lower concentrations (1–100 nM) of Aβ oligomers (data not shown).
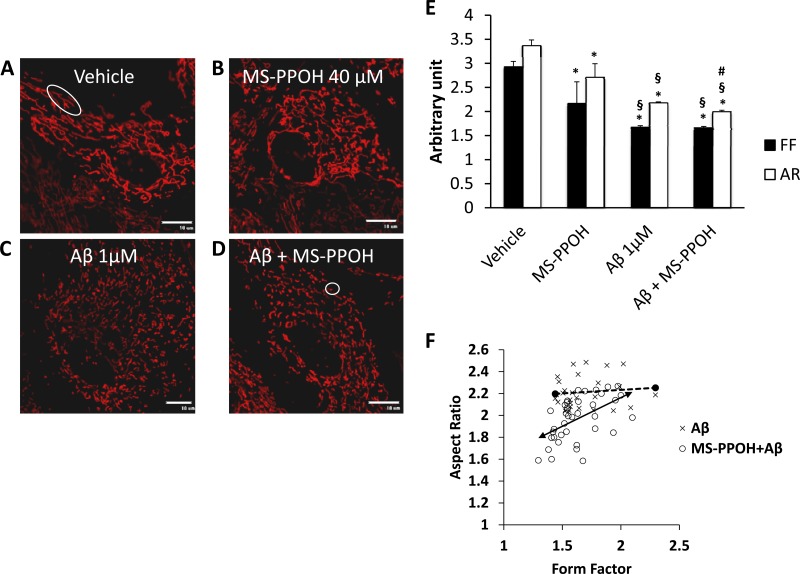
Fig. 3.
Preincubation with MS-PPOH aggravates Aβ-induced mitochondrial fragmentation. Astrocytes were loaded with EET after being treated with vehicle (A), MS-PPOH (B), Aβ (C), and MS-PPOH + Aβ (D). Mitochondrial length is determined by aspect ratio (AR) and length and degree of branching as indicated by form factor (FF). A fragmented, round mitochondrion will have both AR and FF value closer to 1 (circle; D), whereas longer and more branched mitochondria will have higher AR and FF values (ellipsoid; A). Aβ causes a decrease in both AR and FF compared with vehicle; AR is further decreased by incubation with MS-PPOH (E). Individual values for mitochondrial AR are plotted against its corresponding FF (scale bar, 10 μm). each point denotes the mean AR and FF values of a single cell, which is derived from cumulating AR and FF values of all the mitochondrion present in that focal plane (F). Distribution pattern demonstrates a large number of cells from MS-PPOH + Aβ group have a lower AR than cells treated with Aβ alone, but the FF does not differ significantly between the groups. Vehicle, not shown, ranges from 2.2 to 5.7 in FF and 2.8 to 3.1 in AR. *P < 0.001 vs. vehicle, #P < 0.05 vs. Aβ, §P < 0.001 vs. MS-PPOH; n = 24–39 cells.
Aβ affected both length and shape of mitochondria as expressed by a decrease in AR from 3.3 ± 0.1 arbitrary units (AU) in vehicle to 2.17 ± 0.02 AU in Aβ. The FF, an index of length as well as branching, was also affected in Aβ-treated cells (1.6 ± 0.03 AU) compared with vehicle (2.9 ± 0.1 AU). We next investigated the effect of a 12-h preincubation with MS-PPOH with or without exposure to Aβ. MS-PPOH alone (n = 24) caused a reduction in both AR (2.7 ± 0.2 AU) and FF (2.5 ± 0.4 AU), which was lower than that of the vehicle (P < 0.001) but higher compared with that of Aβ alone (P < 0.001). Further reduction in the AR (1.9 ± 0.03 AU, n = 39, P <0.05) was observed when cells were exposed to both MS-PPOH and Aβ (Fig. 3D), but no significant reduction in FF (1.6 ± 0.03 AU) was seen compared with that in Aβ alone (Fig. 3E). A distribution of the individual cellular values (Fig. 3F) for AR and FF demonstrates that cells treated with both MS-PPOH and Aβ tend to have a lower AR compared with Aβ-treated group, indicating that EET blockade can further affect mitochondrial dynamics in astrocytes.
EET preincubation prevents mitochondrial fragmentation in Aβ-treated cells.
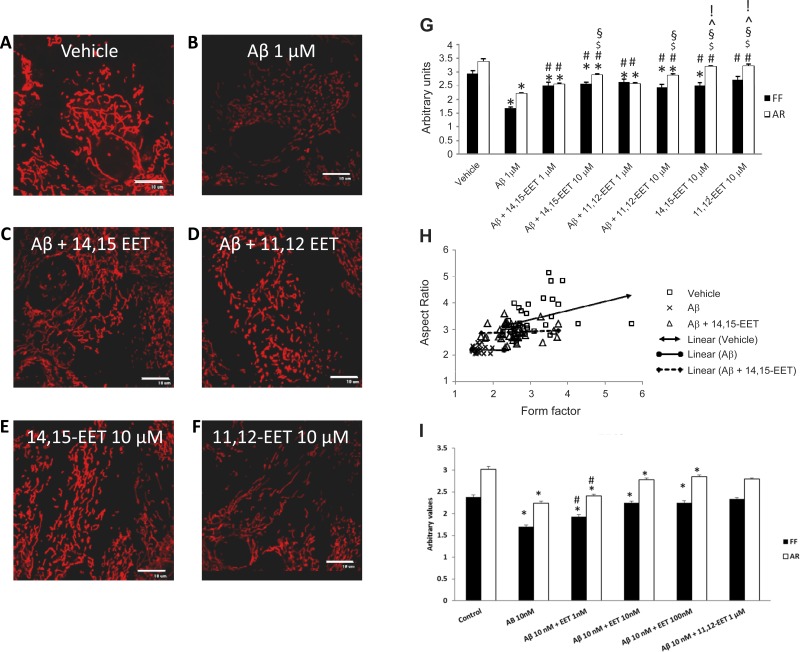
We next evaluated the effect of exogenous EETs on mitochondrial morphology in the presence of Aβ (1 μM). A 12-h preincubation with either EET regioisomer improved mitochondrial morphology, although a complete reversal, as was evident with membrane potential, was not seen. Preincubation with 14,15-EET (10 μM, Fig. 4C, n = 40 cells) decreased fragmentation (P < 0.001 vs. Aβ alone), which was observed as an increase in both AR (2.8 ± 0.04 AU) and FF (2.5 ± 0.07 AU). Similarly, 11,12-EET (10 μM, Fig. 4D; n = 31) also increased AR (2.8 ± 0.07 AU) and FF (2.4 ± 0.1 AU) values in the presence of Aβ. This increment is evident in Fig. 4H, as the distribution of the cells in the EET preincubation group clearly indicates an upward and horizontally rightward shift in AR and FF values compared with the Aβ group. Lower doses (1 μM) of both EETs also prevented mitochondrial fragmentation in the presence of Aβ (Fig. 4G), but the increase in AR was higher in the 10-μM group (2.8 AU) compared with the lower dose (2.5 AU). Additionally, we also observed a dose effect over a range of EET concentrations on mitochondrial fragmentation induced by Aβ and noted that even doses as low as 1 nM EETs can prevent Aβ-induced fragmentation (4I).
Fig. 4.
Preincubation with EETs prevents Aβ-induced mitochondrial fragmentation. Astrocytes were loaded with EET after being treated with vehicle (A), 1 μM Aβ for 24 h (B), Aβ with 12-h reincubation of 10 μM 14,15-EET (C) or 10 μM 11,12-EET (D) and EETs alone (E and F) (scale bar = 10 μm). Mitochondrial length is determined by aspect ratio (AR) and length and degree of branching indicated by form factor (FF). Aβ causes a decrease in both mean (G) and individual (H) AR and FF values compared with vehicle, which is prevented by EET preincubation. Similar preventive effect was also observed with nanomolar concentrations of EETs (I). *P < 0.05 vs. vehicle; #P < 0.005 vs. Aβ; $P < 0.001 vs. Aβ + 14,15-EET 1 μM; §P < 0.005 vs. Aβ + 11,12-EET (1 μM); ^P < 0.005 vs. Aβ + 14,15-EET (10 μm); and !P < 0.002 vs. Aβ + 11,12-EET (10 μM); n = 27–40 cells.
Metabolic impairment induced by Aβ treatment in astrocytes is rescued by preincubation with EETs.
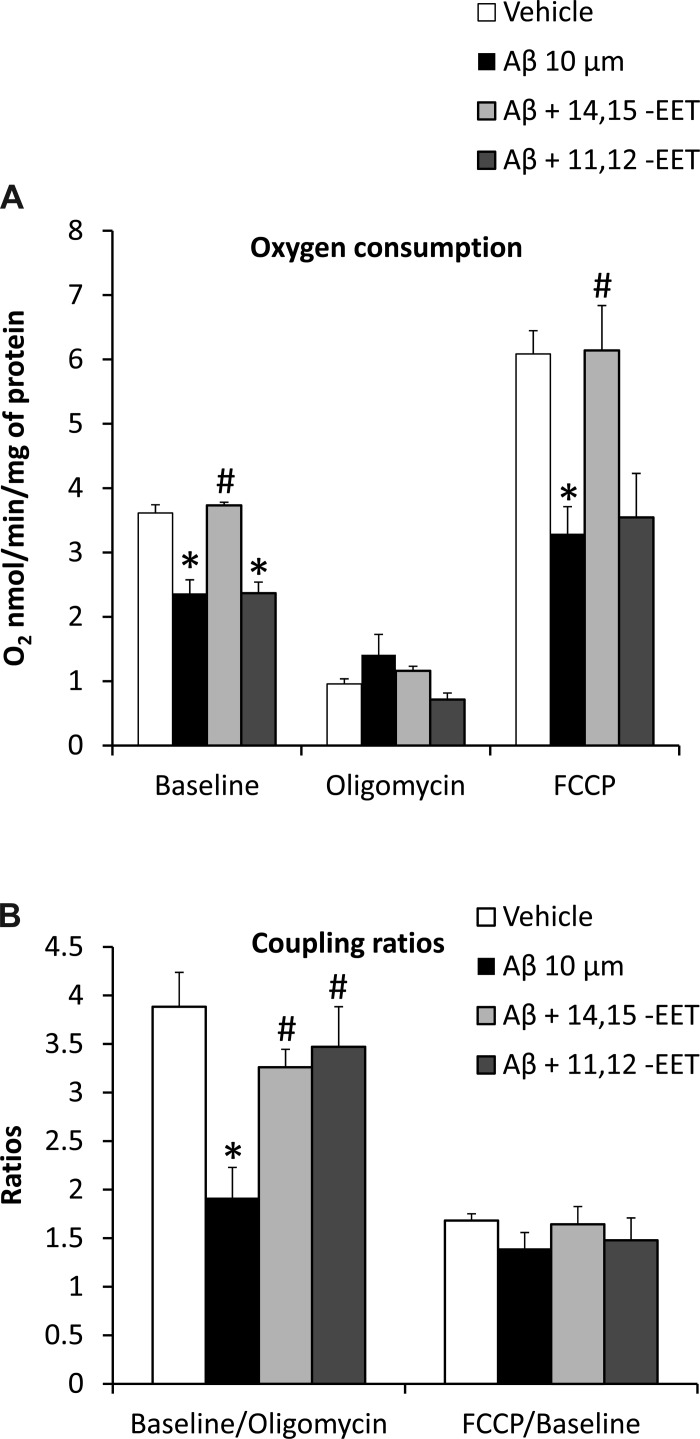
Since changes in mitochondrial membrane potential are known to affect oxidative phosphorylation (26) and Aβ causes a dramatic loss of both membrane polarity and oxygen consumption capacity in astrocytes, we investigated the effect of EETs on mitochondrial oxygen consumption in the presence of Aβ. Incubation (24 h) with Aβ (10 μM) decreased oxygen consumption (Fig. 5A) at a much lower concentration than that previously reported (1). At baseline, in the presence of Aβ (n = 5 independent experiments), O2 utilization was 2.7 ± 0.2 nmol O2·min−1·mg−1 of cell suspension protein compared with 3.6 ± 0.1 nmol·min−1·mg−1 of protein in vehicle (n = 5, P < 0.005). Preincubation with 14,15-EET (10 μM, n = 5) completely blocked this effect of Aβ, and O2 utilization averaged 3.7 ± 0.04 nmol O2·min−1·mg−1 of protein (P < 0.005 vs. Aβ alone). However, preincubation with 11,12-EET (n = 4) failed to restore oxygen consumption in the presence of Aβ (2.3 ± 0.2 nmol O2·min−1·mg−1 of protein, P < 0.005 vs. vehicle). Aβ also caused a reduction in the FCCP-induced maximal respiratory state (3.2 ± 0.4 nmol O2·min−1·mg−1 of protein) compared with vehicle (6.0 ± 0.3 nmol O2·min−1·mg−1 of protein, P < 0.005). 14,15-EET preincubation effectively prevented this reduction (6.1 ± 0.6 nmol O2·min−1·mg−1 of protein, P < 0.005 vs. Aβ alone), whereas 11,12-EET did not (3.5 ± 0.6 nmol O2·min−1·mg−1 of protein, P < 0.005 vs. vehicle). No significant changes were observed in the FCCP-to-baseline ratio since the inhibitory effect on mitochondrial parameters is present in both with and without FCCP (Fig. 5B).
Fig. 5.
Effect of EET preincubation on Aβ-induced metabolic impairment. Cells were treated with 10 μM Aβ for 24 h with and without 12-h EET preincubation, and oxygen consumption of these cells was measured using an Oxygraph instrument. Aβ causes a reduction in oxygen consumption compared with vehicle at basal and maximal respiratory states, which is inhibited by preincubation with 10 μM 14,15-EET (A). The uncoupling of the electron transport chain from ATP synthesis is blocked by both regioisomers of EETs (B). *P < 0.001 vs. vehicle; #P < 0.05 vs. Aβ; n = 4 to 5 independent experiments.
In the presence of the ATP-synthase inhibitor oligomycin, cells in vehicle-treated group exhibited the expected drop in oxygen consumption (0.95 ± 0.08 nmol O2·min−1·mg−1 of protein) compared with the vehicle at baseline. The oligomycin-induced drop in respiration was less in Aβ-treated groups (1.4 ± 0.3 nmol O2·min−1·mg−1 of protein), supporting the uncoupling action of Aβ. To further analyze the effect of oligomycin, we looked at the ratio of respiration at baseline to respiration in the presence of oligomycin (Fig. 5B). This ratio represents the extent of coupling between the electron transport chain and ATP synthase whereby a higher ratio indicates more effective coupling. Vehicle-treated cells showed a higher baseline-to-oligomycin ratio (3.8 ± 0.3) compared with Aβ-treated cells (1.9 ± 0.3, P < 0.001), indicating that ATP synthase is partially uncoupled from the electron transport chain in the presence of Aβ. This uncoupling was at least partially reversed by both 14,15- and 11,12-EETs, which brought the ratios closer to the vehicle-treated group (3.2 ± 0.1 and 3.4 ± 0.4, respectively, P < 0.05 vs. Aβ alone).
Aβ-induced ROS generation is prevented by EETs.
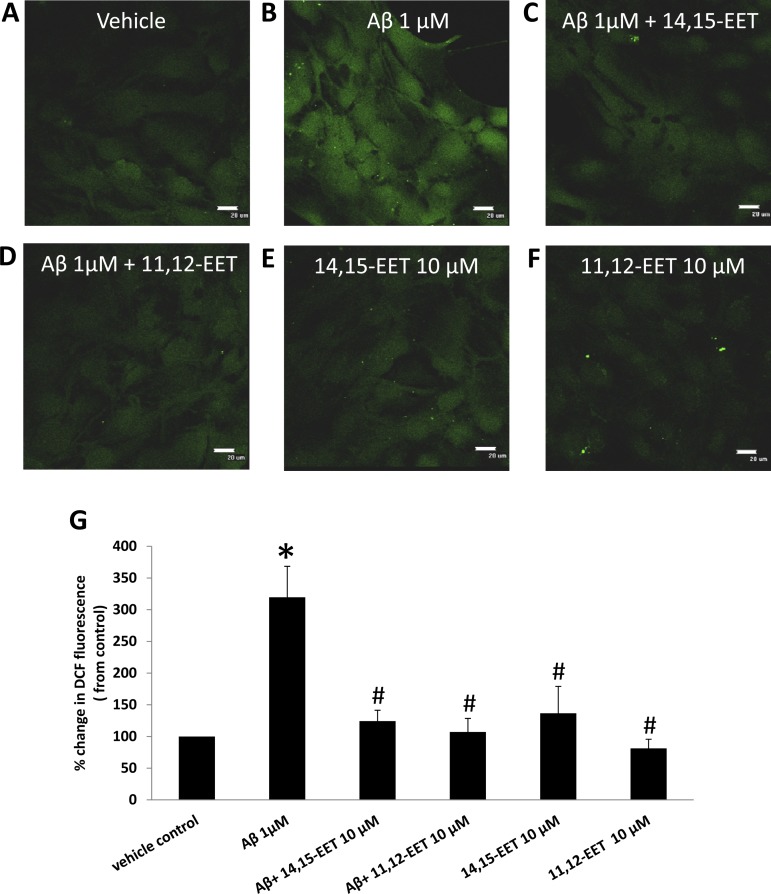
Generation of ROS is one of the most prominent and well-established mechanisms of Aβ-induced toxicity (9, 40). Thus we evaluated the potential of EETs to counteract this effect of Aβ using the intracellular ROS marker DCF (1 μM). Cells treated with Aβ showed a threefold increase (319 ± 48% of control, n = 7 independent experiments, P < 0.001) in ROS production compared with vehicle (Fig. 6, A and B). Preincubation with either 14,15- or 11,12-EET inhibited ROS production in the presence of Aβ (Fig. 6, C and D, respectively). The relative fluorescence intensity of the intracellular ROS marker DC, was 124 ± 17% of vehicle for 14,15-EET (n = 8, P < 0.001 vs. Aβ alone) and 107 ± 21% of vehicle for 11,12-EETs (n = 7, P < 0.001 vs. Aβ alone). Groups treated with EETs without Aβ incubation did not show a significant change in DCF staining compared with vehicle (Fig. 6, E and F).
Fig. 6.
Effect of EET preincubation on ROS generation by Aβ. Astrocytes were loaded with DCF after being treated with vehicle (A), 1 μM Aβ for 24 h (B), and Aβ with 12 h preincubation of 10 μM 14,15-EET (C) or 10 μM 11,12-EET (D) and EETs alone (E and F) (×60 magnification; scale bar = 20 μm). The relative change in fluorescence intensity is expressed as percent change from vehicle set at 100%. Aβ causes an increase in generation of reactive oxygen species compared with vehicle as seen as an increase in fluorescence, which is prevented by incubation with both regioisomers of EETs (G). *P < 0.001 vs. vehicle; #P < 0.001 vs. Aβ; n = 7–9.
DISCUSSION
We have demonstrated that exogenous EETs can prevent Aβ-induced mitochondrial dysfunction in hippocampal astrocytes. Also, reductions in endogenous EET production have the potential to further aggravate Aβ-induced injury. In particular, we report that 1) blockade of endogenous EET production by MS-PPOH can further increase mitochondrial depolarization in the presence of Aβ and that supplementation of EETs to the cells prevented this loss of membrane potential; 2) Aβ can induce mitochondrial fragmentation in the astrocytes, which is aggravated by preincubation with MS-PPOH and reversed in the presence of both regioisomers of EETs; 3) disruption of mitochondrial oxygen consumption and uncoupling in the presence of Aβ is reversed by 14,15-EET pretreatment; and 4) ROS production due to Aβ exposure is blocked by both 11,12- and 14,15-EET. We have previously shown that Aβ affects epoxygenase activity in cultured astrocytes and different areas of the brain (46). In the current study, we report for the first time the involvement of EETs in the downstream pathway of Aβ-induced injury and the therapeutic potential of EETs in restoring mitochondrial function.
Oxidative stress induced by Aβ plays a central role in AD pathology (13), and it has been recently shown that ROS production in astrocytes leads to neuronal death in mixed hippocampal astrocyte-neuron culture. Interestingly, the glial cells experience very little cell death because of Aβ exposure, whereas the neurons show over 50% cell death (2). The mechanism underlying this effect of astrocytes has been attributed to a disturbance in calcium homeostasis (3) and simultaneous upregulation of NADPH oxidase activity in response to Aβ exposure (2), leading to mitochondrial membrane depolarization, reduced oxygen consumption, and increased ROS production (1). These effects cause calcium perturbations and depletion of the antioxidant glutathione in surrounding neurons, leading to cell death (4).
Mitochondrial depolarization can occur because of a lack of substrate availability or opening of the permeability transition pore in response to oxidative stress. Aβ affects glucose transporters (55), thus limiting substrate availability as well as activates NADPH oxidase to induce ROS generation. Inhibition of EET production led to further mitochondrial depolarization, which could be blocked by EET supplementation, indicating that EET reduction may be a yet-unknown pathway through which Aβ induces toxicity. Previously, it has been reported in cardiomyocytes that EETs do not interfere with the permeability pore complex directly (31) but activate mitoK+ channels (12), which prevent the dissipation of mitochondrial potential following cellular stress. Therefore, it is possible that this action of EETs may be mediated through mitoK+ channels and may be blocked by the appropriate K+ channel blocker.
In healthy cells, mitochondrial fission and fusion is a dynamic process that increases the effectiveness of mitochondrial function. Under cellular stress and decreased respiration, mitochondria undergo more fission than fusion, leading to cytochrome-c release, which signals apoptosis (58). The change in fission/fusion balance impacts mitochondrial function, and Aβ has been shown to affect this dynamic by upregulating the fission proteins dynamin-related protein 1 (Drp1) and human fission protein 1 (Fis1), whereas downregulating the fusion protein optic atrophy 1 (OPA1), which results in disrupted distribution and fragmentation of mitochondria (54). Upregulation of the fission protein dynamin-like protein 1 (DLP1) by Aβ also causes a complete collapse in the mitochondrial network that leads to reduced mitochondrial function (53). This imbalance in mitochondrial structural proteins is also evident in AD patients (37), where Drp1 upregulation is seen in both neurons and astrocytes, though no distinct information is available regarding the astrocytic mitochondrial network in the presence of Aβ. We report here for the first time that Aβ induces fragmentation in mitochondria of astrocytes, which not only affects mitochondrial shape but also branching. Addition of MS-PPOH with Aβ caused more fragmentation, leading to further deterioration of mitochondrial shape, but did not have any significant effect on branching. Addition of exogenous EETs prevented the fragmentation and restored the normal mitochondrial network as evidenced by increased branching. Although we recognize that the limitation of our study lies in using a relatively higher concentration of EETs (10 μM), the protective effect of EETs is concentration dependent. As we have shown in Fig. 4I, the efficacy of EETs to block Aβ-induced injury is in the range of nanomolar concentrations, justifying its physiological relevance and therapeutic potential. At this point the pathway through which EETs exert this effect is not known; hence, it would be interesting to investigate the interactions of different mitochondrial fission and fusion proteins with EETs in future studies.
Since degradation in mitochondrial potential and structural network indicates metabolic deficit and EET pretreatment shows a significant improvement in both aspects, our current observation that EETs prevent astrocytic metabolic failure was expected. The surprising observation is the failure of 11,12-EET to prevent the reduction in oxygen consumption under basal and at maximal respiratory state (as seen following the treatment with FCCP), but the ability to block the uncoupling of ATP synthase. 14,15-EET, on the other hand, was successful in rescuing the astrocytes from Aβ-induced reduction in oxygen consumption and uncoupling. Aβ affects mitochondrial respiratory enzyme activity (15), and particularly inhibits complex IV in isolated mitochondria (14). In an AD mouse model, Aβ and τ affect oxygen consumption and inhibit complex I and IV, although Aβ primarily affects complex IV and induces a drop in ATP production (complex V) in neurons (45). This is in line with our finding that supports the direct action of Aβ on the electron transport chain since the effect is observed even in the presence of FCCP. This bioenergetics deficit precedes memory loss (57). Alterations in the respiratory enzyme genes were also found in AD patients (39). Substrate unavailability has been proposed to be another mechanism for Aβ-induced downregulation of cellular respiration (1, 55). At this point, it is unclear what specific effect EETs have on mitochondrial respiration. Based on these studies, it can be concluded that 11,12-EET can reduce uncoupling but might not have any effect on the electron transport chain, whereas 14,15-EET might be able to exert a dual effect since it reverses Aβ-induced inhibition even in the presence of FCCP. Improvement in substrate availability may be an additional or alternative pathway through which EETs prevent metabolic impairment.
The disruption of the electron transport chain by Aβ increases mitochondrial ROS production, which eventually leads to cellular release of ROS (36). Whereas mitochondrial ROS may trigger an increase in Aβ processing (33), cellular ROS activate several apoptotic pathways (48). Recently, it has been shown that local ROS induces apoptotic pathways by upregulating Bid [Bcl-2 homology 3-interacting domain death agonist], which in turn promotes mitochondrial fragmentation (11). EETs have been previously reported to reduce ROS production in several injury models associated with mitochondrial damage (23, 31). In our study, we also found that EETs were very effective in reducing ROS production induced by Aβ, implying that EETs may block the onset of Aβ-induced apoptosis. It will be interesting to further investigate the effect of EETs on Aβ production and accumulation, as generation of ROS creates a positive feedback loop for Aβ processing.
In conclusion, EETs were effective in reducing all markers associated with Aβ-induced mitochondrial dysfunction in astrocytes, which strongly suggests the neuroprotective potential of EETs. Further experiments in neurons are needed to prove the therapeutic potential of EETs against Aβ injury. As mitochondria-targeted therapeutic models emerge and continue to increase in number (20, 38, 41, 56), shown here in our study, EETs, a group of physiologically occurring fatty acids, hold strong potential as novel therapeutic targets in AD pathology.
GRANTS
Funding for this project was provided by National Heart, Lung, and Blood Institute Grants RO1-HL-033833, HL-092105, and HL-105997 and by the VA Research Career Scientist Award (to D. R. Harder); GM-31278, the Robert A. Welch Foundation (GL625910) (to J. R. Falck), and GM-066730 and HL-034708 (to Dr. Zeljko Bosnjak).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
P.S. and D.R.H. conception and design of research; P.S., I.Z., M.B., K.R.R., and S.C. performed experiments; P.S., I.Z., M.B., K.R.R., and M.T. analyzed data; P.S. and M.B. interpreted results of experiments; P.S. prepared figures; P.S. drafted manuscript; P.S., I.Z., M.B., S.C., J.R.F., and D.R.H. edited and revised manuscript; P.S. and D.R.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We sincerely thank Dr. Debebe Gebremedhin for help with editing the manuscript.
REFERENCES
- 1.Abeti R, Abramov AY, Duchen MR. Beta-amyloid activates PARP causing astrocytic metabolic failure and neuronal death. Brain 134: 1658–1672, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci 24: 565–575, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramov AY, Canevari L, Duchen MR. Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture. Biochim Biophys Acta 1742: 81–87, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Abramov AY, Canevari L, Duchen MR. Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. J Neurosci 23: 5088–5095, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abramov AY, Duchen MR. Impaired mitochondrial bioenergetics determines glutamate-induced delayed calcium deregulation in neurons. Biochim Biophys Acta 1800: 297–304, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Alikhani N, Guo L, Yan S, Du H, Pinho CM, Chen JX, Glaser E, Yan SS. Decreased proteolytic activity of the mitochondrial amyloid-beta degrading enzyme, PreP peptidasome, in Alzheimer's disease brain mitochondria. J Alzheimers Dis 27: 75–87, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer's amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol 161: 41–54, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ansari MA, Joshi G, Huang Q, Opii WO, Abdul HM, Sultana R, Butterfield DA. In vivo administration of D609 leads to protection of subsequently isolated gerbil brain mitochondria subjected to in vitro oxidative stress induced by amyloid beta-peptide and other oxidative stressors: relevance to Alzheimer's disease and other oxidative stress-related neurodegenerative disorders. Free Radic Biol Med 41: 1694–1703, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell 77: 817–827, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Blass JP, Gibson GE, Hoyer S. The role of the metabolic lesion in Alzheimer's disease. J Alzheimers Dis 4: 225–232, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Bobinski M, de Leon MJ, Tarnawski M, Wegiel J, Reisberg B, Miller DC, Wisniewski HM. Neuronal and volume loss in CA1 of the hippocampal formation uniquely predicts duration and severity of Alzheimer disease. Brain Res 805: 267–269, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Bodiga S, Zhang R, Jacobs DE, Larsen BT, Tampo A, Manthati VL, Kwok WM, Zeldin DC, Falck JR, Gutterman DD, Jacobs ER, Medhora MM. Protective actions of epoxyeicosatrienoic acid: dual targeting of cardiovascular PI3K and KATP channels. J Mol Cell Cardiol 46: 978–988, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butterfield DA. Amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review. Free Radic Res 36: 1307–1313, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Canevari L, Clark JB, Bates TE. beta-Amyloid fragment 25–35 selectively decreases complex IV activity in isolated mitochondria. FEBS Lett 457: 131–134, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Casley CS, Canevari L, Land JM, Clark JB, Sharpe MA. Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J Neurochem 80: 91–100, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Casley CS, Land JM, Sharpe MA, Clark JB, Duchen MR, Canevari L. Beta-amyloid fragment 25–35 causes mitochondrial dysfunction in primary cortical neurons. Neurobiol Dis 10: 258–267, 2002 [DOI] [PubMed] [Google Scholar]
- 17.de la Monte SM, Wands JR. Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer's disease. J Alzheimers Dis 9: 167–181, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Devi L, Ohno M. Mitochondrial dysfunction and accumulation of the beta-secretase-cleaved C-terminal fragment of APP in Alzheimer's disease transgenic mice. Neurobiol Dis 45: 417–424, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer's disease brain is associated with mitochondrial dysfunction. J Neurosci 26: 9057–9068, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med 14: 1097–1105, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckert A, Hauptmann S, Scherping I, Rhein V, Muller-Spahn F, Gotz J, Muller WE. Soluble beta-amyloid leads to mitochondrial defects in amyloid precursor protein and tau transgenic mice. Neurodegener Dis 5: 157–159, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Eckert A, Schmitt K, Gotz J. Mitochondrial dysfunction–the beginning of the end in Alzheimer's disease? Separate and synergistic modes of tau and amyloid-beta toxicity. Alzheimers Res Ther 3: 15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Sikhry HE, Miller GG, Madiyalakan MR, Seubert JM. Sonodynamic and photodynamic mechanisms of action of the novel hypocrellin sonosensitizer, SL017: mitochondrial cell death is attenuated by 11, 12-epoxyeicosatrienoic acid. Invest New Drugs 29: 1328–1336, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Falkevall A, Alikhani N, Bhushan S, Pavlov PF, Busch K, Johnson KA, Eneqvist T, Tjernberg L, Ankarcrona M, Glaser E. Degradation of the amyloid beta-protein by the novel mitochondrial peptidasome, PreP. J Biol Chem 281: 29096–29104, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Gross GJ, Hsu A, Falck JR, Nithipatikom K. Mechanisms by which epoxyeicosatrienoic acids (EETs) elicit cardioprotection in rat hearts. J Mol Cell Cardiol 42: 687–691, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol Cell Physiol 258: C755–C786, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Hauptmann S, Keil U, Scherping I, Bonert A, Eckert A, Muller WE. Mitochondrial dysfunction in sporadic and genetic Alzheimer's disease. Exp Gerontol 41: 668–673, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Hauptmann S, Scherping I, Drose S, Brandt U, Schulz KL, Jendrach M, Leuner K, Eckert A, Muller WE. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol Aging 30: 1574–1586, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci 21: 3017–3023, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoyer S. Brain glucose and energy metabolism abnormalities in sporadic Alzheimer disease. Causes and consequences: an update. Exp Gerontol 35: 1363–1372, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Katragadda D, Batchu SN, Cho WJ, Chaudhary KR, Falck JR, Seubert JM. Epoxyeicosatrienoic acids limit damage to mitochondrial function following stress in cardiac cells. J Mol Cell Cardiol 46: 867–875, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Koopman WJ, Visch HJ, Verkaart S, van den Heuvel LW, Smeitink JA, Willems PH. Mitochondrial network complexity and pathological decrease in complex I activity are tightly correlated in isolated human complex I deficiency. Am J Physiol Cell Physiol 289: C881–C890, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Leuner K, Schutt T, Kurz C, Eckert SH, Schiller C, Occhipinti A, Mai S, Jendrach M, Eckert GP, Kruse SE, Palmiter RD, Brandt U, Drose S, Wittig I, Willem M, Haass C, Reichert AS, Mueller WE. Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation. Antioxid Redox Signal 16: 1421–1433, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol 155: 853–862, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science 304: 448–452, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet 15: 1437–1449, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Hum Mol Genet 20: 2495–2509, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer's disease neurons. J Alzheimers Dis 20, Suppl 2: S609–S631, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer's disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med 5: 147–162, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Markesbery WR, Carney JM. Oxidative alterations in Alzheimer's disease. Brain Pathol 9: 133–146, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McManus MJ, Murphy MP, Franklin JL. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer's disease. J Neurosci 31: 15703–15715, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morais Cardoso S, Swerdlow RH, Oliveira CR. Induction of cytochrome c-mediated apoptosis by amyloid beta 25–35 requires functional mitochondria. Brain Res 931: 117–125, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Nithipatikom K, Moore JM, Isbell MA, Falck JR, Gross GJ. Epoxyeicosatrienoic acids in cardioprotection: ischemic versus reperfusion injury. Am J Physiol Heart Circ Physiol 291: H537–H542, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Peng X, Carhuapoma JR, Bhardwaj A, Alkayed NJ, Falck JR, Harder DR, Traystman RJ, Koehler RC. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol 283: H2029–H2037, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H, Drose S, Brandt U, Savaskan E, Czech C, Gotz J, Eckert A. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer's disease mice. Proc Natl Acad Sci USA 106: 20057–20062, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarkar P, Narayanan J, Harder DR. Differential effect of amyloid beta on the cytochrome P450 epoxygenase activity in rat brain. Neuroscience 194: 241–249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selkoe DJ, Schenk D. Alzheimer's disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol 43: 545–584, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Serrano F, Chang A, Hernandez C, Pautler RG, Sweatt JD, Klann E. NADPH oxidase mediates beta-amyloid peptide-induced activation of ERK in hippocampal organotypic cultures. Mol Brain 2: 31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seubert JM, Sinal CJ, Graves J, DeGraff LM, Bradbury JA, Lee CR, Goralski K, Carey MA, Luria A, Newman JW, Hammock BD, Falck JR, Roberts H, Rockman HA, Murphy E, Zeldin DC. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ Res 99: 442–450, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shetty PK, Galeffi F, Turner DA. Age-induced alterations in hippocampal function and metabolism. Aging Dis 2: 196–218, 2011 [PMC free article] [PubMed] [Google Scholar]
- 51.Terashvili M, Sarkar P, Nostrand MV, Falck JR, Harder DR. The protective effect of astrocyte-derived 14,15-epoxyeicosatrienoic acid on hydrogen peroxide-induced cell injury in astrocyte-dopaminergic neuronal cell line co-culture. Neuroscience 223: 68–76, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trudeau K, Molina AJ, Guo W, Roy S. High glucose disrupts mitochondrial morphology in retinal endothelial cells: implications for diabetic retinopathy. Am J Pathol 177: 447–455, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer's disease patients. Am J Pathol 173: 470–482, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci USA 105: 19318–19323, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao J, Chen S, Mao Z, Cadenas E, Brinton RD. 2-Deoxy-d-glucose treatment induces ketogenesis, sustains mitochondrial function, and reduces pathology in female mouse model of Alzheimer's disease. PLoS One 6: e21788, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao J, Du H, Yan S, Fang F, Wang C, Lue LF, Guo L, Chen D, Stern DM, Gunn Moore FJ, Xi Chen J, Arancio O, Yan SS. Inhibition of amyloid-beta (Abeta) peptide-binding alcohol dehydrogenase-Abeta interaction reduces Abeta accumulation and improves mitochondrial function in a mouse model of Alzheimer's disease. J Neurosci 31: 2313–2320, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci USA 106: 14670–14675, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol 6: 657–663, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, DeBarber AE, Koop DR, Alkayed NJ. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke 39: 2073–2078, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]