Abstract
Transient receptor potential cation channels have been implicated in the regulation of cardiovascular function, but only recently has our laboratory described the vanilloid-2 subtype (TRPV2) in the cardiomyocyte, though its exact mechanism of action has not yet been established. This study tests the hypothesis that TRPV2 plays an important role in regulating myocyte contractility under physiological conditions. Therefore, we measured cardiac and vascular function in wild-type and TRPV2−/− mice in vitro and in vivo and found that TRPV2 deletion resulted in a decrease in basal systolic and diastolic function without affecting loading conditions or vascular tone. TRPV2 stimulation with probenecid, a relatively selective TRPV2 agonist, caused an increase in both inotropy and lusitropy in wild-type mice that was blunted in TRPV2−/− mice. We examined the mechanism of TRPV2 inotropy/lusitropy in isolated myocytes and found that it modulates Ca2+ transients and sarcoplasmic reticulum Ca2+ loading. We show that the activity of this channel is necessary for normal cardiac function and that there is increased contractility in response to agonism of TRPV2 with probenecid.
Keywords: ion channels, cardiac myocytes, contractility, calcium, blood flow, blood pressure, hemodynamics, vascular resistance, probenecid
the transient receptor potential (TRP) family is comprised of Ca2+ selective channels (18) that have been primarily described in regard to their many roles in the nervous (2, 24), immune (23, 24), and renal systems (22), but information regarding their potential role in the cardiovascular system is less well defined. These cation channels have been implicated in the regulation of cardiovascular function (3, 5, 19), and our laboratory has found that the TRP vanilloid-2 subtype (TRPV2) is present and active in the cardiomyocyte (9, 10). The TRPV2 subtype has also been described in the vascular system, where it appears to be stimulated by osmotic and stretch stimuli (13), and investigations via quantitative real-time PCR (qRT-PCR) determined that expression levels of TRPV2 were second only to TRPV4 in deendothelialized rat aorta (25). Interestingly, Iwata et al. (6) found that overexpression of TRPV2 in the heart resulted in cardiomyopathy because of Ca2+ overload; however, this study did not evaluate the role or mechanism of endogenous channel expression under baseline conditions. Therefore, the present study was performed to explore the mechanism of action of this receptor and the role that it plays in regulating Ca2+ within the cardiomyocyte. Specifically, we examined the effects of TRPV2 activation in wild-type (WT) and TRPV2 null mice at the whole animal, organ, and cellular level.
The uricosuric drug probenecid has been described as a potent, relatively selective, TRPV2 agonist in sensory neurons (1) and more recently as a positive inotrope in live mice (9). In previous studies, we showed that inotropic responses to probenecid were absent in TRPV2 null mice, suggesting an important role of endogenously expressed channels in modulating myocyte contractile function (9, 10). Therefore, in this study, we sought to elucidate the cardiovascular sequellae of TRPV2 gene deletion both at baseline and in response to activation by probenecid and to examine the calcium handling mechanisms that may participate in the observed responses.
MATERIALS AND METHODS
Animals and reagents.
All animal procedures were performed with the approval of the Institutional Animal Care and Use Committee of the University of Cincinnati and in accordance with the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health (NIH Publication No. 85-23, Revised 1996). All WT and TRPV2−/− littermates (breeding pairs on a B6129SF2/J background were provided by Dr. M. Caterina, John's Hopkins, Baltimore, MD) were males 12–16 wk of age (17). A water soluble preparation of probenecid (Molecular Probes, Life Technologies, Eugene, OR) was used for all of the myocyte experiments and was administered in vivo at doses of 30 and 100 mg/kg (the lower dose being similar with the highest human dose of 2 g/daily) and in vitro at concentrations of 10−7 to 10−2 mol/l.
Echocardiography.
Echocardiographic studies were performed with a Vevo 2100 Ultrasound system (Visualsonics, Toronto, Canada) using a MS400 probe (30-MHz centerline frequency) and were postprocessed with Vevostrain software (Visualsonic, Vevo 2100, v. 1.1.1 B1455). Mice were anesthetized with isoflurane (1.5–2%), and images were obtained from a parasternal long axis view between 2 and 10 mm in depth in both M-mode and B-mode and measured as previously described (10).
Cardiovascular function in vivo.
Measurements of left ventricular (LV) performance and carotid artery blood flow were determined for WT and TRPV2−/− mice as previously described (12). Mice were anesthetized with intraperitoneal ketamine (50 μg/g body wt) and thiobutabarbtial (100 μg/g bodt wt, Sigma), a tracheotomy was performed (polyethylene-90 tubing), and body temperature was monitored and maintained with a feedback-controlled heating table. The right femoral artery and vein were cannulated with polyethylene tubing for measurement of blood pressure (COBE Cardiovascular, Arvada, CO) and for delivery of drugs. A high-fidelity, 1.2-Fr Scisense pressure catheter (Scisense, London, ON, Canada) was inserted into the right carotid artery and advanced into the LV to measure pressure. For carotid blood flow measurements, the left carotid artery was isolated and fitted with a 0.5-PSB perivascular flow probe connected to a TS420 flowmeter (Transonic Systems, Ithaca, NY). Cerebral blood flow was chosen since it represents a highly regulated and autoregulated vasculature that can be readily assessed with minimal invasiveness. Pressure and flow signals were recorded and analyzed using a PowerLab system (ADInstruments). Hemodynamic measurements were taken at baseline and after the administration of 30 and 100 μg/g body wt iv doses of probenecid, with 5 min between each dose. Measurements were taken from the final 30–40 s of each dosage period. Maximum first derivative of pressure (dP/dtmax) and dP/dt at 40 mmHg of developed pressure (dP/dt40) were calculated from the first derivative of the pressure waveforms. Cerebral vascular resistance (CVR) was calculated from recorded channels of mean arterial pressure (MAP) and mean cerebral blood flow.
Vascular smooth muscle reactivity in isolated aorta.
Analyses of contractile properties of isolated vascular smooth muscle from WT and TRPV2−/− mice and the vasoactive effects of probenecid were performed in both intact (+E) and endothelium-denuded (−E) thoracic aortae, using a DMT myograph (Danish Myo Technology, Marietta, GA), as previously described (12). The bath solution contained (in mmol/l) 118 NaCl, 4.73 KCl, 1.2 MgCl2, 0.026 EDTA, 1.2 KH2PO4, 2.5 CaCl2, and 5.5 glucose, buffered with 25 NaH2CO3 (pH, when bubbled with 95% O2-5% CO2, was 7.4 at 37°C). Data were collected and analyzed using a PowerLab system (ADInstruments). Resting length of each aorta was set to 90% of the estimated circumference at an estimated transmural pressure of 100 mmHg using the ADInstruments DMT normalization module. Before the start of the experiment, each aortic segment was challenged with 100 mM KCl and 10 μM phenylephrine to ensure reproducible forces. Cumulative concentration-force relationships for increasing doses of probenecid, from 10−7 M to 10−2 M, were first tested to examine whether probenecid can induce contraction. In separate experiments to examine the relaxing effects of probenecid, vessel rings were first contracted with 3 μM phenylephrine and then exposed to increasing concentrations of probenecid from 10−7 M to 10−2 mol/l. The EC50 for the relaxing effects of probenecid were determined using a logistic nonlinear, curve-fitting routine on mean data (OriginLab, Northampton, MA).
Histology.
Following euthanasia, body weights were taken and whole hearts dissected out and weighed to estimate heart weight-to-body weight ratio. Hearts were then rinsed with PBS to remove excess blood before fixation. Hearts (n = 4) were fixed with 3.7% buffered formaldehyde and delivered to the Cincinnati Children's Hospital Medical Center's Department of Pathology Research Core (Cincinnati, OH), where they were routinely processed and embedded in paraffin, cut into 6-μm sections, and individually stained with hemotoxylin and eosin and Masson's trichrome. Hemotoxylin and eosin-stained slides were evaluated by a board-certified veterinary pathologist (American College of Veterinary Pathologists) to identify potential end points of cardiomyopathy.
Histomorphometry.
Hemotoxylin and eosin-stained slides were imaged at ×40 magnification with a Zeiss Axio Scope.A1, equipped with an AxioCamERc5s and Zeiss' Zen Blue Edition software. Myocyte size was determined by measuring the circumference of 30 myocytes, from 10 nonoverlapping LV images for each heart, by using the NIH ImageJ 1.47 software. Measurements were taken in triplicate by a blinded researcher, averaged for each mouse, then averaged for the two groups. Masson's trichrome-stained sections were visually accessed for the degree of collagen deposition.
qRT-PCR.
Smooth muscle (aorta) was obtained for RNA isolation and qRT-PCR was performed from WT and TRPV2−/− mice as previously described (9). Tissues were flash frozen in liquid nitrogen and stored at −80°C until processed for RNA isolation (Qiazol, Qiagen) and cDNA synthesis (high-capacity RNA-to-cDNA kit; Applied Biosystems, Carlsbad, CA) per manufacturer's instructions. Assessment of TRPV2 transcript levels was done using COOH-terminal located primers 5′-CTACTGCTCAACATGCTC-3′ (sense) and 5′-CTCATCAGGTATACCATCC-3′ (antisense), which generate a 198-base pair product. qRT-PCR amplification and product detection was performed using SYBR Green with ROX reference dye (Fisher Scientific) on a Stratagene Mx3000p qPCR instrument. All samples were performed in triplicate, with a minimum of three independent replicates and expression differences calculated using the ΔΔCt approximation method with 18S mRNA as a loading control.
Isolated cardiomyocytes.
Cardiac myocytes from WT and TRPV2−/− mice were isolated according to a protocol previously described (15). Briefly, adult mice were anesthetized with pentobarbital sodium (50 mg/kg); hearts were excised and perfused on a Langendorff apparatus with oxygenated solution containing 0.65 U/ml Liberase TH (Roche, Indianapolis, IN). Following digestion, the LV tissue was excised, minced, pipette dissociated, and filtered through a 240-μm screen. The cell suspension was then sequentially washed in 25, 100, 200 μm and 1 mM Ca2+-Tyrode and resuspended in 1.8 mM Ca2+-Tyrode for further analysis. All experiments were carried out at room temperature (22–25°C) in standard Tyrode solution containing (in mM) 140 NaCl, 5 KCl, 1 MgCl2, 10 glucose, 10 HEPES, and 1.8 CaCl2 (pH 7.4 adjusted with NaOH).
Cell mechanics and intracellular calcium concentration measurements.
Isolated myocytes were paced with a stimulation frequency of 0.5 Hz, and myocyte contractions were measured using a video edge detector (model VED-105, Crescent Electronics) as previously described (16). For Ca2+ signal measurements, myocytes were loaded with the membrane-permeable fluorescent Ca2+ indicator fura-2 (fura-2-AM; 2 μM) and alternately excited at 340 and 380 nm by a delta scan dual-beam spectrophotofluorometer (Photon Technology International, Birmingham, NJ) at baseline conditions and after application of 10 mmol/l caffeine to induce release of sarcoplasmic reticulum (SR) Ca2+ and assess of SR Ca2+ load. Ca2+ transients were expressed as the 340/380 nm ratio of the resulting 510 nm emissions. The decline intracellular calcium concentration during a caffeine-induced Ca2+ transient was taken as a measure of Na+/Ca2+ exchange (NCX). Data were analyzed by Felix software (Photon Technology International).
Western blot analysis.
Total protein lysate was prepared from flash-frozen hearts and blotting was performed as previously described (9, 11). Aliquots of total protein were separated on Novex gels (Life Technologies, NY), transferred to nitrocellulose membrane (Life Technologies, NY) and blocked with 5% milk-TBS-Tween. Protein (20 μg) was loaded on 12% gels for the phospholamban (PLN) antibodies: total (t)-PLN (Thermo Scientific), phospho (p)-PLN S16 (Millipore, MA), and p-PLN T17 (Badrilla). Protein (50 μg) was loaded on 4–12% gradient gels for the ryanodine receptor (RyR) antibodies: RyR2a (Thermo Scientific), p-RyR S2808 (Badrilla), and p-RyR S2814 (gift of Dr. Arnold Schwartz, University of Cincinnati). Protein (50 μg) was loaded on a 10% gel for the (NCX, Swant) and sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2, Thermo Scientific) antibodies. Proteins bands were visualized using Western Lightning reagents (Perkin Elmer) and the FluorChemE (ProteinSimple). The densitometry of the bands was determined using AlphaEase software (ProteinSimple) and normalized to GAPDH for loading control.
Data analysis.
Statistical analysis was performed by ANOVA using either a single factor within-subjects design or a two-factor mixed design with repeated measures as appropriate. Where significance was indicated, post hoc testing was performed using the Holm-Sidak method for comparing individual means and correcting for family-wise error (SigmaPlot v. 11.0, Systat Software, San Jose, CA). Data are presented as means ± SE, and differences were regarded as significant at P ≤ 0.05.
RESULTS
TRPV2 deficiency results in depressed myocardial function in vivo.
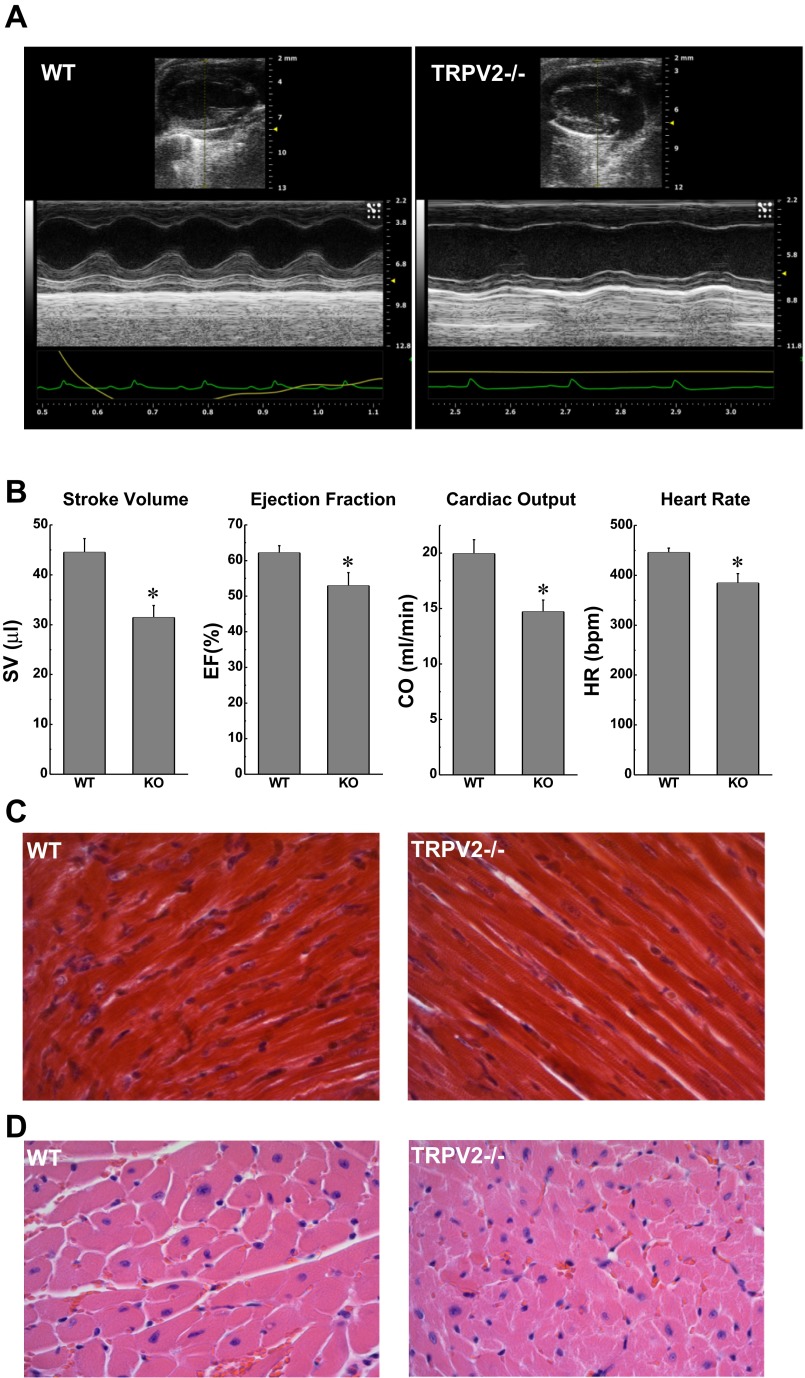
We first used echocardiography to examine baseline cardiac function and geometry noninvasively in male, age-matched WT (n = 8), and TRPV2−/− mice (n = 12) under light anesthesia. Representative images are shown in Fig. 1A. All indexes of cardiac function were significantly lower in the TRPV2−/− compared with those of the WT mice and summary data are shown in Fig. 1B. Histological analysis in Masson's trichrome-stained hearts (Fig. 1C) indicated no overt differences in myocardial collagen content or distribution, and there was no microscopic evidence of myocardial pathology such as inflammation, myofiber degeneration, or necrosis. Measurements of myocyte area in hemotoxylin and eosin-stained sections (representative image, Fig. 1D) revealed no evidence of hypertrophy (273 ± 28 in WT vs. 290 ± 20 μm2 in TRPV2−/−; n = 4). Heart weight normalized to body weight was also not different between the two groups (heart weight-to-body weight ratio, 5.78 ± 0.17 in WT vs. 5.74 ± 0.19 mg/g in TRPV2−/−; n = 7 and 8, respectively).
Fig. 1.
Echocardiographic and morphological indexes of cardiac phenotype in wild-type (WT) and transient receptor potential vanilloid-2 subtype knockout (TRPV2−/−) mice. A: representative echocardiographic M-mode images for WT and TRPV2−/− mice. B: M-mode measurements of stroke volume (SV), ejection fraction (EF), cardiac output (CO), and heart rate [HR, in beats/min (bpm)] demonstrated diminished contractile function in TRPV2−/− mice compared with WT (n = 8 and 12, respectively, *P < 0.05). C: histological analysis of heart sections did not show evidence of increased fibrosis in TRPV2−/− mice; Masson's trichrome stain, ×40. D: there was no histological evidence of cardiac pathology and no change in cardiomyocyte cell area (see text); hematoxylin and eosin, ×40.
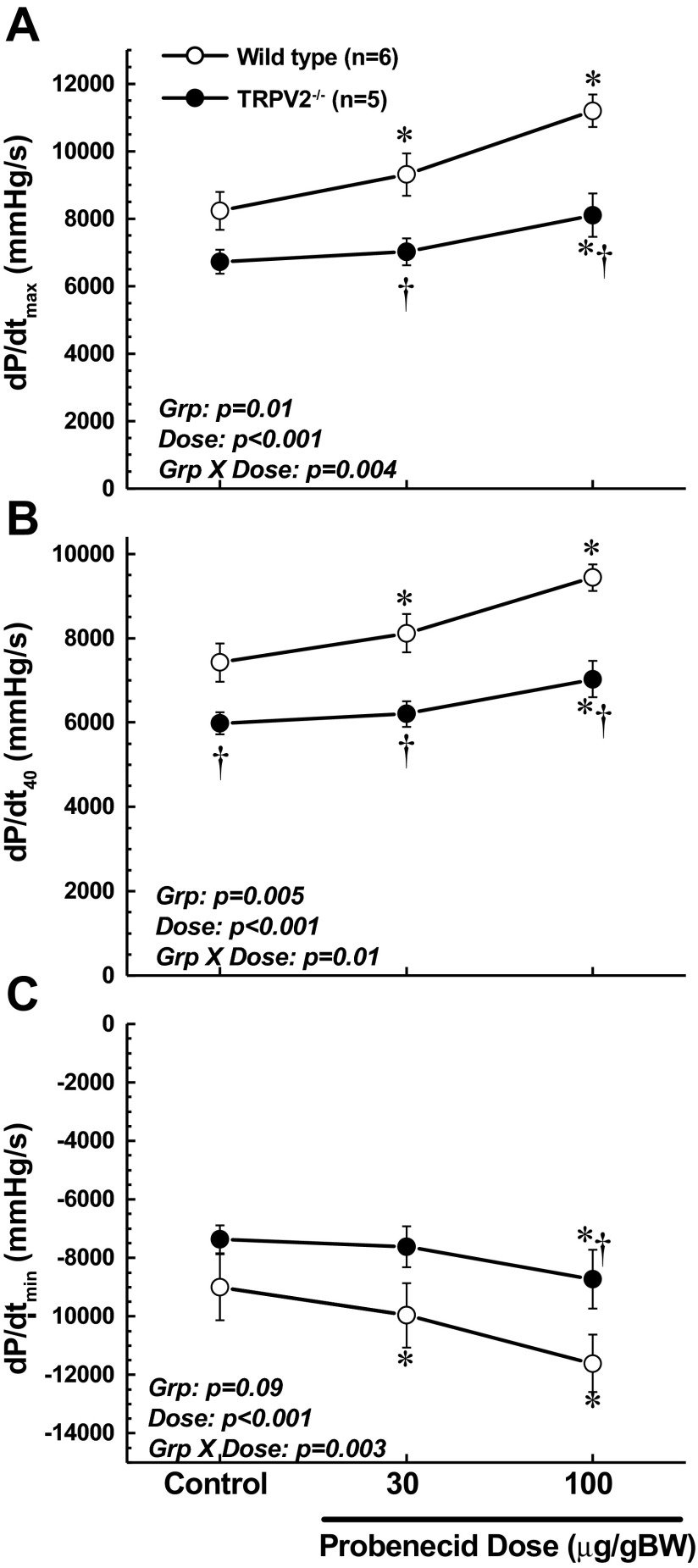
Using the closed-chest, anesthetized mouse model to further evaluate hemodynamic function in these mice, we confirmed that baseline cardiac contractile function, as assessed by LV dP/dtmax and dP/dt40, was significantly reduced in TRPV2−/− mice compared with WT (Fig. 2, A and B). Furthermore, whereas administration of probenecid resulted in a robust, dose-dependent stimulation of contractile function in WT mice, this response was blunted in the TRPV2−/− mice (significant group × dose interaction, P < 0.004 and P < 0.01, respectively). These highly sensitive pressure-phase indexes of cardiac function confirm and extend the noninvasive ejection-phase echo measurements and illustrate important deficits in the TRPV2−/− mice in terms of both baseline function and the response to probenecid. The observed effects of probenecid on LV dP/dt were generally maximal within 1 min of bolus administration and were stable for at least 5 min (at the time of reported measurement). A similar pattern of responses was observed for diastolic function, with slower rates of relaxation in the TRPV2−/−, as assessed by dP/dtmin (Fig. 2C).
Fig. 2.
Invasive measurements of cardiac function in WT vs. TRPV2−/− mice at baseline and in response to low (30 μg/g body wt) and high (100 μg/g body wt iv) injections of probenecid. The maximum rate of contraction (dP/dtmax; A) and the rate of contraction at 40 mmHg of developed pressure (dP/dt40; B) show that TRPV2−/− mice have lower baseline function and blunted responses to probenecid. C: measurements of the maximum rate of relaxation (dP/dtmin) also show blunted responses. *P < 0.01 compared with baseline; †P < 0.05 compared with corresponding value in WT. Grp, group; BW, body weight.
Cardiac contractile effects of TRPV2 deficiency are not dependent on changes in preload or afterload.
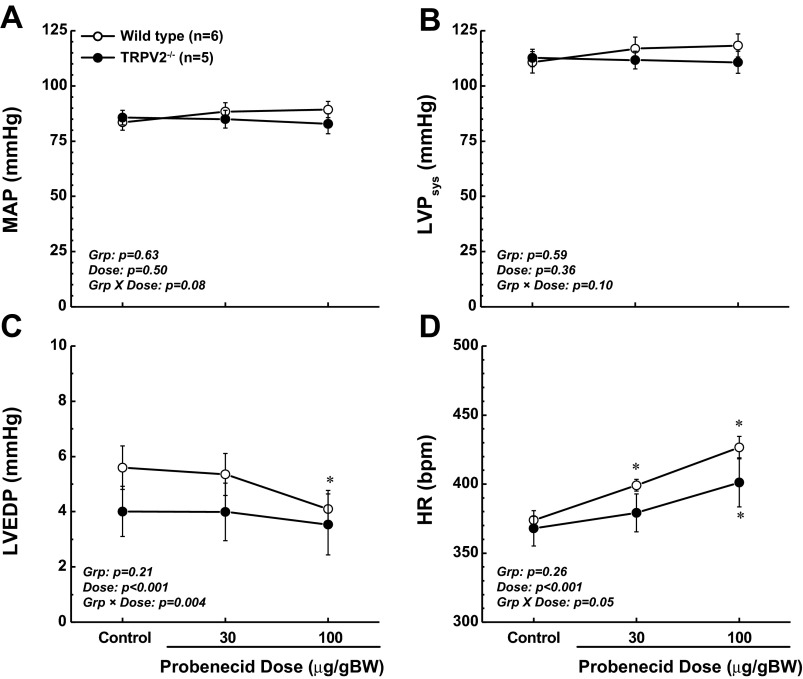
MAP and LV systolic pressure were not significantly different between WT and TRPV2−/− mice under baseline conditions or during administration of probenecid, and probenecid did not alter these pressures in either group (Fig. 3, A and B). Likewise, LV end-diastolic pressure (LVEDP) was not different between the two genotypes at baseline or during treatment with probenecid (Fig. 3C), though there was a significant decrease (P < 0.01) in LVEDP in WT mice at the highest dose of probenecid which was not present in the TRPV2−/− mice (group × dose interaction, P < 0.004). Overall, these data indicate that changes in afterload and preload were not responsible for the observed differences in myocardial function between the WT and TRPV2−/− mice. Finally, in contrast to our echocardiographic results, we found no differences in heart rate (HR) between the two genotypes at baseline, and probenecid administration modestly increased HR in both cohorts. Though the overall differences were small, a modest chronotropic effect of probenecid in WT mice was blunted in TRPV2−/− (group × dose interaction, P = 0.05; Fig. 3D).
Fig. 3.
Invasive hemodynamic measurements in WT vs. TRPV2−/− mice at baseline and in response to probenecid. Mean arterial pressure (MAP; A) and left ventricular (LV) systolic pressure (LVPsys; B) revealed no differences between the 2 genotypes as indexes of afterload. C: LV end-diastolic pressure (LVEDP) was not significantly different between the genotypes, though it was decreased in WT mice, but not in TRPV2−/−, in response to the high dose of probenecid (100 μg/g body wt iv). D: HR was not different at baseline but increased to a greater extent in response to probenecid in WT compared with TRPV2−/− mice (significant group × dose interaction). *P < 0.01 compared with baseline.
TRPV2 deficiency does not alter vascular responsiveness.
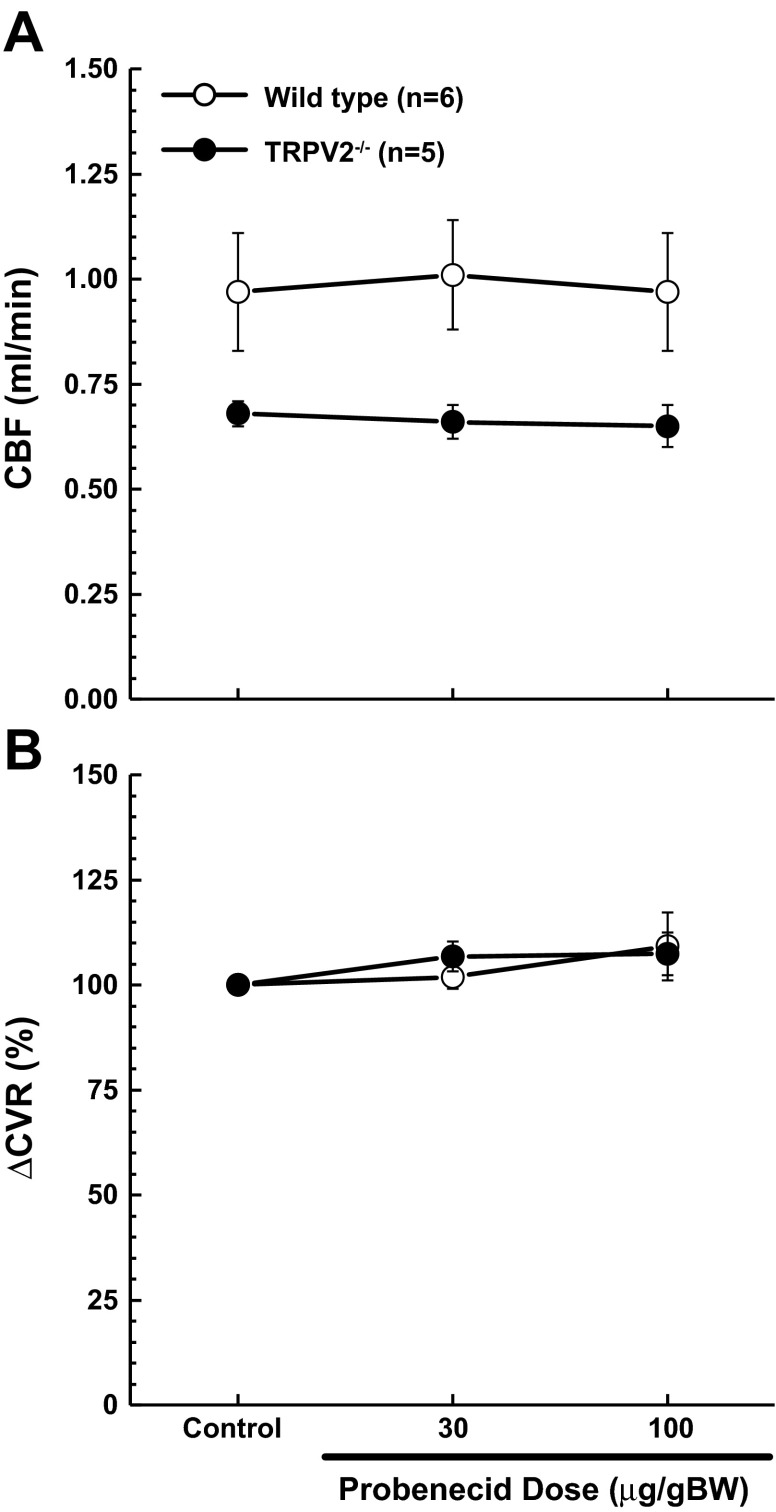
As an initial examination of vascular reactivity, we assessed carotid artery blood flow at baseline and during administration of probenecid. Although carotid blood flow appeared to be somewhat lower in TRPV2−/− mice compared with WT (Fig. 4A), the difference was not significant (P = 0.09). This level of uncertainty is likely due to a high level of variability in these measurements, which in our experience is not unusual, and likely reflects individual differences in relative blood flow through the carotid and vertebral arteries from mouse to mouse. Nonetheless, a meaningful difference cannot be ruled out. Calculated values for CVR were of a similar nature (WT = 94 ± 12% vs. TRPV2−/− = 124 ± 5%; P = 0.09). Because of this high level of variability, changes in CVR in response to probenecid were calculated as a percentage of baseline levels, as shown in Fig. 4B. It is apparent from these data that administration of probenecid at these doses had no effect on the cerebral vasculature in WT and TRPV2−/− mice.
Fig. 4.
Invasive blood flow measurements in WT and TRPV2−/− mice at baseline and in response to probenecid. A: there were no significant differences in carotid blood flow (CBF) between WT and TRPV2−/− mice at baseline or during treatment with probenecid. B: relative changes in cerebral vascular resistance (ΔCVR) indicated that probenecid had no effect on cerebral vascular tone.
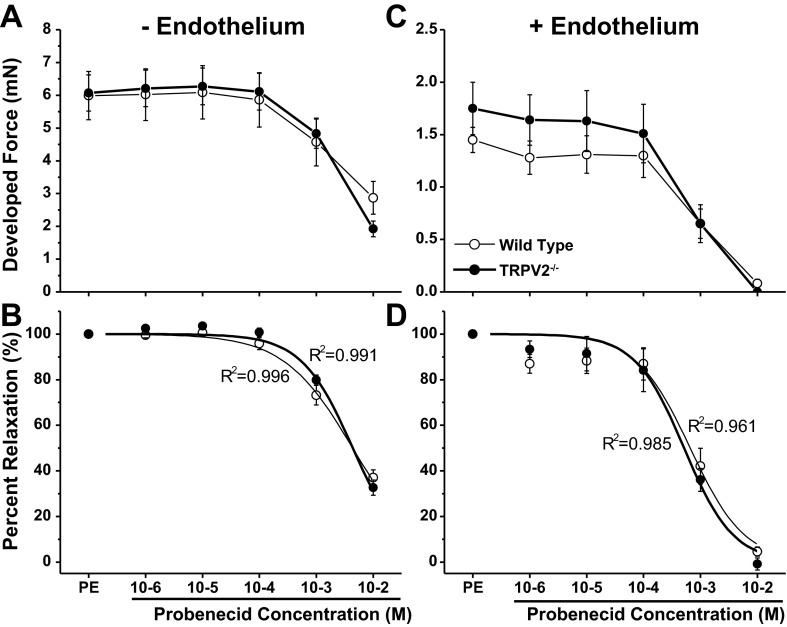
To further examine vascular responsiveness ex vivo, we examined force production in isolated aortic segments from the two groups of mice. We first determined whether there were any vasoconstrictor effects of probenecid over a wide concentration range (10−6 to 10−2 mM), and we found no evidence of increased force production, with or without intact endothelium (data not shown). We then examined the relaxing effects of increasing concentrations of probenecid after precontracting the vessels segments with 3 μM phenylephrine. In endothelium-denuded vessels (−E, Fig. 5, A and B), there were no differences in maximal developed tension in response to phenylephrine between WT (n = 8) and TRPV2−/− (n = 8) mice (5.99 ± 0.74 vs. 6.07 ± 0.55 mN, respectively). In response to increasing probenecid in these precontracted vessels, there were no significant dilatory responses until the concentration reached 1 mM. At the highest concentration of 10 mM, the developed force was reduced to about 25% of maximum in both groups of mice (Fig. 5B). These concentrations are several orders greater than the relevant pharmacological dose of 1 g (orally administered), which results in an approximate serum concentration of 246 μmol/l in humans (21). Finally, there were no differences in the concentration-response characteristics between WT and TRPV2−/− mice without endothelium (EC50: 4.56 ± 0.34 vs. 4.59 ± 0.52 mM, respectively).
Fig. 5.
Measurements of force production in isolated aortic ring preparations from WT (thin line, ○) and TRPV2−/− (thick line, ●; n = 8/group) mice after precontraction with phenylephrine (PE) and in response to escalating doses of probenecid. Measurements were made in vessels in which the endothelium had been removed (−E; A and B) and intact vessels (+E; C and D). Top: developed force following precontraction with 3 μM PE and cumulative addition of probenecid. Bottom: percent relaxation in response to probenecid with logistic curve fit y = {[A1 − A2]/[1 + (x/x0)p]}. In both −E and +E vessels, there were no observed differences in vascular smooth muscle function between the 2 genotypes. Very high concentrations of probenecid (1 mM and above) caused a significant vasodilation that was not different between WT and TRPV2−/− mice.
In WT and TRPV2−/− vessels with intact endothelium (+E, Fig. 5, C and D), maximal developed tension in response to phenylephrine was substantially less than in −E vessels but was not different between the two genotypes (1.45 ± 0.12 vs. 1.75 ± 0.25 mN in WT and TRPV2−/− mice, respectively, Fig. 5C). Vessels with intact endothelium were somewhat more sensitive to probenecid than −E vessels, but again there were no differences in the concentration-response characteristics between the two genotypes (EC50, 0.67 ± 0.21 vs. 0.52 ± 0.11 mM, respectively, Fig. 5D). The increased responsiveness of +E vessels to probenecid suggests some participation of the endothelium in the dilatory response to probenecid, but through a TRPV2−/− independent mechanism. This is confirmed by qRT-PCR of smooth muscle from WT and TRPV2−/− mice which demonstrated no significant expression of TRPV2 (data not shown).
TRPV2 deficiency is associated with compromised contractile function and Ca2+ signaling in isolated cardiomyocytes.
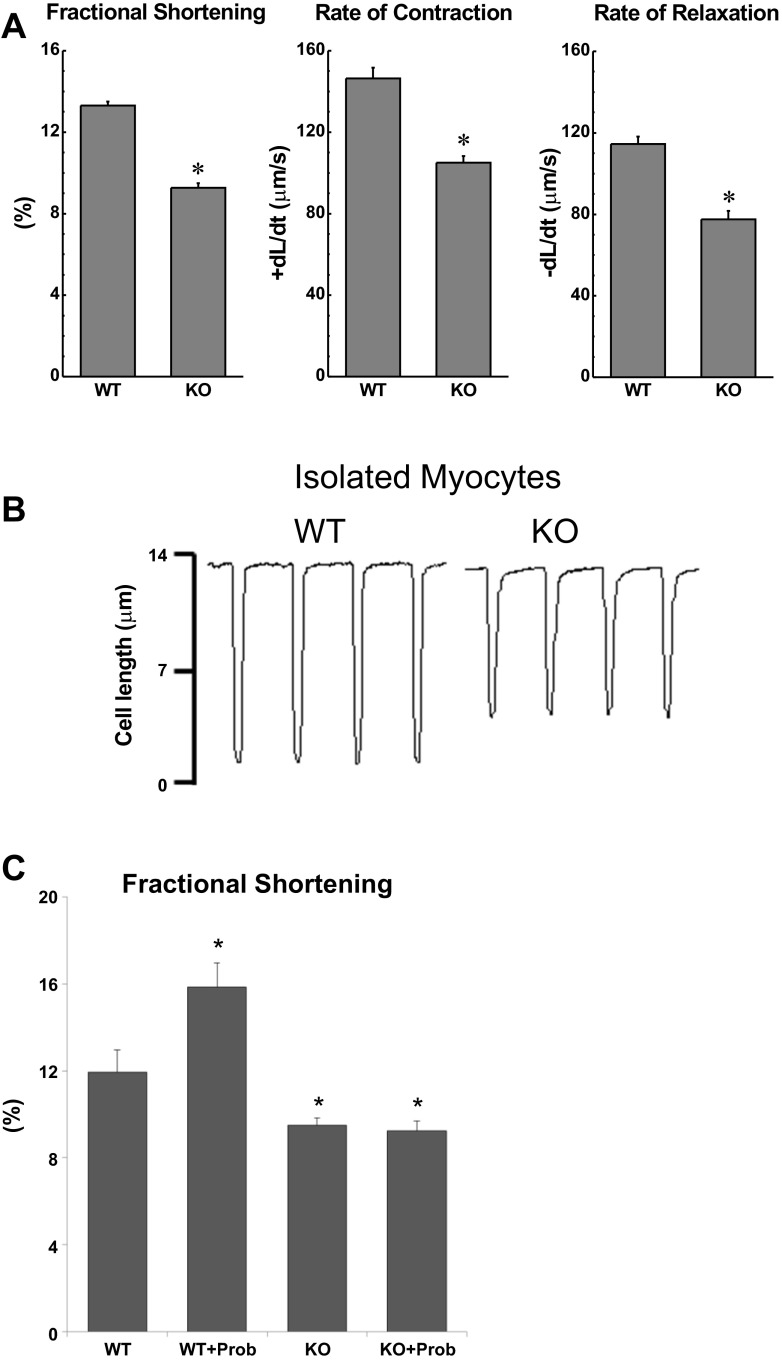
To further examine the mechanisms associated with decreased contractility in TRPV2−/− mice in vivo, we evaluated the mechanical properties in isolated cardiomyocytes, representing a load-independent system. When compared with WT, TRPV2−/− myocytes had significantly reduced fractional shortening (FS, P < 0.001), shortening rate (+dL/dt, P < 0.001), and relengthening rate (−dL/dt, P < 0.001) (Fig. 6A, representative trace in 6B). Treatment with probenecid significantly increased fractional shortening in WT cells (P < 0.001) but had no effect in TRPV2−/− myocytes (Fig. 6C). Similarly, after exposure to probenecid, the WT myocytes increased their shortening rate by 25.4% (+dL/dt, P < 0.001) and relengthening rate by 24.5%(−dL/dt, P < 0.001), whereas the TRPV2−/− myocytes exhibited no significant change.
Fig. 6.
Isolated cardiomyocyte studies of single cell contractile function in WT and TRPV2 knockout (KO) mice. A: FS, rate of contraction (+dL/dt), and rate of relengthening (−dL/dt) were each significantly decreased in the TRPV2−/− myocytes (n = 15 cells/3 hearts) compared with those measurements in WT myocytes (n = 14 cell/3 hearts). B: representative tracings of cell length changes. *P < 0.01 vs. WT. C: FS increased in WT myocytes after exposure to probenecid (Prob) but not in TRPV2−/− myocytes (n = 15 cells/3 hearts). *P < 0.01 vs. WT.
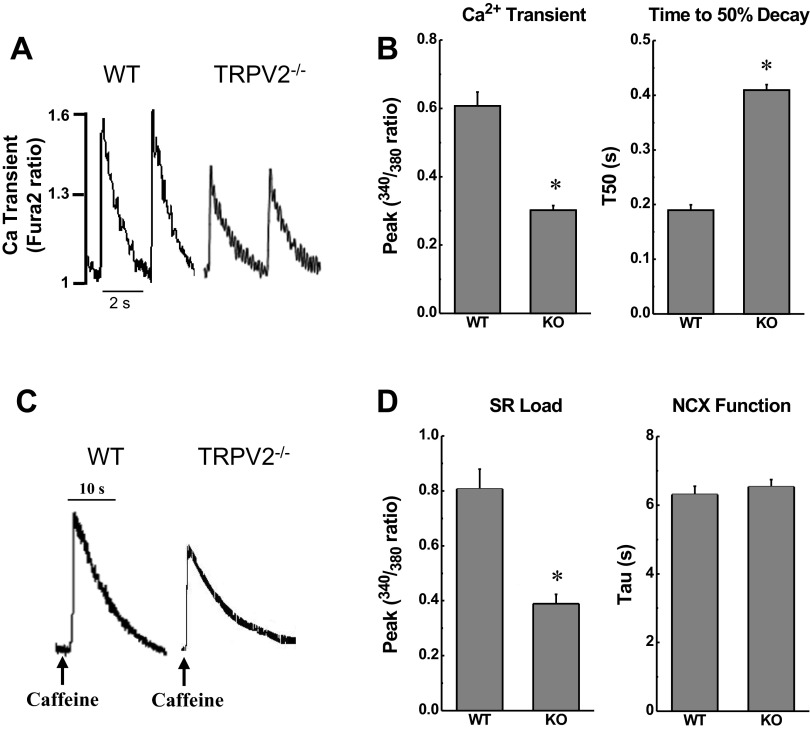
Calcium handling and kinetics were also measured in these isolated cardiomyocytes. The peak of the Ca2+ transient was significantly reduced in TRPV2−/− cardiomyocytes (P < 0.001; representative trace in Fig. 7A; summary data in Fig. 7B). Critically, the time to 50% decay of the Ca2+ peak (T50, Fig. 7B) and the τ were prolonged in TRPV2−/− myocytes relative to WT (P < 0.001), indicating impaired SR Ca2+ cycling. Furthermore, the caffeine-induced Ca2+ transient peak, as an index of SR load, was reduced in TRPV2−/− myocytes (P < 0.001; representative trace in Fig. 7C; summary data in Fig. 7D). Assessment of NCX function by measuring the time constant (τ) of Ca2+ decline during caffeine-induced Ca2+ transients indicated no difference between WT and TRPV2−/− myocytes (P = 0.49; Fig. 7D) (11). There were no significant differences in resting Ca2+ concentration between WT and TRPV2−/− myocytes.
Fig. 7.
Calcium handling in isolated myocyte from TRPV2−/− and WT mice. A: representative tracings of Ca2+ transients in myocytes paced at 0.5 Hz. B: summary data for Ca2+ transient peak amplitude and time to 50% decay (T50) of Ca2+ transient in myocytes from WT (n = 22 cells/3 hearts) and TRPV2−/− mice (n = 24 cells/3 hearts), as indicated by the 340 nm-to-380 nm fura-2 ratio. C: representative tracings of caffeine-induced Ca2+ release in isolated myocytes. D: the amplitude of the caffeine-induced Ca2+ transient was measured as an index of sarcoplasmic reticulum (SR) Ca2+ load, and the time constant of the pulse decay (τ) was used as an index of Na+/Ca2+ exchanger (NCX) activity in myocytes from WT (n = 15 cells/3 hearts) and TRPV2−/− mice (n = 16 cells/3 hearts). *P < 0.05 vs. WT.
TRPV2−/− mice exhibit no changes in expression of traditional calcium handling proteins.
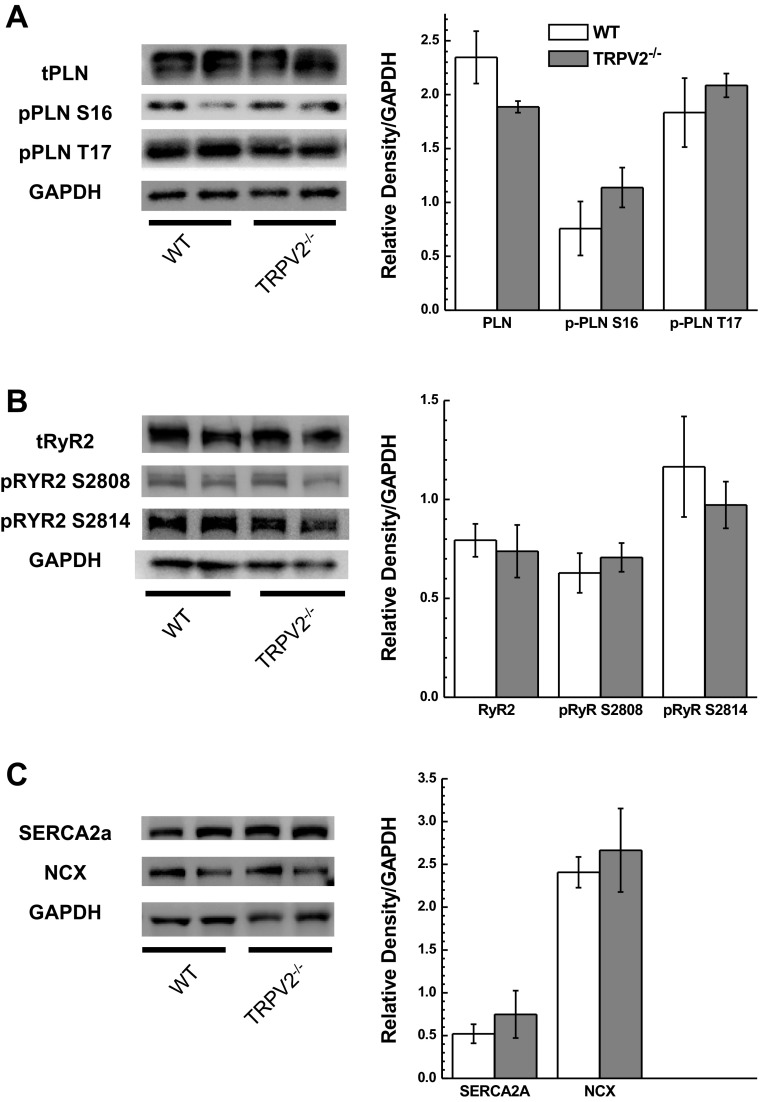
We investigated various proteins known to be involved in the regulation of physiological cardiac function and which have been shown to change expression levels and/or phosphorylation status in heart failure. These included p-PLN and t-PLN, p-RyR2 and t-RyR2, NCX, and the cardiac isoform of the SERCA2. Western blot analysis of typical Ca2+ handling proteins showed no significant changes in protein levels or phosphorylation status between the WT and TRPV2−/− mouse hearts (Fig. 8, A–C).
Fig. 8.
Western blot analysis Ca2+ handling proteins from hearts of WT (n = 4) and TRPV2−/− (n = 5) mice. Left: representative blots. Right: summary data for densitometry readings normalized to GAPDH. A: protein expression levels for total phospholamban (t-PLN) and phosphorylated phospholamban at serine-16 (p-PLN S16) and threonine-17 (p-PLN T17). B: protein expression levels for ryanodine receptor 2A (RyR2A) and phosphorylated ryanodine receptor 2A at serine-2808 (p-RyR2 S2808) or serine-2814 (p-RyR2 S2814). C: protein expression level for sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2A) and the NCX. No differences were observed between WT and TRPV2−/− mice.
DISCUSSION
Data presented in this study implicate TRPV2 channels in the regulation of myocardial function under physiological conditions. TRPV2-deficient mice consistently showed depressed myocardial performance, both in vivo and ex vivo, and inotropic and lusitropic responses to the TRPV2 agonist probenecid were blunted in TRPV2 knockout mice. Despite previous evidence that TRPV2 channels might play a role in regulating vascular tone (13, 25), our results indicated that the myocardial effects of TRPV2 activation were not secondary to differences in loading conditions between the WT and TRPV2−/− mice. Data from isolated myocytes show that Ca2+ handling is altered in TRPV2-deficient myocytes and suggest that the inotropic effects of TRPV2 activation are primarily intrinsic to cardiomyocytes. Overall, our findings confirm TRPV2 as an important component of the Ca2+ handling machinery in cardiomyocytes in vivo.
Initial examination of the TRPV2−/− phenotype using echocardiography revealed depressed systolic function (i.e., stroke volume, ejection fraction, and cardiac output) as estimated from M mode-derived measurements. These data were supported and confirmed by invasive measurements of LV systolic and diastolic function, which showed depressed values of ±dP/dt in TRPV2-deficient mice as well as blunted responsiveness to probenecid. Although the data from these different approaches yielded consistent results with respect to contractility, we found that HR was slightly lower in the TRPV2-deficient mice using echocardiography under light anesthesia and not different under the heavier anesthetic load required for invasive catheterization. Thus a chronotropic influence of TRPV2 channels cannot be ruled out. Consistent with this notion, probenecid increased HR mildly in the WT mice, and this effect was blunted in TRPV2 knockouts. This TRPV2-dependent positive chronotropic effect would be consistent with data shown in Fig. 1B. The lack of a difference in HR between the groups at baseline may be due to the deeper level of anesthesia in this set of experiments (a bottom effect). Finally, morphological studies indicate that there are no significant differences in cardiac histopathology or cardiomyocyte size to account for or reflect on the observed alterations in function.
We examined the participation of TRPV2 in the regulation of vascular tone, in vivo and ex vivo, and we found little evidence to support a substantial role. In particular, data from isolated aortae revealed no differences in force production between genotypes, and there were no apparent vasoactive effects of probenecid in either intact or deendothelialized vessels until superpharmacological concentrations were administered (i.e., ≥1 mmol/l). These ex vivo data are supported by intact measurements which showed that cerebral blood flow and vascular resistance were unaffected by administration of probenecid in both WT and TRPV2−/− mice. We also saw no differences in MAP or LV systolic pressure between the two groups, indicating that there were no primary disparities in afterload. LVEDP was not different between the WT and TRPV2−/− mice at baseline, despite the apparent tendency for lower baseline values in the TRPV2−/− mice (Fig. 3C). Moreover, whereas probenecid caused a significant decrease in LVEDP in WT mice at the higher dose, this effect was abolished in the TRPV2−/− mice. Thus the aforementioned increase in cardiac performance in response to probenecid (Fig. 2, A and B) occurred despite, rather than because of, a decrease in preload.
The effects of TRPV2-deficiency on cerebral blood flow might be interpreted in light of other roles that TRPV2 has been shown to play in the nervous system (14), as well as with possible effects in the microvasculature that were not studied in these experiments. TRPV2 channels are highly expressed in both the forebrain and hindbrain, including areas that have important roles in regulating body fluid homeostasis, autonomic function, and metabolism (14). Furthermore, though the brains of TRPV2−/− mice are evidently not grossly abnormal, a detailed analysis has not been reported and an influence of TRPV2 gene deletion on brain development and morphology cannot be ruled out. We did not measure brain weight, and we were therefore unable to express blood flow normalized to tissue weight, as would be the convention. Additional studies will focus on these possibilities and will also attempt to put in perspective the findings by Muraki et al. (13) who previously described TRPV2 as an active player in vascular tone with a series of experiments using murine aortic myocytes. Based on experiments that stretched and swelled mouse aortic myocytes in a number of different manners and in transiently transfected cells, they concluded that TRPV functions as an important stretch sensor in vascular smooth muscles. Though our studies did not reveal a difference in vascular function between WT and TRPV2−/−, we did not examine the stretch-dependent myogenic responses in these mice. Therefore, our data neither support nor refute the earlier findings described, under stress conditions, by Muraki and colleagues, and future studies are needed to ascertain the role that TRPV2 plays in the vasculature under physiological and stress conditions.
The isolated myocyte studies provide additional information regarding the role that TRPV2 plays in cardiomyocyte contractility and relaxation. We show with a load- independent system that intrinsic myocyte function is reduced in the TRPV2−/− cells. This confirms our in vivo findings of the importance of TRPV2 at the cardiac level as this unloaded system allows the differences in cardiomyocyte function to become more apparent. The studies of Ca2+ dynamics further clarify the role of TRPV2 by demonstrating lower Ca2+ transients and Ca2+ SR load in the TRPV2 knockout mice, and this occurs without affecting NCX function. This implicates, though does not prove, a role of TRPV2 in SR Ca2+ cycling. We speculate that this is an indirect role, due to moderate uptake of Ca2+ through these sarcolemmal channels, and that small increases in cytosolic Ca2+ would lead to increased SR load and release. This conclusion is supported by the protein expression analysis of Ca2+ handling proteins in WT and TRPV2−/− mice, where we found no difference for PLN, p-PLN (S16 and T17), RyR2, p-RyR2 (S2808 and S2814), NCX, and SERCA2.
With respect to the mechanism of TRPV2 activation, we previously reported that probenecid administration, putatively through TRPV2 stimulation, results in increased cytosolic Ca2+ concentrations in isolated WT cardiomyocytes (9). It has also been shown that TRPV2 (described as growth factor-regulated chain) translocates to the cytosolic membrane (8) and that cardiac-specific overexpression results in cardiomyopathy because of Ca2+ overload (6). This latter finding led the authors to suggest that growth factor-regulated chain (i.e., TRPV2) is a “key player” in myocyte degeneration, since it was elevated in the sarcolemma of humans with muscular dystrophy as well as in the dilated heart samples of transgenic mice. Based on our findings, we would extend this argument to suggest that under physiological conditions, TRPV2 plays a clinically relevant and readily assessed role in myocyte contractility through small changes in Ca2+ transients and that under experimental conditions of marked overexpression of TRPV2, the exaggerated influx of Ca2+ is associated with the development of cardiomyopathy. A future direction of study is needed to evaluate the role that TRPV2 plays under stress conditions such as pressure overload as its activity may be modulated under tonic stress.
Probenecid, a Food and Drug Administration-approved drug with a very safe clinical profile (20), has been previously demonstrated to be a TRPV2 agonist (1). Based on this information, we recently showed that probenecid has inotropic properties, which for decades have been overlooked (9), and that this effect was secondary to transient increases in cytosolic Ca2+ through SR release and not through the traditional inotropic pathway of β-adrenergic stimulation (10). This is a crucial finding, since clinically available positive inotropes generally increase metabolic demand, activate proapoptotic signaling pathways, and promote malignant arrhythmias, which result in increased mortality (7). In this study, we not only confirm our previous findings of probenecid as an inotrope with invasive measurements, but we also demonstrate inotropic and lusitropic effects at both high (100 mg/kg) and low (30 mg/kg) doses. This latter dose likely reflects the higher end of the dose range that can be safely administered to humans, since it represents ∼2 to 3 g/day dosage in an average person (4).
In conclusion, our data provide evidence that the TRPV2 channel participates in the Ca2+ handling machinery of the cardiomyocyte and contributes to normal baseline function. Activation of this channel increased function though modulation of the Ca2+ transient and perhaps through an indirect effect on SR Ca2+ load. This mechanism is quite different from that of clinically used inotropes and opens the possibility for clinical applications for patients with heart failure.
GRANTS
This study was supported by the Rehn Family grant and the CVCoE Pilot Grant program (to J. Rubinstein); by National Heart, Lung, and Blood Institute Grant HL-64018 (to E. G. Kranias); and the American Heart Association postdoctoral fellowship (to V. P. Singh) and Beginning Grant-in-Aid 13BGIA17140069 (to J. Rubinstein).
DISCLOSURES
J. Rubinstein and W. K. Jones are founding members of TRP Therapeutics with the goal of commercializing TRP channel modulators. Neither has received fees, honoraria, or any income from this venture.
AUTHOR CONTRIBUTIONS
J.R., S.E.K., E.G.K., W.K.J., and J.N.L. conception and design of research; J.R., V.M.L., S.E.K., V.P.S., V.C., N.R., A.R.P., M.J., and P.B. performed experiments; J.R., V.M.L., S.E.K., V.P.S., V.C., N.R., A.R.P., M.J., P.B., E.G.K., and J.N.L. analyzed data; J.R., V.M.L., S.E.K., V.P.S., V.C., N.R., A.R.P., M.J., P.B., E.G.K., W.K.J., and J.N.L. interpreted results of experiments; J.R., V.M.L., S.E.K., V.P.S., N.R., A.R.P., P.B., and J.N.L. prepared figures; J.R. and J.N.L. drafted manuscript; J.R., S.E.K., V.P.S., N.R., E.G.K., W.K.J., and J.N.L. edited and revised manuscript; J.R., V.M.L., S.E.K., V.P.S., V.C., N.R., A.R.P., M.J., E.G.K., W.K.J., and J.N.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. K. Haghighi and C. Lam for thoughtful discussions. We gratefully acknowledge Dr. Michael Caterina for technical support and providing the initial breeding pairs of TRPV2−/− mice. We are also grateful to Dr. Arnold Schwartz and Michael Tranter for scientific input and Mindi Naticchioni, Corin N. Newman, and Rajiv Karani for technical support.
REFERENCES
- 1.Bang S, Kim KY, Yoo S, Lee SH, Hwang SW. Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci Lett 425: 120–125, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398: 436–441, 1999 [DOI] [PubMed] [Google Scholar]
- 3.González-Cobos JC, Zhang X, Motiani RK, Harmon KE, Trebak M. TRPs to cardiovascular disease. In: TRP Channels in Drug Discovery. Totowa, NJ: Humana, 2012, p. 3–40 [Google Scholar]
- 4.Gutman AB, Yu TF. Benemid (p-[di-n-propylsulfamyl]-benzoic acid) as uricosuric agent in chronic gouty arthritis. Trans Assoc Am Physicians 64: 279–288, 1951 [PubMed] [Google Scholar]
- 5.Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res 99: 119–131, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Iwata Y, Katanosaka Y, Arai Y, Komamura K, Miyatake K, Shigekawa M. A novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel. J Cell Biol 161: 957–967, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamouh A, Francis GS. Contemporary management and research directions in advanced heart failure: where are we going? Congest Heart Fail 17: 241–247, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol 1: 165–170, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Koch SE, Gao X, Haar L, Jiang M, Lasko VM, Robbins N, Cai W, Brokamp C, Varma P, Tranter M, Liu Y, Ren X, Lorenz JN, Wang HS, Jones WK, Rubinstein J. Probenecid: novel use as a non-injurious positive inotrope acting via cardiac TRPV2 stimulation. J Mol Cell Cardiol 53: 134–144, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koch SE, Tranter M, Robbins N, Luther K, Singh U, Jiang M, Ren X, Tee T, Smith L, Varma P, Jones WK, Rubinstein J. Probenecid as a noninjurious positive inotrope in an ischemic heart disease murine model. J Cardiovasc Pharmacol Ther 18: 280–289, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Chu G, Kranias EG, Bers DM. Cardiac myocyte calcium transport in phospholamban knockout mouse: relaxation and endogenous CaMKII effects. Am J Physiol Heart Circ Physiol 274: H1335–H1347, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Lorenz JN, Loreaux EL, Dostanic-Larson I, Lasko V, Schnetzer JR, Paul RJ, Lingrel JB. ACTH-induced hypertension is dependent on the ouabain-binding site of the α-Na+-K+-ATPase subunit. Am J Physiol Heart Circ Physiol 295: H273–H280, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res 93: 829–838, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Nedungadi TP, Dutta M, Bathina CS, Caterina MJ, Cunningham JT. Expression and distribution of TRPV2 in rat brain. Exp Neurol 237: 223–237, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol 357: 271–296, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Park CS, Chen S, Lee H, Cha H, Oh JG, Hong S, Han P, Ginsburg KS, Jin S, Park I, Singh VP, Wang HS, Franzini-Armstrong C, Park WJ, Bers DM, Kranias EG, Cho C, Kim do H. Targeted ablation of the histidine-rich Ca2+-binding protein (HRC) gene is associated with abnormal SR Ca2+-cycling and severe pathology under pressure-overload stress. Basic Res Cardiol 108: 344, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park U, Vastani N, Guan Y, Raja SN, Koltzenburg M, Caterina MJ. TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J Neurosci 31: 11425–11436, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium 38: 233–252, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Robbins N, Koch SE, Rubinstein J. Targeting TRPV1 and TRPV2 for potential therapeutic interventions in cardiovascular disease. Transl Res 161: 469–476, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Robbins N, Koch SE, Tranter M, Rubinstein J. The history and future of probenecid. Cardiovasc Toxicol 12: 1–9, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Selen A, Amidon GL, Welling PG. Pharmacokinetics of probenecid following oral doses to human volunteers. J Pharm Sci 71: 1238–1242, 1982 [DOI] [PubMed] [Google Scholar]
- 22.Sharif-Naeini R, Ciura S, Zhang Z, Bourque CW. Contribution of TRPV channels to osmosensory transduction, thirst, and vasopressin release. Kidney Int 73: 811–815, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Stokes AJ, Shimoda LM, Koblan-Huberson M, Adra CN, Turner H. A TRPV2-PKA signaling module for transduction of physical stimuli in mast cells. J Exp Med 200: 137–147, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci 24: 6410–6415, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang XR, Lin MJ, McIntosh LS, Sham JS. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol 290: L1267–L1276, 2006 [DOI] [PubMed] [Google Scholar]