Abstract
Langendorff-perfused hearts and working hearts are established isolated heart preparation techniques that are advantageous for studying cardiac physiology and function, especially when fluorescence imaging is a key component. However, oxygen and energy requirements vary widely between isolated heart preparations. When energy supply and demand are not in harmony, such as when oxygen is not adequately available, the imbalance is reflected in NADH fluctuations. As such, NADH imaging can provide insight into the metabolic state of tissue. Hearts from New Zealand white rabbits were prepared as mechanically silenced Langendorff-perfused hearts, Langendorff-perfused hearts, or biventricular working hearts and subjected to sudden changes in workload, instantaneous global ischemia, and gradual hypoxia while heart rate, aortic pressure, and epicardial NADH fluorescence were monitored. Fast pacing resulted in a dip in NADH upon initiation and a spike in NADH when pacing was terminated in biventricular working hearts only, with the magnitude of the changes greatest at the fastest pacing rate. Working hearts were also most susceptible to changes in oxygen supply; NADH was at half-maximum value when perfusate oxygen was at 67.8 ± 13.7%. Langendorff-perfused and mechanically arrested hearts were the least affected by low oxygen supply, with half-maximum NADH occurring at 42.5 ± 5.0% and 23.7 ± 4.6% perfusate oxygen, respectively. Although the biventricular working heart preparation can provide a useful representation of mechanical in vivo heart function, it is not without limitations. Understanding the limitations of isolated heart preparations is crucial when studying cardiac function in the context of energy supply and demand.
Keywords: Langendorff, biventricular working, blebbistatin, nadh imaging, workload
the heart is responsible for pumping blood with vital supplies to the entire body and therefore requires a constant high rate of energy supply. Additionally, the heart has a large dynamic range with the capacity to increase its workload 10-fold and maintain this increase for extended periods of time. This metabolic requirement necessitates acute regulation of energy supply to match demand, as well as a constant adequate oxygen supply. Any limitation in energy supply is rapidly detrimental to cardiac function.
The demand for ATP and oxygen varies, depending on the amount of work being performed (12a, 17). The amount of available ATP to the heart at any given time is enough for only a few beats and must constantly be resynthesized to keep up with utilization (16). In the heart, 80–90% of energy production occurs via oxidative phosphorylation (25). Fats and carbohydrates are broken down and electrons are harvested, reducing NAD+ to NADH, (and, to a lesser extent, FAD+ to FADH2). In the presence of oxygen, NADH (and FADH2) are oxidized by the electron transport chain (ETC) where oxygen at Complex IV is the terminal electron acceptor. The proton motive force that is developed across the inner mitochondrial membrane during electron transfer is then used to synthesize ATP.
Study of ex vivo hearts provides many advantages: precise control of fuel supply and pressures, elimination of confounding variables such as hormonal changes, and the ability to image various fluorescent parameters, both endogenous and exogenous. Excised hearts are most commonly perfused retrogradely via the aorta as originally described by Langendorff (22). In this way, the heart is not required to provide its own coronary perfusion as it must in vivo. Additionally, in many investigations, often those using fluorescence imaging, blebbistatin, or other electromechanical uncouplers are administered to suppress contraction. This results in a dramatic reduction in energy utilization, since actomyosin-ATPases account for ∼75% of myocardial energy expenditure (27). The biventricular working heart, introduced by Demmy et al. in 1992 (10), contracts in the presence of both preload and afterload pressures and must provide its own coronary perfusion; thus work and oxygen demands are high. Due to these inherent differences in work demand, each heart preparation (biventricular working, Langendorff, and variations thereof) has distinct oxygen requirements. Oxygen can be a limiting factor in heart tissue that becomes ischemic and/or hypoxic, and insufficient oxygen availability can lead to an accumulation of NADH. Demand-induced ischemia may also occur during an episode of tacchycardia, when a fast heart rate is accompanied by a drop in aortic pressure that is inadequate for full coronary perfusion (23, 30).
Fluorescent imaging of epicardial NADH (fNADH) has been demonstrated as a useful tool to gain insight into the redox state of cardiac tissue (1, 2, 14, 15, 17, 18, 20). However, the effect of heart preparation on fNADH within the context of myocardial work load and oxygen supply is not well understood. fNADH imaging can provide critical information for defining the relationship between workload and oxygen deprivation that is essential for gauging the severity of ischemic events. The goal of this study was to explain how sudden changes in workload, hypoxia, and ischemia can affect NADH in different heart preparations, specifically in noncontracting Langendorff-perfused, Langendorff-perfused, and biventricular working hearts. This information is relevant to the optical mapping community where excitation-contraction uncouplers are routinely used for high-speed fluorescence imaging studies. Understanding how mechanically uncoupled hearts behave differently from working hearts is important when the results of optical mapping studies are used to explain in vivo function.
The present study subjected Langendorff-perfused mechanically silenced hearts, Langendorff-perfused contracting hearts, and biventricular working hearts to three different pacing rates and observed the effect on fNADH. Additionally, the hearts were subjected to instantaneous ischemia and gradual hypoxia while imaging fNADH. This study is novel in its direct comparison of isolated hearts with varying degrees of mechanical functionality, time course fNADH data, and the effects of oxygen limitations resulting from both no-flow ischemia and full-flow hypoxia. Previous studies examined NADH changes during workload fluctuations in trabeculae (5, 6) and in left ventricular working hearts; however, the latter did not provide a time course for these changes (14). It was predicted in the present study that biventricular working hearts would be the most sensitive to oxygen deprivation, as reflected by increases in fNADH, whereas mechanically arrested hearts would be the least susceptible to changes in fNADH during reduced oxygen availability. Due to the low levels of ATP utilization in mechanically arrested hearts, it was hypothesized that perfusate oxygen concentration must be reduced to levels lower than 50% before a marked increase in fNADH occurs since this would be observed almost immediately upon reduced perfusate oxygen concentration in working hearts. Investigators also hypothesized work jumps would result in a temporary oxidation of NADH, whereas return to baseline would result in a slight increase in fNADH as work demand decrease, indicating a momentary mismatch between energy supply and demand as cellular processes readjusted to the new workload. The findings demonstrate the greatest sensitivity of biventricular working hearts to oxygen deprivation, either by ischemia or hypoxia, whereas noncontracting hearts were the least sensitive. Interestingly, NADH was only affected by workload increases in biventricular hearts, with the highest heart rates resulting in an oxygen limitation.
MATERIALS AND METHODS
Heart Excision and Data Acquisition
Animal protocols were approved by the George Washington University's Animal Care and Use Committee. New Zealand white rabbits (n = 18) were anesthetized with an intramuscular injection of ketamine (44 mg/kg) and xylazine (10 mg/kg). Heparin (2,000 units) was administered via an intravenous ear vein injection, followed by pentobarbital sodium (50 mg/kg). After the cessation of pain reflexes, hearts were quickly excised and Langendorff perfused within 1 min at 55 mmHg and 37°C on a biventricular working heart system (Hugo Sachs Elektronik) with a Krebs-Henseleit solution containing (in mM) 118 NaCl, 3.3 KCl, 2.0 CaCl2, 1.2 MgSO4, 24.0 NaHCO3, 1.2 KH2PO4, 10.0 glucose, 2.0 sodium pyruvate, 10 HEPES, and 20.0 mg/l albumin (13). Unless otherwise noted, perfusate was oxygenated with 95% O2-5% CO2. While in Langendorff mode, 0.66 μmol of 5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate, succinimidyl ester (CICF) (Life Technologies) dissolved in DMSO was diluted in 5 ml perfusate and injected into the aortic chamber. Upon entry into cells, the acetoxy groups of CICF are cleaved, trapping the fluorescent marker. CICF fluorescence was then used to calculate a ratio with NADH to account for motion artifact and changes in epicardial illumination (due to contractions), changes in heart shape (due to swelling and shrinking during hypoxia and ischemia), and intrinsic fluorescence heterogeneities (due to epicardial fat, vessels, and connective tissue) (14). Electrodes placed on the apex of the heart and the right and left atria were used to acquire ECG, and a bipolar stimulus electrode was placed on the right atrium for pacing. Pressure transducers measured aortic pressure, right and left atrial preload, and right ventricular afterload. Perfusate oxygen concentration was measured using flow-through optical oxygen sensors (Polestar, Needham Heights, MA). Data acquisition was via PowerLab (AD Instruments).
Heart Preparation
Hearts were prepared one of three ways: as biventricular working hearts, as Langendorff-perfused hearts, or as Langendorff-perfused hearts mechanically uncoupled with blebbistatin. To prepare working hearts, after cardiac function stabilized under Langendorff perfusion, the left atrium was cannulated and the heart was transitioned into left working heart mode. The inferior vena cava and pulmonary artery were then cannulated, and the heart was put into full working heart mode, as described previously (1). Preloads and afterloads for working hearts were set based on individual heart performance. Only hearts able to maintain aortic pressures at or above 50 mmHg were used in analysis. To mechanically silence Langendorff-perfused hearts, 1 μmol blebbistatin was dissolved in 30 ml perfusate and slowly injected into the aortic chamber (28) just before imaging. If cessation of contraction did not occur, an additional 0.5 μmol was administered. Blebbistatin is an excitation-contraction uncoupler that eliminates contraction without affecting calcium cycling or electrical activity (11, 12). No additional preparation was needed for Langendorff-perfused hearts.
Dual Epicardial NADH and CICF Imaging
Two UV LEDs with a peak wavelength of 365 nm (Mightex, Pleasanton, CA) were used to excite the epicardium for fluorescence imaging. Emitted light was separated using a 500-nm dichroic mirror. Reflected light was band pass filtered at 460 ± 20 nm to image endogenous NADH fluorescence and transmitted light was band pass filtered at 585 ± 10 nm to image CICF fluorescence. Because NADH bound to Complex I of the ETC results in an amplification of the NADH fluorescence, captured fluorescence was assumed to be of mitochondrial origin (3). Addition of CICF did not increase the 460 ± 20 nm filtered fluorescence signal, indicating no crosstalk of CICF into the NADH signal. Complete reduction of NADH during ischemia resulted in an increase in the 585 ± 10 nm filtered fluorescence signal that was less than one third. This indicates the maximal contamination by NADH into the CICF filtered signal. As these signals were divided (see Data Analysis), this crosstalk would result in dampening, not amplification, of NADH changes. Aligned charge-coupled devices cameras (iXon DV860; Andor, Technology Belfast, UK) fitted with a dual-port adaptor were used to image NADH fluorescence (fNADH) and CICF fluorescence (fCICF) signals. SOLIS software (Andor) was used to capture images at 2 frames per second throughout the experimental protocols.
Experimental Protocols
Pacing protocol.
While ECG, pressure, and imaging data were collected, hearts were allowed to beat at normal sinus rhythm (NSR) for 25 s. Hearts were then paced at one of three pacing rates (330-, 220-, or 170-ms intervals) for 1 min after which pacing was terminated, and the heart was allowed to return to NSR while data were recorded for an additional 2 min. Hearts were allowed an additional 3 min of recovery time before the above was repeated at the remaining pacing rates, with the order of the rates randomized between studies. In working hearts, the protocol above was repeated; however, instead of pacing at 25 s, aortic pressure was dropped to 30 mmHg.
Hypoxia and ischemia protocols.
After the completion of the pacing protocol, hearts were subjected to either gradual global hypoxia or instantaneous global ischemia. Gradual hypoxia was achieved by bubbling the perfusate with nitrogen gas to stepwise decrease oxygen concentration while perfusate oxygen content was continually monitored. Global ischemia was attained by physically shutting off flow to the aorta for Langendorff-perfused hearts. In working hearts, aortic pressure was reduced to 0 mmHg to induce global ischemia. Hearts were paced at 220 ms during the ischemia protocol.
Data Analysis
Data analysis was performed in SOLIS (Andor Technology) and Matlab (Mathworks, Natick, MA) to analyze images for changes in fNADH and fCICF during pacing, gradual hypoxia, and instantaneous ischemia. To analyze changes in fNADH and fCICF, a large region of interest excluding areas of epicardial fat was selected on the ventricle in SOLIS. Average fluorescence from the selected regions was exported to Matlab. A ratio of fNADH and fCICF signals (rNADH) was calculated to remove motion artifact and account for changes in heart size during ischemia in Langendorff-perfused and working hearts: rNADH = fNADH/fCICF.
Because only working hearts experienced changes in rNADH during pacing, these studies warranted a method of normalization. The signals for pacing rates were normalized from 0 to 1, where 0 was the minimum rNADH value and 1 was the maximum NADH value reached for a given heart, yielding normalized rNADH values (nNADH): nNADH = (rNADH − rNADHmin)/(rNADHmax − rNADHmin).
Ischemia and hypoxia data from all hearts were normalized from 0 to 1 in the same manner, where 0 was the baseline minimum rNADH value and 1 corresponded to rNADH during maximum ischemia or hypoxia.
Statistical analysis was performed using unpaired Student's t-test after verifying data normality with a Kolmogorov-Smirnov test. Differences were considered significant when P < 0.05.
RESULTS
Effect of Pacing on fNADH
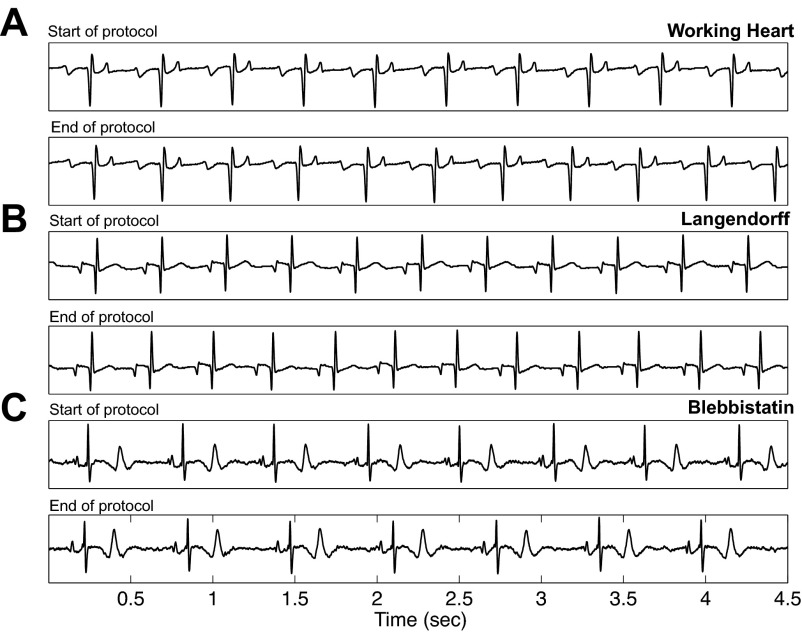
Pacing protocols were used to investigate the effects of increased workload on biventricular working and Langendorff-perfused hearts. Three pacing rates were chosen to mimic varying degrees of work: 330 ms (182 beats/min, approximate NSR), 220 ms (273 beats/min, a 50% rate increase over NSR), and 170 ms (353 beats/min, simulation of extreme tachycardia). Average NSR for working hearts, Langendorff-perfused hearts, and noncontracting hearts were 141 ± 50, 147 ± 24, and 159 ± 18 beats/min, respectively. Heart preparations were very stable over the experimental time period of ∼3 h. The data in Table 1, reported as end points expressed as a percentage of initial values, demonstrate a stability of heart rates, pressures, and fNADH. ECG traces and parameters are provided in Fig. 1 and Table 2 to show stability of electrical signals for the duration of the pacing protocol. Additionally, Table 2 provides a comparison of ECG parameters between published in vivo data and the excised preparations used in the present study (21). Under Langendorff perfusion, in contracting or mechanically silenced hearts, increased pacing rates had no effect on NADH fluorescence (ΔfNADH = 3.76 ± 1.16 and 2.89 ± 1.41 arbitrary units, respectively; Fig. 2, A and B). NADH remained stable even when pacing at 170 ms, a rate higher than the maximal heart rate of New Zealand rabbits (Fig. 2B). In contrast, increased workload resulted in drastic NADH changes in biventricular working hearts compared with blebbistatin (P < 0.001) and Langendorff hearts (P < 0.001) (Figs. 2, A and B, and 3A).
Table 1.
Functional stability of heart preparations
| Heart Rate, % | Fluorescent Imaging of Epicardial NADH, % | Mean Aortic Pressure, % | Mean Pulmonary Pressure, % | |
|---|---|---|---|---|
| Blebbistatin | 96.0 ± 11.2 | 98.9 ± 4.0 | 102.2 ± 1.7 | N/A |
| Langendorff | 100.2 ± 0.7 | 95.2 ± 4.9 | 102.1 ± 0.8 | N/A |
| Biventricular Working | 97.2 ± 5.9 | 99.1 ± 6.9 | 98 ± 6.3 | 104.5 ± 17.9 |
Values are means ± SD; n = 3. Parameters (heart rate, fluorescent imaging of epicardial NADH, mean aortic pressure, and mean pulmonary pressure) are expressed as a percentage of initial values after complete experimental protocol, typically <3 h. N/A, not applicable.
Fig. 1.
Electrical stability before and after pacing protocol. Representative ECG signals from hearts in each of the 3 preparations: working hearts (A), Langendorff hearts (B), and mechanically arrested hearts (C). Signals at the beginning of the protocol for each preparation are above signals acquired at the end of the entire pacing protocol.
Table 2.
Electrical stability of heart preparations
| Interval, s |
||||
|---|---|---|---|---|
| R-R | P-R | QRS | Q-T | |
| Working heart | ||||
| Start of protocol | 0.422 ± 0.029 | 0.078 ± 0.010 | 0.045 ± 0.002 | 0.180 ± 0.017 |
| End of protocol | 0.412 ± 0.005 | 0.078 ± 0.022 | 0.046 ± 0.001 | 0.158 ± 0.024 |
| Langendorff | ||||
| Start of protocol | 0.403 ± 0.054 | 0.071 ± 0.014 | 0.044 ± 0.012 | 0.177 ± 0.005 |
| End of protocol | 0.404 ± 0.056 | 0.066 ± 0.011 | 0.034 ± 0.002 | 0.175 ± 0.025 |
| Blebbistatin | ||||
| Start of protocol | 0.438 ± 0.113 | 0.072 ± 0.010 | 0.032 ± 0.000 | 0.167 ± 0.017 |
| End of protocol | 0.469 ± 0.236 | 0.065 ± 0.025 | 0.031 ± 0.001 | 0.166 ± 0.009 |
| In vivo data (Kour et al. 2013) | 0.240–0.359 | 0.040–0.080 | 0.040–0.120 | 0.080–0.160 |
Values are means ± SD; n = 2. ECG parameters (R-R interval, P-R interval, QRS interval, and Q-T interval) for working, Langendorff, and mechanically arrested hearts are compared at the beginning and at the end of pacing protocols. In vivo values are also included for comparison.
Fig. 2.
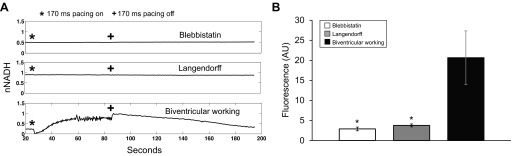
Effect of fast pacing on NADH. A: representative normalized NADH fluorescence (fNADH)/CICF fluorescence (fCICF) during a 170-ms pacing protocol for a blebbistatin heart, Langendorff heart, and biventricular working heart; pacing was initiated at (*) and terminated at (+). B: dynamic range of fNADH during a 170-ms pacing protocol, calculated as fNADHmax − fNADHmin as described in materials and methods for blebbistatin hearts (white, n = 6), Langendorff hearts (gray, n = 7), and biventricular working (black, n = 4). *P < 0.05 statistically different from working hearts. nNADH, normalized ratio of fNADH and fCICF signal values; AU, arbitrary units.
Fig. 3.
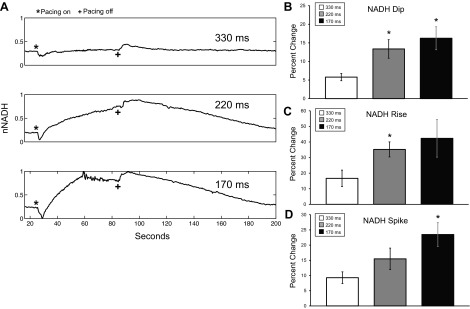
Effect of pacing rate on NADH in biventricular working hearts (n = 5). A: nNADH during 330-, 220-, and 170-ms pacing protocols in a biventricular working heart, where pacing was initiated at (*) and terminated at (+). Percent change in the magnitude of the nNADH change from baseline to dip at the onset of pacing (B), from baseline to maximal before pacing is terminated (C), and from the maximal to the spike offset of pacing (D) is shown. For all bar graphs: 330 ms (white), 220 ms (gray), and 170 ms (black). *P < 0.05 statistically different from 330-ms pacing.
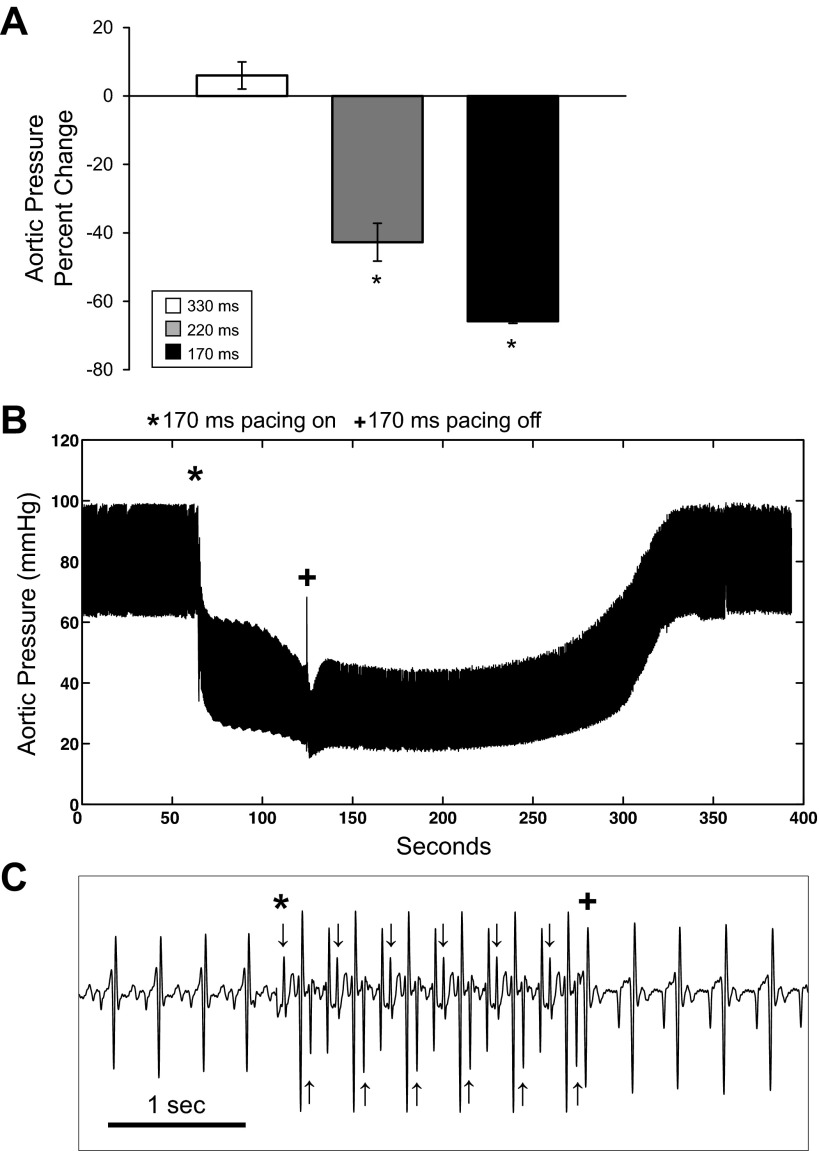
Immediately at the onset of pacing, a dip in fNADH was observed in working hearts at any pacing rate (Fig. 3A). The fNADH dip was smallest in hearts being paced at 330 ms, near NSR (5.8 ± 1.9%), and greatest in hearts that were paced at 170 ms (16.3 ± 5.4%; P < 0.05; Fig. 3B). After the initial dip, fNADH rose in hearts paced at 220 and 170 ms (Fig. 3A). The increase in fNADH was greater in hearts paced at 170 vs. 220 ms (P < 0.05; Fig. 3C). Upon termination of pacing, fNADH demonstrated a small spike before decreasing back to the initial baseline value. Return to baseline fNADH occurred within 4 min in all hearts. Hearts subjected to a greater workload took longer to recover to fNADH baseline once work was suspended. Similar to the dips at the onset of pacing, the magnitude of the spikes in fNADH when pacing stopped increased with increasing pacing rate (P = 0.01 for 170 ms; Fig. 3D). A drop in aortic pressure accompanied the changes in fNADH at the onset of pacing at 170 ms (P < 0.005) and 220 ms (P < 0.05) (65.8 ± 0.6% and 42.7 ± 5.5%, respectively; Fig. 4, A and B).
Fig. 4.
Effect of pacing rate on aortic pressure in biventricular working hearts (n = 5). A: percent change in aortic pressure at the onset of pacing for pacing at 330 ms (white), 220 ms (gray), and 170 ms (black). B: representative aortic pressure data from a working heart during a 170-ms pacing protocol, where pacing was initiated at (*) and terminated at (+). C: confirmation of pacing capture via ECG, where pacing was initiated at (*) and terminated at (+). Arrows correspond to pacing spikes. *P < 0.05 statistically different from 330-ms pacing.
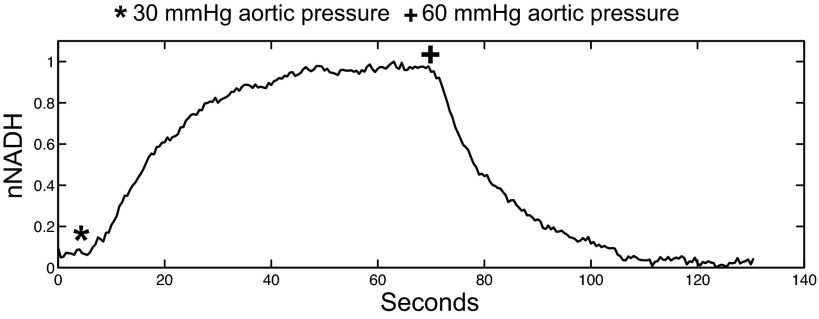
To compare the effect of the work jump with the effect of the decrease in aortic pressure that accompanied pacing at 170 ms, aortic pressure was manually decreased to 30 mmHg in working hearts. This resulted in a gradual rise in NADH that mirrored the rise that occurred during pacing at 170 ms; however, the dips and spikes that occurred during pacing were absent (Fig. 5).
Fig. 5.
Effect of decreased aortic pressure on nNADH in a biventricular working heart. Aortic pressure was reduced to 30 mmHg at (*) and returned to 60 mmHg at (+).
Effect of Gradual Hypoxia on fNADH
To observe the effect of decreased oxygen availability on NADH fluorescence, hearts were subjected to gradual hypoxia by decreasing oxygen in the perfusate while monitoring fNADH. Figure 6A shows a rise in fNADH and fCICF as the tissue becomes more hypoxic over time and expands in shape. The increase in NADH fluorescence as well as change in heart shape can clearly be seen in Fig. 6B. The fNADH/fCICF signal, rNADH, corrected for this change in tissue shape and as well as any movement. rNADH steadily increased as perfusate oxygen concentration decreased over time (Fig. 6C). The data in Fig. 6C were then plotted to show rNADH as a function of oxygen percentage in Fig. 6D. rNADH clearly increases as perfusate oxygen concentration decreases (Fig. 6D).
Fig. 6.
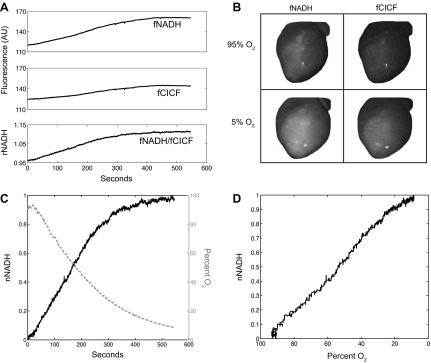
Representative data for a Langendorff heart during gradual hypoxia protocol. A: fNADH and fCICF over time, with the ratio signal, rNADH, calculated after data acquisition. B: fNADH and fCICF images before (95% O2) and at the end (5% O2) of gradual hypoxia protocol. C: rNADH normalized from 0 to 1 as described in materials and methods and perfusate oxygen percentage over time. D: nNADH as a function of perfusate oxygen percentage. AU, arbitrary units.
As oxygen concentration approached zero, NADH plateaued in biventricular working hearts and contracting Langendorff hearts (Fig. 7A). A linear fit was applied to the last five points of each data set to determine the slope of each plateau at lowest oxygen concentrations, producing negligible slopes for both biventricular working (0.002 ± 0.002; P < 0.05) and contracting Langendorff (0.008 ± 0.004; P < 0.05) hearts. rNADH did not reach a plateau in hearts mechanically silenced with blebbistatin, even when perfusate oxygen reached 15%, maintaining a slope of 0.063 ± 0.015 (Fig. 7D). rNADH was normalized to maximum rNADH reached after 15 min of hypoxia (nNADH).
Fig. 7.
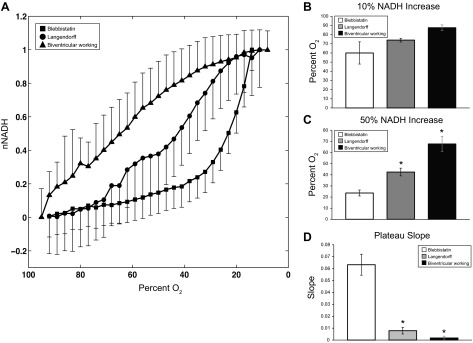
Effect of gradual hypoxia on NADH. A: nNADH as a function of perfusate oxygen percentage for biventricular hearts (▲, n = 4), Langendorff hearts (●, n = 2), and blebbistatin hearts (■, n = 3). Perfusate oxygen concentration when NADH increases 10% of full range (NADH = 0.1; B) and 50% of full range (NADH = 0.5; C) is shown. D: slope of NADH at plateau as described in results. For all bar graphs: blebbistatin hearts (white), Langendorff hearts (gray), and biventricular working (black). *P < 0.05 statistically different from blebbistatin hearts.
Biventricular working hearts were the most sensitive to the decrease in oxygen availability; as soon as oxygen concentration began to decrease, nNADH began to rise (Fig. 7A). A 10% increase in nNADH was seen when oxygen concentration fell to 87.5 ± 6.4%. The rise in nNADH in Langendorff and Langendorff hearts with blebbistatin tracked each other until oxygen concentration decreased below 75% at which point the two signals diverged. A 10% increase in nNADH occurred at an oxygen concentration of 74.0 ± 2.8% in Langendorff-perfused hearts and at 60.0 ± 21.0% in hearts with blebbistatin (Fig. 7B). The half-maximal value of nNADH in working hearts (P < 0.005) occurred when oxygen concentration reached 67.8 ± 13.7%, whereas oxygen concentration had to decrease much more to reach half-maximal nNADH in Langendorff-perfused hearts (42.5 ± 5.0%; P < 0.05) (Fig. 7C). Mechanically uncoupled hearts were very insensitive to oxygen deprivation, with oxygen concentration decreasing to 23.7 ± 4.6% before nNADH was half-maximal (Fig. 7C).
Effect of Instantaneous Ischemia on fNADH
fNADH was monitored in real time while hearts were subjected to global ischemia and then normalized to the maximum fNADH value reached. τ is defined as time to 66% rise in nNADH. Global ischemia resulted in a much faster increase in nNADH in biventricular working and Langendorff-perfused hearts than in mechanically arrested hearts (τ = 17.3 ± 10.3, 43.5 ± 21.2, and 100.0 ± 64.4 s, respectively). Global ischemia was achieved in Langendorff and blebbistatin hearts by simply shutting off flow to the aorta. In working hearts, however, global ischemia was mimicked by reducing left ventricular afterload to 0 mmHg to terminate coronary perfusion. Figure 8B shows the drop in aortic pressure as LV afterload was reduced to 0 mmHg. Pressure was held at zero until fNADH reached a plateau, and then LV afterload was increased back to 60 mmHg for coronary perfusion and recovery of fNADH.
Fig. 8.
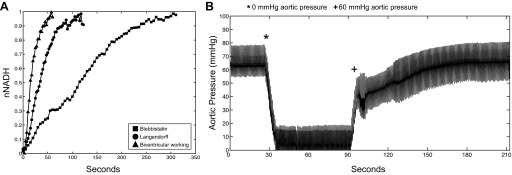
Effect of instantaneous ischemia on nNADH. A: representative rise in nNADH after termination of flow at time = 0 for a biventricular working heart (▲), Langendorff heart (●), and blebbistatin heart (■). B: representative trace of aortic pressure for a biventricular working heart as left ventricular afterload was decreased to 0 mmHg (*) to achieve global ischemia and then returned to 60 mmHg (+) for recovery.
DISCUSSION
The present study demonstrates significant differences between heart preparations with respect to the effects of work jumps and hypoxia on NADH. First, the energetic demands of Langendorff-perfused mechanically silenced hearts are much lower than those of contracting Langendorff-perfused hearts, and these two models do not reproduce the metabolic demands of biventricular working hearts. The use of heart preparations that do not depend on their own function for sufficient coronary perfusion and have minimized energy requirements could result in misinterpretation of results when studying cardiac work, metabolism, and temporal changes in function that result from ischemia. Second, oxygen availability is of critical importance when studying cardiac tissue, and even a small reduction in perfusate oxygenation results in extreme consequences in ex vivo biventricular working hearts. Physiologically relevant perturbations, such as a 50% increase in heart rate, result in NADH accumulation.
NADH Dynamics During Changes in Workload
In biventricular working hearts, high pacing rates lead to a mismatch in energy supply and demand that is reflected in NADH. This is manifested as quick dips and spikes in NADH as work is applied and removed (Fig. 2A). The initial, rapid dips in NADH at the onset of pacing are similar to those seen in rabbit trabeculae upon an increase in stimulation frequency (5, 6) and in isolated heart mitochondria upon the addition of ADP (4). When ATP utilization increases, NADH is oxidized as a result of the increase in the rate of oxidative phosphorylation. At first, the increase in NADH utilization via ETC flux is not matched by an increase in NADH production. With increased workload, cytosolic and mitochondrial Ca2+ concentrations increase. This increase in Ca2+ stimulates the enzymes involved in NADH production, specifically pyruvate dehydrogenase, 2-oxogluterate dehydrogenase, and isocitrate dehydrogenase (9, 24). The dip in NADH recovers when the Ca2+ stimulation of NADH production allows supply to again match demand (6). Interestingly, the magnitude of the work-related decreases in NADH correlate with the amount of work being performed; faster pacing results in a greater dip at the onset of pacing (Fig. 3, A and B).
The rise in NADH seen after the initial drop when pacing at 220 and 170 ms is attributed to an oxygen limitation at these workloads. The drop in aortic pressure that accompanies high pacing rates results in a limitation in coronary perfusion. The energy required could not be met with the oxygen available to the cardiac tissue, causing a demand-induced ischemia (Fig. 3, A and B). The decrease in coronary perfusion, resulting in a decrease in oxygen availability, combined with an increased oxygen demand due to increased workload, results in severe hypoxia. The rise in NADH is greater at faster pacing rates, reflecting the severity of the hypoxia (Fig. 3, A and C).
Upon the termination of pacing, there is a spike in NADH (Fig. 3, A and D). This is consistent with findings in loaded rat trabeculae (5, 6) and can be explained by the Ca2+ activation of NADH production during increased workload. When the heart returns to normal sinus rhythm, NADH production momentarily outpaces utilization, resulting in a transient increase in NADH. As mitochondrial [Ca2+] decreases to baseline levels, NADH production and utilization are again matched. Aortic pressure returns to baseline, resuming adequate perfusion and oxygenation. Together, these result in a restoration of NADH to the initial baseline value. Interestingly, the 330-ms pacing rate, which is only slightly faster than sinus rhythm, displayed the work dip and spike associated with an increase and decrease in workload. We conclude this pacing rate results in small, transient mismatching of NADH supply and demand. However, this rate does not result in a drop in aortic pressure and is therefore not fast enough rhythm to cause demand-induced ischemia (Fig. 4A).
Consistent with the conclusions that NADH dips and spikes are a result of changes in workload and the gradual rise in NADH during pacing is a result of inadequate coronary perfusion, when aortic pressure is manually reduced to the level achieved during pacing at 170 ms, there is a steady rise in NADH without NADH dips and spikes (Fig. 5).
Both contracting and noncontracting Langendorff-perfused hearts are the most resistant to changes in NADH during pacing. Because 70–80% of energy utilization in the heart is allocated for contraction (27), noncontracting Langendorff-perfused hearts require energy only for Ca2+ cycling, the firing of action potentials, and the maintenance of ionic gradients. Because little energy is required, oxygen demands are also very low, and it is not surprising there are no changes in NADH when heart rate increases (Fig. 2A). This was also true for Langendorff-perfused hearts. They are contracting, but do not have to sustain their own coronary flow. This constant perfusion provides adequate oxygen supply for changes in workload. It is also important to note that in the present study, Langendorff hearts did not contract against resistance, and it is reasonable to assume that the inclusion of a left ventricular balloon would increase the amount of work done by the ventricle, which would be reflected as changes in NADH during pacing.
NADH Dynamics During Ischemia and Hypoxia
The relative insensitivity of mechanically silenced hearts to changes in oxygenation is apparent since NADH did not rise dramatically during hypoxia until perfusate oxygen fell below about 30% (Fig. 7A). Additionally, NADH took three times longer to reach a plateau during no-flow ischemia than any other preparation, demonstrating the extremely low energy utilization rate of noncontracting hearts (Fig. 8A). Contracting Langendorff-perfused hearts are slightly more sensitive to changes in oxygen availability, with NADH rising more dramatically with a given decrease in oxygen concentration (Fig. 7A). Langendorff-perfused hearts were sensitive to flow-off ischemia, however, and NADH rose almost as quickly as in working hearts (Fig. 8A). Working hearts require the most energy to sustain contraction and are extremely sensitive to changes in oxygen availability. This was apparent since even a slight decrease in perfusate oxygenation resulted in a dramatic increase in NADH (Fig. 7A). Additionally, when aortic pressure was dropped to 0 mmHg to produce global ischemia, the rise in NADH was extremely rapid, indicating the highest energy utilization in this preparation (Fig. 8A).
A Shortcoming of the Biventricular Working Heart Preparation and Study Limitations
Although biventricular working hearts share functional similarities with their in vivo counterparts, fNADH imaging has revealed an important difference: biventricular working hearts are highly sensitive to perfusate oxygen level. We observed fNADH elevations when perfusate oxygenation was not maintained above a certain threshold (∼80%-90% in the present study), indicating a deficit in myocardial oxygenation. Increasing the oxygen carrying capacity of perfusate would likely improve biventricular mechanical function. Indeed, perfusion of a working rabbit heart with red blood cells at 15% hematocrit improved mechanical function over that of K-H buffer alone (8). However, red blood cell perfusion has concomitant limitations as hemoglobin interferes with fluorescence imaging. Other differences between excised biventricular working hearts and hearts in vivo are the presence of vasodilators that adapt vasculature to changes in blood pressure and the availability of fatty acid substrates for fuel. Blood flow can increase with increasing heart rate to keep tissue adequately perfused (15, 19, 29). Inclusion of a vasodilator such as nitroprusside and fatty acids such as palmitate may prove better at simulating in vivo conditions with an ex vivo working heart preparation and could improve overall function (2).
Several possible limitations of our studies include the removal of the pericardium and slight cooling of the epicardial surface during global ischemia. Removal of the pericardium has advantages for epicardial imaging; however, if it is intact it may counteract heart chamber overdistension during diastole. Aortic flow was stopped to induce ischemia. Because hearts were not submerged in a temperature-controlled environment, the lack of heating from coronary flow during ischemia would result in gradual cooling of the epicardial layer. The potential risk of ischemic damage during the heart excision and cannulation heart procedure is also a limitation, so procedure times were minimized. Finally, a cautionary note: the wavelengths at which NADH is imaged should be carefully selected to prevent signal contamination by absorbance changes of other tissue components, such a myoglobin (26).
Conclusions
Mechanically silenced hearts have very low ATP and oxygen demands, which is reflected in no measureable changes in NADH upon increases in workload and extremely slow rises in NADH when coronary oxygen is limited. Although beneficial for fluorescence imaging studies, this preparation does not mimic mechanical in vivo heart function. Unloaded contracting Langendorff-perfused hearts demonstrate a higher sensitivity to oxygen limitations during ischemia and hypoxia; however, they are not affected by changes in workload. Biventricular working hearts have much higher ATP and oxygen demands, and even a slight decrease in oxygen availability results in a measureable accumulation of NADH. Fast pacing results in an initial dip in NADH and a spike upon return to NSR, consistent with increased ATP turnover and Ca2+ activation of NADH production. This preparation, however, is subject to demand-induced ischemia, since fast pacing decreases aortic pressure and results in an accumulation of NADH. The working heart contracts against physiologic preload and afterload pressures and supplies its own coronary perfusion, making it mechanically suitable for certain heart studies. It is not, however, without shortcomings. Because optical mapping studies are performed to attain a more complete picture of mechanical, electrical, and metabolic components, it is important to consider the benefits and limitations of isolated heart preparations when choosing a technique and interpreting data.
GRANTS
This study was support by National Heart, Lung, and Blood Institute Grant HL-095828 (to M. W. Kay), a George Washington University Presidential Merit Fellowship (to A. M. Wengrowski), and an Achievement Rewards for College Scientists Graduate Fellowship (to A. M. Wengrowski).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.M.W. and S.K.-G. performed experiments; A.M.W. analyzed data; A.M.W., S.K.-G., and R.J. interpreted results of experiments; A.M.W. prepared figures; A.M.W. drafted manuscript; A.M.W., S.K.-G., R.J., and M.W.K. approved final version of manuscript; S.K.-G. and M.W.K. conception and design of research; S.K.-G., R.J., and M.W.K. edited and revised manuscript.
REFERENCES
- 1.Asfour H, Wengrowski AM, Jaimes R, III, Swift LM, Kay MW. NADH fluorescence imaging of isolated biventricular working rabbit hearts. J Vis Exp. In print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashruf JF, Coremans JM, Bruining HA, Ince C. Increase of cardiac work is associated with decrease of mitochondrial NADH. Am J Physiol Heart Circ Physiol 269: H856–H862, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Blinova K, Levine RL, Boja ES, Griffiths GL, Shi ZD, Ruddy B, Balaban RS. Mitochondrial NADH fluorescence is enhanced by complex I binding. Biochemistry 47: 9636–9645, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bose S, French S, Evans FJ, Joubert F, Balaban RS. Metabolic network control of oxidative phosphorylation: multiple roles of inorganic phosphate. J Biol Chem 278: 39155–39165, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Brandes R, Bers DM. Intracellular Ca2+ increases the mitochondrial NADH concentration during elevated work in intact cardiac muscle. Circ Res 80: 82–87, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Brandes R, Bers DM. Simultaneous measurements of mitochondrial NADH and Ca2+ during increased work in intact rat heart trabeculae. Biophys J 83: 587–604, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen V, Chen YH, Downing SE. An improved isolated working rabbit heart preparation using red cell enhanced perfusate. Yale J Biol Med 60: 209–219, 1987 [PMC free article] [PubMed] [Google Scholar]
- 9.Crompton M. The role of Ca2+ in the function and dysfunction of heart mitochondria. In: Calcium and the Heart, edited by Langer G. New York: Raven Press, 1990, p. 167–198 [Google Scholar]
- 10.Demmy TL, Magovern GJ, Kao RL. Isolated biventricular working rat heart preparation. Ann Thorac Surg 54: 915–920, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Farman GP, Tachampa K, Mateja R, Cazorla O, Lacampagne A, de Tombe PP. Blebbistatin: use as inhibitor of muscle contraction. Pflügers Arch 455: 995–1005, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, Efimov IR. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Hear Rhythm 4: 619–626, 2007 [DOI] [PubMed] [Google Scholar]
- 12a.Gibbs C. The cytoplasmic phosphorylation potential. Its possible role in the control of myocardial respiration and cardiac contractility. J Mol Cell Cardiol 17: 727–731, 1985 [DOI] [PubMed] [Google Scholar]
- 13.Gillis AM, Kulisz E, Mathison HJ. Cardiac electrophysiological variables in blood-perfused and buffer-perfused, isolated, working rabbit heart. Am J Physiol Heart Circ Physiol 271: H784–H789, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Heineman FW, Balaban RS. Effects of afterload and heart rate on NAD(P)H redox state in the isolated rabbit heart. Am J Physiol Heart Circ Physiol 264: H433–H440, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Ince C, Ashruf JF, Avontuur JA, Wieringa PA, Spaan JA, Bruining HA. Heterogeneity of the hypoxic state in rat heart is determined at capillary level. Am J Physiol Heart Circ Physiol 264: H294–H301, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res 81: 412–419, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz aL, Koretsky AP, Balaban RS. Respiratory control in the glucose perfused heart. A 31P NMR and NADH fluorescence study. FEBS Lett 221: 270–276, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Kay MW, Swift LM, Sangave A, Zderic V. High resolution contrast ultrasound and NADH fluorescence imaging of myocardial perfusion in excised rat hearts. Conf Proc IEEE Eng Med Biol Soc 2008: 969–972, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Kedem J, Breuer J, Acad BA, Sonn J. Coronary vasodilation produced by tachycardia under various basal flow conditions. Arch Int Physiol Biochim 89: 287–294, 1981 [DOI] [PubMed] [Google Scholar]
- 20.Koretsky AP, Katz LA, Balaban RS. Determination of pyridine nucleotide fluorescence from the perfused heart using an internal standard. Am J Physiol Heart Circ Physiol 253: H856–H862, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Kour J, Ahmed JA, Aarif O, Kour J, Ahmed JA, Aarif O. Impact of heat stress on electrocardiographic changes in New Zealand white rabbits impact of heat stress on electrocardiographic changes in New Zealand white rabbits. 9: 242–252, 2013 [Google Scholar]
- 22.Langendorff O. [Investigations on the surviving mammalian heart.] Arch Gesante Physiol 61: 291–332, 1895 [Google Scholar]
- 23.Masuyama T, Uematsu M, Doi Y, Yamamoto K, Mano T, Naito J, Kondo H, Nagano R, Hori M, Kamada T. Abnormal coronary flow dynamics at rest and during tachycardia associated with impaired left ventricular relaxation in humans: implication for tachycardia-induced myocardial ischemia. J Am Coll Cardiol 24: 1625–1632, 1994 [DOI] [PubMed] [Google Scholar]
- 24.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev 70: 391–425, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Opie LH. Metabolism of the heart in health and disease. Am Heart J 76: 685–698, 1968 [DOI] [PubMed] [Google Scholar]
- 26.Rothstein EC, Carroll S, Combs CA, Jobsis PD, Balaban RS. Skeletal muscle NAD(P)H two-photon fluorescence microscopy in vivo: topology and optical inner filters. Biophys J 88: 2165–2176, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schramm M, Klieber HG, Daut J. The energy expenditure of actomyosin-ATPase, Ca2+-ATPase and Na+,K+-ATPase in guinea-pig cardiac ventricular muscle. J Physiol 481.3: 647–662, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swift LM, Asfour H, Posnack NG, Arutunyan A, Kay MW, Sarvazyan N. Properties of blebbistatin for cardiac optical mapping and other imaging applications. Pflügers Arch Eur J Physiol 464: 503–512, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tune JD. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol 97: 404–415, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Wégria R, Frank CW, Wang H. The Effect of Atrial and Ventricular Tachycardia on Cardiac Output, Coronary Blood Flow and Mean Arterial Pressure. Circ Res 6: 624–632, 1958 [DOI] [PubMed] [Google Scholar]