Abstract
Diagnosis of myocardial infarction (MI) is based on ST-segment elevation on electrocardiographic evaluation and/or elevated plasma cardiac troponin (cTn) levels. However, troponins lack the sensitivity required to detect the onset of MI at its earliest stages. Therefore, to confirm its viability as an ultra-early biomarker of MI, this study investigates the release kinetics of cardiac myosin binding protein-C (cMyBP-C) in a porcine model of MI and in two human cohorts. Release kinetics of cMyBP-C were determined in a porcine model of MI (n = 6, pigs, either sex) by measuring plasma cMyBP-C level serially from 30 min to 14 days after coronary occlusion, with use of a custom-made immunoassay. cMyBP-C plasma levels were increased from baseline (76 ± 68 ng/l) at 3 h (767 ± 211 ng/l) and peaked at 6 h (2,418 ± 780 ng/l) after coronary ligation. Plasma cTnI, cTnT, and myosin light chain-3 levels were all increased 6 h after ligation. In a cohort of patients (n = 12) with hypertrophic obstructive cardiomyopathy undergoing transcoronary ablation of septal hypertrophy, cMyBP-C was significantly increased from baseline (49 ± 23 ng/l) in a time-dependent manner, peaking at 4 h (560 ± 273 ng/l). In a cohort of patients with non-ST segment elevation MI (n = 176) from the SYNERGY trial, cMyBP-C serum levels were significantly higher (7,615 ± 4,514 ng/l) than those in a control cohort (416 ± 104 ng/l; n = 153). cMyBP-C is released in the blood rapidly after cardiac damage and therefore has the potential to positively mark the onset of MI.
Keywords: biomarker, acute coronary syndrome, myocardial infarction, MYBPC3, cMyBP-C, cardiac troponin I
each year, ∼915,000 americans will suffer from acute coronary syndrome (ACS) and myocardial infarctions (MI) (11). Central in the pathogenesis of ACS is a reduction in coronary flow, resulting in ischemia of the supplied myocardium with/without subsequent infarction. Annually, about 2.5 million people are hospitalized for ACS in the United States. Many of the hospitalized patients are admitted at a very high level of hospital care. Despite the advent of modern treatment options, mortality and morbidity levels post-MI are still high. The current diagnostic approach to ACS is based on initial ECG analysis. Significant ST-segment elevation has a high positive predictive value for MI (30), but it has poor sensitivity. In many patients with ACS, particularly in those with non-ST-segment elevation MI (NSTEMI), ECG findings are inconclusive and diagnosis must rely primarily on the use of circulating biomarkers (18). The gold standard biomarkers for the detection of infarction are cardiac troponin I (cTnI) and T (cTnT), which are released from the damaged myocardium (29). Despite careful history-taking and ECG interpretation, a fair proportion of MIs currently cannot be timely diagnosed (3, 17, 22, 28). Early detection of MI might reduce the high mortality by earlier medical treatment and coronary intervention as a result of timely identification of patients with myocardium at risk. Currently, the presence of NSTEMI is determined by measuring the amount of plasma proteins released from necrotic cardiomyocytes, but the earliest biomarkers now available can only detect such plasma proteins anywhere from 4 to 12 h after MI (23). Hence, it is important to find biomarkers able to rule out ACS, as well as detect myocardial ischemia, before irreversible MI occurs (24). Moreover, although ultrasensitive cTnl assays are available, their positive predictive value still has to be established (1). Furthermore, cardiac troponin plasma levels can be increased from causes other than MI, such as myocarditis or renal failure (15). Delays in diagnosis are not inconsequential, since early intervention can reduce mortality and morbidity in patients with ACS (15). Thus there is a need for additional biomarkers enabling more accurate and timely diagnosis.
Cardiac myosin binding protein-C (cMyBP-C) is a cardiac-specific sarcomere protein involved in regulating cardiac structure and function (6). We have recently found that plasma cMyBP-C levels were elevated in a rat model and a small group of patients with MI (12, 13), suggesting that plasma cMyBP-C level is a potential biomarker of MI (26). Building from our previous data, we assessed the future potential of cMyBP-C as an early-stage biomarker. We determined the release kinetics following coronary artery ligation of cMyBP-C compared with the existing biomarkers cTnI, cTnT, and myosin light chain-3 (MYL3) in a porcine model of MI. We also determined the time-dependent release of cMyBP-C in patients undergoing transcoronary ablation of septal hypertrophy (TASH), which is a procedure to relieve left ventricular outflow obstruction in hypertrophic cardiomyopathy (HCM) patients. Additionaly the serum levels of cMyBP-C were determined in NSTEMI patient cohort. Data from these studies determined the release kinetics of cMyBP-C during MI as a potential early indicator of myocardial injury. Our findings are anticipated to open up new avenues of diagnostic investigation.
METHODS
Animal utilization protocols complied with recommendations of the Canadian Council of Animal Care, were approved by the Institutional Animal Care Committee, and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. The Institutional Review Board at Loyola University Chicago approved the protocol for the use of de-identified human serum and plasma samples previously used for research studies.
Porcine MI Model
Animal preparation.
Adult (25–45 kg) Yucatan miniature swine of either sex were issued from an inbred colony maintained by the Animal Resources of Memorial University of Newfoundland, Canada. Animal utilization protocols complied with recommendations of the Canadian Council of Animal Care. The full protocol was approved by the Institutional Animal Care Committee of Memorial University and was monitored by the university's veterinary services. The animals were anesthetized by intramuscular injection of ketamine (Ketalar, 22 mg/kg) and xylazine hydrochloride (2 mg/kg), intubated, and maintained under deep anesthesia with isoflurane (2–2.5%). An ear catheter was installed for drug administration during the surgery and maintained for postoperative medication. Aseptic condition of the left side of the thorax was obtained by treatment with providone-iodine (PVP-I) 75% (Proviodine). The body temperature was maintained with a homeothermic blanket, and rectal body temperature was monitored throughout the surgery. Cardiac function was monitored electrocardiographically with bipolar and unipolar leads. Needle electrodes were inserted subcutaneously into the limbs according to Einthoven's triangle. Signals were continuously recorded on precordial leads on a Gould ECG Signal Conditioner (Model 8188-2202-00).
Open-chest surgery and coronary ligation.
The entire surgical procedure was implemented under strict aseptic conditions. The region of incision was further anesthetized by a series of local intramuscular injections of lidocaine in the intercostal space. A 20-cm posterolateral incision through skin and fat layer was made along the underlying rib down to subcutaneous tissue and superficial fascia. The chest cavity was opened with a rib spreader to expose the lungs and heart. The pericardium was incised linearly in caudal direction. The left anterior descending (LAD) coronary artery was identified, and vascular branches were selected for coronary ligature so that sizable infarct was created without complete akinesia of the ventricle. A silk suture was placed around the artery with a curved needle, and the ligature was smoothly tightened on the artery. The heart was repositioned into the chest cavity. The intercostal space was closed with a series of pericostal sutures. Negative pressure was created in the rib cage before complete closure.
Blood sampling.
A catheter was placed in the left jugular vein and maintained for 14 days postsurgery for blood sampling. The preparation of the surgical area for jugular catheterization was identical to that described above for chest opening. The left jugular vein was located via blunt dissection through the left ventral tissues of the neck. The proximal and distal parts of the vein were lifted carefully with silk knots to maintain permanent blood flow and prevent collapse of the vessel. The vein was cannulated with a 14-gauge catheter, and 10 cm of a microbore tube was introduced into the vein. The jugular catheter was directed to the neck region and positioned dorsally through a tunnel made under the skin with a trocar. A plastic cannula and IV adapter were added to the catheter and filled with heparin to avoid clotting and obstruction.
Validation of MI in Swine
The presence of a typical MI was validated through two different approaches: electrocardiography and morphometric analysis.
Electrocardiography.
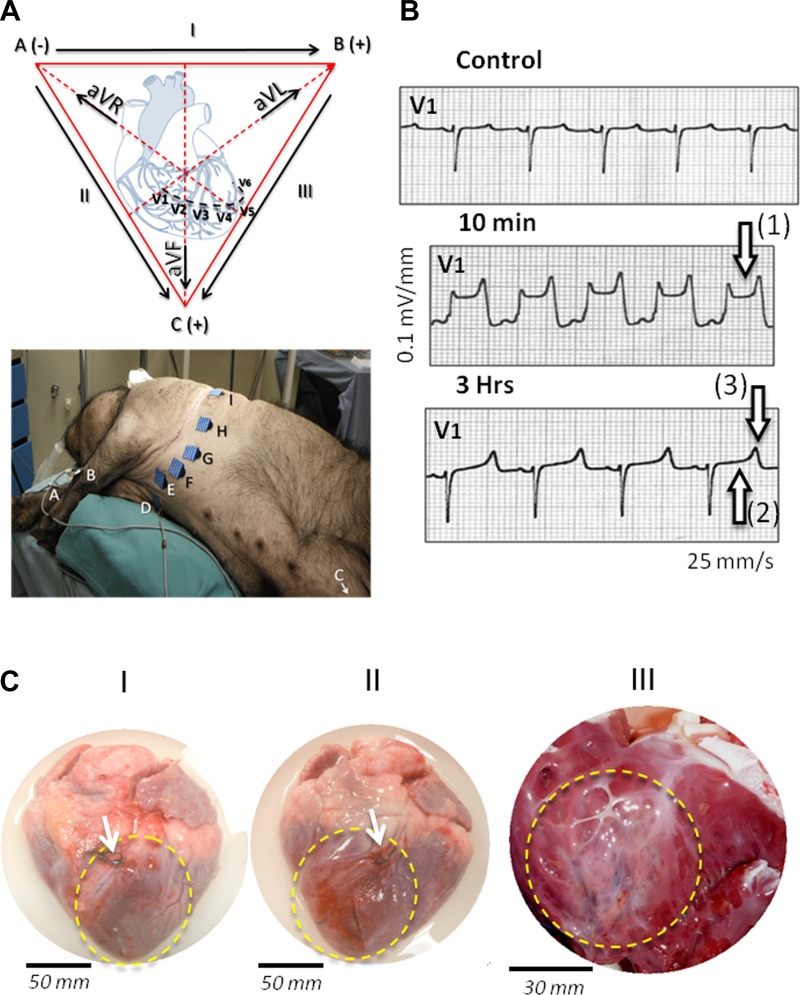
After MI surgery, cardiac electrical activity and heart rate were monitored by standard 12-lead ECG. Six chest (precordial; V1–6) and six limb leads were recorded from nine electrodes positioned on the animal as indicated in Fig. 2A. Under our experimental conditions, the most reproducible signs of MI were a significant elevation (cut-off, 0.15 mV) of the ST segment and presence of peaked T wave on two consecutive precordial leads. Under our measurement conditions in the swine, these alterations were visible most systematically on V1 and monitored up to 3 h after ligation. The location of the infarct was finalized by analysis of leads DI, DII, DIII (bipolar), and AVR, AVL, AVF (unipolar). This in vivo pre-analysis of the MI was confirmed visually postmortem as indicated below.
Fig. 2.
Development of myocardial infarction (MI) after left anterior descending (LAD) occlusion in Yucatan miniature swine. A: cardiac electrical activity was monitored by standard 12-leads ECG during the first hours following the coronary ligation. Bottom: positions of the electrodes on the animal; to facilitate the identification of the leads, letters on the electrodes refer to the Einthoven's triangle represented at top. B: under our experimental conditions, in swine (n = 6), evident signs of severe ischemia were detected on precordial leads (V1-V6, see A). The representative example of tracing on lead V1 is shown here before (control), 10 min, and 3 h after complete LAD coronary ligation; 10 min after ligation, significant ST elevation (≥0.1 mV) indicates ongoing infarction [see arrow (1)], whereas 3 h after ligation pseudonormalization of ST segment [arrow (2)] with peaked T wave [arrow (3)] and prolonged QT interval confirms an established myocardial injury. C: this first electrocardiographic estimate was confirmed later (here 72 h after coronary ligation) by visual inspection of the heart on 4 separate animals; these animals went through the same surgical procedure but were euthanized early in the 14-days blood sampling protocol. As shown in representative cardiac morphological changes (C, I and II), necrotic area (dashed yellow line) was readily visible downstream LAD coronary ligation (see white arrows). The infarction affected 20% to 30% of the left ventricular free wall, extending from the permanently occluded coronary segment down to the apex. As shown in C (III), the interior examination of the ventricular chamber confirmed the presence of large transmural myocardial injury in the left anterior wall.
Morphometry of the infarct.
After 14 days, the heart was extracted from the animal under deep anesthesia and placed in cold (5–10°C) saline solution (Tyrode) to which 4 ml heparin was added. Atria and vessels were trimmed off. The infarction was identified in the free wall of the ventricles and septum by a clearly delimited necrotic region (see Fig. 2C). The MI region was carefully isolated. The volume of fresh necrotic tissue was evaluated from the surface area and thickness of the infarct. An estimate of the extent of MI was given by the volume and mass of the infarct relative to total volume and mass of the ventricles (including septum).
Transcoronary Ablation of Septal Hypertrophy
Serum samples from HCM patients (n = 12) with left ventricular outflow tract obstruction undergoing TASH were taken at various time points after ethanol infusion in Bad Nauheim, Germany, as described before (20). The Institutional Review Board at Loyola University Chicago approved the protocol for the use of de-identified human samples previously used for research studies, and all studies were in accordance with the declaration of Helsinki.
Blood Sampling from MI Patients and Control Group
Blood samples from NSTEMI patients were used and the control group consisted of samples without known cardiovascular disease, which were banked blood samples used in previous studies. Cases with NSTEMI (n = 176) were from those patients who participated in the SYNERGY library study (1). The SYNERGY study compared nonfractionated heparin versus enoxaparin in high-risk NSTEMI patients. Inclusion criteria patients included those showing ischemic symptoms, along with at least two other high-risk factors (age >60, + troponin, + CKMB, ECG changes). The parent SYNERGY study and a subset of the SYNERGY library studies have been published (9, 28a), and the TexGen study populations were included as controls (n = 153) (7).
cMyBP-C Immunoassay
cMyBP-C level in serum and plasma samples from MI pigs and patients with HCM and NSTEMI was measured using a custom immunoassay based on electrochemiluminescence (Meso Scale Discovery, Rockville, MD) (19). The anti-cMyBP-C antibodies used in this study are specific to cMyBP-C and were generated against the C0 domain of cMyBP-C, which is exclusively present in the cardiac isoform and does not cross-react with skeletal MyBP-C isoforms (21). The assay is comparable with the ELISA described previously (12, 13) and uses the same capture and detection antibodies. This immunoassay has improved sensitivity, compared with ELISA. Capture antibody (5 μg/ml monoclonal cMyBP-C, raised against amino acids 1–120, E-7; Santa Cruz Biotechnologies) was coated onto a multi-array 96-well plate (Meso Scale Discovery) overnight at 4°C. Nonspecific binding to the plate was blocked by incubating the plate with 150 μl 5% (wt/vol) BSA/PBS for 1 h at room temperature while shaking. The plate was washed three times with 150 μl 0.05% (vol/vol) Tween-20/PBS. Standard series was prepared by diluting recombinant cMyBP-C (13) (amino acids 1–271 of mouse, human, or pig sequence of cMyBP-C domains C0 to C1) in 1% BSA/PBS at a starting concentration of 200,000 ng/l and serially diluted by a factor 5. Samples were used either undiluted or diluted 1:1 with 1% BSA/PBS. Samples and standards were incubated for 1 h at room temperature while shaking. After wash, the plate was incubated with detection antibody [custom antibody with epitope amino acids 2–14 of cMyBP-C (13), concentration 1 μg/ml] for 1 h at room temperature. The detection antibody was labeled with MSD SULFO-TAG, enabling chemiluminescent detection. After wash, read-buffer (Meso Scale Discovery) was added to each well, and the plate was read on a Sector Imager 2400 (Meso Scale Discovery). An electrical signal to the bottom of the plate causes a local production of light (in conjunction with the read-buffer) that is directly proportional to the amount of SULFO-TAG present and, thus, the amount of bound detection antibody.
cTnI, cTnT, and MYL3 Multiplex Immunoassay
cTnI, cTnT, and essential myosin light chain-3 (MYL3) levels in pig MI serum samples were determined using a multiplex assay (K15161C; Meso Scale Discovery). These 96-well plates are precoated with capture antibodies against cTnI, cTnT, and MYL3. The assay was performed according to the manufacturer's instructions. Briefly, a standard series was made by serially diluting cTnI (23,500 ng/l), cTnT (47,400 ng/l), and MYL3 (51,300 ng/l) by dilution factor 4 in diluent 7 (Meso Scale Discovery) + 30 mM EDTA + 0.25 mM DTT. Pig MI serum samples were four-time diluted (1:4) in diluent 7/EDTA/DTT. Standards and samples were added to the plate and incubated for 2 h at room temperature while shaking. The plate was washed three times with 150 μl 0.05% (vol/vol) Tween-20/PBS. A mixture of SULFO-TAG-labeled cTnI, cTnT, and MYL3 detection antibodies was added to each well, and the plate was incubated for 2 h at room temperature with shaking. After wash, read-buffer (Meso Scale Discovery) was added to each well, and the plate was read on a Sector Imager 2400 (Meso Scale Discovery). In hypertrophic obstructive cardiomyopathy patient samples, both cTnT and creatine kinase muscle brain (CK-MB) were measured using the highly sensitive electrochemiluminescence immunoassay (hs-cTnT assay, Elecsys Analyzer 2010; Roche Diagnostics) and the chemiluminescent microparticle immunoassay (ARCHITECT_STAT CK-MB; Abbott Laboratories), respectively, as described previously (20).
Standard Curve for the Immunoassays
A luminescent signal from the standard series was used to construct a standard curve (4 parameter curve fit), using the following equation: signal = D + (A − D)/(1 + (Concentration/C)B), where A = minimum, B = Hill slope, C = EC50, and D = maximum. The same formula was used for all standard curves used. Lower limit of detection (LLOD; the lowest amount of signal that can be measured above a blank value) is defined as the calculated concentration of blank + three times the SD of the blank value. Lower limit of quantification (LLOQ) is defined as the lowest measured concentration that is ≥ LLOD and has recovery [defined as 100% * (calculated concentration/actual concentration)] between 80% and 120% and a coefficient of variance <20%. Upper limit of quantification (ULOQ) is defined as the highest measured concentration that has recovery between 80% and 120% and a coefficient of variance <20%.
Statistics
Data are presented as means ± SE unless indicated otherwise. Data were considered significantly different at P < 0.05. Data analysis was performed by GraphPad Prism version 6 (GraphPad Software, San Diego, CA). For the pig MI and TASH time point study, difference in biomarker serum concentration levels was measured by ANOVA, followed by Dunnett's multiple comparison post hoc test, comparing all time points to baseline. Because not all MI-induced animals had a complete set of time point samples, paired measurements over the whole time course could not be performed. For the first three time points, all samples were collected; thus, time points at 30 min and 3 h were compared with the 0 time point using repeated-measures ANOVA, followed by Bonferroni post hoc test. Difference in serum levels of cMyBP-C in NSTEMI patients and controls was tested by a Mann-Whitney nonparametric test. Correlation between cMyBP-C and cTnI levels in NSTEMI patients was determined using nonparametric Spearman's rank correlation.
RESULTS
Biomarker Immunoassays
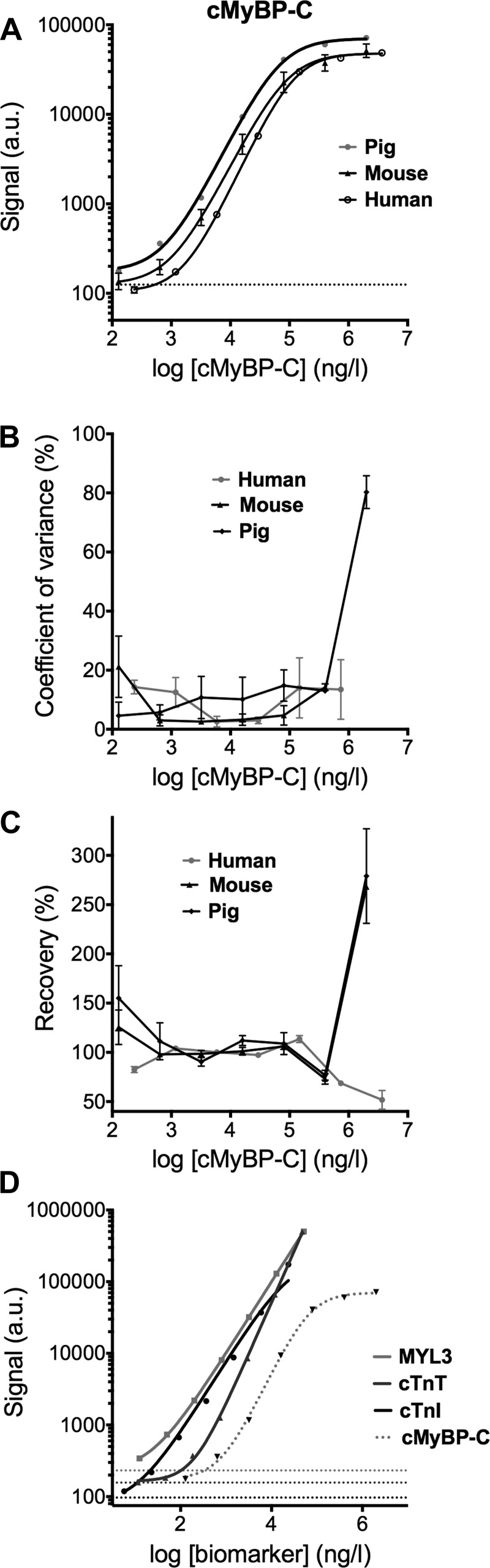
Electrochemiluminescence immunoassays were performed to determine levels of cMyBP-C, cTnI, cTnT, and MYL3 in serum samples. cMyBP-C immunoassay (19) has a detection range from 116 to 2,000,000 ng/l. Quantification of the cMyBP-C recombinant standards showed a recovery between 80% and 120% and coefficient of variance <20% for the concentration range of 640 to 400,000 ng/l (Fig. 1). Sensitivity (Fig. 1A), variability (Fig. 1B), and recovery (Fig. 1C) for mouse, pig, and human cMyBP-C were all similar, indicating that the assay detects cMyBP-C of different species with the same sensitivity and performance. cTnI, cTnT, and MLC1v were measured simultaneously using a multiplex immunoassay and could be quantified with high sensitivity (Fig. 1D and Table 1).
Fig. 1.
Characterization of immunoassays. A: sensitivity of an immunoassay originally optimized using mouse cardiac myosin binding protein-C (cMyBP-C) standards was tested using porcine and human standard curves of 40-kDa recombinant protein. Chemiluminescent signal was plotted against log cMyBP-C concentration. Dashed lines indicate the lowest signal detectable above blank. B: coefficient of variance and recovery of the assay (C) was plotted against the log cMyBP-C concentration. D: cardiac troponin I (cTnI), cardiac troponin T (cTnT), and MYL3 detection was performed in multiplex immunoassay with high sensitivity. Dashed lines indicate the lowest signal detectable above blank for each assay. Data are presented as means ± SE. Au, arbitrary units.
Table 1.
Upper and lower limits of detection/quantification for biomarker immunoassays
| Lower Limit of Detection, ng/l | Lower Limit of Quantification, ng/l | Upper Limit of Quantification, ng/l | |
|---|---|---|---|
| cMyBP-C | 116.0 | 640.0 | 400,000 |
| Cardiac troponin I | 5.0 | 6 | 23,500 |
| Cardiac troponin T | 5.4 | 741 | 47,400 |
| Myosin light chain-3 | 4.0 | 13 | 51,300 |
cMyBP-C, cardiac myosin binding protein-C.
Induction of MI in Pig and Time Course of cMyBP-C Release
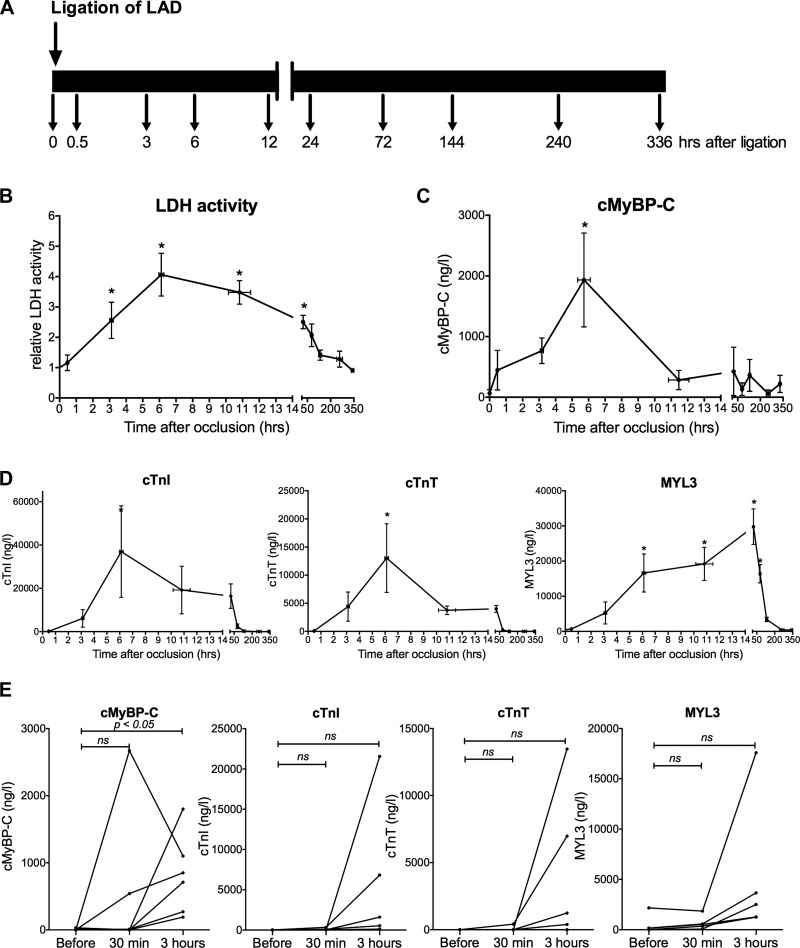
To determine the release of cMyBP-C following coronary artery occlusion, acute MI was induced by permanent ligation of a branch of the left anterior descending coronary artery using a silk suture in six Yucatan pigs. Typical ST-segment elevation appeared on precordial leads, indicating the presence of ischemic injury in the left anterior wall within minutes after ligation (Fig. 2A). Peaked T waves and pseudo-normalization were observed after 3 h, confirming the presence of an established MI (Fig. 2B). Large transmural infarcts, representing 12.0 ± 1.5% of total ventricular mass, were anatomically confirmed postmortem at 72 h after coronary occlusion (Fig. 2C). Venous blood samples were taken at predetermined time points (Fig. 3A) to measure lactate dehydrogenase (Fig. 3B), cMyBP-C (Fig. 3C), cTnI, cTnT, and MYL3 levels (Fig. 3D). Release of the nonspecific cell-damage marker lactate dehydrogenase confirmed the presence of cell injury (Fig. 3B). cMyBP-C was detectable after 30 min, peaked at 6 h, and returned to baseline at 12 h. However, cTnI and cTnT appeared after 3 h, and MYL3 serum levels did not peak until 72 h after LAD ligation (Fig. 3C). Repeated-measures ANOVA followed by a Bonferroni post hoc test showed that cMyBP-C was significantly increased after 3 h (P = 0.02), whereas the other biomarkers were not significantly elevated at this time point (Fig. 3E).
Fig. 3.
Biomarker release in porcine post-MI model. A: time course of venous blood sampling following LAD ligation in swine. B: circulating lactate dehydrogenase (LDH) activity in pigs after LAD ligation (n = 5) normalized to baseline, confirming cell damage. *P < 0.05 vs. t = 0, ANOVA followed by Dunnett's multiple comparison post hoc test. C: cMyBP-C levels measured by immunoassay (n = 6). D: cTnI, cTnT, and MYL3 serum concentrations measured in a multiplex immunoassay in swine (n = 5). *P < 0.05 vs. t = 0, ANOVA followed by Dunnett's multiple comparison post hoc test. E: from all animals, paired measurements for the first 3 time points were possible. cMyBP-C significantly increased after 3 h, whereas the increase in cTnI, cTnT, and MYL3 was not significant. Data are presented as means ± SE.
Release Kinetics of cMyBP-C in Hypertrophic Obstructive Cardiomyopathy Patients Undergoing TASH
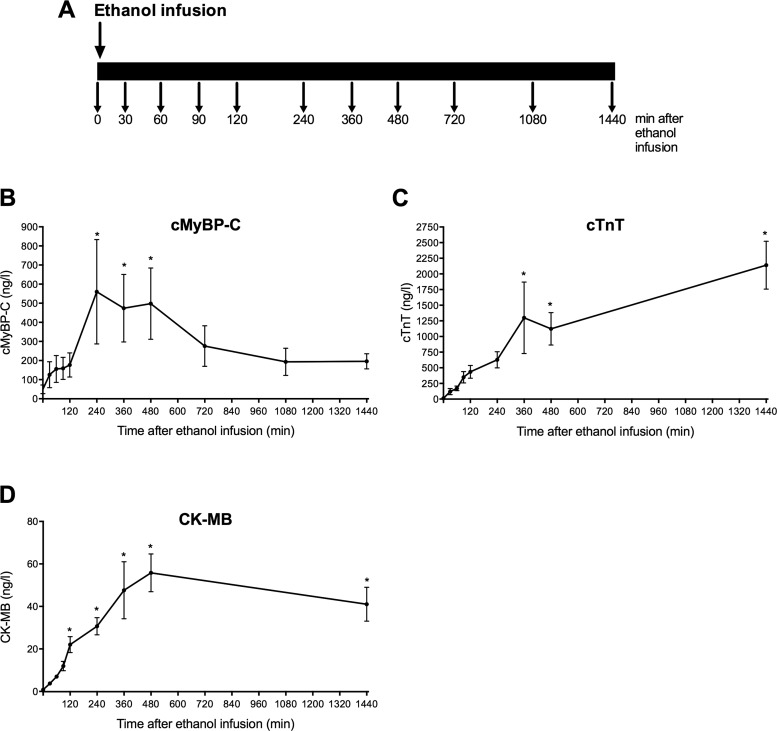
To validate the above findings in humans, sequential release of cMyBP-C, cTnT, and CK-MB was determined in a group of HCM patients who were treated for left ventricular outflow obstruction with TASH (n = 12). Blood sampling was performed at 10 different time points beginning at the start of procedure and ending 1,440 min after coronary ethanol infusion (Fig. 4A), and cMyBP-C was measured in serum, as well as plasma (Fig. 4B). Recovery of cMyBP-C was comparable between serum and plasma (Table 2). cMyBP-C was increased at 2 h after TASH, peaked at 4 h, and remained elevated until the last time point (24 h) (Fig. 4B), indicating cMyBP-C time-dependent release following cardiac necrosis in humans. We further measured cTnT release, which showed a slightly different pattern. In this case, cTnT serum levels also increased early and were significantly increased after 6 h, but kept on increasing until the final time point (Fig. 4C). In contrast, CK-MB showed significant early release within 2 h and remained elevated up to 24 h (Fig. 4D).
Fig. 4.
cMyBP-C release kinetics after myocardial injury in hypertrophic cardiomyopathy (HCM) patients undergoing transcoronary ablation of septal hypertrophy (TASH). A: sequential blood samples were taken from HCM patients (n = 12) with left ventricular outflow obstruction undergoing TASH. B: cMyBP-C levels measured by immunoassay. Peak value of cMyBP-C plasma levels reached significance after 240 min and remained high until the last time point. *P < 0.05 vs. t = 0. C: cTnT levels measured in the same patients with the high-sensitivity cardiac troponin T assay. *P < 0.05 vs. t = 0. D: levels of creatine kinase muscle brain (CK-MB) in the same patients. *P < 0.05 vs. t = 0.
Table 2.
Percentage of cMyBP-C recovery in plasma and serum
| Plasma | Serum | |
|---|---|---|
| Percentage of cMyBP-C recovery | 108.1 ± 9.7 | 106.8 ± 11.1 |
Values are means ± SE and represent an average of 4 experiments; n = 4 for plasma and serum. cMyBP-C was spiked into cMyBP-C-negative serum at 200,000 ng/l and 1,600 ng/l, and recovery was calculated as 100 *(calculated concentration/measured concentration).
cMyBP-C Levels in NSTEMI Patients
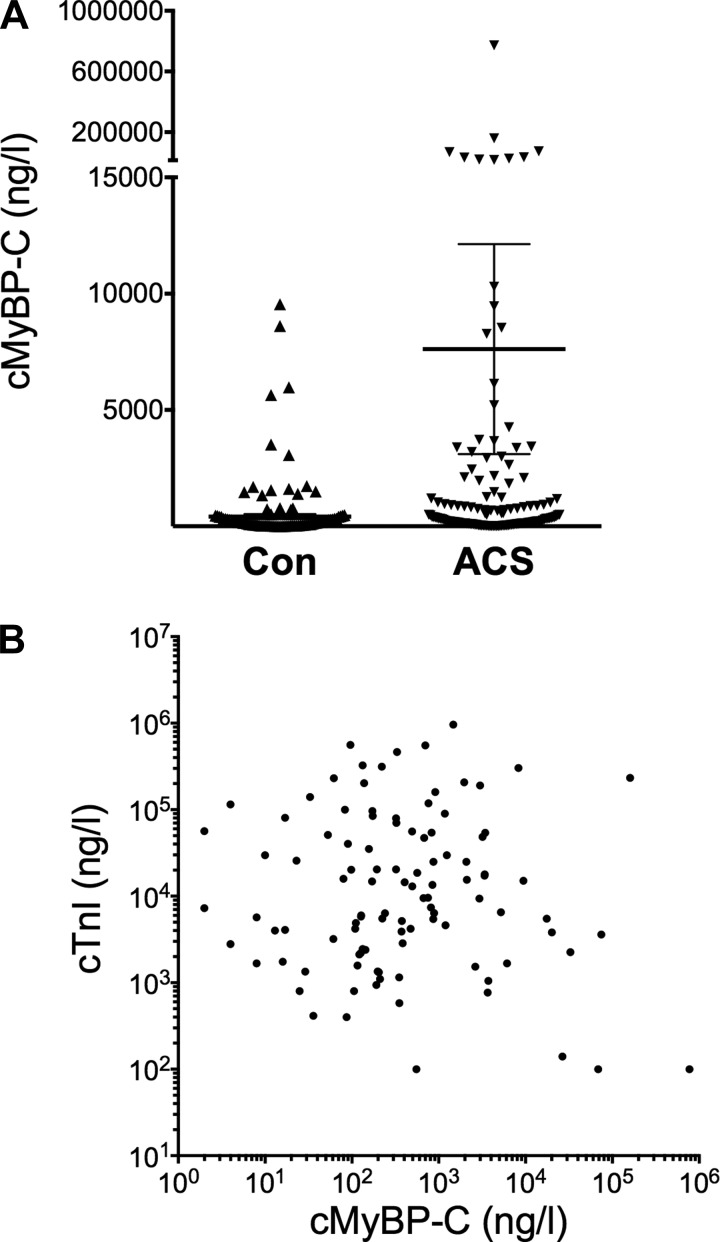
To determine potential utility of cMyBP-C levels in the diagnosis of NSTEMI, cMyBP-C levels were measured in the SYNERGY Library study subpopulation (n = 176) (28a) and 153 individuals without known cardiovascular diseases from the TEXGen study (7). Blood samples were collected during hospital admission, but the time of the onset of symptoms was not documented. However, despite late sample collection in some patients, the mean cMyBP-C level was higher in patients with NSTEMI compared with the control group (Fig. 5A). In a subset of study patients (n = 134), cTnI levels were measured (mean, 54,739 ± 10,846 ng/l). Interestingly, no significant correlation between cMyBP-C plasma levels and cTnI (Spearman, r = 0.014; P = 0.096) was observed (Fig. 5B).
Fig. 5.
Levels of serum cMyBP-C in patients with non-ST-segment elevation MI (NSTEMI). A: cMyBP-C measured in NSTEMI patients (n = 176) was increased compared with a control population (Con; n = 153; P < 0.001). B: no significant correlation between cMyBP-C and cTnI plasma levels in the NSTEMI patient population was noted (Spearman r = 0.014; P = 0.096). Data are presented as means ± SE. ACS, acute coronary syndrome.
DISCUSSION
The present study aims to determine the release kinetics of cMyBP-C post-myocardial injury. The findings in three models of MI, namely a porcine model of permanent LAD branch ligation, TASH in patients with HCM with left ventricular outflow obstruction, and NSTEMI patients confirm that cMyBP-C is released very early after cardiac injury. When compared with other cardiac proteins, cMyBP-C level was significantly elevated within 3 h after ligation of a branch of the LAD, peaked after 6 h, and returned to the baseline values at 12 h. In contrast, levels of cTnI, cTnT, and MLC1v were not significantly different within the first 3 h and were increased at later time points. Similarly, plasma cMyBP-C level was significantly elevated within 4 h after TASH, whereas cTnT levels in the same samples were significantly increased only after 6 h. These findings suggest that cMyBP-C has potential as an ultra-early biomarker for the diagnosis of MI, but this still needs to be validated using a large cohort study. Guidelines from the American Heart Association and the American College of Cardiology state that cardiac troponins (cTn) should be used as biomarkers for the diagnosis of acute MI (1, 2). When cytosolic proteins, such as creatine kinase and myoglobin, are compared with cardiac sarcomeric proteins, the presence of cTnI and cTnT in the circulatory system is nearly always cardiac specific (96%) (5). However, cardiac troponin plasma levels can also be increased in non-MI pathologies, such as myocarditis and renal failure (15). Whether cMyBP-C is also released under these circumstances needs to be established. It is likely that disease states that cause damage to cardiomyocytes will also lead to the release of cMyBP-C. Cardiac Tnl, as a marker of myocardial necrosis, is only detectable in the blood 6 to 12 h after the onset of ischemia (2, 16). This delayed rise in cTnl precludes earlier treatment of NSTEMI patients. In contrast, our pilot studies in this report indicated the presence of cMyBP-C in the plasma within 30 min of ischemia, suggesting that cMyBP-C could be an earlier sign of myocardial necrosis than the current gold standards cTnl and cTnT. Addition of cMyBP-C in a multimarker regime with cardiac troponins might lead to an earlier diagnosis in patients who present at the emergency department shortly after coronary artery blockade. However, a systematic prospective investigation is required to establish such data for clinical use.
Based on the data from the porcine MI model and the TASH study, cMyBP-C seems to have earlier release and clearance kinetics in the blood as compared with cardiac troponins. Because cMyBP-C, cTnI, and cTnT are all myofilament proteins, we might ask why cMyBP-C would be released earlier [16] than the cardiac troponins. This could be explained by the known susceptibility of cMyBP-C to proteolysis following cardiac stress (8, 27), leading to the production of a 40 kDa NH2-terminal fragment (27). Because this fragment is no longer attached to the myofilament, it would be more easily released from cardiomyocytes into the bloodstream (13), causing toxicity to the cardiomyocytes (14) and, ultimately, heart failure (25). Skeletal muscle injury causing false positives is improbable, since our immunoassay uses capture and detection antibodies that both recognize the cardiac isoform-specific C0 domain, which is not present in either skeletal isoform. Therefore, cross-reactivity with skeletal MyBP-C isoforms is highly unlikely. We have recently shown that cMyBP-C is not detectable in either fast- or slow-twitch skeletal muscle fibers and that the detection antibody does not recognize fast and slow MyBP-C isoforms (21).
In addition to its more rapid release, cMyBP-C was cleared from the blood within 12 h after MI. In contrast, clearance of cardiac troponins was slower, as levels returned to baseline between 72 and 132 h. Cardiac troponins typically have a delayed release pattern, peaking around 10 h (10) from the onset of ACS symptoms. In contrast, cMyBP-C is easily degradable upon ischemia and is released earlier following MI (13). HCM patients undergoing TASH have been proposed as a model for precise determination of release kinetics following infarction. A recent study showed a significant elevation in cTnT 60 min after TASH (20). In the present study, we saw a time-dependent increase of cMyBP-C, but this failed to reach statistical significance until 4 h after ethanol infusion. Measuring cTnT release in the same set of samples revealed that cTnT levels increased early and that this reached statistical significance at 6 h. In contrast with cMyBP-C, release did not peak early on, but rather kept rising until the last time point (1,440 min) measured. Contrary to instantaneous necrosis following infusion of ethanol during TASH, cell death reported in MI occurs over the course of hours. It is conceivable that ischemia results in quick release of cMyBP-C via calpain-mediated cleavage, whereas necrosis does not (13). Another possible explanation is that the infarction created with this procedure is relatively small compared with the porcine MI model. This could explain the lower amount of cMyBP-C that is released after TASH compared with that released after MI.
NSTEMI is currently determined by measuring released proteins from necrotic cardiomyocytes, but such detection is only apparent from 4 to 12 h post-MI. In ACS patients without STEMI, cardiac biomarkers are a vital part of diagnosis and help to distinguish between NSTEMI and unstable angina. We used samples from a NSTEMI patient subpopulation of the SYNERGY library study (28a) to measure the levels of cMyBP-C. When compared with control samples, cMyBP-C levels were higher in the NSTEMI group. Unfortunately, the time from onset of ACS symptoms to venipuncture for blood collection was not documented in the SYNERGY library subpopulation. Therefore, the performance characteristics of cMyBP-C levels as a time-dependent biomarker for early detection of NSTEMI could not be determined in this study cohort. In a fraction of NSTEMI patients, plasma cMyBP-C was not increased compared with that of the control group. This might be explained by delayed blood collection in these patients. As seen in both the porcine MI models and the TASH samples, circulating cMyBP-C levels decreased more rapidly than circulating troponins. Nevertheless, when mean values were compared, cMyBP-C levels were significantly higher in patients with NSTEMI than in controls, despite considerable variation within each group. In our porcine MI model, cMyBP-C levels increased and returned to baseline quickly and returned to baseline. This result, coupled with the lack of correlation between cTnI and cMyBP-C levels, agrees with the notion that 1) these biomarkers have different mechanisms of release and clearance and 2) under these circumstances, cMyBP-C levels might be an important complementary biomarker for the diagnosis of NSTEMI.
Limitations of the Study
This article seeks to determine the release kinetics of cMyBP-C following cardiac injury in three study populations (MI pig models, TASH, and NSTEMI patients). Although the porcine MI model suggests that cMyBP-C has faster release in comparison with cTnI and cTnT, the levels are highly variable, suggesting the need for a larger cohort study to confirm these findings. Part of the variation at the later time points might also be explained by differences in clearance form the circulation. Nothing is yet known about the cMyBP-C clearance from the circulation, but it seems to happen quicker than clearance of troponins or MYL3. In the porcine MI model and human TASH samples, this variation in serum levels was also seen in the other markers, implying variation in the level of tissue damage following MI in porcine and ethanol infusion in TASH study. A small number of samples from control patients free from overt cardiovascular disease also showed high cMyBP-C levels (Fig. 3B), possibly because of undetected heart disease. A recent study shows that cMyBP-C degradation can also be detected in end-stage failing hearts from nonischemic cardiomyopathy (25), indicating that other forms of cardiac stress could lead to cMyBP-C degradation and release.
These data provide a proof-of-concept to support the central hypothesis that cMyBP-C is an early biomarker of MI and beckon the need for large-scale prospective clinical studies to test the potential utility of addition of cMyBP-C to a multimarker regime with cardiac troponin will increase the early diagnostic and prognostic biomarker in patients with ACS.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01HL-105826 and K02HL-114749 (to Dr. Sadayappan), Canadian Institutes of Health Research Grant 81150 (to Dr. Stuyvers), and American Heart Association Midwest Fellowship 13POST17220009 (to Dr. Kuster).
DISCLOSURES
A full patent application is pending (Application Serial No. 13/464,466, Pub. No. US 2012/0282618 A1 and Date: 05/04/12) to determine the risk factors associated with cMyBP-C degradation and release into human body fluid.
AUTHOR CONTRIBUTIONS
Author contributions: D.K., A.C.-O., L.M., C.L., C.T., H.M.N., H.M., C.W.H., K.S.P., K.W.M., B.D.S., A.J.M., and S.S. conception and design of research; D.K., A.C.-O., L.M., C.L., H.M.N., H.M., C.W.H., K.S.P., K.W.M., N.S.K., B.D.S., and S.S. performed experiments; D.K., A.C.-O., L.M., C.L., C.T., H.M.N., H.M., C.W.H., K.S.P., K.W.M., N.S.K., B.D.S., A.J.M., and S.S. analyzed data; D.K., A.C.-O., C.L., C.T., H.M.N., H.M., C.W.H., K.S.P., K.W.M., N.S.K., B.D.S., A.J.M., and S.S. interpreted results of experiments; D.K., A.C.-O., B.D.S., and S.S. prepared figures; D.K., B.D.S., and S.S. drafted manuscript; D.K., B.D.S., A.J.M., and S.S. edited and revised manuscript; D.K., A.C.-O., L.M., C.L., C.T., H.M.N., H.M., C.W.H., K.S.P., N.S.K., B.D.S., A.J.M., and S.S. approved final version of manuscript.
REFERENCES
- 1.Alcalai R, Planer D, Culhaoglu A, Osman A, Pollak A, Lotan C. Acute coronary syndrome vs. nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med 167: 276–281, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr, Writing Group M, ACCF/AHA Task Force M 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 123: e426–e579, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Apple FS, Christenson RH, Valdes R, Jr, Andriak AJ, Berg A, Duh SH, Feng YJ, Jortani SA, Johnson NA, Koplen B, Mascotti K, Wu AH. Simultaneous rapid measurement of whole blood myoglobin, creatine kinase MB, and cardiac troponin I by the triage cardiac panel for detection of myocardial infarction. Clin Chem 45: 199–205, 1999 [PubMed] [Google Scholar]
- 5.Babuin L, Jaffe AS. Troponin: the biomarker of choice for the detection of cardiac injury. CMAJ 173: 1191–1202, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barefield D, Sadayappan S. Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J Mol Cell Cardiol 48: 866–875, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen SN, Ballantyne CM, Gotto AM, Jr, Tan Y, Willerson JT, Marian AJ. A common PCSK9 haplotype, encompassing the E670G coding single nucleotide polymorphism, is a novel genetic marker for plasma low-density lipoprotein cholesterol levels and severity of coronary atherosclerosis. J Am Coll Cardiol 45: 1611–1619, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decker RS, Nakamura S, Decker ML, Sausamuta M, Sinno S, Harris K, Klocke FJ, Kulikovskaya I, Winegrad S. The dynamic role of cardiac myosin binding protein-C during ischemia. J Mol Cell Cardiol 52: 1145–1154, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Ferguson JJ, Califf RM, Antman EM, Cohen M, Grines CL, Goodman S, Kereiakes DJ, Langer A, Mahaffey KW, Nessel CC, Armstrong PW, Avezum A, Aylward P, Becker RC, Biasucci L, Borzak S, Col J, Frey MJ, Fry E, Gulba DC, Guneri S, Gurfinkel E, Harrington R, Hochman JS, Kleiman NS, Leon MB, Lopez-Sendon JL, Pepine CJ, Ruzyllo W, Steinhubl SR, Teirstein PS, Toro-Figueroa L, White H, Investigators ST Enoxaparin vs. unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA 292: 45–54, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Ferraro S, Ardoino I, Boracchi P, Santagostino M, Ciardi L, Antonini G, Braga F, Biganzoli E, Panteghini M, Bongo AS. Inside ST-elevation myocardial infarction by monitoring concentrations of cardiovascular risk biomarkers in blood. Clin Chim Acta 413: 888–893, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 127: e6–e245, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Govindan S, Kuster DW, Lin B, Kahn DJ, Jeske WP, Walenga JM, Leya F, Hoppensteadt D, Fareed J, Sadayappan S. Increase in cardiac myosin binding protein-C plasma levels is a sensitive and cardiac-specific biomarker of myocardial infarction. Am J Cardiovasc Dis 3: 60–70, 2013 [PMC free article] [PubMed] [Google Scholar]
- 13.Govindan S, McElligott A, Muthusamy S, Nair N, Barefield D, Martin JL, Gongora E, Greis KD, Luther PK, Winegrad S, Henderson KK, Sadayappan S. Cardiac myosin binding protein-C is a potential diagnostic biomarker for myocardial infarction. J Mol Cell Cardiol 52: 154–164, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govindan S, Sarkey J, Ji X, Sundaresan NR, Gupta MP, de Tombe PP, Sadayappan S. Pathogenic properties of the N-terminal region of cardiac myosin binding protein-C in vitro. J Muscle Res Cell Motil 33: 17–30, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaffe AS. Chasing troponin: how low can you go if you can see the rise? J Am Coll Cardiol 48: 1763–1764, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Jaffe AS, Apple FS. High-sensitivity cardiac troponin: hype, help, and reality. Clin Chem 56: 342–344, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Jurlander B, Clemmensen P, Wagner GS, Grande P. Very early diagnosis and risk stratification of patients admitted with suspected acute myocardial infarction by the combined evaluation of a single serum value of cardiac troponin-T, myoglobin, and creatine kinase MB(mass). Eur Heart J 21: 382–389, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Kontos MC, Roberts BD, Tatum JL, Roberts CS, Jesse RL, Ornato JP. Mortality based on the presenting electrocardiogram in patients with myocardial infarction in the troponin era. Am J Emerg Med 27: 146–152, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Kuster DW, Barefield D, Govindan S, Sadayappan S. A sensitive and specific quantitation method for determination of serum cardiac myosin binding protein-C by electrochemiluminescence immunoassay. J Vis Exp. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liebetrau C, Mollmann H, Nef H, Szardien S, Rixe J, Troidl C, Willmer M, Hoffmann J, Weber M, Rolf A, Hamm C. Release kinetics of cardiac biomarkers in patients undergoing transcoronary ablation of septal hypertrophy. Clin Chem 58: 1049–1054, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Lin B, Govindan S, Lee K, Zhao P, Han R, Runte KE, Craig R, Palmer BM, Sadayappan S. Cardiac myosin binding protein-C plays no regulatory role in skeletal muscle structure and function. PLoS One 8: e69671, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCord J, Nowak RM, McCullough PA, Foreback C, Borzak S, Tokarski G, Tomlanovich MC, Jacobsen G, Weaver WD. Ninety-minute exclusion of acute myocardial infarction by use of quantitative point-of-care testing of myoglobin and troponin I. Circulation 104: 1483–1488, 2001 [DOI] [PubMed] [Google Scholar]
- 23.McMahon CG, Lamont JV, Curtin E, McConnell RI, Crockard M, Kurth MJ, Crean P, Fitzgerald SP. Diagnostic accuracy of heart-type fatty acid-binding protein for the early diagnosis of acute myocardial infarction. Am J Emerg Med 30: 267–274, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Morrow DA, de Lemos JA, Sabatine MS, Antman EM. The search for a biomarker of cardiac ischemia. Clin Chem 49: 537–539, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Razzaque MA, Gupta M, Osinska H, Gulick J, Blaxall BC, Robbins J. An endogenously produced fragment of cardiac myosin-binding protein C is pathogenic and can lead to heart failure. Circ Res 113: 553–561, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadayappan S. Cardiac myosin binding protein-C: a potential early-stage, cardiac-specific biomarker of ischemia-reperfusion injury. Biomark Med 6: 69–72, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadayappan S, Osinska H, Klevitsky R, Lorenz JN, Sargent M, Molkentin JD, Seidman CE, Seidman JG, Robbins J. Cardiac myosin binding protein-C phosphorylation is cardioprotective. Proc Natl Acad Sci USA 103: 16918–16923, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stork TV, Wu AH, Muller-Bardorff M, Gareis R, Muller R, Hombach V, Katus H, Mockel M. Diagnostic and prognostic role of myoglobin in patients with suspected acute coronary syndrome. North-Wurttemberg Infarction Study (NOWIS) Group. Am J Cardiol 86: 1371–1374, 2000 [DOI] [PubMed] [Google Scholar]
- 28a.SYNERGY Executive Committee. Superior Yield of the New strategy of Enoxaparin, Revascularization and GlYcoprotein IIb/IIIa inhibitors The SYNERGY trial: study design and rationale. Am Heart J 143: 952–960, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BR, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Gabriel Steg P, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Morais J, Aguiar C, Almahmeed W, Arnar DO, Barili F, Bloch KD, Bolger AF, Botker HE, Bozkurt B, Bugiardini R, Cannon C, de Lemos J, Eberli FR, Escobar E, Hlatky M, James S, Kern KB, Moliterno DJ, Mueller C, Neskovic AN, Pieske BM, Schulman SP, Storey RF, Taubert KA, Vranckx P, Wagner DR. Third universal definition of myocardial infarction. Eur Heart J 33: 2551–2567, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Yusuf S, Pearson M, Sterry H, Parish S, Ramsdale D, Rossi P, Sleight P. The entry ECG in the early diagnosis and prognostic stratification of patients with suspected acute myocardial infarction. Eur Heart J 5: 690–696, 1984 [DOI] [PubMed] [Google Scholar]