Abstract
The polyphenolic 1,2,3,4,6-penta-O-galloyl-beta-d-glucose from several medicinal herbs triggers apoptosis and has, thus, been proposed for treatment of malignancy. The substance is at least partially effective through caspase activation. In analogy to apoptosis of nucleated cells, erythrocytes may enter suicidal death or eryptosis, which is characterized by cell shrinkage and by phosphatidylserine translocation to the erythrocyte surface. Eryptosis is triggered by increase of cytosolic Ca2+-activity ([Ca2+]i). The sensitivity to [Ca2+]i is enhanced by ceramide. The present study explored whether penta-O-galloyl-β-d-glucose stimulates eryptosis. Cell volume was estimated from forward scatter, phosphatidylserine exposure from annexin V binding, hemolysis from hemoglobin-release, [Ca2+]i from Fluo3-fluorescence and ceramide abundance from fluorescent antibodies. A 48-h exposure of human erythrocytes to penta-O-galloyl-β-d-glucose significantly decreased forward scatter (50 µM) and significantly increased annexin V binding (10 µM). Up to 50 µM penta-O-galloyl-β-d-glucose did not significantly modify [Ca2+]i. However, the effect of penta-O-galloyl-β-d-glucose (25 µM) induced annexin V binding was slightly, but significantly, blunted by removal of extracellular Ca2+, pointing to sensitization of erythrocytes to the scrambling effect of Ca2+. Penta-O-galloyl-β-d-glucose (25 µM) further increased ceramide formation. In conclusion, penta-O-galloyl-β-d-glucose stimulates suicidal erythrocyte death or eryptosis, an effect partially due to stimulation of ceramide formation with subsequent sensitization of erythrocytes to Ca2+.
Keywords: cell membrane scrambling, phosphatidylserine, calcium, cell volume, eryptosis
1. Introduction
1,2,3,4,6-Penta-O-galloyl-beta-d-glucose, a polyphenolic compound from medicinal herbs [1], has been proposed for the treatment of several disorders including cancer and diabetes [1]. Its activity against malignancy has been attributed to inhibition of angiogenesis [1,2], cell proliferation [1,3,4], DNA replication [1,2,3], inflammation [1,5], oxidative stress [1,6,7], as well as induction of cell cycle arrest [1,2,3,4,5,8] and apoptosis [1,2,3,8,9,10,11,12,13,3,8]. Signaling mediating the effects of penta-O-galloyl-beta-d-glucose include p53 [1,7], JAK-Stat [1,11], COX-2 [1,12], VEGFR1 [1,12], AP-1 [1], SP-1 [1], Nrf-2 [1], MMP-9 [1], jun kinase [9], MAP kinases [12], and caspases [3,9,12,13]. In addition to its stimulatory effect penta-O-galloyl-beta-d-glucose has been shown to counteract apoptosis and cell injury [6,7,10,14].
Similar to apoptosis of nucleated cells, erythrocytes may undergo suicidal death or eryptosis, which is characterized by erythrocyte shrinkage and phosphatidylserine scrambling of the erythrocyte membrane [15]. Triggers of eryptosis include increase of cytosolic Ca2+ concentration ([Ca2+]i), resulting from Ca2+ entry through Ca2+-permeable cation channels [15,16]. The channels are activated by oxidative stress [15]. Increased [Ca2+]i activates Ca2+-sensitive K+ channels [17,18] leading to cell shrinkage due to K+ exit, hyperpolarization and Cl− exit followed by osmotically obliged water [19]. Increased [Ca2+]i further triggers phospholipid scrambling of the cell membrane with phosphatidylserine translocation to the erythrocyte surface [20]. The Ca2+ sensitivity of cell membrane scrambling is enhanced by ceramide [15]. Further triggers of eryptosis include energy depletion [15], caspase activation [15,21,22], and deranged activity of AMP activated kinase (AMPK) [16], cGMP-dependent protein kinase [23], Janus-activated kinase 3 (JAK3) [24], casein kinase 1α [25,26], p38 kinase [27], as well as sorafenib- [28] and sunitinib- [29] sensitive kinases.
The present study explored the effect of penta-O-galloyl-β-d-glucose on cell volume and phosphatidylserine abundance at the erythrocyte surface. As a result, penta-O-galloyl-β-d-glucose is a powerful stimulator of eryptosis.
2. Results and Discussion
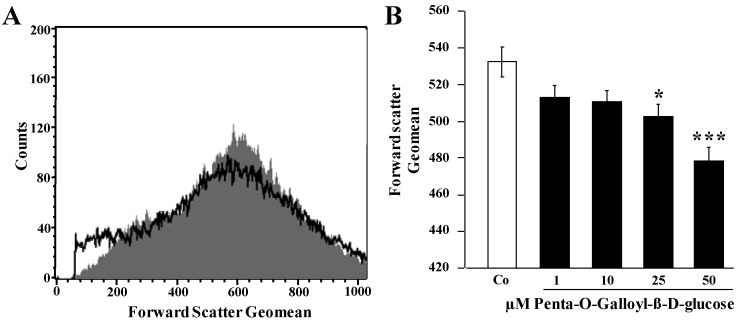
Flow cytometry was employed to explore whether penta-O-galloyl-β-d-glucose triggers eryptosis, the suicidal erythrocyte death characterized by cell shrinkage and cell membrane scrambling. Cell volume of human erythrocytes was estimated from forward scatter. As illustrated in Figure 1, a 48-h exposure to penta-O-galloyl-β-d-glucose led to a decrease of forward scatter, an effect reaching statistical significance at 25 µM penta-O-galloyl-β-d-glucose concentration. Accordingly, penta-O-galloyl-β-d-glucose treatment was followed by erythrocyte shrinkage.
Figure 1.
Effect of penta-O-galloyl-β-d-glucose (PGG) on erythrocyte forward scatter (A) Original histogram of forward scatter of erythrocytes following exposure for 48 h to Ringer solution without (grey) and with (black) the presence of 25 µM penta-O-galloyl-β-d-glucose; (B) Arithmetic means ± SEM (n = 8) of the normalized erythrocyte forward scatter (FSC) following incubation for 48 h to Ringer solution without (white bar) or with (black bars) penta-O-galloyl-β-d-glucose (1–50 µM). * (p < 0.05), *** (p < 0.001) indicates significant difference from the absence of penta-O-galloyl-β-d-glucose (ANOVA).
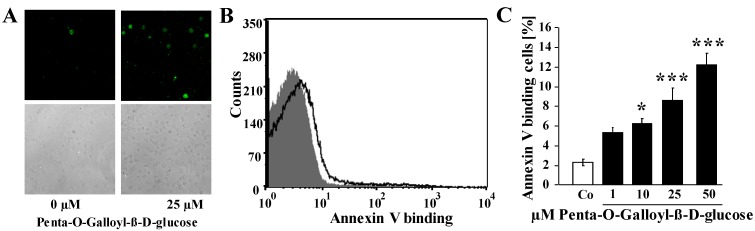
In order to elucidate whether penta-O-galloyl-β-d-glucose stimulates cell membrane phospholipid scrambling with phosphatidylserine exposure at the erythrocyte surface, phosphatidylserine exposing erythrocytes were identified from annexin V binding determined in flow cytometry. As illustrated in Figure 2, a 48-h exposure to penta-O-galloyl-β-d-glucose increased the percentage of annexin V binding erythrocytes, an effect reaching statistical significance at a concentration of 10 µM penta-O-galloyl-β-d-glucose. Accordingly, penta-O-galloyl-β-d-glucose triggered erythrocyte cell membrane scrambling with phosphatidylserine exposure at the cell surface.
Figure 2.
Effect of penta-O-galloyl-β-d-glucose (PGG) on phosphatidylserine exposure (A) Confocal images of FITC-dependent fluorescence (upper panels) and light microscopy (lower panels) of human erthrocytes following exposure for 48 h to Ringer solution without (left panel) and with (right panel) the presence of 25 µM penta-O-galloyl-β-d-glucose; (B) Original histogram of annexin V binding of erythrocytes following exposure for 48 h to Ringer solution without (grey) and with (black) the presence of 25 µM penta-O-galloyl-β-d-glucose. (C) Arithmetic means ± SEM of erythrocyte annexin V binding (n = 8) following incubation for 48 h to Ringer solution without (white bar) or with (black bars) the presence of penta-O-galloyl-β-d-glucose (1–50 µM). * (p < 0.05), *** (p < 0.001) indicates significant difference from the absence of penta-O-galloyl-β-d-glucose (ANOVA).
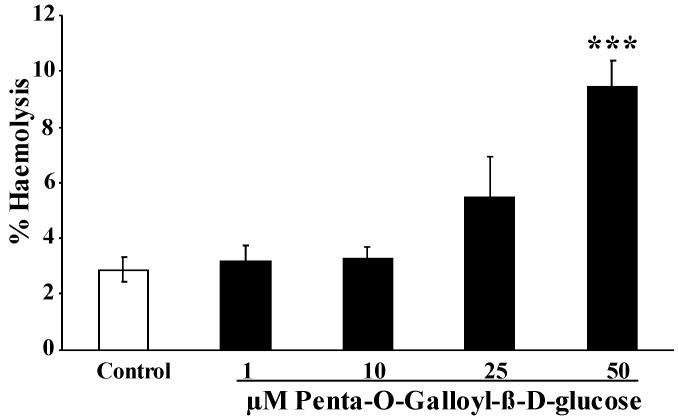
To explore whether penta-O-galloyl-β-d-glucose exposure triggers hemolysis, the percentage of hemolysed erythrocytes was estimated from hemoglobin concentration in the supernatant. As illustrated in Figure 3, the percentage erythrocytes releasing hemoglobin increased following a 48-h exposure to penta-O-galloyl-β-d-glucose concentration, an effect reaching statistical significance at 50 µM penta-O-galloyl-β-d-glucose concentration.
Figure 3.
Effect of penta-O-galloyl-β-d-glucose (PGG) on hemolysis. Arithmetic means ± SEM of the percentage hemolytic erythrocytes (n = 8) following incubation for 48 h to Ringer solution without (white bar) or with (black bars) the presence of penta-O-galloyl-β-d-glucose (1–50 µM). *** (p < 0.001) indicates significant difference from the absence of penta-O-galloyl-β-d-glucose (ANOVA).
Fluo3 fluorescence was employed in order to test, whether penta-O-galloyl-β-d-glucose influences the cytosolic Ca2+ activity ([Ca2+]i). To this end, human erythrocytes were loaded with Fluo3-AM and the Fluo3 fluorescence determined by flow cytometry. Prior to determination of Fluo3-fluorescence erythrocytes were incubated in Ringer solution without or with penta-O-galloyl-β-d-glucose (1–50 µM). As a result, a 48-h exposure of erythrocytes to penta-O-galloyl-β-d-glucose did not significantly modify [Ca2+]i. The respective arbitrary units (a.u.) approached (n = 8 each) 20.29 ± 1.19 a.u., 18.96 ± 1.05 a.u., 17.81 ± 0.93 a.u., 17.61 ± 0.83 a.u., and 22.58 ± 1.37 a.u. after a 48-h exposure to 0, 1, 10, 25, and 50 µM, respectively, of penta-O-galloyl-β-d-glucose.
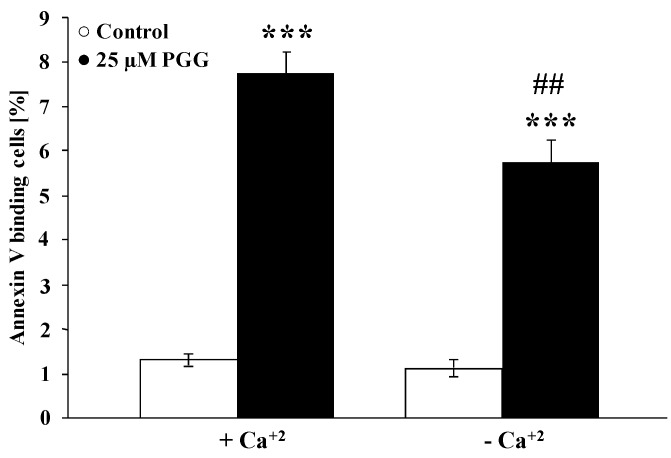
Even though penta-O-galloyl-β-d-glucose did not significantly modify cytosolic Ca2+ activity, the effect of the substance could still be dependent on the presence of Ca2+. In order to test this possibility, erythrocytes were exposed to 25 µM penta-O-galloyl-β-d-glucose for 48 h in the presence and in the nominal absence of extracellular Ca2+. As illustrated in Figure 4, the effect of penta-O-galloyl-β-d-glucose on annexin V binding was slightly but significantly blunted in the nominal absence of Ca2+. Thus, the effect of penta-O-galloyl-β-d-glucose required in part the presence of Ca2+.
Figure 4.
Effect of penta-O-galloyl-β-d-glucose (PGG) on phosphatidylserine exposure in the presence and absence of extracellular Ca2+. Arithmetic means ± SEM (n = 8) of the percentage of annexin V binding erythrocytes after a 48-h treatment with Ringer solution without (white bar) or with (black bars) 25 µM penta-O-galloyl-β-d-glucose in the presence (left bars, Plus Calcium) and absence (right bars, Minus Calcium) of calcium. *** (p < 0.001) indicates significant difference from the absence of penta-O-galloyl-β-d-glucose (ANOVA), ## (p < 0.01) indicates significant difference from the respective values in the presence of Ca2+ (ANOVA).
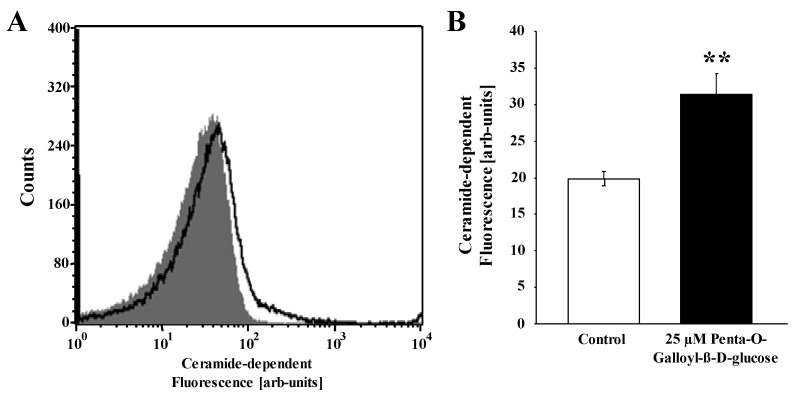
The sensitivity of cell membrane scrambling to cytosolic Ca2+ could be enhanced by ceramide, which is known to trigger eryptosis even at constant [Ca2+]i. In order to test, whether ceramide is enhanced by penta-O-galloyl-β-d-glucose, ceramide abundance at the cell surface was elucidated utilizing FITC-labeled anti-ceramide antibodies. As shown in Figure 5, penta-O-galloyl-β-d-glucose significantly increased ceramide-dependent fluorescence.
Figure 5.
Effect of penta-O-galloyl-β-d-glucose (PGG) on ceramide formation. (A) Original histogram of anti-ceramide FITC-fluorescence in erythrocytes following exposure for 48 h to Ringer solution without (grey) and with (black) the presence of 25 µM penta-O-galloyl-β-d-glucose; (B) Arithmetic means ± SEM (n = 8) of ceramide abundance after a 48 h incubation in Ringer solution without (white bar) or with (black bars) penta-O-galloyl-β-d-glucose (25 µM). ** (p < 0.01) indicates significant difference from control (t test).
The present study discloses a novel xenobiotic triggering suicidal erythrocyte death or eryptosis. Treatment of human erythrocytes with penta-O-galloyl-β-d-glucose is followed by erythrocyte shrinkage and erythrocyte cell membrane scrambling, the two hallmarks of eryptosis. The concentrations required (10–50 µM) are similar to those inhibiting hepatocyte apoptosis [14].
Penta-O-galloyl-β-d-glucose is not paralleled by and thus not due to increase of cytosolic Ca2+ activity. Most triggers of eryptosis exert their effect on annexin V binding and cell volume by activation of Ca2+ permeable non-selective cation channels in erythrocytes, which involve the transient receptor potential channel TRPC6 [15]. Even though penta-O-galloyl-β-d-glucose treatment did not significantly modify cytosolic Ca2+ activity, the full effect of penta-O-galloyl-β-d-glucose was dependent on the presence of extracellular Ca2+. Thus, penta-O-galloyl-β-d-glucose appeared to sensitize the erythrocytes to the effects of cytosolic Ca2+. A substance known to sensitize erythrocytes to the scrambling effect of enhanced cytosolic Ca2+ activity is ceramide [15]. As a matter of fact, penta-O-galloyl-β-d-glucose did stimulate ceramide formation.
In nucleated cells, penta-O-galloyl-β-d-glucose has been shown to either stimulate [1,2,3,8,9,10,11,12,13] or inhibit apoptosis [6,7,10,14]. A powerful regulator of nuclear cell fate is ceramide, which stimulates death of a wide variety of cells [30]. To the best of our knowledge, penta-O-galloyl-β-d-glucose has never been shown before to stimulate the formation of ceramide. Thus, the present observations disclose a mechanism, which could well contribute to triggering of apoptosis.
The present study may further disclose an effect of penta-O-galloyl-β-d-glucose limiting its therapeutic use. Phosphatidylserine exposing erythrocytes adhere to CXCL16/SR-PSO expressed at the luminal cell membrane of endothelial cells [31]. The adherence of the phosphatidylserine exposing erythrocytes to endothelial cells is expected to compromise blood flow and thus to impair microcirculation [31,32,33,34,35,36]. In addition, phosphatidylserine, exposing erythrocytes, may foster blood clotting and, thus, trigger thrombosis [32,37,38]. Phosphatidylserine exposing erythrocytes are further cleared from circulating blood [15]. To the extent that the clearance of erythrocytes exceeds the formation of new erythrocytes, the stimulation of eryptosis may lead to anemia [15]. The therapeutic use of penta-O-galloyl-β-d-glucose may, thus, be limited by triggering of eryptosis with subsequent impairment of microcirculation and development of anemia. It should be kept in mind that the sensitivity of erythrocytes to penta-O-galloyl-β-d-glucose may be increased by other xenobiotics [15,24,27,39,40,41,42,43,44,45,46,47,48,49,50] and be enhanced in diseases associated with enhanced eryptosis, such as diabetes [15,22,51], renal insufficiency [52], hemolytic uremic syndrome [53], sepsis [54], sickle cell disease [55], malaria [15,56,57], Wilson’s disease [57], iron deficiency [58], phosphate depletion [59], and, presumably, metabolic syndrome [60].
3. Experimental Section
3.1. Erythrocytes, Solutions, and Chemicals
Leukocyte-depleted erythrocytes were kindly provided by the blood bank of the University of Tübingen. The volunteers (age range 18–68 years) providing blood were tested for HIV, syphilis, and Hepatitis A, B, and C before donation. The study is approved by the ethics committee of the University of Tübingen (184/2003V). Erythrocytes were incubated in vitro at a hematocrit of 0.4% in Ringer solution containing (in mM) 125 NaCl, 5 KCl, 1 MgSO4, 32 N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (HEPES), 5 glucose, 1 CaCl2; pH 7.4 at 37 °C for 48 h. Where indicated, erythrocytes were exposed to penta-O-galloyl-β-d-glucose (Sigma, Freiburg, Germany) at the indicated concentrations. In Ca2+-free Ringer solution, 1 mM CaCl2 was substituted by 1 mM glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid (EGTA).
3.2. Confocal Microscopy and Immunofluorescence
For the visualization of eryptotic erythrocytes, 20 μL erythrocytes were incubated under the respective experimental conditions and then stained with FITC-conjugated Annexin V (1:100 dilution; ImmunoTools, Friesoythe, Germany) in 200 μL Ringer solution containing 5 mM CaCl2. Then, the erythrocytes were washed twice and finally resuspended in 100 μL Ringer solution containing 5 mM CaCl2. Forty microliters were placed with Prolong Gold antifade reagent (Invitrogen, Darmstadt, Germany) onto a glass slide, covered with a coverslip, and images were subsequently taken on a Zeiss LSM 5 EXCITER confocal laser-scanning microscope (Carl Zeiss MicroImaging, Oberkochen, Germany) with a water immersion Plan-Neofluar 40/1.3 NA DIC.
3.3. FACS Analysis of Annexin V Binding and Forward Scatter
After incubation under the respective experimental condition, 50 µL cell suspension was washed in Ringer solution containing 5 mM CaCl2 and then stained with Annexin-V-FITC (1:200 dilution; ImmunoTools, Friesoythe, Germany) in this solution at 37 °C for 20 min under protection from light. In the following, the forward scatter (FSC) of the cells was determined, and annexin V fluorescence intensity was measured with an excitation wavelength of 488 nm and an emission wavelength of 530 nm on a FACS Calibur (BD, Heidelberg, Germany).
3.4. Measurement of Intracellular Ca2+
After incubation erythrocytes were washed in Ringer solution and then loaded with Fluo-3/AM (Biotium, Hayward, CA, USA) in Ringer solution containing 5 mM CaCl2 and 5 µM Fluo-3/AM. The cells were incubated at 37 °C for 30 min and washed twice in Ringer solution containing 5 mM CaCl2. The Fluo-3/AM-loaded erythrocytes were resuspended in 200 µL Ringer. Then, Ca2+-dependent fluorescence intensity was measured with an excitation wavelength of 488 nm and an emission wavelength of 530 nm on a FACS Calibur (BD, Heidelberg, Germany).
3.5. Measurement of Hemolysis
For the determination of hemolysis the samples were centrifuged (3 min at 400 g, room temperature) after incubation, and the supernatants were harvested. As a measure of hemolysis, the hemoglobin (Hb) concentration of the supernatant was determined photometrically at 405 nm. The absorption of the supernatant of erythrocytes lysed in distilled water was defined as 100% hemolysis.
3.6. Determination of Ceramide Formation
For the determination of ceramide, a monoclonal antibody-based assay was used. After incubation, cells were stained for 1 h at 37 °C with 1 µg/mL anti-ceramide antibody (clone MID 15B4, Alexis, Grünberg, Germany) in PBS containing 0.1% bovine serum albumin (BSA) at a dilution of 1:10. The samples were washed twice with PBS-BSA. Subsequently, the cells were stained for 30 min with polyclonal fluorescein-isothiocyanate (FITC)-conjugated goat anti-mouse IgG and IgM specific antibody (Pharmingen, Hamburg, Germany) diluted 1:50 in PBS-BSA. Unbound secondary antibody was removed by repeated washing with PBS-BSA. The samples were then analyzed at an excitation wavelength of 488 nm and an emission wavelength of 530 nm on a FACS Calibur (BD, Heidelberg, Germany).
3.7. Confocal Microscopy and Immunofluorescence
For the visualization of eryptotic erythrocytes, 20 μL erythrocytes were incubated under the respective experimental conditions and then stained with FITC-conjugated Annexin V (1:100 dilution; ImmunoTools) in 200 μL Ringer solution containing 5 mM CaCl2. Then, the erythrocytes were washed twice and finally resuspended in 100 μL Ringer solution containing 5 mM CaCl2. Forty microliters were placed with Prolong Gold antifade reagent (Invitrogen, Darmstadt, Germany) onto a glass slide, covered with a coverslip, and images were subsequently taken on a Zeiss LSM 5 EXCITER confocal laser-scanning microscope (Carl Zeiss MicroImaging, Oberkochen, Germany) with a water immersion Plan-Neofluar 40/1.3 NA DIC.
3.8. Statistics
Data are expressed as arithmetic means ± SEM. As indicated in the figure legends, statistical analysis was made using ANOVA with Tukey’s test as post-test and t test as appropriate. n denotes the number of different erythrocyte specimens studied. As different erythrocyte specimens used in distinct experiments are differently susceptible to triggers of eryptosis, the same erythrocyte specimens have been used for control and experimental conditions.
4. Conclusions
Penta-O-galloyl-β-d-glucose triggers cell shrinkage and cell membrane scrambling of human erythrocytes and thus eryptosis, the suicidal death of erythrocytes. The effect is in part dependent on the presence of extracellular Ca2+ and is at least in part due to stimulation of ceramide formation.
Acknowledgments
The authors acknowledge the meticulous preparation of the manuscript by Tanja Loch. The study was supported by the Deutsche Forschungsgemeinschaft and the Open Access Publishing Fund of Tuebingen University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Zhang J., Li L., Kim S.H., Hagerman A.E., Lu J. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm. Res. 2009;26:2066–2080. doi: 10.1007/s11095-009-9932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu H., Zhang J., Lee H.J., Kim S.H., Lu J. Penta-O-galloyl-beta-d-glucose induces s- and g(1)-cell cycle arrests in prostate cancer cells targeting DNA replication and cyclin d1. Carcinogenesis. 2009;30:818–823. doi: 10.1093/carcin/bgp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chai Y., Lee H.J., Shaik A.A., Nkhata K., Xing C., Zhang J., Jeong S.J., Kim S.H., Lu J. Penta-O-galloyl-beta-d-glucose induces g1 arrest and DNA replicative s-phase arrest independently of cyclin-dependent kinase inhibitor 1a, cyclin-dependent kinase inhibitor 1b and p53 in human breast cancer cells and is orally active against triple negative xenograft growth. Breast Cancer Res. 2010;12:R67. doi: 10.1186/bcr2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh G.S., Pae H.O., Oh H., Hong S.G., Kim I.K., Chai K.Y., Yun Y.G., Kwon T.O., Chung H.T. In vitro anti-proliferative effect of 1,2,3,4,6-penta-O-galloyl-beta-d-glucose on human hepatocellular carcinoma cell line, sk-hep-1 cells. Cancer Lett. 2001;174:17–24. doi: 10.1016/S0304-3835(01)00680-2. [DOI] [PubMed] [Google Scholar]
- 5.Kiss A.K., Filipek A., Zyzynska-Granica B., Naruszewicz M. Effects of penta-O-galloyl-beta-d-glucose on human neutrophil function: Significant down-regulation of l-selectin expression. Phytother. Res. 2013;27:986–992. doi: 10.1002/ptr.4822. [DOI] [PubMed] [Google Scholar]
- 6.Lee H.J., Jeong S.J., Lee H.J., Lee E.O., Bae H., Lieske J.C., Kim S.H. 1,2,3,4,6-penta-O-galloyl-beta-d-glucose reduces renal crystallization and oxidative stress in a hyperoxaluric rat model. Kidney Int. 2011;79:538–545. doi: 10.1038/ki.2010.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bing S.J., Kim M.J., Park E., Ahn G., Kim D.S., Ko R.K., Lee N.H., Shin T., Park J.W., Jee Y. 1,2,3,4,6-Penta-O-galloyl-beta-d-glucose protects splenocytes against radiation-induced apoptosis in murine splenocytes. Biol. Pharm. Bull. 2010;33:1122–1127. doi: 10.1248/bpb.33.1122. [DOI] [PubMed] [Google Scholar]
- 8.Huang C., Lee S.Y., Lin C.L., Tu T.H., Chen L.H., Chen Y.J., Huang H.C. Co-treatment with quercetin and 1,2,3,4,6-penta-O-galloyl-beta-d-glucose causes cell cycle arrest and apoptosis in human breast cancer mda-mb-231 and au565 cells. J. Agric. Food Chem. 2013;61:6430–6445. doi: 10.1021/jf305253m. [DOI] [PubMed] [Google Scholar]
- 9.Kwon T.R., Jeong S.J., Lee H.J., Lee H.J., Sohn E.J., Jung J.H., Kim J.H., Jung D.B., Lu J., Kim S.H. Reactive oxygen species-mediated activation of jnk and down-regulation of daxx are critically involved in penta-O-galloyl-beta-d-glucose-induced apoptosis in chronic myeloid leukemia k562 cells. Biochem. Biophys. Res. Commun. 2012;424:530–537. doi: 10.1016/j.bbrc.2012.06.150. [DOI] [PubMed] [Google Scholar]
- 10.Ryu H.G., Jeong S.J., Kwon H.Y., Lee H.J., Lee E.O., Lee M.H., Choi S.H., Ahn K.S., Kim S.H. Penta-O-galloyl-beta-d-glucose attenuates cisplatin-induced nephrotoxicity via reactive oxygen species reduction in renal epithelial cells and enhances antitumor activity in caki-2 renal cancer cells. Toxicol. in Vitro. 2012;26:206–214. doi: 10.1016/j.tiv.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Lee H.J., Seo N.J., Jeong S.J., Park Y., Jung D.B., Koh W., Lee H.J., Lee E.O., Ahn K.S., Ahn K.S., et al. Oral administration of penta-O-galloyl-beta-d-glucose suppresses triple-negative breast cancer xenograft growth and metastasis in strong association with jak1-stat3 inhibition. Carcinogenesis. 2011;32:804–811. doi: 10.1093/carcin/bgr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huh J.E., Lee E.O., Kim M.S., Kang K.S., Kim C.H., Cha B.C., Surh Y.J., Kim S.H. Penta-O-galloyl-beta-d-glucose suppresses tumor growth via inhibition of angiogenesis and stimulation of apoptosis: Roles of cyclooxygenase-2 and mitogen-activated protein kinase pathways. Carcinogenesis. 2005;26:1436–1445. doi: 10.1093/carcin/bgi097. [DOI] [PubMed] [Google Scholar]
- 13.Pan M.H., Lin J.H., Lin-Shiau S.Y., Lin J.K. Induction of apoptosis by penta-O-galloyl-beta-d-glucose through activation of caspase-3 in human leukemia hl-60 cells. Eur. J. Pharmacol. 1999;381:171–183. doi: 10.1016/S0014-2999(99)00549-X. [DOI] [PubMed] [Google Scholar]
- 14.Park E.J., Zhao Y.Z., An R.B., Kim Y.C., Sohn D.H. 1,2,3,4,6-Penta-O-galloyl-beta-d-glucose from galla rhois protects primary rat hepatocytes from necrosis and apoptosis. Planta Med. 2008;74:1380–1383. doi: 10.1055/s-2008-1081300. [DOI] [PubMed] [Google Scholar]
- 15.Lang E., Qadri S.M., Lang F. Killing me softly—Suicidal erythrocyte death. Int. J.Biochem. Cell.Biol. 2012;44:1236–1243. doi: 10.1016/j.biocel.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Foller M., Sopjani M., Koka S., Gu S., Mahmud H., Wang K., Floride E., Schleicher E., Schulz E., Munzel T., et al. Regulation of erythrocyte survival by amp-activated protein kinase. FASEB J. 2009;23:1072–1080. doi: 10.1096/fj.08-121772. [DOI] [PubMed] [Google Scholar]
- 17.Bookchin R.M., Ortiz O.E., Lew V.L. Activation of calcium-dependent potassium channels in deoxygenated sickled red cells. Prog. Clin. Biol. Res. 1987;240:193–200. [PubMed] [Google Scholar]
- 18.Brugnara C., De Franceschi L., Alper S.L. Inhibition of Ca(2+)-dependent K+ transport and cell dehydration in sickle erythrocytes by clotrimazole and other imidazole derivatives. J. Clin. Invest. 1993;92:520–526. doi: 10.1172/JCI116597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang P.A., Kaiser S., Myssina S., Wieder T., Lang F., Huber S.M. Role of Ca2+-activated K+ channels in human erythrocyte apoptosis. Am. J. Physiol. Cell. Physiol. 2003;285:C1553–C1560. doi: 10.1152/ajpcell.00186.2003. [DOI] [PubMed] [Google Scholar]
- 20.Berg C.P., Engels I.H., Rothbart A., Lauber K., Renz A., Schlosser S.F., Schulze-Osthoff K., Wesselborg S. Human mature red blood cells express caspase-3 and caspase-8, but are devoid of mitochondrial regulators of apoptosis. Cell. Death Differ. 2001;8:1197–1206. doi: 10.1038/sj.cdd.4400905. [DOI] [PubMed] [Google Scholar]
- 21.Lau I.P., Chen H., Wang J., Ong H.C., Leung K.C., Ho H.P., Kong S.K. In vitro effect of ctab- and peg-coated gold nanorods on the induction of eryptosis/erythroptosis in human erythrocytes. Nanotoxicology. 2011 doi: 10.3109/17435390.2011.625132. [DOI] [PubMed] [Google Scholar]
- 22.Maellaro E., Leoncini S., Moretti D., Del Bello B., Tanganelli I., De Felice C., Ciccoli L. Erythrocyte caspase-3 activation and oxidative imbalance in erythrocytes and in plasma of type 2 diabetic patients. Acta Diabetol. 2011 doi: 10.1007/s00592-011-0274-0. [DOI] [PubMed] [Google Scholar]
- 23.Foller M., Feil S., Ghoreschi K., Koka S., Gerling A., Thunemann M., Hofmann F., Schuler B., Vogel J., Pichler B., et al. Anemia and splenomegaly in cgki-deficient mice. Proc. Natl. Acad. Sci. USA. 2008;105:6771–6776. doi: 10.1073/pnas.0708940105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhavsar S.K., Gu S., Bobbala D., Lang F. Janus kinase 3 is expressed in erythrocytes, phosphorylated upon energy depletion and involved in the regulation of suicidal erythrocyte death. Cell. Physiol. Biochem. 2011;27:547–556. doi: 10.1159/000329956. [DOI] [PubMed] [Google Scholar]
- 25.Kucherenko Y.V., Huber S.M., Nielsen S., Lang F. Decreased redox-sensitive erythrocyte cation channel activity in aquaporin 9-deficient mice. J. Membr. Biol. 2012;245:797–805. doi: 10.1007/s00232-012-9482-y. [DOI] [PubMed] [Google Scholar]
- 26.Zelenak C., Eberhard M., Jilani K., Qadri S.M., Macek B., Lang F. Protein kinase ck1alpha regulates erythrocyte survival. Cell. Physiol. Biochem. 2012;29:171–180. doi: 10.1159/000337598. [DOI] [PubMed] [Google Scholar]
- 27.Gatidis S., Zelenak C., Fajol A., Lang E., Jilani K., Michael D., Qadri S.M., Lang F. P38 mapk activation and function following osmotic shock of erythrocytes. Cell. Physiol. Biochem. 2011;28:1279–1286. doi: 10.1159/000335859. [DOI] [PubMed] [Google Scholar]
- 28.Lupescu A., Shaik N., Jilani K., Zelenak C., Lang E., Pasham V., Zbidah M., Plate A., Bitzer M., Foller M., et al. Enhanced erythrocyte membrane exposure of phosphatidylserine following sorafenib treatment: An in vivo and in vitro study. Cell. Physiol. Biochem. 2012;30:876–888. doi: 10.1159/000341465. [DOI] [PubMed] [Google Scholar]
- 29.Shaik N., Lupescu A., Lang F. Sunitinib-sensitive suicidal erythrocyte death. Cell. Physiol. Biochem. 2012;30:512–522. doi: 10.1159/000341434. [DOI] [PubMed] [Google Scholar]
- 30.Morad S.A., Cabot M.C. Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer. 2013;13:51–65. doi: 10.1038/nrc3398. [DOI] [PubMed] [Google Scholar]
- 31.Borst O., Abed M., Alesutan I., Towhid S.T., Qadri S.M., Foller M., Gawaz M., Lang F. Dynamic adhesion of eryptotic erythrocytes to endothelial cells via cxcl16/sr-psox. Am. J. Physiol. Cell. Physiol. 2012;302:C644–C651. doi: 10.1152/ajpcell.00340.2011. [DOI] [PubMed] [Google Scholar]
- 32.Andrews D.A., Low P.S. Role of red blood cells in thrombosis. Curr. Opin. Hematol. 1999;6:76–82. doi: 10.1097/00062752-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Closse C., Dachary-Prigent J., Boisseau M.R. Phosphatidylserine-related adhesion of human erythrocytes to vascular endothelium. Br. J. Haematol. 1999;107:300–302. doi: 10.1046/j.1365-2141.1999.01718.x. [DOI] [PubMed] [Google Scholar]
- 34.Gallagher P.G., Chang S.H., Rettig M.P., Neely J.E., Hillery C.A., Smith B.D., Low P.S. Altered erythrocyte endothelial adherence and membrane phospholipid asymmetry in hereditary hydrocytosis. Blood. 2003;101:4625–4627. doi: 10.1182/blood-2001-12-0329. [DOI] [PubMed] [Google Scholar]
- 35.Pandolfi A., Di Pietro N., Sirolli V., Giardinelli A., Di Silvestre S., Amoroso L., Di Tomo P., Capani F., Consoli A., Bonomini M. Mechanisms of uremic erythrocyte-induced adhesion of human monocytes to cultured endothelial cells. J. Cell. Physiol. 2007;213:699–709. doi: 10.1002/jcp.21138. [DOI] [PubMed] [Google Scholar]
- 36.Wood B.L., Gibson D.F., Tait J.F. Increased erythrocyte phosphatidylserine exposure in sickle cell disease: Flow-cytometric measurement and clinical associations. Blood. 1996;88:1873–1880. [PubMed] [Google Scholar]
- 37.Chung S.M., Bae O.N., Lim K.M., Noh J.Y., Lee M.Y., Jung Y.S., Chung J.H. Lysophosphatidic acid induces thrombogenic activity through phosphatidylserine exposure and procoagulant microvesicle generation in human erythrocytes. Arterioscler. Thromb. Vasc. Biol. 2007;27:414–421. doi: 10.1161/01.ATV.0000252898.48084.6a. [DOI] [PubMed] [Google Scholar]
- 38.Zwaal R.F., Comfurius P., Bevers E.M. Surface exposure of phosphatidylserine in pathological cells. Cell. Mol. Life Sci. 2005;62:971–988. doi: 10.1007/s00018-005-4527-3. [DOI] [PubMed] [Google Scholar]
- 39.Braun M., Foller M., Gulbins E., Lang F. Eryptosis triggered by bismuth. Biometals. 2009;22:453–460. doi: 10.1007/s10534-008-9180-5. [DOI] [PubMed] [Google Scholar]
- 40.Felder K.M., Hoelzle K., Ritzmann M., Kilchling T., Schiele D., Heinritzi K., Groebel K., Hoelzle L.E. Hemotrophic mycoplasmas induce programmed cell death in red blood cells. Cell. Physiol. Biochem. 2011;27:557–564. doi: 10.1159/000329957. [DOI] [PubMed] [Google Scholar]
- 41.Ghashghaeinia M., Toulany M., Saki M., Bobbala D., Fehrenbacher B., Rupec R., Rodemann H.P., Ghoreschi K., Rocken M., Schaller M., et al. The nfkb pathway inhibitors bay 11-7082 and parthenolide induce programmed cell death in anucleated erythrocytes. Cell. Physiol. Biochem. 2011;27:45–54. doi: 10.1159/000325204. [DOI] [PubMed] [Google Scholar]
- 42.Lang E., Jilani K., Zelenak C., Pasham V., Bobbala D., Qadri S.M., Lang F. Stimulation of suicidal erythrocyte death by benzethonium. Cell. Physiol. Biochem. 2011;28:347–354. doi: 10.1159/000331751. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen D.B., Wagner-Britz L., Maia S., Steffen P., Wagner C., Kaestner L., Bernhardt I. Regulation of phosphatidylserine exposure in red blood cells. Cell. Physiol. Biochem. 2011;28:847–856. doi: 10.1159/000335798. [DOI] [PubMed] [Google Scholar]
- 44.Qadri S.M., Bauer J., Zelenak C., Mahmud H., Kucherenko Y., Lee S.H., Ferlinz K., Lang F. Sphingosine but not sphingosine-1-phosphate stimulates suicidal erythrocyte death. Cell. Physiol. Biochem. 2011;28:339–346. doi: 10.1159/000331750. [DOI] [PubMed] [Google Scholar]
- 45.Qadri S.M., Kucherenko Y., Lang F. Beauvericin induced erythrocyte cell membrane scrambling. Toxicology. 2011;283:24–31. doi: 10.1016/j.tox.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 46.Qadri S.M., Kucherenko Y., Zelenak C., Jilani K., Lang E., Lang F. Dicoumarol activates ca-permeable cation channels triggering erythrocyte cell membrane scrambling. Cell. Physiol. Biochem. 2011;28:857–864. doi: 10.1159/000335800. [DOI] [PubMed] [Google Scholar]
- 47.Zbidah M., Lupescu A., Shaik N., Lang F. Gossypol-induced suicidal erythrocyte death. Toxicology. 2012;302:101–105. doi: 10.1016/j.tox.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Abed M., Towhid S.T., Shaik N., Lang F. Stimulation of suicidal death of erythrocytes by rifampicin. Toxicology. 2012;302:123–128. doi: 10.1016/j.tox.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Lupescu A., Jilani K., Zbidah M., Lang F. Induction of apoptotic erythrocyte death by rotenone. Toxicology. 2012;300:132–137. doi: 10.1016/j.tox.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Lang E., Modicano P., Arnold M., Bissinger R., Faggio C., Abed M., Lang F. Effect of thioridazine on erythrocytes. Toxins. 2013;5:1918–1931. doi: 10.3390/toxins5101918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calderon-Salinas J.V., Munoz-Reyes E.G., Guerrero-Romero J.F., Rodriguez-Moran M., Bracho-Riquelme R.L., Carrera-Gracia M.A., Quintanar-Escorza M.A. Eryptosis and oxidative damage in type 2 diabetic mellitus patients with chronic kidney disease. Mol. Cell. Biochem. 2011;357:171–179. doi: 10.1007/s11010-011-0887-1. [DOI] [PubMed] [Google Scholar]
- 52.Myssina S., Huber S.M., Birka C., Lang P.A., Lang K.S., Friedrich B., Risler T., Wieder T., Lang F. Inhibition of erythrocyte cation channels by erythropoietin. J. Am. Soc. Nephrol. 2003;14:2750–2757. doi: 10.1097/01.ASN.0000093253.42641.C1. [DOI] [PubMed] [Google Scholar]
- 53.Lang P.A., Beringer O., Nicolay J.P., Amon O., Kempe D.S., Hermle T., Attanasio P., Akel A., Schafer R., Friedrich B., et al. Suicidal death of erythrocytes in recurrent hemolytic uremic syndrome. J. Mol. Med. 2006;84:378–388. doi: 10.1007/s00109-006-0058-0. [DOI] [PubMed] [Google Scholar]
- 54.Kempe D.S., Akel A., Lang P.A., Hermle T., Biswas R., Muresanu J., Friedrich B., Dreischer P., Wolz C., Schumacher U., et al. Suicidal erythrocyte death in sepsis. J. Mol. Med. 2007;85:273–281. doi: 10.1007/s00109-006-0123-8. [DOI] [PubMed] [Google Scholar]
- 55.Lang P.A., Kasinathan R.S., Brand V.B., Duranton C., Lang C., Koka S., Shumilina E., Kempe D.S., Tanneur V., Akel A., et al. Accelerated clearance of plasmodium-infected erythrocytes in sickle cell trait and annexin-a7 deficiency. Cell. Physiol. Biochem. 2009;24:415–428. doi: 10.1159/000257529. [DOI] [PubMed] [Google Scholar]
- 56.Foller M., Bobbala D., Koka S., Huber S.M., Gulbins E., Lang F. Suicide for survival—Death of infected erythrocytes as a host mechanism to survive malaria. Cell. Physiol. Biochem. 2009;24:133–140. doi: 10.1159/000233238. [DOI] [PubMed] [Google Scholar]
- 57.Lang P.A., Schenck M., Nicolay J.P., Becker J.U., Kempe D.S., Lupescu A., Koka S., Eisele K., Klarl B.A., Rubben H., et al. Liver cell death and anemia in wilson disease involve acid sphingomyelinase and ceramide. Nat. Med. 2007;13:164–170. doi: 10.1038/nm1539. [DOI] [PubMed] [Google Scholar]
- 58.Kempe D.S., Lang P.A., Duranton C., Akel A., Lang K.S., Huber S.M., Wieder T., Lang F. Enhanced programmed cell death of iron-deficient erythrocytes. FASEB J. 2006;20:368–370. doi: 10.1096/fj.05-4872fje. [DOI] [PubMed] [Google Scholar]
- 59.Birka C., Lang P.A., Kempe D.S., Hoefling L., Tanneur V., Duranton C., Nammi S., Henke G., Myssina S., Krikov M., et al. Enhanced susceptibility to erythrocyte “apoptosis” following phosphate depletion. Pflugers. Arch. 2004;448:471–477. doi: 10.1007/s00424-004-1289-y. [DOI] [PubMed] [Google Scholar]
- 60.Zappulla D. Environmental stress, erythrocyte dysfunctions, inflammation, and the metabolic syndrome: Adaptations to CO2 increases? J. Cardiometab. Syndr. 2008;3:30–34. doi: 10.1111/j.1559-4572.2008.07263.x. [DOI] [PubMed] [Google Scholar]