Abstract
Several reviews have analyzed the factors that affect the change in soil organic C (SOC) when forest is converted to agricultural land; however, the effects of forest type and cultivation stage on these changes have generally been overlooked. We collated observations from 453 paired or chronosequential sites where forests have been converted to agricultural land and then assessed the effects of forest type, cultivation stage, climate factors, and soil properties on the change in the SOC stock and the SOC turnover rate constant (k). The percent decrease in SOC stocks and the turnover rate constants both varied significantly according to forest type and cultivation stage. The largest decrease in SOC stocks was observed in temperate regions (52% decrease), followed by tropical regions (41% decrease) and boreal regions (31% decrease). Climate and soil factors affected the decrease in SOC stocks. The SOC turnover rate constant after the conversion of forests to agricultural land increased with the mean annual precipitation and temperature. To our knowledge, this is the first time that original forest type was considered when evaluating changes in SOC after being converted to agricultural land. The differences between forest types should be considered when calculating global changes in SOC stocks.
More than 40% of the total organic C in terrestrial ecosystems is stored in forest soil1. Converting natural forests to agricultural land results in the mineralization of soil organic C (SOC), thus reducing SOC stocks and increasing atmospheric CO2 concentrations2,3. The decreases in SOC following a land-use change are difficult to predict due to variations in the factors that drive SOC mineralization, e.g., forest type, climate, and soil properties1. Understanding the effects of these factors is important for assessing the C flux between the soil and the atmosphere and for understanding the feedbacks within the global C cycle4,5.
The conversion of forest to agricultural land destroys the soil's aggregate structure, which enhances organic matter mineralization and CO2 emissions. Generally, SOC stocks decrease rapidly and then stabilize after a land-use change2,6. However, previous reviews have largely ignored the effects of forest type and cultivation stage on SOC changes2,6,7,8, which is especially true in temperate and boreal regions. The SOC turnover rate constant is often unreported following a land-use change even though it is essential for predicting SOC changes and understanding the feedbacks between C cycling and the climate system.
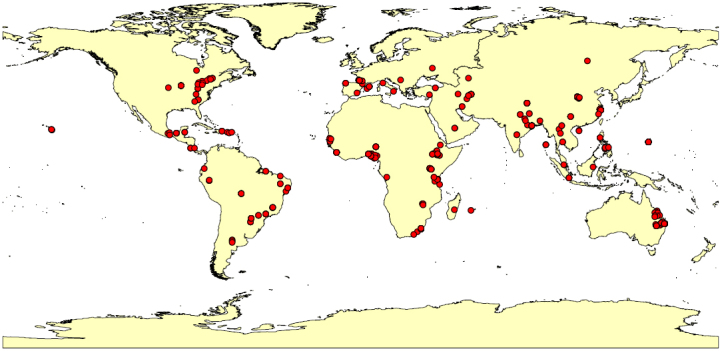
The objectives of this study were to learn more about how global SOC stocks change in response to the conversion of forests to agricultural land and to understand how the changes depend on climate and soil properties. We collated observations from 119 peer-reviewed publications to create a global dataset of 453 paired or chronosequential sites where forests have been converted to agricultural land (Fig. 1). We then assessed the effects of forest type, cultivation stage, climate, and soil properties on SOC stocks and the SOC turnover rate constant (k).
Figure 1. Distribution of the study sites in which forests have been converted to agricultural land (created in ArcGis 10.0).
Results
1. Changes in SOC stocks
The SOC stocks decreased at 98% of the sites following the conversion of forests to agricultural land; the mean decrease in SOC stocks was 44.5 ± 1.0% (Fig. 2). The SOC stocks increased after the land-use change at the other 2% of the sites. The mean increase at these sites was 23.6 ± 8.9%. Regardless, the SOC stocks decreased by an average of 43.1 ± 1.1% for all sites. Moreover, the mean rate of decrease was 1.31 ± 0.10 mg ha−1 yr−1, and the mean turnover rate constant was 0.016 ± 0.002 yr−1 (corresponding to a mean turnover time of 62 years).
Figure 2. Percent decrease in SOC after the conversion of forests to agricultural land.
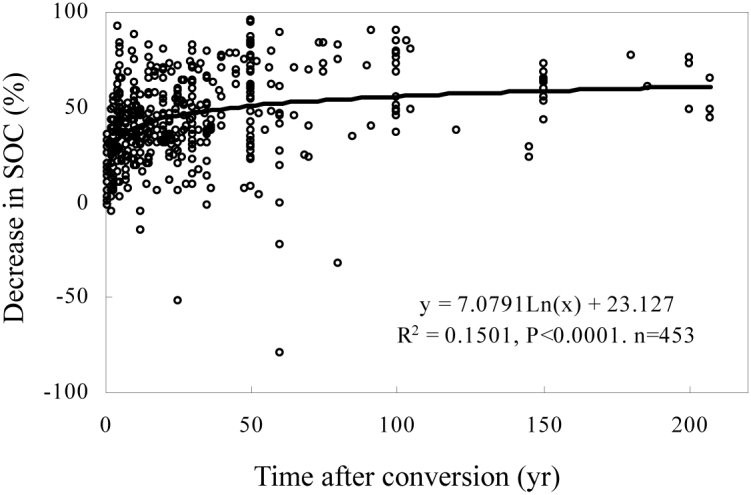
Negative values indicate that SOC stocks increased after the land-use change.
Both forest type and cultivation stage exhibited significant effects on the percent decrease in SOC; however, their interaction did not. Averaged across all forest types, the SOC stocks decreased by an average of 34.7 ± 1.6% at sites in the early stage (≤10 yr) of cultivation, 45.3 ± 1.4% at sites in the middle stage (11–50 yr) of cultivation, and 53.2 ± 3.4% at sites in the late stage (>50 yr) of cultivation (P < 0.05, Fig. 3A). Averaged across cultivation stages, the SOC stocks decreased by 31.1 ± 5.3% in boreal regions, 40.8 ± 1.3% in tropical regions, and 52.3 ± 2.0% in temperate regions (P < 0.05, Fig. 3A). The effect of cultivation stage varied with forest type (Fig. 3A). Specifically, in boreal regions, SOC decreased more at sites in the middle cultivation stage than at sites in the late stage. Moreover, in the temperate and tropical regions, more SOC was lost as the cultivation time increased.
Figure 3. The effects of cultivation age and forest type on the percent decrease in SOC stocks (A) and turnover rate constants (k) (B) after the conversion of forests to agricultural land.
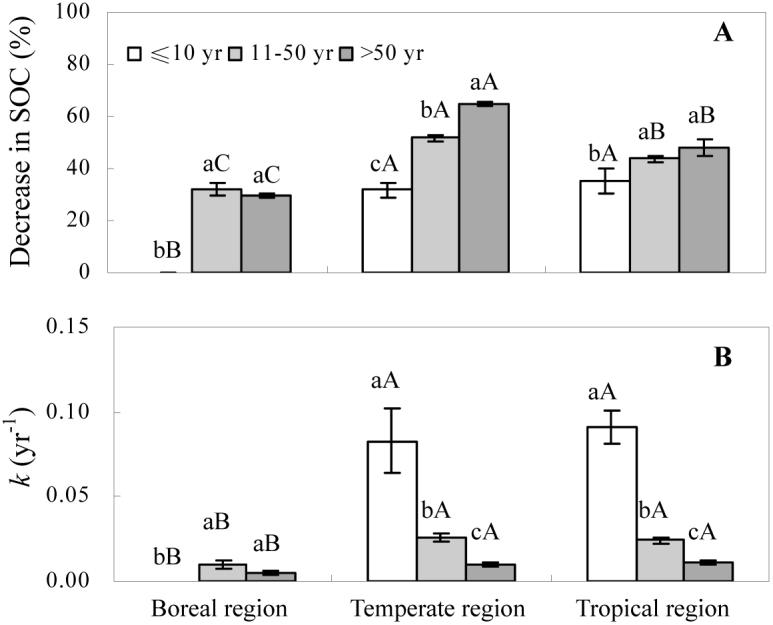
Negative values indicate that SOC stocks increased after the land-use change. The error bars represent the standard errors for each group. The boreal region had 0, 12, and 10 sites in the early (≤10 yr), middle (11–50 yr), and late (>50) stages of cultivation, respectively. The temperate region had 19, 58, and 34 sites in the early, middle, and late stages of cultivation, respectively. The tropical region had 128, 162, and 30 sites in the early, middle, and late stages of cultivation, respectively. Values with different lowercase letters are different at a significance level of P < 0.05 for each cultivation stage. Values with different uppercase letters are different at a significance level of P < 0.05 for each forest type. The interaction between forest type and cultivation stage had no significant effect on the turnover rate constant (P = 0.3073) or the percent decrease in SOC (P = 0.0793).
Cultivation stage and forest type also had significant effects on the SOC turnover rate constant. Averaged across all cultivation stages, the turnover rate constants were 0.020 ± 0.003 yr−1 in tropical regions, 0.020 ± 0.003 yr−1 in temperate regions, and 0.006 ± 0.003 yr−1 in boreal regions (P < 0.05, Fig. 3B). Averaged across all forest types, the turnover rate constants were 0.090 ± 0.018 yr−1 at sites in the early stage, 0.023 ± 0.003 yr−1 at sites in the middle stage, and 0.010 ± 0.001 yr−1 at sites in the late stage of cultivation (P < 0.05, Fig. 3B). For each cultivation stage, the turnover rate constants were higher in both temperate and tropical regions than in boreal regions (Fig. 3B).
2. Effects of soil properties and climate
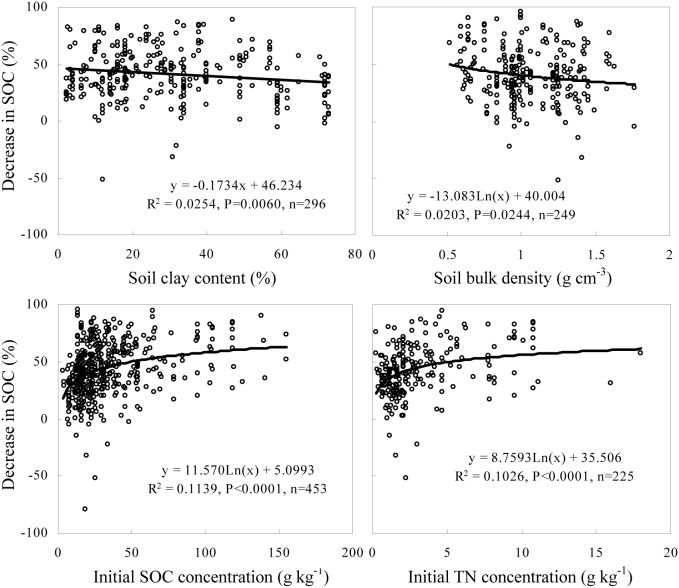
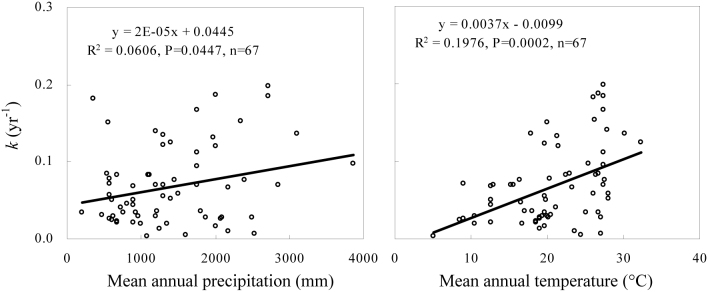
The percent decrease in SOC stocks after the land-use change was significantly and positively correlated with the initial SOC concentration and with the initial total soil N concentration; however, a negative correlation was found with the soil clay content and the bulk density (Fig. 4). The turnover rate constants were significantly and positively correlated with the mean annual precipitation and mean annual temperature (Fig. 5). There were no significant interactive effects between the mean annual precipitation and the mean annual temperature or among soil properties (Table S1) in this study. Additionally, the effects of climate and soil properties on the percent decrease in SOC stocks varied with cultivation age and forest type (Tables S2–3).
Figure 4. The effects of initial soil clay content, bulk density, initial SOC concentration, and initial N concentration on the percent decrease in SOC stock after the conversion of forests to agricultural land.
Negative values indicate that SOC stocks increased after the land-use change.
Figure 5. The effects of mean annual precipitation and mean annual temperature on the SOC turnover rate constant (k) after the conversion of forests to agricultural land.
During the early stage of cultivation, the percent decrease in SOC stocks was significantly affected by the mean annual precipitation, mean annual temperature, soil clay content, initial SOC concentration, and initial total N concentration (Table S2). Moreover, the percent decrease in SOC stocks was significantly affected by the soil bulk density, initial SOC concentration, and initial total N concentration during the middle stage of cultivation (Table S2). During the late stage of cultivation, the percent decrease in SOC stocks was significantly affected by the soil bulk density, initial SOC concentration, and initial total N concentration (Table S2).
In boreal regions, the percent decrease in SOC stocks was significantly affected by the soil bulk density, initial SOC concentration, and the initial total N concentration. Furthermore, the decrease in SOC stocks was significantly affected by the soil clay content, soil pH, and initial total N concentration in temperate regions (Table S3). The mean annual temperature and precipitation exhibited no significant effects on the decrease in SOC stocks in either boreal or temperate regions (Tables S3). In tropical regions, the decrease in SOC stocks was significantly affected by the mean annual precipitation, soil clay content, bulk density, initial SOC concentration, and initial total N concentration (Table S3).
Discussion
The conversion of forests to agricultural land caused a rapid initial decrease in SOC stocks, followed by a slow decline. This description is consistent with reports that most SOC losses occur within the first few years after the cultivation of previously untilled soils6,9,10. For example, Davidson and Ackerman (1993) observed that 20% of SOC was lost within the first 5 yr of cultivation and that an additional 5% of SOC was lost within the next 15 yr6.
Across all of the cultivation stages in our study, the SOC stocks decreased by 43.1 ± 1.1%. This decline was much higher than the 30% decrease reported by Davidson and Ackerman (1993)6 and Murty et al. (2002)8 but similar to the 42% decrease observed by Guo and Gifford (2002)7. Cultivation stage should be considered when comparing datasets. For example, 58% of the sites in the review by Murty et al. (2002)8 were in the early stage of cultivation. However, in our study, 68% of the sites were in the middle or late stages of cultivation. This difference is important because the percent decrease in SOC stocks increases with time. Therefore, decreases in SOC stocks will be underestimated in recently converted land. Soil depth is another important consideration in estimating SOC stocks. Our dataset focused on changes in stocks within the topsoil (0–30 cm) because that is where the largest changes in SOC occur. Therefore, the percent decrease in SOC stocks was expected to be higher than in studies that considered a depth of 0–100 cm.
The decrease in SOC following the land-use change was largest in temperate regions, which was followed by tropical regions and then boreal regions. The turnover rate constants followed a slightly different order, i.e., higher in tropical and temperate regions than in boreal regions. This finding was expected because the higher mean annual temperatures in temperate and tropical regions accelerate organic matter mineralization compared with boreal regions11,12,13,14. Moreover, SOC should decrease more after a forest is converted to agricultural land in tropical regions compared to temperate regions due to the higher mean annual temperatures; however, we observed greater changes in temperate regions. One explanation for this discrepancy is that the sites in temperate regions have generally been cultivated longer than the sites in tropical areas (i.e., 83% of the temperate sites have been cultivated for more than 10 yr, whereas only 60% of the tropical sites have been cultivated for more than 10 yr).
The percent decrease in SOC during the early stage of cultivation was significantly affected by both climate (i.e., mean annual precipitation and mean annual temperature) and soil properties (i.e., soil clay content, pH and initial SOC and total N concentrations). Rapid decreases in SOC during the early stage of cultivation are likely caused by the destruction of soil aggregates by tillage15. Rainfall during this period can accelerate the destruction of aggregates. Moreover, high temperatures and the greater availability of SOC and N can increase the SOC mineralization rates14,16,17,18. During the middle and late stages of cultivation, the percent decrease in SOC was significantly affected by soil properties (i.e., soil bulk density, initial SOC concentration, and initial total N concentration) and not by climate.
Comparisons within forest types indicated that the decreases in SOC after conversion to agricultural land were determined by the mean annual precipitation in tropical regions. The SOC loss was expected to depend on climate conditions in tropical regions because the moisture and higher temperatures enhance soil organic matter decomposition and thus the loss of SOC13,14. However, climatic factors in neither boreal nor temperate areas significantly affected the percent decrease in SOC. One explanation for this finding is that the dataset contained fewer observations from boreal (22 sites) and temperature regions (111 sites) than from tropical regions (320 sites). The analysis may have been affected by the narrow gradients in the mean annual precipitation and mean annual temperature at the temperate and boreal sites. The effects of forest cultivation on SOC losses and the dependence of the losses on climate conditions should be studied further, especially in boreal regions.
Regardless of the cultivation stage or forest type, the largest decreases in SOC stocks occurred for high initial SOC or total N concentrations. The smallest decreases in SOC stocks were observed for high soil clay content or bulk density. Our results agree with previous findings that mineralization rates generally increase as the concentrations of either SOC or total N increase16,17,18 and as soil clay content decreases19. Previous studies have indicated linear relationships between SOC loss and initial SOC and N concentrations8. This indicates that the SOC loss would continue to increase as the initial SOC and N concentration increased. In contrast, our results exhibited nonlinear relationships between SOC loss and initial SOC and N concentrations. The nonlinear relationship in which SOC losses eventually stop increasing with continually increasing initial SOC and N concentrations is more realistic. Accordingly, the SOC stocks should decrease more rapidly in soils with high sand or initial SOC levels than in soils with low sand or initial SOC levels. Moreover, atmospheric N deposition and the application of nitrogenous fertilizers could also result in greater decreases in SOC stocks. All of these factors should be considered when predicting the SOC response to the conversion of forests to agricultural land.
In our study, the turnover rates increased (turnover times decreased) significantly as the mean annual temperature increased. This observation is consistent with previous findings that SOC decomposition rates increased with mean annual temperature at both regional and global scales13,14,20. In contrast, Giardina and Ryan (2000)21 observed that the SOC decomposition rates in undisturbed forest soils were remarkably similar across a wide range of mean annual temperatures. This result, suggests that the mechanism behind SOC losses after the conversion of forests to agricultural land is different from the mechanism causing SOC mineralization in undisturbed soil with natural vegetation.
The dependence of SOC turnover on the mean annual temperature indicates not only that future temperature increases will result in greater SOC losses but also that there is a positive feedback between SOC mineralization and global warming. Most SOC in undisturbed land is occluded within aggregates and thereby physically protected from microbial decomposition22,23. Converting forests to agricultural land damages the aggregate structure, making SOC more available for microbial attacks. Plants and soil microorganisms may acclimate to changes in temperature, minimizing the feedback between CO2 release and global warming24,25,26,27. However, the disruption of aggregates by tillage is not affected by temperature or moisture. Conversion of forests to agricultural land could thus enhance the feedback between CO2 emission and global warming and should be carefully managed.
Most previous studies have emphasized the loss of SOC in tropical areas2,3. However, 35% of the global decrease in forest cover has occurred in boreal regions28. There is more organic C in boreal forest soil than in either temperate or tropical forest soils29,30. Furthermore, some models predict that the largest increases in temperature due to global warming will occur in boreal regions1. Therefore, the SOC loss following the conversion of boreal forests to agricultural land deserves more attention in future scenarios of global warming. If the loss of SOC due to the conversion of grassland to agricultural land depends on temperature, then any conversion from natural vegetation to agriculture would accelerate the positive feedback between the C cycle and the climatic system.
Methods
Data acquisition
The data were obtained from 119 peer-reviewed publications that described 453 sites in 36 countries (Table S4). To be included in this dataset, the data had to satisfy the following criteria: (1) the cultivated plots must have been converted from primary forest, (2) the cultivated and forest plots must be adjacent to ensure similar pedological conditions, and (3) the time of forest conversion must be known or derivable. Only studies with paired plots or chronosequences were included. The variables that were obtained included the following: forest type (i.e., tropical, temperate, and boreal forests), cultivation stage, SOC concentration or stock, total N concentration or stock, soil texture (i.e., percent sand and clay), bulk density, pH, sampling depth, and the mean annual precipitation and temperature. We classified the data into three stages based on the time since the conversion of forests to agricultural land: early (≤10 yr), middle (11–50 yr), and late (>50 yr). The analytical methods for measuring SOC varied among the studies. However, these differences should not have affected our analysis because we compared either k or the relative change in SOC within a specific study.
The SOC stocks were calculated based on the SOC concentration, soil depth, and bulk density at each site. The bulk density of forest soil often changes after land-use change; therefore, many researchers have emphasized that corrections for changes in bulk density should be made before comparing SOC stocks in forest and cultivated soils2,8,31,32,33.
The corrected SOC stocks were calculated in this study as31,33
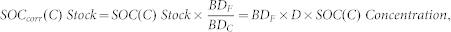 |
where SOCcorr(C) Stock is the corrected SOC stock based on the equivalent soil mass in the cultivated soils, SOC(C) Stock is the uncorrected SOC stock in cultivated soils, BDF and BDC are the bulk densities of forest and cultivated soils, respectively, D is the sampling depth, and SOC(C) Concentration is the SOC concentration in cultivated soils.
In this study, we only considered SOC in the upper 0–30 cm because the largest changes in SOC occur there and because more data are available compared to subsoil depths. Among the 453 sites, 20 characterized the soil depth as the surface layer. We assumed a soil depth of 20 cm for these sites because we could not verify the exact soil depth. To simplify the comparisons, we reported the SOC stocks per 10 cm depth of soil at each site. The organic horizon was excluded from this study.
When the data presented in the original publications were presented as concentrations, we calculated the SOC stock by accounting for the bulk density at each depth. However, a few studies did not report bulk densities. In these cases, the bulk density was estimated using a relationship between the bulk density and the SOC concentration that was derived from the overall dataset. For forest soil, the relationship was
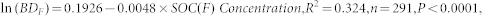 |
where ln(BDF) is the natural logarithm of the forest soil bulk density and SOC(F) Concentration is the SOC concentration in the forest soil.
For agricultural land, the relationship was
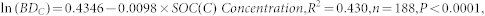 |
where ln(BDC) is the natural logarithm of the bulk density in the agricultural soil and SOC(C) Concentration is the SOC concentration in the agricultural soil.
Data analysis
The relative decrease in SOC (ΔSOC, %) after forest cultivation was calculated as
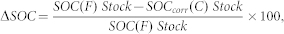 |
where SOC(F) Stock is the SOC stock in forest soil.
A first-order model was used to assess the turnover of SOC after the conversion from forest to agricultural land as suggested by Six and Jastrow (2002)34:
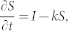 |
where k is the turnover rate constant (yr−1), t is the time since the land-use change (yr), S is the SOC stock at time t (SOCcorr(C) in this study), and I is the input rate. This formulation represents a mono-exponential decay model and has been commonly used to describe the turnover of SOC. This model assumes a constant zero-order input and a first-order SOC loss rate. The solution to the model is35
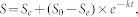 |
where S0 is the SOC stock before conversion (SOC(F) in this study), and Se is the equilibrium SOC stock. This method has been used to estimate the turnover of SOC following land-use changes23,34,36. In this study, the model was fitted to the entire dataset to obtain a general turnover rate constant and then fitted to each forest type across and within stages and to each cultivation stage across and within forest types to obtain turnover rate constants for each forest type and cultivation stage. We also calculated the turnover rate constant for all of the sites that were sampled at least 3 times since conversion in the same study (including pre-conversion forest as year 0). In total, turnover rate constants were determined for 67 sites. These 67 turnover rate constants were used to establish the relationships with climate and soil properties.
A two-way analysis of variance was performed to test the primary and interactive effects of the forest type and cultivation stage on the percent decrease in SOC. A one-way variance analysis was conducted to test the effects of forest type within each cultivation stage and to test the effects of cultivation stage within each forest type.
We used a 95% confidence interval (CI) to assess the statistical differences in the turnover rate constants within forest types or cultivation stages. The 95% CI was calculated as
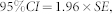 |
where SE is the standard error of the turnover rate constant and was obtained from the model fitting. The differences between groups (forest types and cultivation stages) were identified as significant when the 95% CIs did not overlap.
General linear models were used to test the primary and interactive effects of climate factors (i.e., mean annual precipitation and mean annual temperature) and the primary and interactive effects of soil characteristics (i.e., of soil clay content, soil pH, soil bulk density, and initial SOC or N concentrations) on the percent decrease in SOC and the turnover rate constant. Nitrogenous fertilizers are often applied to cultivated soil. Therefore, we did not analyze the changes in soil N after forest cultivation. Generally, the decrease in SOC is a function of both climate and soil physicochemical properties. Therefore, we used linear and nonlinear regressions to test relationships across and within forest types and cultivation stages between the percent decrease in SOC and the mean annual precipitation, mean annual temperature, soil clay content, soil pH, bulk density, or initial SOC and N concentrations.
We also used linear and nonlinear regressions to test whether the turnover rate constant was related to the mean annual precipitation, mean annual temperature, soil clay content, soil pH, bulk density, or initial SOC and N concentrations for all forest types and cultivation stages. We did not analyze the effects of climate and soil properties on the turnover rate constant for each forest type or cultivation stage due to the limited sample sizes for each group. Data analysis was performed using JMP 10 software (SAS Institute, Cary, NC, USA).
Author Contributions
X.W., M.S. designed the study, X.W. conducted the study, X.W., W.G. wrote the paper, X.W., W.G., L.L. revised the paper.
Supplementary Material
Supplementary Info
Supplementary Table S4 The compiled global dataset-Wei
Acknowledgments
This research was supported by National Natural Science Foundation of China (41271315, 40901145, 40801111), the Program for New Century Excellent Talents in University (NCET-13-0487) and the Programs from the Institute of Soil and Water Conservation of CAS & MWR (A315021381).
References
- IPCC. Climate change 2007: The physical science basis (Cambridge University Press, Cambridge, UK and New York, USA, 2007). [Google Scholar]
- Don A., Schumacher J. & Freibauer A. Impact of tropical land-use change on soil organic carbon stocks–a meta-analysis. Global Change Biol. 17, 1658–1670 (2011). [Google Scholar]
- Harris N. L. et al. Baseline map of carbon emissions from deforestation in tropical regions. Science 336, 1573–1576 (2012). [DOI] [PubMed] [Google Scholar]
- Heimann M. & Reichstein M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451, 289–292 (2008). [DOI] [PubMed] [Google Scholar]
- Mahecha M. D. et al. Global convergence in the temperature sensitivity of respiration at ecosystem level. Science 329, 838–840 (2010). [DOI] [PubMed] [Google Scholar]
- Davidson E. A. & Ackerman I. L. Changes in soil carbon inventories following cultivation of previously untilled soils. Biogeochemistry 20, 161–193 (1993). [Google Scholar]
- Guo L. B. & Gifford R. M. Soil carbon stocks and land use change: a meta analysis. Global Change Biol. 8, 345–360 (2002). [Google Scholar]
- Murty D., Kirschbaum M. F., McMurtrie R. E. & McGilvray H. Does conversion of forest to agricultural land change soil carbon and nitrogen? A review of the literature. Global Change Biol. 8, 105–123 (2002). [Google Scholar]
- Houghton R. A., Skole D. L. & Lefkowitz D. S. Changes in the landscape of Latin America between 1850 and 1985 II. Net release of CO2 to the atmosphere. Forest Ecol. Manag. 38, 173–199 (1991). [Google Scholar]
- Mann L. K. Changes in soil carbon after cultivation. Soil Sci. 142, 279–288 (1986). [Google Scholar]
- Trumbore S. E., Chadwick O. A. & Amundson R. Rapid exchange between soil carbon and atmospheric carbon dioxide driven by temperature change. Science 272, 393–396 (1996). [Google Scholar]
- Cao M. K. & Woodward F. I. Dynamic responses of terrestrial ecosystem carbon cycling to global climate change. Nature 393, 249–252 (1998). [Google Scholar]
- Knorr W., Prentice J. C., House J. I. & Holland E. A. Long-term sensitivity of soil carbon turnover to warming. Nature 433, 298–302 (2005). [DOI] [PubMed] [Google Scholar]
- Bond-Lamberty B. & Thomson A. Temperature-associated increases in the global soil respiration record. Nature 464, 579–582 (2010). [DOI] [PubMed] [Google Scholar]
- Grandy A. S. & Robertson G. P. Aggregation and organic matter protection following tillage of a previously uncultivated soil. Soil Sci. Soc. Am. J. 70, 1398–1406 (2006). [Google Scholar]
- Bellamy P. H., Loveland P. J., Bradley R. I., Lark R. M. & Kirk G. J. D. Carbon losses from all soils across England and Wales 1978–2003. Nature 437, 245–248 (2005). [DOI] [PubMed] [Google Scholar]
- Mack M. C., Schuur E. A. G., Bret-Harte M. S., Shaver G. R. & Chapin F. S. III Ecosystem carbon storage in arctic tundra reduced by long-term nutrient fertilization. Nature 431, 440–443 (2004). [DOI] [PubMed] [Google Scholar]
- Nadelhoffer K. J., Giblin A. E., Shaver G. R. & Laundre J. A. Effects of temperature and substrate quality on element mineralization in six arctic soils. Ecology 72, 242–253 (1991). [Google Scholar]
- Torn M. S., Trumbore S. E., Chadwick O. A., Vitousek P. M. & Hendricks D. M. Mineral control of soil organic carbon storage and turnover. Nature 389, 170–173 (1997). [Google Scholar]
- Raich J. W., Russell A. E., Kitayama K., Parton W. J. & Vitousek P. M. Temperature influences carbon accumulation in moist tropical forests. Ecology 87, 76–87 (2006). [DOI] [PubMed] [Google Scholar]
- Giardina C. P. & Ryan M. G. Evidence that decomposition rates of organic carbon in mineral soil do not vary with temperature. Nature 404, 858–861 (2000). [DOI] [PubMed] [Google Scholar]
- Von Lützow M. et al. SOM fractionation methods: relevance to functional pools and to stabilization mechanisms. Soil Biol. Biochem. 39, 2183–2207 (2007). [Google Scholar]
- Wei X. R., Shao M. A., Gale W. J., Zhang X. C. & Li L. H. Dynamics of aggregate-associated organic carbon following conversion of forest to cropland. Soil Biol. Biochem. 57, 876–883 (2013). [Google Scholar]
- Oechel W. C. et al. Acclimation of ecosystem CO2 exchange in the Alaskan Arctic in response to decadal climate warming. Nature 406, 978–981 (2000). [DOI] [PubMed] [Google Scholar]
- Luo Y., Wan S., Hui D. & Wallace L. Acclimatization of soil respiration to warming in a tall grass prairie. Nature 413, 622–625 (2001). [DOI] [PubMed] [Google Scholar]
- King A. W., Gunderson C. A., Post W. M., Weston D. J. & Wullschleger S. D. Plant respiration in a warmer world. Science 312, 536–537 (2006). [DOI] [PubMed] [Google Scholar]
- Atkin O., Millar H. & Turnbull M. Plant respiration in a changing world. New Phytol. 187, 268–272 (2010). [DOI] [PubMed] [Google Scholar]
- Hansen M. C., Stehman S. V. & Potapov P. V. Quantification of global gross forest cover loss. Proc. Natl. Acad. Sci. USA 107, 8650–8655 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- House J. I., Prentice I. C. & Quéré C. L. Maximum impacts of future reforestation or deforestation on atmosphere CO2. Global Change Biol. 8, 1047–1052 (2002). [Google Scholar]
- Ping C. et al. High stocks of soil organic carbon in the North American Arctic region. Nat. Geosci. 1, 615–619 (2008). [Google Scholar]
- Ellert B. H. & Bettany J. R. Calculation of organic matter and nutrients stored in soils under contrasting management regimes. Can. J. Soil Sci. 75, 529–538 (1995). [Google Scholar]
- Lee J., Hopmans J. W., Rolston D. E., Baer S. G. & Six J. Determining soil carbon stock changes: Simple bulk density corrections fail. Agr. Ecosys. Environ. 134, 251–256 (2009). [Google Scholar]
- Poeplau C. et al. Temporal dynamics of soil organic carbon after land-use change in the temperate zone-carbon response functions as a model approach. Global Change Biol. 17, 2415–2427 (2011). [Google Scholar]
- Six J. & Jastrow J. D. in Encyclopedia of Soil Science. (ed Lal R.) 936–942 (USA Marcel Dekker, New York, 2002). [Google Scholar]
- Jastrow J. D. Soil aggregate formation and the accrual of particulate and mineral-associated organic matter. Soil Biol. Biochem. 28, 665–676 (1996). [Google Scholar]
- Preger A. C., Kösters R., Du Preez C. C., Brodowski S. & Amelung W. Carbon sequestration in secondary pasture soils: a chronosequence study in the South African Highveld. Eur. J. Soil Sci. 61, 551–562 (2010). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Info
Supplementary Table S4 The compiled global dataset-Wei