Abstract
Much of the activity of the macrophage as an effector cell is performed within its phagocytic compartment. This ranges from the degradation of tissue debris as part of its homeostatic function, to the generation of the superoxide burst as part of its microbicidal response to infection. We have developed a range of real-time readouts of phagosomal function that enables these activities to be rigorously quantified. This chapter contains the description of several of these assays assessed by different methods of quantitation; including a Fluorescence Resonance Emission Transfer (FRET) assay for phagosome/lysosome fusion measured by spectrofluorometer, a fluorogenic assay for the superoxide burst measured by flow cytometry, and a fluorogenic assay for bulk proteolysis measure by confocal microscope. These assays illustrate both the range parameters that can be quantified as well as the flexibility of instrumentation that can be exploited for their quantitation.
Keywords: Macrophage, phagosome, phagocytosis, phagosome maturation
Introduction
This unit details quantitative, real-time assays that measure a variety of physiological parameters within the phagosomes of professional phagocytes. Phagocytes fulfill diverse functions within the body, ranging from the clearance of dead and dying cells, through the killing of invading microbes, to the processing and presentation of antigens to T cells. The majority of these functions are performed, at least in part, within the phago-lysosome of the cell. The assays detailed in this unit measure parameters that are important to these cellular functions. To date we have developed assays that measure pH, a Fluorescence Resonance Energy Transfer (FRET) assay for phagosome-lysosome fusion, and assays that measure the following enzymatic activities; bulk proteolysis, cysteine proteinase activity, lipolysis, β-galactosidase activity, lipid peroxidation, and NADPH oxidase activity (Yates, Hermetter et al. 2005; Yates and Russell 2008; Russell, Vanderven et al. 2009; VanderVen, Yates et al. 2009; Yates, Hermetter et al. 2009). In this unit we provide detailed descriptions of three assays, one that measures phagosome/lysosome fusion, one that quantifies the superoxide burst generated within the phagosomal cup, and one that measures bulk proteolytic activity in the maturating phagosome. Each assay is quantified using different instrumentation to illustrate the flexibility of the reporter platform.
The assays all exploit small (3μm diameter) particles that are modified to carry a fluorogenic reporter together with a calibration fluorochrome. For all of the enzyme-based assays the readout is expressed as a ratio of the substrate fluorescence divided by the calibration fluor, thus providing an internal correction for dosage.
All the assays were developed using a PTI SM4 QE Spectrofluorometer, however the methods are extremely flexible and can be analyzed by fluorescent plate reader, confocal microscope and by flow cytometry with Fluorescent Activated Cell Sorters (FACS) instruments.
Strategic Planning
Documentation of all of the phagosome assays we have developed is beyond the purview of this chapter and for that reason we have decided to present three different assays analyzed by three different approaches. The choice of analytical method is just as important as the choice of assay.
The first assay is the FRET assay for phagosome/lysosome fusion. This is an assay that has broad application for any questions relating to phagosome maturation. It is a sustained assay that can be performed over an extended time window because it is not reliant on an enzymatic readout that could be substrate limiting. This assay is recorded using a spectrofluorometer, which generates averaged data across all the cells in the excitation field of the spectrofluorometer.
The second assay is a substrate-based assay that measures the activity of the NADPH oxidase complex within the phagosome. This is a short-term assay that measures a transient event that occurs in the first 20 minutes following initiation of phagosome formation. This assay is analyzed by flow cytometry, which does not give the fine temporal resolution of the spectrofluorometer but it does provide an excellent method for assessing heterogeneity in the response at the cellular level.
The third assay measure bulk proteolytic activity in the maturing phagosome. This is an assay of longer duration, 1–4 hrs, that measures the degradation and unquenching of fluorescently-derivatized BSA. The assay has the appeal that it actually reports the sum total of multiple steps in the maturation process because total proteolytic activity is influenced by pH, acquisition and activation of lysosomal hydrolases, and fusion with pre-existing lysosomal compartments. In the approach documented in this Chapter we quantify the increasing fluorescence by confocal microscopy at the level of the individual phagosome to reveal the heterogeneity in the process.
We feel that the provision of these three assays and diverse methods of analysis will aid investigators in deciding which platform is most suitable to their own experiments. The other assays that we have developed but do not detail here are described in depth in the following papers (Yates, Hermetter et al. 2005; Yates and Russell 2008; Russell, Vanderven et al. 2009; VanderVen, Yates et al. 2009; Yates, Hermetter et al. 2009).
Basic Protocol 1. Measurement of phagosome/lysosome fusion
This protocol describes a rigorous, quantitative assay that measures the concentration of fluid phase lysosomal cargo in the phagosome following internalization of the reporter particle, which provides a functional read-out of the amount of phagosome/lysosome fusion. IgG-opsonized reporter particles derivatized with a donor fluorochrome are fed to murine bone marrow-derived macrophages (Mø), the endosomal system of which was loaded previously with an acceptor fluorochrome. The degree of mixing between the nascent phagosomes and the pre-existing lysosomes is measured by the quenching of the donor fluorochrome on the particle and the excitation of the acceptor fluorochrome in the lysosomes through quantitation of the resulting FRET signal.
Materials List
Solutions and Reagents
Macrophages, bone-marrow–derived murine macrophages (Mø) have been used (see reagents).
Cover slips: Sterile 13 mm × 25 mm cover glass from Knittel Glaser.
Cuvette Buffer (see recipe)
IgG-opsonized FRET Reporter Particles particles consisting of 3.0 μm carboxylate-modified silica particles (Si-COOH) (Kisker Biotech) (see reagents).
Donor Flurochrome: Alexa Fluor 488 carboxylic acid, succinimidyl ester (Alexa 488-SE) (Molecular Probes).
Acceptor Fluorochrome: Alexa Fluor 594 hydrazide, sodium salt (Alexa 594-HA) (Molecular Probes).
Cyanamide (Sigma).
Coupling Buffer (see recipe).
Special Equipment
Spectrofluorometer
The spectrofluorometer should be set up according to manufacturer’s directions such that optimal measurements can be taken using the desired wavelengths. We use a PTI QMSE4 spectrofluorometer with monochrometer adjustment of the excitation and emission wavelengths. The instrument has a 4 position cuvette holder in an environmental chamber that is equilibrated to 37°C prior to initiation of the assay. Clean quartz cuvettes containing Cuvette Buffer are inserted into the cuvette holders and warmed to 37°C prior to the loading of the monolayers
Prior to initiation of the assay the Mø need to be loaded with acceptor fluorochrome. Using sterile forceps, transfer the Mø monolayers on glass cover slips (prepared as per reagents), to a Petri dish containing 100 μg/ml of the acceptor fluor Alexa Fluor 594 hydrazide (Alexa 594-HA) in 6 ml Mø media. The monolayers are incubated at 37°C for 3–5 h to allow sufficient pinocytic uptake of the membrane impermeable acceptor fluor. Remove the Mø monolayers, dip them in fresh medium to remove excess fluor, and place them in a Petri dish containing fresh, warm growth medium. Incubate the monolayers at 37°C for a further 4–10 h to ensure the acceptor fluor is chased into the lysosomes.
Remove 20 μL of IgG-opsonized FRET reporter particles from the stock solution (this contains sufficient particles to give a dose of 2–3 particles per cell on a fully confluent coverslip, if your format differs from the one detailed here you will need to recalculate the particle dose). Wash the particles twice with Cuvette Buffer to remove the Na azide then resuspend in 400 μL of Cuvette Buffer to achieve a concentration of approximately 107 particles/ml.
Place the 4 coverslips with Mø monolayers into the 4 cuvettes each containing 3 ml of Cuvette Buffer equilibrated to 37°C, as illustrated in Figure 1. Prior to adding the particle you need to establish the background fluorescent values by recording the following 3 measurements: First, measure the total acceptor fluorochrome emission at 620 nm with excitation at 594 nm. While this measurement is not used in any further calculation, it is a necessary control to ensure equivalent acceptor fluorochrome loadings and Mø monolayer densities of the samples. Second, obtain accurate background values for both the donor and FRET wavelengths at 488|520 nm and 488|620 nm (excitation|emission) respectively.
Remove the Mø monolayers from the spectrofluorometer, place cell side up on Parafilm and add 85 μL of the FRET reporter particle suspension to each coverslip. Incubate for 3 min at rt, rinse off unbound particles by dipping the coverslips into a 50 ml Falcon tube filled with warmed Cuvette Buffer then replace the Mø monolayers into the same cuvettes and sample positions used to establish the preliminary measurements.
-
Record both the donor fluorochrome and FRET-generated emissions at 520 and 620 nm, respectively, following excitation at 488 nm. Typically an integration time of 1 s per data point is optimal. Collect data for at least 3 h to allow all the phagosomes to reach equilibrium with respect to accumulation of lysosomal constituents. At the conclusion of the assay, examine the monolayers by microscopy with trypan blue to ensure that cell viability has not been affected during the assay.
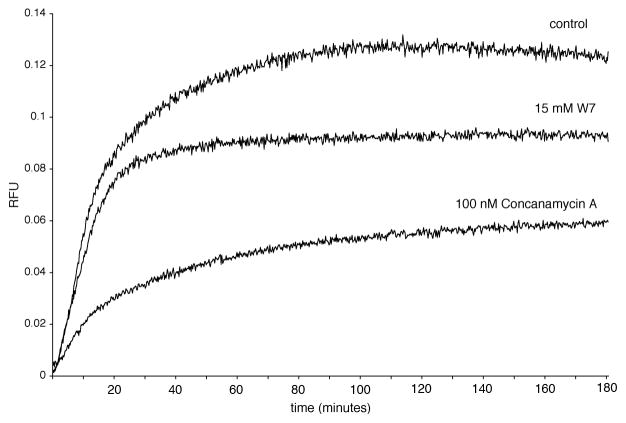
The rates and degree of lysosomal mixing with the phagosomes can be manipulated by drugs such as the calmodulin inhibitor W7 (15 μM) and the V-ATPase inhibitor concanamycin A (100 nM), see Figure 2.
At the conclusion of the assay, determine the proportion of the “FRET” signal due to potential bleed-through signal from the particles themselves. This is a constant used in later calculations. Background measurements at 488|520 nm and 488|620 nm are made with a clean cuvette containing Cuvette Buffer. Add 5 μL of FRET particle suspension to the cuvette and record emissions at 520 and 620 nm following excitation at 488 nm.
Export all data into a standard spreadsheet application such as Microsoft Excel. Deduct the appropriate background values (if not already deducted by the acquisition software) and define the relative FRET units (RFU) by the following equation: RFU = FRT/DRT − FB/DB (where FRT = FRET generated fluorescent emission in real time, DRT = donor emission in real time, FB = “FRET” signal contribution of the particles alone, DB = donor emission of the particles alone) plot these values against time (see Figure 2). The RFU at time zero should be close to zero.
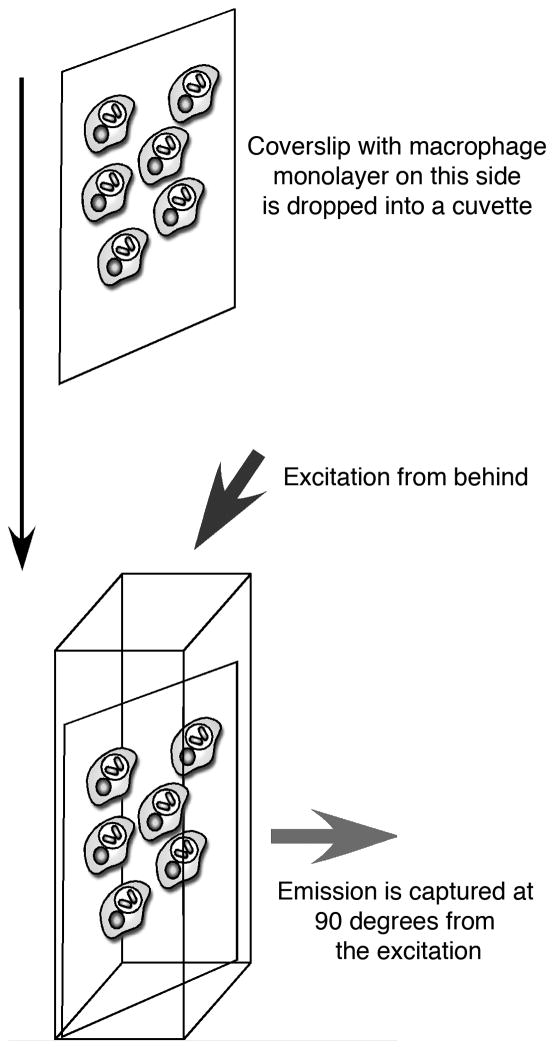
Figure 1.
Macrophages are established as confluent monolayers on coverslips sized to fit diagonally when dropped into cuvettes seated in the spectrofluorometer. The cells are excited from behind and the emission read from the cell side at a angle of 90 degrees to the excitation.
Figure 2.
FRET-based phagosome/lysosome fusion profiles. Lysosomal fusion profiles for IgG-coupled bead containing phagosomes were generated using the equation FU=FRT/DRT −FBO/DBO. Fluorescent measurements were taken every two seconds for three hours. Diminished phagosome/lysosome fusion was achieved with the calmodulin inhibitor W7 (15 μM) and the V-ATPase inhibitor concanamycin A (100 nM).
Basic Protocol 2. Intraphagosomal measurement of the magnitude and duration of the superoxide burst
This protocol describes the real-time measurement of both the magnitude and duration of the superoxide burst from within the phagosomal compartment. Existing assays measure the extracellular accumulation of byproducts of the NADPH oxidase activity. These assays have been extremely useful in defining the activity of the complex but they do not provide any spatial information regarding the intensity or duration of the burst within the phagosome, which is its site of action. The assay detailed here exploits reporter particles derivatized with both a calibration fluorochrome and the reporter fluorochrome, Oxyburst SE, which is thought to respond predominantly to H2O2.
Although these assays were developed using the spectrofluorometer, most have also been modified to facilitate analysis by FACS. This is to enable their use in immunology laboratories or in a clinical setting, as detailed in the following protocol.
Materials List
Solutions and Reagents
Macrophages, bone-marrow–derived murine macrophages (Mø) have been used (see reagents).
IgG-opsonized Superoxide Burst Reporter Particles particles consisting of 3.0 μm carboxylate-modified silica particles (Si-COOH) (Kisker Biotech) (see reagents).
Calibration Flurochrome: Alexa Fluor 633 carboxylic acid, succinimidyl ester (Alexa 633-SE) (Molecular Probes).
Reporter Fluorochrome: Oxyburst Green, succinimidyl ester (H2DCFDA-SE) (Molecular Probes).
Cyanamide (Sigma).
Coupling Buffer (see recipe).
1% paraformaldehyde in PBS
Special Equipment
Fluorescent Activated Cell Sorter (FACS) or Flow Cytometer
H2DCFDA-SE fluoresces at 520 nm following excitation at 490 nm and the Alexa 633-SE fluoresces at 650 nm following excitation at 630 nm. These fluorochromes were chosen because they are common settings for FACS instruments such as the BD FACScalibur, which was used in the protocol described.
Plate the Mø in 6 well plates to full confluency (approximately 106 cells per well).
Remove 5 μl of reporter particle suspension per experimental well (add an extra 10 μl to provide sufficient particles to calibrate the FACS instrument). Wash the particles three times with 1 ml medium (+FCS) in a microfuge prior to use. The FCS prevents the particles from sticking to the sides of the microfuge tube.
Resuspend the particles in 100 μl medium + FCS per experimental well (ie. 2 wells, 200 μl suspension), and keep in the dark until ready for use
Take the 6 well plate, remove the medium and non-adherent cells, and add fresh medium pre-warmed to 37°C (2 ml per well). Add 100 μl of particles per well, agitate gently and place in incubator at 37°C. This is T=0 min. Ensure that you leave one well with no reporter particles to enable the gates on the FACS instrument to be set prior to analysis of the experimental cells.
After 10 minutes take the first timepoint (t=10 min), and remove unbound particles from the other wells as follows: Agitate the plate and remove the medium from the 10 min well, add 1 ml cold PBS and scrape the cells into suspension, pipette and add to a tube with 1 ml PBS with 1% paraformaldehyde. Cap the tube and place at 4°C in dark. Gently pipette the medium in the remaining wells to resuspend unbound particles, remove and discard the medium. Replace with 2 ml warmed medium.
-
At t=60 min, remove the medium from the second well. Put 1 ml PBS in the well, scrape the cells, remove by pipette and put in a tube that has 1 ml of PBS + 1% paraformaldehyde. This sample can be stored at 4°C in the dark.
We are only detailing two time points in this assay. Additional time points or experimental conditions can be added as required.
At the conclusion of the assay take the unused reporter particles and add 50 μl of this suspension to 2 ml of PBS with 1% paraformaldehyde. Place in the dark at 4°C with the other samples.
The samples are analyzed by flow cytometry. First, run the reporter particles with no cells and record their relative fluorescence graphing the calibration fluorochrome (vertical axis, 650 nm emission) against the reporter fluorochrome (horizontal axis, 520 nm emission), see Figure 3A. You will have to reset the gates for the cells. Secondly, run the control cells with no particles to set the forward and side scatter gates at an appropriate level. Record the background fluorescence signal for the control cells in both channels, then record the fluorescence values for the experimental particles at the different time points, and graph the data as illustrated in Figure 3A.
-
Comparison between samples is achieved readily through calculation of the mean activity ratio, which is calculated from the average substrate signal divided by the average calibration fluorochrome signal as shown in Figure 3B.
The mean activity ratio can be used to compare different experimental conditions, or, as is shown in Figure 3, the relative activity in macrophages isolated from the lungs of different patients, discussed in reference (Russell, Vanderven et al. 2009).
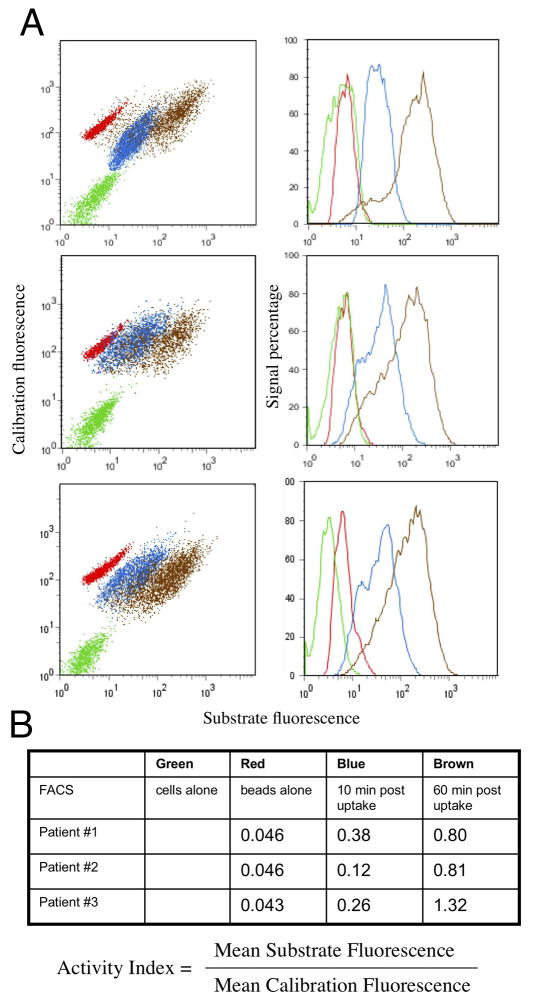
Figure 3.
Functional assays of phagosomes may be used in a clinical setting both as diagnostic and investigative tools. (A) shows the flow cytometric profiles from alveolar macrophages from three individual patients with no particles, the particles alone, and the macrophages with particles at 10 and 60 minutes post-internalization. Because the particles also carry the calibration fluorochrome Alexa 633-SE, the result can be expressed as an Activity Index to facilitate comparison between patients (B). In this instance the alveolar macrophages from one patient shows a higher superoxide burst indicating either that these cells may be activated, or that the other two patients’ cells may be depressed. Further analysis using antibodies against cell surface activation markers or HIV proteins can be done to associate functional phenotype with the infection or activation status of the macrophages.
Basic Protocol 3. Measurement of the extent of proteolytic activity in phagosomes
This protocol describes a real-time quantitative assay that measures the extent of bulk proteolytic activity in the phagosome. Bulk proteolysis is a useful indicator of progression of phagosome maturation since it is dependent upon efficient acidification and delivery of lysosomal enzymes to the maturing phagosome for optimal activity. IgG-opsonized reporter particles used for this assay are derivatized with both a calibration fluorochrome and the reporter fluorochrome, DQ Green-BSA, which upon hydrolysis by proteases produces brightly fluorescent product. As this is a substrate-based assay, it does suffer from possible substrate limitation, however for the majority of phagosomes, substrate limitation was not an issue for at least 2–3 hours of assay duration.
This assay can be performed using a spectrofluorometer, fluorescence plate reader or flow cytometer, and, like most other assays of phagosome function, has been adapted for quantitative analysis by real-time live cell confocal microscopy. This assay enables quantitative characterization of the onset and extent of proteolytic activity in individual phagosomes, and variation thereof, with high temporal detail. This confocal-based approach was exploited recently to probe the modulation of phagosome function in individual phagosomal compartments in Mycobacterium-tuberculosis-infected macrophages. The study highlights the differences between population-based analysis across a cell monolayer versus measurement at the level of the individual phagosome (Podinovskaia, Lee et al. 2012).
Materials list
Solutions and reagents
Macrophages, bone-marrow–derived murine macrophages (Mø) have been used (see reagents).
Chamber dishes: 35 mm μ-Dishes from Ibidi.
Cuvette Buffer (see recipe).
IgG-opsonized DQ Green-BSA Reporter Particles particles consisting of 3.0 μm carboxylate-modified silica particles (Si-COOH) (Kisker Biotech) (see reagents).
Calibration Flurochrome: Alexa Fluor 633 carboxylic acid, succinimidyl ester (Alexa 633-SE) (Molecular Probes).
Reporter Fluorochrome: DQ Green-BSA (Molecular Probes).
Cyanamide (Sigma).
Coupling Buffer (see recipe).
Special equipment
Confocal microscope
The microscope should have a stage heating system and the chamber should be pre-heated to 37°C. The microscope should be set up with excitation wavelengths of 488 and 633 nm, and emission range of 505–535 nm and 643–673 nm, for reporter and calibration fluorophore, respectively. We use a Leica SP5 confocal laser-scanning system with an inverted microscope and Leica Application Suite Advanced Fluorescence (LAS-AF) software (Leica Microsystems GmbH, Germany) for image acquisition and analysis. Imaging should be performed using a x63 oil objective.
Quantification software
Volocity image analysis software (PerkinElmer Life Sciences) is used for reporter particle tracking and quantification in the protocol described.
Plate Mø 35 mm Ibidi μ-Dishes to full confluency (approximately 106 cells per well). Plate an extra dish for calibration purposes.
Remove 2 μl of reporter particle suspension per dish. Wash the particles three times with 1 ml Cuvette Buffer to remove the Na azide. Resuspend in 40 μl Cuvette Buffer per dish (e.g. 2 wells, 200 μl suspension). This will provide a dose of 1–2 particles per cell. Keep in the dark until ready to use.
When ready to image, remove medium from the established Mø monolayer, wash with Cuvette Buffer pre-warmed to 37°C once, and add 1.5 mL Cuvette Buffer to the dish. Do this for one dish at a time, so as to avoid leaving cells in Cuvette Buffer for longer than 2 hours prior to imaging. Transfer the dish to the pre-heated chamber of the microscope.
Add 40 μl of particles to the dish reserved for calibration purposes and incubate for 40–80 min. Since the particles start out negative for green fluorescence, this late time point will provide a reference for the fluorescence emission intensity to set up the microscope accordingly.
Set up the microscope excitation wavelengths to 488 and 633 nm, and emission range of 505–535 nm and 643–673 nm, for reporter and calibration fluorochrome, respectively, and image bright-field in parallel. Ensure that fluorescence of the particles in the calibration dish is within the detectable range for both fluorochromes. Program the microscope to take fifteen 512×512 optical slices at 1.4 μm z-axis intervals every 1–2 min for the intended time period. Ensure that the z-stack captures the desired volume range. For the proteolysis assay, 80–120 min total time per assay is recommended.
Once the microscope is set up, perform step 3 for an experimental dish. Add 40 μl of particles to the dish. Immediately transfer the dish to the stage, adjust the focus and start recording. It is essential to note the time of particle addition and the time the imaging is started, to adjust for the time variations when comparing experimental sets.
After the assay is complete, merge the optical slices of the z-stack together, using maximum projection option. Select for particles using the calibration fluorochrome as a reference for finding regions of interest. This can be done by manually drawing regions of interest around the particles for each required time point, or for more precise and automated quantification, using measurements option in Volocity software.
Record maximum fluorescence for each region of interest in both, the reporter and the calibration fluorochrome channels. Volocity software will do this for every region and time point of interest. The ratio of the two will provide calibrated fluorescence of each particle at a specified time. See Figure 4A. Care must be taken to exclude beads that are not in direct contact with macrophages and hence do not get internalized.
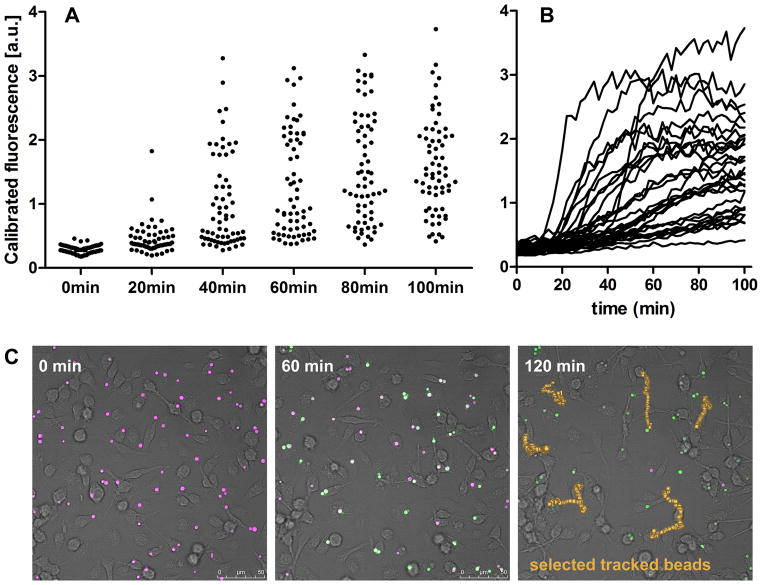
Figure 4.
Individual phagosomes display heterogeneity in the onset and extent of proteolytic activity. Fluorescence of the reporter and the calibration fluorochromes was recorded by confocal microscopy every 2 min for 2 hours following addition of reporter particles to macrophages. (A) Calibrated fluorescence of individual reporter particles at selected time points. (B) Calibrated fluorescence of individual reporter particles tracked over 100 min. (C) Confocal images of reporter particles in macrophages at selected time points, with reporter and calibration fluorochromes displayed in green and purple, respectively. Upon proteolysis, reporter particles fluoresce green. Paths taken by selected beads over 120 min are displayed in yellow.
The particles can be tracked over time using Track Objects option in Volocity software to produce a time kinetic for individual particles, as shown in Figure 4B. Care must be taken to ensure that every particle has been tracked correctly through manual monitoring. Figure 4C illustrates a typical set of micrographs achieved in the proteolysis assay and an example of particles tracked over 120 min using Volocity software.
Reagents and solutions
Mø are derived from the bone marrow extracted from the femurs, tibias, and iliums of euthanized mice and maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS), 5% horse serum, 2 mM l-glutamine, 1 mM sodium pyruvate, and 20% L929-cell conditioned media.
Macrophage monolayers: Fully differentiated Mø are grown to confluency in untreated Petri dishes. Growth media is removed and replaced with cold PBS pH7.2 prior to gently scraping the cells loose with a rubber policeman (Sarstedt). Sterile, clean 13 mm × 25 mm cover slips are placed in a sterile 4 chamber Petri dish with sterile forceps (2 coverslips per chamber). Mø are gently resuspended in media to a density of 1.0 × 106 Mø/ml. 3 ml of Mø suspension is added to each chamber of the Petri dish and incubated at 37°C for 24 h to allow a monolayer to establish on the cover slips. Finally, for the proteolysis assays, Mø are plated into 6-well plates or 35 mm Ibidi μ-Dishes.
Cuvette Buffer: Tissue culture tested phosphate-buffered saline (PBS) pH 7.2 (Gibco) adjusted to contain 1 mM CaCl2, 2.7 mM KCl, 0.5 mM MgCl2, 5 mM dextrose, 10 mM HEPES, and 0.1 % calf skin gelatin.
Coupling Buffer: 0.1 M sodium borate in ddH2O Adjust pH to 8.0 with 10 M NaOH. Filter sterilize through 0.22 μm filter.
FRET Reporter particles: 5 mg of carboxylate-modified silica particles is washed three times in 1 ml of PBS by brief vortexing and centrifugation in a tabletop microfuge at 2000g for 60 s. Particles are resuspended in PBS (pH 7.2) with 25 mg/ml of the heterobifunctional crosslinker cyanamide (freshly dissolved) and incubated at room temperature with agitation for 15 min. The particles are then washed twice with Coupling Buffer. The particles are resuspended in 1 ml of Coupling Buffer with 2 mg of defatted BSA (Sigma) and 50 μg murine IgG (Sigma), and incubated with agitation overnight at rt. Particles are washed twice with PBS. The particles are resuspended in 1 ml of Coupling Buffer, and 10 μl of the 5 mg/mL stock of Alex Fluor 488-SE in DMSO is added followed by incubation for 1 hr at rt. The fluorescent particles are washed three times with PBS and stored at 4°C in the dark following addition of 0.01% Na azide.
Superoxide Burst Reporter particles: 5 mg of carboxylate-modified silica particles is washed three times in 1 ml of PBS by brief vortexing and centrifugation in a tabletop microfuge at 2000g for 60 s. Particles are resuspended in PBS (pH 7.2) with 25 mg/ml of the heterobifunctional crosslinker cyanamide (freshly dissolved) and incubated at room temperature with agitation for 15 min. The particles are then washed twice with Coupling Buffer. The particles are resuspended in 1 ml of Coupling Buffer with 2 mg of defatted BSA (Sigma) and 50 μg murine IgG (Sigma), and incubated with agitation overnight at room temperature. Particles are washed twice with Coupling Buffer. The particles are resuspended in 1 ml of Coupling Buffer. 10 μl of the 5 mg/ml stock of Oxyburst-SE (H2DCFDA-SE) in DMSO is diluted to 100 μl with fresh DMSO and added to the particle suspension. The tube is purged with argon and incubated for 1 hr at rt. The H2DCFDA-SE particles are washed three times with Coupling Buffer, and resuspended in 1 ml of Coupling Buffer. 2 μl for a 5 mg/ml stock of Alexa 633-SE is added to the suspension, which is purged with argon and incubated for 60 min at rt. The particle are finally washed three times with PBS and stored at 4°C in the dark under argon following addition of 0.01% Na azide.
DQ Green BSA Reporter Particles: 5 mg of carboxylate-modified silica particles is washed three times in 1 ml of PBS by brief vortexing and centrifugation in a tabletop microfuge at 2000g for 60 s. Particles are resuspended in PBS (pH 7.2) with 25 mg/ml of the heterobifunctional crosslinker cyanamide (freshly dissolved) and incubated at room temperature with agitation for 15 min. The particles are then washed twice with coupling Buffer. The particles are resuspended in 1 ml of Coupling Buffer with 2 mg of DQ Green-BSA (Molecular Probes) and 50 μg murine IgG (Sigma), and incubated with agitation overnight at 4°C. Particles are washed twice with Coupling Buffer. The particles are resuspended in 1 ml of Coupling Buffer. 2 μl for a 5 mg/ml stock of Alexa 633-SE is added to the suspension and incubated for 60 min at rt. The particles are finally washed three times with PBS and stored at 4°C in the dark following addition of 0.01% Na azide.
Commentary
Macrophages are plastic cells that are required to fulfill a broad range of functions in the body. In their resting state their primary role is one of tissue homeostasis where they are required to ingest and degrade dead and dying cells without causing trauma to the tissue, or activating an innate or acquired immune response. However, in their activated state these cells are capable of efficient antigen presentation and of killing microbes through a range of antimicrobial mechanisms that can be highly inflammatory. The site of the majority of these activities is the phagosome, yet our appreciation of the physiology of this key organelle has been limited by the paucity of assays available to probe its functionality. The majority of published studies rely on immunofluoresent co-localization, which is based on on implied function through presence. When enzyme activities rely on activation, the pH optima, and the presence or absence of regulatory proteins, a simple measure of presence is a grossly inadequate readout.
Over the past few years we have developed assays the measure pH, phagosome/lysosome fusion and a range of enzymatic activities including bulk proteinase and cysteine proteinase activites, lipolysis and β-galactosidase activity, as well as the superoxide burst and lipid peroxidation (Yates, Hermetter et al. 2005; Yates and Russell 2008; VanderVen, Yates et al. 2009; Yates, Hermetter et al. 2009). We have applied these assays to probe the biology of the macrophage phagosome following activation of the cell with either innate or acquired immune stimulation.
Activation of macrophages with innate immune stimuli such as the TLR agonists LPS and Pam3Cys failed to influence the short-term maturation of the phagosome (up to 90 min), which was assayed by real-time readouts of the rate of phagosomal acidification and the kinetics of phagosome/lysosome fusion (measure by the FRET assay detailed here) (Yates and Russell 2005). These data are in contrast to the report of Blander and Medzhitov, which relied on observational analysis by immunofluorescent co-localization (Blander and Medzhitov 2004). The discrepancies between these two studies have been discussed in the literature (Russell and Yates 2007).
In contrast, long-term activation of macrophages (more than 120 minutes) with either TLR agonists of cytokines such as interferon-γ led to some major re-alignment in phagosomal function that would never have been detected by immunofluorescence analysis. The most striking difference was the down-regulation of proteolytic activity in the phagosomes of macrophages activated by interferon-γ (Yates, Hermetter et al. 2007). This might appear counterintuitive because the activated macrophage is regarded as a more aggressive phagocyte capable of killing microbes, however the reduced proteolytic capacity appears to correlate with the increased ability of the macrophage to process and present antigens. An activated macrophage expresses levels of intraphagosomal proteolysis comparable to a dendritic cell (Russell, Vanderven et al. 2009). In contrast, there is an increase in other anti-microbial activities such as the superoxide burst (VanderVen, Yates et al. 2009). Macrophage activation leads to an enhancement of the superoxide burst with respect to its intensity but not its duration. Our assays demonstrate that within the phagosome the superoxide burst is relatively short lived and only generates oxygen radicals over the first 20 minutes following the induction of phagosome formation. Recently these assays have been exploited to demonstrate that the reduced proteolysis found in the dendritic cell phagosomes is achieved through inactivation of hydrolases via the activity of the NADPH oxidase indicating that the superoxide burst cam modulate phagosome function in addition to its microbicidal activities (Rybicka, Balce et al. 2012).
The methods that we have developed clearly afford both new insights into the biology of the phagosome but also generate quantitative data capable of revealing subtle alterations in the kinetics of phagosomal physiology. Furthermore they have wide application and have been exploited recently to perform a high-throughput screen of 90,000 small molecules in 384 well plates to identify inhibitors of intra-phagosomal lipolysis. The assay exploited a quenched-pyrene substrate delivered on small C18 beads from a reverse phase HPLC column (VanderVen, Hermetter et al. 2010). We feel that these assays offer a marked improvement over immunofluorescent methods, and are readily transferable to other laboratories and other systems. These methods are extremely accessible.
Critical Parameters and Trouble Shooting
There are several factors that can impact negatively on the efficiency of these assays and these are discussed in the following section. Firstly, one of the most frequent problems is autofluorescence in the macrophages. We use bone-marrow-derived macrophages and these cells show increased autofluorescence as they age, particularly in the green channel, so we use the cells from a first passage following 5 days maturation in the presence of L929 cell-conditioned medium. Researchers need to check the autofluorescence of their specific phagocyte cell type prior to embarking on experiments.
The density of cells on the glass coverslip is also very important for assays employing a spectrofluorometer, such as Protocol 1. The cells need to be fully confluent to limit the background generated by particles sticking to the exposed glass. The cell number given in the method is for bone marrow-derived macrophages and will undoubtedly vary with the cell type employed.
The sensitivity of the assays is determined in part by the density of labeling of the reporter particles with the reporter flurochrome or substrate. The methods detail conditions that should provide the flurochrome or the substrate in excess thus maximizing the labeling process. An idea of the possible dynamic range can be obtained by suspending the FRET reporter particle in a solution containing acceptor fluorochrome (Alexa 594 hydrazide) and measuring the FRET versus donor signals at excitation 490 nm and emission 520 nm (donor) and 620 nm (acceptor). The sensitivity range of the Oxyburst reporter particles can be determined by treating the particles with H2O2 and measuring the increase in fluorescence at excitation 490 nm and emission 520 nm. The dynamic range of the proteolysis assay can be measured similarly by treating the beads with trypsin, however, this will yield an artificially lower signal in fluid phases versus inside the phagosome, where the highly fluorescen peptides are retained more efficiently.
Anticipated Results
As discussed in the Background Information, modulation of the activation status of the macrophage can impact on phagosome maturation generating both changes in the kinetics of maturation as well the “final”, or steady state, status of the differentiated phagosome. These assays are new, only a few activation conditions have been sampled, and the full impact of macrophage modulation on phagosome physiology remains to be elucidated.
Time Considerations
The time required to perform the assays, once the reporter particles have been generated, is not extensive but the limitation will be the number of experimental variables or cell types that need to be assayed. Analysis by spectrofluorometer or by confocal microscope limits the number of samples that can be processed at any one time. In contrast, adapting the assays to a multi-well plate format for analysis in a fluorescent plate reader, or using FACS analysis of a series of fixed time points, will enable the investigator to process many more samples within the time frame of the assay. The investigator needs to assess whether it is more important to generate a high resolution, kinetic analysis of a few experimental parameters, or a limited series of time points from a greater number of samples. Of course, for most of the methods that we have developed, these choices are not mutually exclusive so long as the laboratory has access to the appropriate instrumentation.
Contributor Information
Maria Podinovskaia, Email: Mp552@cornell.edu.
Brian C. VanderVen, Email: bcv8@cornell.edu.
Robin M. Yates, Email: rmyates@ucalgary.ca.
Sarah Glennie, Email: Sarah.glennie@liverpool.ac.uk.
Duncan Fullerton, Email: Duncan.fullerton@liverpool.ac.uk.
Henry C. Mwandumba, Email: Henry.mwandumba@liverpool.ac.uk.
Literature Cited
- Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304(5673):1014–8. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- Podinovskaia M, Lee W, et al. Infection of macrophages with Mycobacterium tuberculosis induces global modifications to phagosomal function. Cell Microbiol. 2012 doi: 10.1111/cmi.12092. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DG, Vanderven BC, et al. The macrophage marches on its phagosome: dynamic assays of phagosome function. Nat Rev Immunol. 2009;9(8):594–600. doi: 10.1038/nri2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DG, Yates RM. Toll-like receptors and phagosome maturation. Nat Immunol. 2007;8(3):217. doi: 10.1038/ni0307-217a. author reply 217–8. [DOI] [PubMed] [Google Scholar]
- Rybicka JM, Balce DR, et al. Phagosomal proteolysis in dendritic cells is modulated by NADPH oxidase in a pH-independent manner. EMBO J. 2012;31(4):932–44. doi: 10.1038/emboj.2011.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderVen BC, Hermetter A, et al. Development of a novel, cell-based chemical screen to identify inhibitors of intraphagosomal lipolysis in macrophages. Cytometry A. 2010;77(8):751–60. doi: 10.1002/cyto.a.20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderVen BC, Yates RM, et al. Intraphagosomal measurement of the magnitude and duration of the oxidative burst. Traffic. 2009;10(4):372–8. doi: 10.1111/j.1600-0854.2009.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates RM, Hermetter A, et al. The kinetics of phagosome maturation as a function of phagosome/lysosome fusion and acquisition of hydrolytic activity. Traffic. 2005;6(5):413–20. doi: 10.1111/j.1600-0854.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- Yates RM, Hermetter A, et al. Recording phagosome maturation through the real-time, spectrofluorometric measurement of hydrolytic activities. Methods Mol Biol. 2009;531:157–71. doi: 10.1007/978-1-59745-396-7_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates RM, Hermetter A, et al. Macrophage activation downregulates the degradative capacity of the phagosome. Traffic. 2007;8(3):241–50. doi: 10.1111/j.1600-0854.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- Yates RM, Russell DG. Phagosome maturation proceeds independently of stimulation of toll-like receptors 2 and 4. Immunity. 2005;23(4):409–17. doi: 10.1016/j.immuni.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Yates RM, Russell DG. Real-time spectrofluorometric assays for the lumenal environment of the maturing phagosome. Methods Mol Biol. 2008;445:311–25. doi: 10.1007/978-1-59745-157-4_20. [DOI] [PMC free article] [PubMed] [Google Scholar]