Abstract
Study Objectives:
To investigate whether subjective sleep quality is associated with brain volume independent of comorbid psychiatric conditions.
Design:
Cross-sectional.
Setting:
Department of Veterans Affairs (VA) Medical Center.
Participants:
One hundred forty-four Gulf War Veterans (mean age 45 years; range: 31-70 years; 14% female).
Interventions:
None.
Measurements and Results:
Total cortical, lobar gray matter, and hippocampal volumes were quantified from 1.5 Tesla magnetic resonance images using Freesurfer version 4.5. Subjective sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI). Multiple linear regressions were used to determine the association of sleep quality with total and regional brain volumes. The global PSQI score was positively correlated with lifetime and current posttraumatic stress disorder (PTSD) and current depressive symptoms (P < 0.001) and was higher in veterans with Gulf War Illness, trauma exposure, and those using psychotropic medication (P ≤ 0.03). After adjusting for these comorbid variables, age, intracranial volume, and multiple comparisons, global PSQI was inversely associated with total cortical and frontal gray matter volume (adjusted P ≤ 0.03). Within the frontal lobe, total PSQI was inversely associated with the superior and middle frontal, orbitofrontal, anterior cingulate, and frontal pole volumes (adjusted P ≤ 0.02). Examination of the 3-factor structure of the PSQI revealed that the associations were driven by perceived sleep quality.
Conclusions:
Poorer subjective sleep quality was associated with reduced total cortical and regional frontal lobe volumes independent of comorbid psychiatric conditions. Future work will be needed to examine if effective treatment of disturbed sleep leads to improved structural and functional integrity of the frontal lobes.
Citation:
Chao LL; Mohlenhoff BS; Weiner MW; Neylan TC. Associations between subjective sleep quality and brain volume in Gulf War Veterans. SLEEP 2014;37(3):445-452.
Keywords: Frontal lobe, structural imaging, Freesurfer, Pittsburgh Sleep Quality Index, Gulf War Veterans
INTRODUCTION
Recent neuroimaging studies suggest that sleep disturbances are associated with structural brain changes, particularly in the frontal lobe. For example, Koenigs et al. reported a significant association between insomnia and left dorsomedial prefrontal damage in a study of patients with focal brain lesions.1 Altena and colleagues reported reduced orbitofrontal cortex volume in elderly insomnia patients relative to matched controls.2 Joo and colleagues found reduced orbitofrontal and superior frontal volumes in patients with obstructive sleep apnea syndrome and narcolepsy with cataplexy, and thinner cortex in the dorsolateral, medial frontal, and anterior cingulate in narcoleptic patients with cataplexy relative to matched controls.3–5 In healthy subjects without insomnia, there have been reports of negative correlations between orbitofrontal cortex volume and early morning awakening and daytime sleepiness.6,7
Sleep complaints are common among patients with post-traumatic stress disorder (PTSD),8,9 major depressive disorder (MDD),10,11 and Gulf War Illness.12–14 Structural brain alterations have also been noted in PTSD and MDD.15–17 The goal of this study was to investigate whether subjective sleep quality is associated with cortical volume independent of comorbid psychiatric conditions. Based on the findings of the neuroimaging studies of sleep disturbances studies cited above, we hypothesized that sleep quality would be inversely associated with reduced gray matter volume in the frontal lobe, particularly in the medial and orbitofrontal cortex. We also examined the relationship between hippocampal volume and subjective sleep quality. Because Reimann et al.18 have reported decreased bilateral hippocampal volumes in patients with primary insomnia and because we previously found a significant, inverse correlation between the Insomnia Severity Index (ISI) and total hippocampal volume,19 we hypothesized that sleep quality would also be inversely associated with hippocampal volume.
METHODS
Participants
We conducted a secondary analysis of imaging and clinical data of 144 Gulf War Veterans. These veterans represent a subset of those used in a previous cross sectional study of the effects of service in the Persian Gulf War on the brain. Clinical and imaging data from the study have been reported in previous publications on relationship between Gulf War Illness, brain N-acetylaspartate, and PTSD,20 the effects of current versus lifetime PTSD symptom severity on hippocampal volume,21 and the effects of suspected low-level sarin exposure on brain structure and function.22 All participants provided written informed consent. Details of the original study design, recruitment, and participant characteristics have been described elsewhere.20,21 All research was approved by the University of California at San Francisco and the Veterans Administrations Committees on Human Research and the Department of Defense Human Subjects Research Review board.
Assessments
Global subjective sleep quality was measured by the Pittsburgh Sleep Quality Index (PSQI).23 We also examined the 3-factor structure of the PSQI,24 which correspond to measures of sleep efficiency, perceived sleep quality, and daily disturbances. Lifetime and current PTSD symptom severity were assessed with the Clinician Administered PTSD scale (CAPS).25 Participants were considered to have adult trauma if they experienced traumatic life events that met Criterion A. The Structured Clinical Interview for DSM-IV (SCID)26 was used to diagnose Axis I disorders: 58 participants (40%) had lifetime major depression disorder (MDD); 18 (13%) had current MDD; 26 (18%) had current PTSD; 28 (19%) had recovered from PTSD (e.g., had lifetime CAPS > 40 but did not meet criteria for current PTSD); 4 had anxiety disorders other than PTSD (3%); 1 had obsessive compulsive disorder (OCD; 1%); 72 (50%) had past alcohol abuse/dependence; and 24 (17%) had past substance abuse/dependence. Participants with bipolar, psychotic, and dissociative disorders were excluded from the study. All diagnoses were made by trained clinical interviewers who calibrated their assessments at weekly case consensus meetings, supervised by an experienced PhD-level clinical psychologist. Depressive symptoms were assessed with the Hamilton Depression Scale (HAMD).27 To determine the presence or absence of childhood trauma before the age of 14 years, we used the interview version of the Life Stressor Checklist (LSC).28 The LSC assesses 21 stressful life events. For each event, the respondent indicated (1) whether the event occurred; (2) whether at the time of exposure the event triggered intense emotions consistent with DSM-IV criterion A2 for PTSD; (3) whether the event occurred once or multiple times; and (4) the ages at which the event first and last occurred. Participants were considered to have childhood trauma if they experienced traumatic life events to the extent that they felt serious personal life threat or physical harm to the self in 5 items assessed by the LSC (i.e., physical neglect, family violence, physical abuse, forced sexual touch, or forced sexual intercourse) before age 14.29–31
MRI: Acquisition
Structural MRI data were acquired with a 1.5-T scanner (Vision, Siemens Medical Systems, Iselin, New Jersey) and a three-dimensional magnetization prepared T1-weighted gradient echo sequence with the following parameters: repetition time/spin-echo time/inversion time = 10/4/300 msec, 1 × 1 mm2 in-plane resolution, and 1.5-mm slab thickness, angulated perpendicular to the long axis of the hippocampus.
MRI: Processing
The publicly available Freesurfer v4.5 (http://surfer.nmr.mgh.harvard.edu/) volumetric segmentation and cortical surface reconstruction methods were used to obtain regional measures of neocortical volumes (mm3). Each cortical surface was spatially normalized to a template cortical surface, allowing for the automatic parcellation of the cortical surface into 34 anatomical ROIs per cortical hemisphere.32 To protect against type I error, homologous parcel volumes were summed across hemispheres and parcels were combined to create volumes for total cortical gray matter, frontal, temporal, parietal, and occipital lobes. In the Desikan nomenclature, the constituents of the frontal lobe parcel included the precentral gyrus, superior frontal, caudal and rostral middle frontal, pars opercularis, pars triangularis, pars orbitalis, lateral and medial orbitofrontal cortex, frontal pole, and caudal and rostral anterior cingulate gyri. The parietal lobe included the postcentral gyrus, inferior parietal, superior parietal, supramarginal, paracentral, precuneus, posterior cingulate, and isthmus of the cingulate. The temporal lobe included the transverse temporal, bank of the superior temporal sulcus, superior, middle, and inferior temporal gyri, temporal pole, entorhinal cortex, fusiform, and parahippocampal gyri. The occipital lobe included the lateral occipital, cuneus, pericalcarine, and lingual gyri. Total cortical gray matter volume included all of these parcels and the insula. The reconstructed cortical surface models for each participant were manually inspected to ensure segmentation accuracy. Because cortical parcellations were combined to derive total and lobar gray matter volumes, subjects who had poor segmentation (i.e., underestimation of gray matter) due to poor image quality or misregistration in any parcellation were excluded from statistical analyses. Cases where the addition of control points did not correct underestimation of the temporal pole (n = 48), superior temporal gyrus (n = 27), fusiform gyrus (n = 25), inferior temporal gyrus (n = 14), middle temporal gyrus (n = 10), entorhinal cortex (n = 7), lateral orbitofrontal (n = 1), and superior frontal cortex (n = 1) were excluded. In many instances, there was underestimation of gray matter in multiple brain regions. The final data set included MRIs with good segmentation accuracy and complete datasets for the relevant sleep and clinical information in 144 veterans of the 247 veterans who participated in the original study.
Statistical Analysis
Pearson correlation was used to examine the relationship between PSQI scores and continuous variables (e.g., age, body mass index, lifetime and current CAPS, HAMD). Independent sample t-tests were used to examine sleep quality differences among dichotomous variables (e.g., gender, Gulf War Illness,33 sarin exposure status,34,35 early life and adult trauma exposure, lifetime alcohol abuse/dependence). Multiple linear regressions were used to test the association of sleep quality with measures of total brain and lobar volume. The regressions were adjusted for age and intracranial volume as well as variables that correlated significantly with PSQI (i.e., lifetime and current CAPS and HAMD) or that resulted in different PSQI scores (e.g., subjects with Gulf War Illness, adult trauma, and those using psychotropic medication had higher PSQI than subjects without). The α level for the main effects was adjusted for multiple comparisons based on the Tukey, Ciminera, and Heyse multiple endpoint adjustment procedure36: with 6 ROIs (total GM, frontal, temporal, parietal, occipital lobes, and hippo-campus) and an average intercorrelation of r = 0.750 among the ROIs, the adjusted α level was P ≤ 0.032.
Post Hoc Analyses
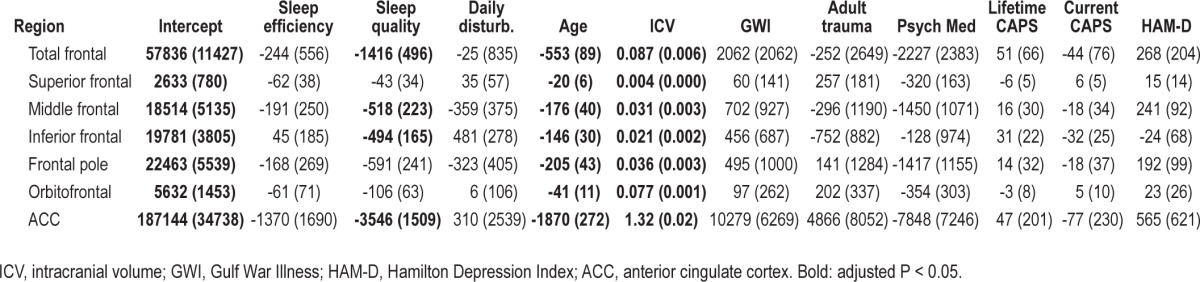
I. Because we found a significant relationship between PSQI and frontal lobe volume, we further examined the association between PSQI and volumetric measures of sub-regions of the frontal lobe. To protect against type I error, the frontal lobe parcels, excluding the precentral gyrus, were combined to create 6 ROIs: superior, middle, and inferior frontal, frontal pole, orbitofrontal cortex, and anterior cingulate cortex (Figure 1). The P-values were adjusted for the 6 ROIs and the average intercorrelation (r = 0.789) among the ROIs. We also examined the relationship between the 3-factor structure of the PSQI (sleep efficiency, perceived sleep quality, and daily disturbances24) with total frontal and regional frontal volumes. Again, the α level for the main effects was adjusted for multiple comparisons based on the Tukey, Ciminera, and Heyse multiple endpoint adjustment procedure36: with 7 ROIs and an average intercorrelation of r = 0.812 among the ROIs, the adjusted α level was P ≤ 0.035.
Figure 1.
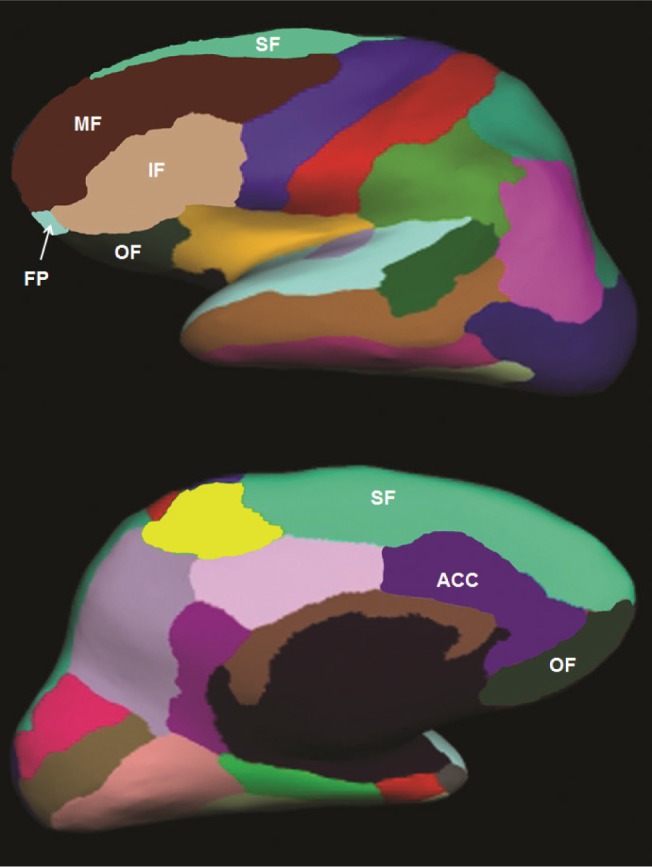
Example of Freesurfer frontal lobe parcels on an inflated brain. The middle frontal (MF; brown), inferior frontal (IF; tan), and frontal pole (FP; light blue) parcels are shown on the lateral surface of the brain. Superior frontal (SF; light green) and orbitofrontal (OF; dark green) parcels are shown on the lateral and medial surfaces of the brain. The anterior cingulate cortex (ACC; purple) is shown on the medial surface.
II. To investigate whether the current findings are related to sleep related breathing disorders (SBD), we included body mass index (BMI) and an estimate of SBD derived from PSQI questions that pertained to snoring and breathing during sleep (Q5: During the past month, how often have you had trouble sleeping because you (d) cannot breathe comfortably, (e) cough or snore loudly; Q10: According to your roommate or bed partner, if you have one, how often in the past week have you had (a) loud snoring; (b) long pauses between breaths while asleep) in the regression models along with the other covariates.
III. Because we22,37 and others38,39 have previously found evidence of morphometric brain changes in GW veterans with suspected low-level sarin exposure, we re-ran the analyses with sarin exposure status in the regression models along with the other covariates.
IV. Because the goal of this study was to investigate the association between subjective sleep quality and cortical volume independent of comorbid psychiatric conditions, we re-ran the analyses in a subset of 108 subjects without current PTSD, MDD, OCD, or anxiety disorders other than PTSD. The regressions were adjusted for age and intracranial volume, Gulf War Illness status, adult trauma, lifetime CAPS, and HAMD.
RESULTS
Detailed characteristics of the participants are shown in Table 1. The veterans were 45 ± 10 years old, had an average of 15 ± 2 years of education, and a mean PSQI global score of 8.6 ± 4.6 (range 1-19). Twenty-two (15%) veterans were women. Twenty-six (18%) veterans satisfied the definition of Weathers et al.40 for PTSD (CAPS ≥ 40). Seventeen (13%) veterans had current major depressive disorder (MDD) and 72 (50%) veterans had past alcohol abuse or dependence according to the SCID. Thirty-two (22%) veterans met the criteria of Fukuda et al.33 for Gulf War Illness, and 18 (13%) veterans had suspected low-level sarin exposure according to the Directorate for Deployment Health Support of the Special Assistant to the Under Secretary of Defense (Personnel and Readiness) for Gulf War Illness Medical Readiness and Military.34
Table 1.
Participant characteristics
There were significant, positive correlations between total PSQI and lifetime (r = 0.32, P < 0.001) and current (r = 0.43, P < 0.001) CAPS and HAMD (r = 0.63, P < 0.001). BMI was not correlated with the total PSQI (r = 0.14, P = 0.09). Subjects with Gulf War Illness (t = 2.24, df = 142, P = 0.03), adult trauma (t = 2.36, df = 142, P = 0.02), and those taking psychotropic medication (t = 3.75, df = 142, P < 0.001) had higher PSQI scores than subjects without Gulf War Illness or adult trauma.
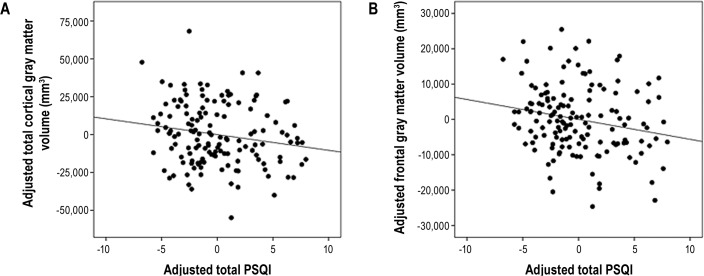
Table 2 shows the relationships between global sleep quality and measures of total and regional cortical gray matter volumes. All fits were significant (all model P < 0.0001; 0.44 ≤ R2 ≤ 0.81). Significant inverse relationships were observed between PSQI and total cortical (standardized β = -0.11, adjusted P = 0.03) and frontal lobe (standardized β = -0.15, adjusted P = 0.02) gray matter volume (Figure 2). As expected, older age was significantly associated with decreased volume, while larger intracranial volume was significantly associated with increased volumes in all regions. Unexpectedly, Gulf War Illness was associated with greater temporal lobe volume (standardized β = 0.11, adjusted P = 0.03). Contrary to our hypothesis, there was no significant relationship between PSQI and total hippocampal volume (standardized β = -0.08, P = 0.37).
Table 2.
Relationships of PSQI to measures of brain volume reported as unstandardized regression coefficient and standard error in parentheses.
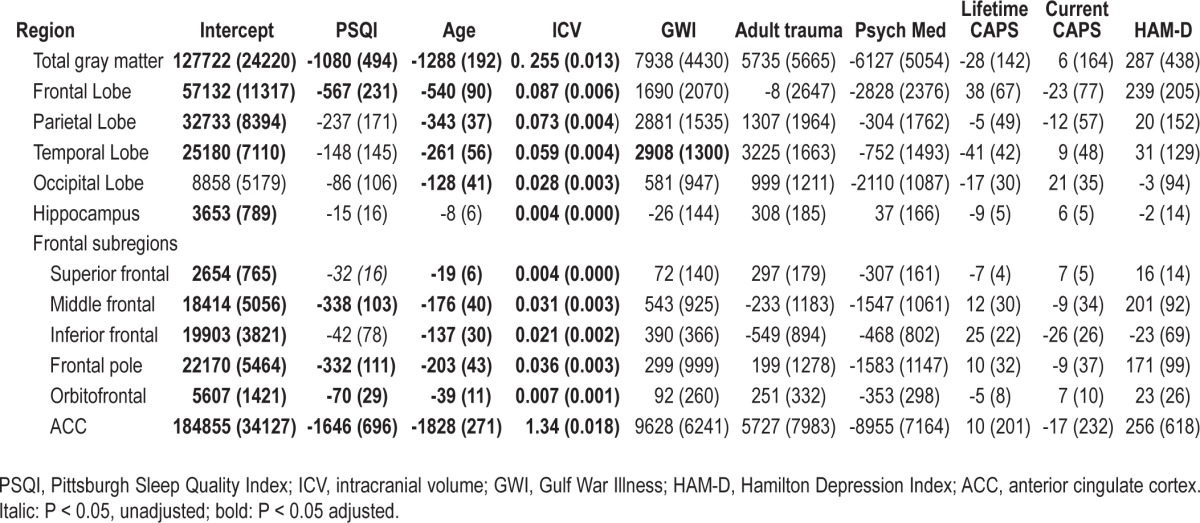
Figure 2.
Scatterplots showing the relationships between (A) total cortical and (B) frontal lobe gray matter volume and total PSQI score. The PSQI score and brain volumes were regressed on age, intracranial volume, adult trauma, lifetime and current CAPS, and HAM-D; the unstandardized residuals were plotted against each other. The slope of the line of best fit is the same as the regression coefficient for total PSQI score in the linear models.
All fits in the post hoc analysis of frontal lobe sub-regions were significant (all model P < 0.0001, 0.48 ≤ R2 ≤ 0.98). There were significant inverse relationships between PSQI and the middle frontal (standardized β = -0.23, adjusted P = 0.001), frontal pole (standardized β = -0.20, adjusted P = 0.003), orbitofrontal (standardized β = -0.19, adjusted P = 0.02), and anterior cingulate cortex (standardized β = -0.04, adjusted P = 0.02) volume. There was also a marginal relationship between PSQI and the superior frontal lobe volume (standardized β = -0.17, unadjusted P = 0.04).
Table 3 shows the relationships between the 3-factor structure of the PSQI and measures of total and regional frontal lobe volumes. Again, all fits were significant (all model P < 0.0001; 0.50 ≤ R2 ≤ 0.98). There were significant inverse relationships between perceived sleep quality and total frontal (standardized β = -0.18, adjusted P = 0.005), middle frontal (standardized β = -0.17, adjusted P = 0.02), inferior frontal (standardized β = -0.23, adjusted P = 0.003), frontal pole (standardized β = -0.17, adjusted P = 0.02), and anterior cingulate (standardized β = -0.04, adjusted P = 0.02) volumes.
Table 3.
Relationships between the 3-factor structure of the PSQI to measures of frontal lobe volume, reported unstandardized regression coefficient, and standard error in parentheses
Accounting for BMI and our estimate of SDB based on PSQI questions that related to snoring and breathing during sleep in post hoc analyses did not alter any of the main findings concerning regional frontal lobe volumes. However, accounting SDB did render the relationship between PSQI and total cortical volume marginally significant (standardized β = -0.10, unadjusted P = 0.05). Accounting for BMI and SDB also rendered the unexpected finding of greater temporal lobe volume in veterans with Gulf War Illness insignificant. Accounting for suspected sarin exposure status, past alcohol and substance abuse/dependence did not alter any of the significant main findings concerning total or regional frontal lobe volumes.
When we re-ran the analyses in a subset of 108 subjects without current comorbid psychiatric conditions, all fits were significant (all model P < 0.0001, 0.42 ≤ R2 ≤ 0.98). As with the entire cohort, there were significant inverse relationships between PSQI and total cortical gray matter (standardized β = -0.14, adjusted P = 0.01), total frontal lobe (standardized β = -0.14, adjusted P < 0.03), middle frontal (standardized β = -0.22, adjusted P = 0.003), frontal pole (standardized β = -0.19, adjusted P = 0.009), and anterior cingulate cortex (standardized β = -0.05, adjusted P = 0.01) volume. There were also trends towards an inverse relationship between PSQI and parietal and temporal lobe volumes (standardized β = -0.12, unadjusted P = 0.07 for both).
DISCUSSION
The main finding of this study is that poor sleep quality was associated with decreased frontal lobe gray matter volume. As expected, current and lifetime PTSD symptom severity and current depressive symptoms were negatively correlated with PSQI scores. Veterans with adult trauma, Gulf War Illness, and those using psychotropic medication also had poorer subjective sleep quality. However, the inverse relationship between subjective sleep quality and frontal lobe volume remained significant even after accounting for these comorbid conditions.
Not only do individuals with insomnia frequently report cognitive difficulties, but there is evidence of impaired working memory and executive function—processes that depend on the integrity of the prefrontal cortex—in insomniacs relative to good sleepers.41–55 Positron emission tomography (PET) studies have demonstrated decreased cerebral glucose metabolism in the frontal, temporal, and parietal cortices of insomniacs relative to good sleepers.56 Finally, there is evidence that suggests functional deficits may exist in the absence of behavioral differences. For example, insomnia patients had less medial prefrontal and inferior frontal gyrus activation than controls during a verbal fluency task despite performing comparably to controls.57 Together, these findings support the frontal lobe hypothesis, which posits that sleep deprivation acts primarily on the frontal lobe to produce frontal cortex dysfunction.58
Data from the present and other morphometric studies extend the frontal lobe hypothesis by linking sleep abnormalities with structural integrity of the frontal lobe. For example voxel-based morphometry studies have reported significant gray matter reductions within the ventral medial prefrontal cortex of patients with narcolepsy and cataplexy,4 obstructive sleep apnea,3 and chronic insomnia.2 There have also been reports that gray matter volume in the left ventromedial prefrontal cortex was significantly related to greater self-reported daytime sleepiness on the Epworth Sleepiness Scale,7 and that gray matter volume in the left inferior orbitofrontal cortex was significantly associated with greater self-reported early morning awakening.6 Together with these previous findings, the current result suggests that morphology of the medial prefrontal and orbitofrontal cortex may be affected by, or may contribute to, some sleep-related difficulties.
Our results extend these findings by showing that poor sleep quality is not only related to atrophy in the orbitofrontal and anterior cingulate cortex, but also globally in the entire frontal lobe and in the middle frontal cortex and frontal pole. Examination of the three-factor structure of the PSQI revealed that the inverse relationship between global and regional frontal lobe volume, and the global PSQI score was driven primarily by perceived sleep quality. It is interesting to note that when we examined the three-factor structure of the PSQI, the inferior frontal ROI was inversely associated with perceived sleep quality, while the orbitofrontal and frontal pole ROIs— which were associated with global PSQI—were not related to perceived sleep quality. This suggests that sleep quality and other measures of sleepiness and wakefulness may have different effects on different regions of the frontal lobe.
Although the mechanisms underlying the relationship between frontal lobe volume and subjective sleep quality remain uncertain, it is noteworthy that a recent magnetoencephalography study localized the greatest activity increases during both REM and deep sleep to left dorsomedial prefrontal cortex.59 Another study using high-density electroencephalography found that sleep slow waves preferentially originate in the left frontoinsular area and cingulate gyrus.60 In a study of 192 patients with focal brain lesions, Koenigs et al. showed a significant association between insomnia and left dorsomedial prefrontal damage.1 Together, these results suggest that left medial prefrontal cortex and insula may play a critical role in sleep maintenance and insomnia.
Frontal lobe volume may also be associated with poorer sleep quality or decreased capacity for sleep to be experienced as restorative. It has been proposed that part of the orbitofrontal cortex (the “orbital network”) is essential for the hedonic evaluation of somatosensory input, including the evaluation of thermal comfort.61–63 Thus, it is possible that compromised thermal comfort, known to be prominent in elderly insomniacs,64 is linked to gray matter volume reduction in the orbitofrontal cortex. Studies that use prospective designs and randomized treatment trials that involve effective treatment for sleep disturbances will be needed to resolve issues related to causality in the association between sleep and frontal lobe structure.
Although we hypothesized that sleep quality would be inversely associated with total hippocampal volume, we found no significant relationship between the global PSQI score and total hippocampal volume in this study and in a previous study that examined structural MRI data acquired on a 4 Tesla scanner.19 While this contradicts a previous report of smaller hippocampal volumes in subjects with insomnia,18 it is noteworthy that other researchers have not been able to replicate that finding,2,65,66 possibly due to differences to sample composition and method of MRI assessment. In our prior study at 4 T, we did observe a significant negative correlation between the PSQI and CA3/dentate subfield. It is possible that the relationship between sleep and hippocampal structure is only found in this subfield. We are not able to examine if this same relationship holds in this study because the structural resolution at 1.5 T is not sufficient to accurately measure subfield volumes.
A number of limitations should be considered in the interpretation of the current findings. First, the cross-sectional design limits our ability to determine a causal relationship between sleep and frontal lobe volume. Second, the study has limited generalizability because our sample consisted solely of Gulf War Veterans, some of whom had Gulf War Illness and/ or suspected sarin exposure. However, it should be noted that neither condition affected frontal lobe volume. Furthermore, accounting for Gulf War Illness and sarin exposure status in the regression models did not alter the effect of PSQI on total brain, frontal lobe, or regional frontal volumes. A third limitation is the lack of objective sleep measures. Fourth, we did not have information about whether or not participants suffered from sleep related disorders (e.g., primary insomnia, obstructive sleep apnea). However, accounting for body mass index and an estimate of possible sleep disordered breathing derived from sub-scores of the PSQI in post hoc analyses did not alter the main findings concerning total or regional frontal lobe volume and subjective sleep quality. Fifth, the imaging data was acquired on a 1.5 Tesla scanner, which has poorer gray/white matter contrast and larger voxel size than images acquired on higher field strength scanners. Finally, the a priori parcellations may be insensitive to regional findings that do not corresponding to parcel boundaries. Future whole-brain or vertex-wise analyses will be needed to examine the possibility of missed foci of volume loss or cortical thinning. These limitations notwithstanding, our results suggest that poor sleep quality, which has been linked to impaired psychosocial, physical, and occupational functioning,41 is associated with frontal lobe atrophy independent of trauma exposure, current and lifetime PTSD symptom severity, current MDD, and Gulf War Illness status. Future work will be needed to examine if effective treatment of disturbed sleep leads to improved frontal lobe structural and functional integrity.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by Department of Defense Grant DAMD17-01-1-0764, entitled, “Magnetic Resonance and Spectroscopy of the Human Brain in Gulf War Illness,” awarded to the Northern California Institute for Research and Education from the Department of Defense Gulf War Illnesses Research Program, US Army Medical Research and Materiel Command. Further support was provided by the Mental Illness Research and Education Clinical Center of the US Veterans Health Administration. Brian S. Mohlenhoff received funding from R25MH060482 and the Mental Illness Research, Education and Clinical Center at VA VISN 21. Resources and the use of facilities were provided by the Veterans Administration Medical Center, San Francisco, California. Dr. Neylan has received study medication (almorexant) from Actelion for a study funded by the Department of Defense and study medication (GSK561679) from Glaxo Smith Kline for a study funded by the Department of Veterans Affairs. Neither is relevant to the content of this report. Dr. Weiner has served on the scientific advisory boards of Pfizer & BOLT international in 2011. He has served as a consultant for Pfizer, Janssen, KLK Associates, Easton Associates, Harvard University, KLJ Associates, in Thought, INC, Research, Inc, University of California (Los Angeles), Alzheimer's Drug Discovery Foundation, Alzheimer's Drug Discovery Foundation, Avid Radio Pharmaceuticals, Eli Lilly, and Sanofi-Aventis. He has received funding for travel from Pfizer, AD PD meeting, Paul Sabatier University, Novartis, Tohoku University, from the MCI Group, France, Travel eDreams, Inc, Neuro-science School of Advanced Studies, Danone Trading, VB, CTAD ANT Congress, ADRC, University of California (Los Angeles), and from AD/PD, ADRC, UCLA. He serves on the editorial advisory boards of Alzheimer's & Dementia and MRI. He has received honoraria from Pfizer, Tohoku University, and from Danone Trading BV. He has received commercial entities research support from Merck and Avid. He has received government entities research support from the Departments of Defense and Veterans' Affairs. He has stock options in Elan. Organizations contributing to the Foundation for NIH and thus to the NIA funded Alzheimer's Disease Neuroimaging Initiative (ADNI) include the following: Abbott, Alzheimer's Association, Alzheimer's Drug Discovery Foundation, Anonymous Foundation, AstraZeneca, Bayer Healthcare, BioClinica Inc, Bristol-Meyers Squibb, Cure Alzheimer's Fund < Eisai, Elan, Gene Network Sciences, Genetech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson & Johnson, Eli Lilly & Company, Medpace, Merck, Novartis, Pfizer Inc, Roche, Schering Plough, Wyeth. The other authors have indicated not financial conflicts of interest. This study does not include any off-label or investigational use.
REFERENCES
- 1.Koenigs M, Holliday J, Solomon J, Grafman J. Left dorsomedial frontal brain damage is associated with insomnia. J Neurosci. 2010;30:16041–3. doi: 10.1523/JNEUROSCI.3745-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altena E, Vrenken H, Van Der Werf YD, van den Heuvel OA, Van Someren EJ. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry. 2010;67:182–5. doi: 10.1016/j.biopsych.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Joo EY, Tae WS, Lee MJ, et al. Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep. 2010;33:235–41. doi: 10.1093/sleep/33.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joo EY, Tae WS, Kim ST, Hong SB. Gray matter concentration abnormality in brains of narcolepsy patients. Korean J Radiol. 2009;10:552–8. doi: 10.3348/kjr.2009.10.6.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joo EY, Jeon S, Lee M, et al. Analysis of cortical thickness in narcolepsy patients with cataplexy. Sleep. 2011;34:1357–64. doi: 10.5665/SLEEP.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoffers D, Moens S, Benjamins J, et al. Orbitofrontal gray matter relates to early morning awakening: a neural correlate of insomnia complaints? Front Neurol. 2012;3:105. doi: 10.3389/fneur.2012.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Killgore WD, Schwab ZJ, Kipman M, DelDonno SR, Weber M. Voxel-based morphometric gray matter correlates of daytime sleepiness. Neurosci Lett. 2012;518:10–13. doi: 10.1016/j.neulet.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Neylan TC, Marmar CR, Metzler TJ, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155:929–33. doi: 10.1176/ajp.155.7.929. [DOI] [PubMed] [Google Scholar]
- 9.Ohayon MM, Shapiro CM. Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Compr Psychiatry. 2000;41:469–78. doi: 10.1053/comp.2000.16568. [DOI] [PubMed] [Google Scholar]
- 10.Thase ME. Antidepressant treatment of the depressed patients with insomnia. J Clin Psychiatry. 1999;60:28–31. [PubMed] [Google Scholar]
- 11.Tsuno N, Besset A. Sleep and depression. J Clin Psychiatry. 2005;66:1254–69. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Unexplained illness among Persian Gulf veterans in an Air National Guard unit: preliminary report--August 1990-March 1995. MMWR Morb Mortal Wkly Rep. 1995;44:443–4. [PubMed] [Google Scholar]
- 13.Persian Gulf Veterans Coordinating Board. Unexplained illness among Desert Storm veterans: a search for causes, treatment and cooperation. Arch Intern Med. 1995;155:262–8. doi: 10.1001/archinte.155.3.262. [DOI] [PubMed] [Google Scholar]
- 14.The Iowa Persian Gulf Study Group. Self-reported illness and health status among Gulf War veterans: a population-based study. JAMA. 1997;277:238–45. [PubMed] [Google Scholar]
- 15.Bremner JD. Stress and brain atrophy. CNS Neurol Disord Drug Targets. 2006;5:503–12. doi: 10.2174/187152706778559309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–31. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Arnone D, McIntosh AM, Ebmeier KP, Munafò MR, Anderson IM. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur Neuropsychopharmacol. 2012;22:1–16. doi: 10.1016/j.euroneuro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Riemann D, Voderholzer U, Spiegelhalder K, et al. Chronic insomnia and MRI-measured hippocampal volumes: a pilot study. Sleep. 2007;30:955–8. doi: 10.1093/sleep/30.8.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neylan TC, Mueller SG, Wang Z, et al. Insomnia severity is associated with a decreased volume of the CA3/dentate gyrus hippocampal subfield. Biol Psychiatry. 2010;68:494–6. doi: 10.1016/j.biopsych.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiner MW, Meyerhoff DJ, Neylan TC, et al. The relationship between Gulf War illness, brain N-acetylaspartate, and post-traumatic stress disorder. Mil Med. 2010;176:896–902. doi: 10.7205/milmed-d-10-00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apfel BA, Ross J, Hlavin J, et al. Hippocampal volume and current versus lifetime PTSD symptoms in Gulf War veterans. Biol Psychiatry. 2011;69:541–8. doi: 10.1016/j.biopsych.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao LL, Rothlind JC, Cardenas VA, Meyerhoff DJ, Weiner MW. Effects of low-level exposure to sarin and cyclosarin during the 1991 Gulf War on brain function and brain structure in US veterans. Neurotoxicology. 2010;31:493–501. doi: 10.1016/j.neuro.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 24.Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, Irwin MR. Validation of a 3-factor scoring model for the Pittsburgh sleep quality index in older adults. Sleep. 2006;29:112–6. doi: 10.1093/sleep/29.1.112. [DOI] [PubMed] [Google Scholar]
- 25.Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinican-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 26.First M, Spitzer R, Gobbon M, Williams J. Structured Clinical Interview for DMS-IV. New York: New York State Psychiatric Institute Biometrics Research Department; 1995. [Google Scholar]
- 27.Hamilton M. Developement of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 28.Wolfe J, Kimerling R, Brown P, Chresman K, Levin K. Psychometric review of the life stressor checklist-revised. In: Stamm BH, editor. Instrumentation in stress, trauma, and adaptation. Lutherville, MD: Sidran Press; 1996. pp. 144–51. [Google Scholar]
- 29.ODonovan A, Epel E, Lin J, et al. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011;70:465–71. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pole N, Neylan TC, Otte C, et al. Associations between childhood trauma and emotion-modulated psychophysiological responses to startling sounds: a study of police cadets. J Abnorm Psychol. 2007;116:352–61. doi: 10.1037/0021-843X.116.2.352. [DOI] [PubMed] [Google Scholar]
- 31.Otte C, Neylan TC, Pole N, et al. Association between childhood trauma and catecholamine response to psychological stress in police academy recruits. Biol Psychiatry. 2005;57:27–32. doi: 10.1016/j.biopsych.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 33.Fukuda K, Niesenbaum R, Stewart G, et al. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA. 1998;280:981–8. doi: 10.1001/jama.280.11.981. [DOI] [PubMed] [Google Scholar]
- 34.Final Report (1996) Washington, DC: U.S. Government Printing Office; Presidential Advisory Committee on Gulf War Veterans' Illness. [Google Scholar]
- 35.Directorate for Deployment Health Support of the Special Assistant to the Under Secretary of Defense (Personnel and Readiness) for Gulf War Illness Medical Readiness and Military Deployments (2002): US demolition operations at the Khamisiyah ammunition point (case narrative) Available at: http://www.gulflink.osd.mil/khamishaii.
- 36.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med. 1997;16:2529–42. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 37.Chao LL, Abadjian L, Hlavin J, Meyerhoff DJ, Weiner MW. Effects of low-level sarin and cyclosarin exposure and Gulf War Illness on brain structure and function: a study at 4 Tesla. Neurotoxicology. 2011;32:814–22. doi: 10.1016/j.neuro.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Proctor SP, Heaton KJ, Heeren T, White RF. Effects of chemical warfare agent exposure on central nervous system functioning in US army veterans of the 1991 Gulf War. Neurotoxicology. 2006;27:931–9. doi: 10.1016/j.neuro.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Heaton KJ, Palumbo CL, Proctor SP, Killiany RJ, Yurgelun-Todd DA, White RF. Quantitative magnetic resonance brain imaging in US army veterans of the 1991 Gulf War potentially exposed to sarin and cyclosarin. Neurotoxicology. 2007;28:761–9. doi: 10.1016/j.neuro.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 40.Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: A review of the first ten years of research. Depress Anxiety. 2001;13:132–56. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 41.Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. II. Sleep Breath. 1999;22(Suppl 2):S354–8. [PubMed] [Google Scholar]
- 42.Linton SJ, Bryngelsson I. Insomnia and its relationship to work and health in a working-age population. J Occup Rehabil. 2000;10:169–83. [Google Scholar]
- 43.Boyle J, Trick L, Johnsen S, Roach J, Rubens R. Next-day cognition, psychomotor function, and driving-related skills following nighttime administration of eszopiclone. Hum Psychopharmacol. 2008;23:385–97. doi: 10.1002/hup.936. [DOI] [PubMed] [Google Scholar]
- 44.Orff HJ, Drummond SP, Nowakowski S, Perils ML. Discrepancy between subjective symptomatology and objective neuropsychological performance in insomnia. Sleep. 2007;30:1205–11. doi: 10.1093/sleep/30.9.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haimov I, Hanuka E, Horowitz Y. Chronic insomnia and cognitive functioning among older adults. Behav Sleep Med. 2008;6:32–54. doi: 10.1080/15402000701796080. [DOI] [PubMed] [Google Scholar]
- 46.Randazzo AC, Schweitzer PK, Stone KL, Compton JD, Walsh JK. Impaired cognitive function in insomniacs vs. normals. Sleep. 2000;23:A4. (Abstract Supplement) [Google Scholar]
- 47.Varkevisser M, Van Dongen HP, Van Amsterdam JG, Kerkhof GA. Chronic insomnia and daytime functioning: an ambulatory assessment. Behav Sleep Med. 2007;5:279–96. doi: 10.1080/15402000701557425. [DOI] [PubMed] [Google Scholar]
- 48.Vignola A, Lamoureux C, Bastien CH, Morin CM. Effects of chronic insomnia and use of benzodiazepines on daytime performance in older adults. J Gerontol B Psychol Sci Soc Sci. 2000;55:54–62. doi: 10.1093/geronb/55.1.p54. [DOI] [PubMed] [Google Scholar]
- 49.Mendelson WB, Garnett D, Gillin JC, Weingartner H. The experience of insomnia and daytime and nighttime functioning. Psychiatry Res. 1984;12:235–50. doi: 10.1016/0165-1781(84)90029-5. [DOI] [PubMed] [Google Scholar]
- 50.Edinger JD, Glenn DM, Bastian LA, Marsh GR. Slow-wave sleep and waking cognitive performance II: findings among middle-aged adults with and without insomnia complaints. Physiol Behav. 2000;70:127–34. doi: 10.1016/s0031-9384(00)00238-9. [DOI] [PubMed] [Google Scholar]
- 51.Lundh LG, Froding A, Gyllenhammar L, Broman JE, Hetta J. Cognitive bias and memory performance in patients with persistent insomnia. Scand J Behav Ther. 1997;26:27–35. [Google Scholar]
- 52.Fang SC, Huang CJ, Yang TT, Tsai PS. Heart rate variability and daytime functioning in insomniacs and normal sleepers: preliminary results. J Psychosom Res. 2008;65:23–30. doi: 10.1016/j.jpsychores.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 53.Braver TS, Barch DM, Kelley WM, et al. Direct comparison of prefrontal cortex regions engaged by working and long-term memory tasks. Neuroimage. 2001;14:48–59. doi: 10.1006/nimg.2001.0791. [DOI] [PubMed] [Google Scholar]
- 54.Funahashi S. Neuronal mechainsms of executive control by the prefrontal cortex. Neurosci Res. 2001;39:147–65. doi: 10.1016/s0168-0102(00)00224-8. [DOI] [PubMed] [Google Scholar]
- 55.Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychon Bull Rev. 2002;9:637–71. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- 56.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 57.Altena E, Van Der Werf YD, Sanz-Arigita EJ, et al. Prefrontal hypoactivation and recovery in insomnia. Sleep. 2008;31:1271–6. [PMC free article] [PubMed] [Google Scholar]
- 58.Horne JA. Human sleep, sleep loss and behaviour: implications for the prefrontal cortex and psychiatric disorder. Br J Psychiatry. 1993;162:413–9. doi: 10.1192/bjp.162.3.413. [DOI] [PubMed] [Google Scholar]
- 59.Ioannides AA, Kostopoulos GK, Liu L, Fenwick PB. MEG identifies dorsal medial brain activations during sleep. Neuroimage. 2009;44:455–68. doi: 10.1016/j.neuroimage.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 60.Murphy M, Riedner B, Huber R, Massimini M, Ferrarelli F, Tononi G. Source modeling sleep slow waves. Proc Natl Acad Sci U S A. 2009;406:1608–13. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–49. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 62.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 63.Dunn BJ, Conover K, Plourde G, Munro D, Kilgour R, Shizgal P. Hedonic valuation during thermal alliesthesia. Paper presented at: Annual Meeting of the Organization for Human Brain Mapping in Minneapolis; 2010; MN. [Google Scholar]
- 64.Raymann RJ, Van Someren EJ. Diminished capability to recognize the optimal temperature for sleep initiation may contribute to poor sleep in elderly people. Sleep. 2008;31:1301–9. [PMC free article] [PubMed] [Google Scholar]
- 65.Winkelman JW, Benson KL, Buxton OM, et al. Lack of hippocampal volume differences in primary insomnia and good sleeper controls: an MRI volumetric study at 3 Tesla. Sleep Med. 2010;11:576–82. doi: 10.1016/j.sleep.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 66.Noh HJ, Joo EY, Kim ST, et al. The relationship between hippocampal volume and cognition in patients with chronic primary insomnia. J Clin Neurol. 2012;8:130–8. doi: 10.3988/jcn.2012.8.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]