Abstract
Study Objective:
To evaluate next-morning driving performance after middle-of-the-night use of zolpidem 3.5 mg in a buffered sublingual formulation (ZST).
Design:
Single-center, four-period, randomized, double-blind, placebo-controlled, crossover study.
Setting:
Maastricht University, The Netherlands.
Participants:
Forty healthy volunteers (20 females).
Interventions:
Single dose of ZST administered in the middle of the night at 3 and 4 h before driving, zopiclone 7.5 mg at bedtime 9 h before driving, and placebo.
Measurements:
Performance in a 100-km standardized highway driving test in normal traffic measuring standard deviation of lateral position (SDLP) — an index of weaving. Drug-placebo changes in SDLP > 2.5 cm were considered to reflect clinically relevant driving impairment.
Result:
For ZST, Max McNemar symmetry analyses showed that the proportion of drivers classified as impaired was increased 3 h after dosing (P < 0.012), but not 4 h after dosing. Mean increases in SDLP from placebo, although statistically significant, were small (1.46 cm [P < 0.0001] at 3 h and 0.83 cm [P = 0.0174] at 4 h). The morning after zopiclone, 45% of the drivers were classified as impaired with a mean increase in SDLP of 2.46 cm (P < 0.0001). There were no significant sex differences in effects of ZST and zopiclone.
Conclusion:
Zolpidem 3.5 mg in a buffered sublingual formulation has a minimal risk of impairing driving performance in the morning ≥ 4 hours after middle-of-the night use. When taken 3 hours before driving, the drug may have impairing effects so caution should be exercised if medication is taken other than as indicated.
Clinical Trial Information:
ClinicalTrials.gov Identifier: NCT01106859; Trial Name: Driving Performance After Middle of the Night Administration of 3.5 mg Zolpidem Tartrate Sublingual Tablet; http://clinicaltrials.gov/ct2/show/NCT01106859.
Citation:
Vermeeren A; Vuurman EF; Leufkens TR; Van Leeuwen CJ; Van Oers AC; Laska E; Rico S; Steinberg F; Roth T. Residual effects of low-dose sublingual zolpidem on highway driving performance the morning after middle-of-the-night use. SLEEP 2014;37(3):489-496.
Keywords: Driving, hypnotics, sex, zopiclone, zolpidem sublingual
INTRODUCTION
A large segment of the general population experiences middle-of-the-night (MOTN) insomnia, i.e., nocturnal awakenings followed by difficulties resuming sleep. Telephone surveys in the United States and Europe have shown that about one-third of the respondents reported waking up during the night for multiple nights per week, and between 25% and 43% of those (7.7% and 15.2% of all respondents) reported difficulty resuming sleep once awakened.1,2 To prevent MOTN awakenings, intermediate- or long-acting hypnotic drugs are typically used, but are often associated with residual effects including impaired driving.3 In addition, patients should use medication only when needed, instead of in a preventive way. Until recently, however, none of the available hypnotic drugs was formulated or approved for use later in the night, due to the potential to produce residual sedation and performance impairment the next morning. Performance impairment is a particular concern for patients driving a car.
Drug-induced driving impairment is a serious public health concern.4 Several epidemiological studies have shown that use of hypnotic drugs increases the risk of traffic accidents.5 Controlled experiments support this finding and show that risk of impairment generally decreases with shorter duration of action of a drug.3 Short half-life hypnotic drugs, such as zopiclone (ZOP) and zolpidem, are therefore considered relatively safe by prescribing physicians and have become the most commonly prescribed hypnotic drugs in Europe and the United States, respectively. Nonetheless, these drugs can cause next-day impairment when taken as indicated or especially when taken against prescribing information.6 Several experimental studies have shown that bedtime doses of ZOP 7.5 mg have effects on driving that are on average comparable to those of blood alcohol concentrations (BACs) of 0.05 %.3,7–14 When taken in the MOTN, 5 h before driving, its average effects were found to be comparable to BACs of 0.08 %.12 Similarly, zolpidem 10 mg used in the MOTN was found to have effects on driving comparable to BACs between 0.05 and 0.08 %.7,15
In November 2011, buffered sublingual formulations of zolpidem (ZST), in doses of 1.75 mg for adult women and 3.5 mg for adult men, were approved in the United States for use as needed to treat insomnia characterized by MOTN awakening followed by difficulty returning to sleep, providing there are at least 4 h of time in bed remaining.16 Studies in patients with insomnia have shown that ZST 3.5 mg significantly shortens sleep latencies after nocturnal awakenings as compared with placebo (PBO).17,18
ZST delivers and facilitates absorption of a part of the dose through the buccal mucosa, while the remainder is swallowed and absorbed in the gastrointestinal tract. A pharmacokinetic study in 24 healthy young volunteers found that peak plasma concentrations after single doses of ZST are on average reached in approximately 38 min, after which they declined with an elimination half-life of approximately 2.5 h.19 ZST plasma levels of 20 to 25 ng/mL were reached within 20 min and maintained for up to 4 h, after both 1.75 mg and 3.5 mg doses. In comparison, after use of ZST in its conventional oral dosage form of 10 mg immediate-release tablets for adults, peak plasma concentration of 125 ng/mL is generally reached within 1 to 2 h, and decline to 20 to 25 ng/mL in 8 h.20,21 Studies in patients with insomnia have shown that ZST 3.5 mg significantly shortens sleep latencies after nocturnal awakenings as compared with PBO.17,18
Based on its formulation, pharmacokinetics, and dose, it was expected that ZST 3.5 mg would have no clinically relevant residual effects 4 h or more after intake. In line with this expectation, no significant residual sedation or psychomotor impairment was found in patients using subjective ratings of daytime alertness and performance on a digit symbol substitution task.17 In addition, a study comparing the effects of single daytime doses of 1.0, 1.75, and 3.5 mg ZST and PBO in healthy volunteers on a battery of psychomotor and memory tests showed that recovery to predrug levels of psychomotor performance occurred within 4 h after the two highest doses of ZST.19 Subjective ratings of sedation were no different from PBO after 3 h, and effects on memory were no longer found 1 h after administration. Together the results of these studies suggest that ZST 3.5 mg could be administered up to about 4 h before awakening without having residual effects on psychomotor performance in the morning. However, it is important to assess its effects on car driving the morning after MOTN dosing to further characterize morning function.
The current study was therefore designed to measure the morning effects of ZST 3.5 mg on driving performance, using an on-the-road driving test that was standardized in the 1980s.22,23 The primary outcome variable of this test is standard deviation of lateral position (SDLP), which is a measure of road-tracking error or the amount of “weaving” of the car. It is a reliable characteristic of individual driving performance; the test-retest reliability of unmedicated drivers is r = 0.85 (range: 0.7-0.9).22–24 It is sensitive to the effects of many sedating agents including alcohol, with blood concentrations as low as 0.035%.25 The test has been calibrated with increasing doses of alcohol sufficient to raise BACs to 0.12%. The alcohol calibration curve demonstrates that drinkers' mean SDLP rises exponentially with increasing BACs, and that the mean increase in SDLP from PBO at a BAC of 0.05% is approximately 2.5 cm. This effect has often been used as a criterion level to quantify effects of drugs other than alcohol. Drug-induced mean increases in SDLP that are significantly different from PBO and exceed this criterion are considered clinically relevant.26 Significant increases in SDLP below this criterion are generally considered minor, and if the 95% confidence interval (CI) is below the criterion as well, the drug is considered unlikely to produce relevant impairment.
To evaluate the probability that a drug will increase the risk of impaired driving rather than simply affecting mean performance, another approach evaluates the proportion of subjects whose change in SDLP during the drug condition compared with the PBO condition is sufficiently large to imply potential risk of impaired driving. Although such a cutoff point for individual performance changes in SDLP has not been formally validated, a first threshold could be set at the same criterion as used for the mean increase in SDLP, i.e., increases exceeding 2.5 cm. It would be concluded that the drug has no effect on driving if changes in SDLP following drug treatment are random, in that numbers of subjects showing changes in SDLP above +2.5 cm and below -2.5 cm are not different. If symmetry analysis shows that significantly more subjects show changes above +2.5 cm than below -2.5 cm, the drug increases the risk of impaired driving performance.27
The objective of this study was to assess the effects of ZST 3.5 mg on highway driving at 4 h after dosing (consistent with patient instructions for appropriate use) and 3 h after dosing (inconsistent with patient instructions). ZOP 7.5 mg taken at bedtime 9 h before driving (consistent with patient instructions for appropriate use) was included in the study as an active control to demonstrate assay sensitivity as has been the case in other studies.3,7–14 Driving impairment was evaluated by symmetry analyses of proportions of impaired and improved drivers,27 and by comparing means and 95% CIs of drug-PBO changes in SDLP. In addition, sex differences in treatment effects were evaluated (post hoc), given the sex differences in zolpidem pharmacokinetics.28,29
METHODS
Subjects
Forty subjects (20 men, 20 women) were recruited via advertisements placed in local newspapers. Healthy volunteers age 21 to 64 y were eligible to enroll if they possessed a valid driving license, had an average driving experience of at least 3,000 km/y over the past 3 y, a body mass index of no more than 29 kg/m2, and normal vision (corrected or uncorrected). Subjects were required to be in good health as confirmed by their medical history questionnaire, physical examination, electrocardiogram, and laboratory tests (blood chemistry and hematology).
Subjects who met any of the following criteria were excluded from the study: history or current evidence of any clinically significant physical, mental, or sleep disorders, alcoholism, or drug abuse; pregnancy or breastfeeding; use of medication known to affect sleep-wake function or driving performance or hepatic drug metabolism; participation in any other clinical trial, oral surgery, tooth extraction, or piercing of the lip or tongue within 60 days prior to screening; excessive smoking (> 10 cigarettes a day); and overconsumption of alcohol (> 21 drinks per w) or caffeine (> 6 cups of coffee per day). All subjects were tested for drug use (amphetamines, benzodiazepines, cannabis, cocaine, MDMA [3,4-methylenedioxy-N-methylamphetamine; Ecstasy], and opiates), and females for pregnancy at prestudy screening and at the start of each test session.
During participation, subjects were required to abstain from prescription and nonprescription medications. They also had to refrain from smoking and/or consuming caffeine and alcohol from the time of arrival at the site on treatment days, until the completion of all tests the next day. In addition, alcoholic drinks, grapefruit juice, and grapefruit were not permitted from 24 h before arrival, and caffeine and food were not permitted from 4 h prior to arrival.
Ethical approval was obtained from the Medical Ethics Committee of Maastricht University, and all volunteers provided written informed consent prior to enrollment. The study was carried out in compliance with the current revision of the Declaration of Helsinki, and the International Conference on Harmonization Guidelines for Good Clinical Practice.
Design and Treatments
The study was conducted according to a randomized, double-blind, PBO-controlled, four-way crossover design. The four treatment conditions were single oral doses of ZST 3.5 mg administered in the MOTN 4 h before the start of the morning driving test; ZST administered in the MOTN 3 h before the start of the driving test; ZOP 7.5 mg administered at bedtime, 9 h before the start of the driving test; and PBO. These treatment conditions will be referred to as ZST 4 h, ZST 3 h, ZOP, and PBO, respectively, hereafter. Bedtime drug administration (23:15) was followed by PBO MOTN administration (04:15 or 05:15) and vice versa, or PBO at both times (Figure 1). Treatment was blinded using a double-dummy technique with two PBOs; a capsule to match ZOP for bedtime dosing, and a sublingual tablet to match ZST for MOTN dosing. Order of treatment conditions and time of MOTN awakening in ZOP and PBO was balanced over subjects using a Williams design. Washout periods between treatments were at least 3 days.
Figure 1.
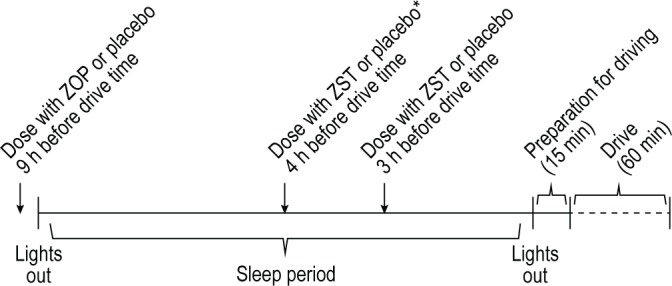
Study design. PBO, placebo; ZOP, zopiclone 7.5mg; ZST, zolpidem 3.5 mg sublingual formulation. *Randomized 1:1 to receive placebo at 3 or 4 h before driving in ZOP and PBO conditions.
Standardized Highway Driving Test
Driving performance was assessed using a standardized highway driving test22,23 recording SDLP as a measure of driver vehicle control (Figure 2).
Figure 2.
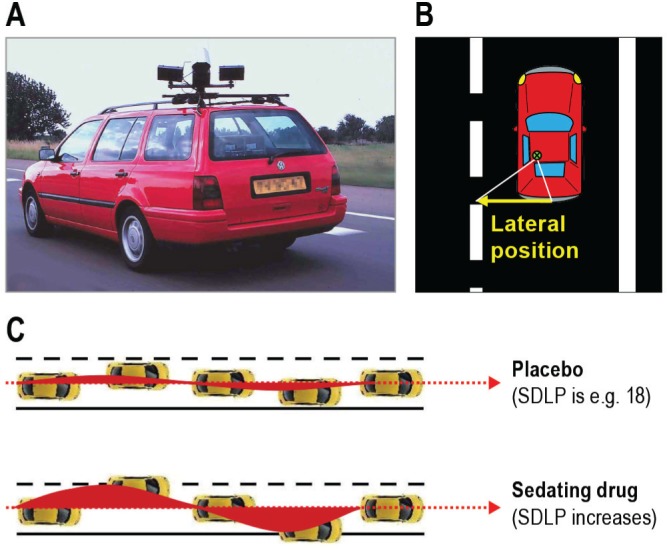
Highway driving test. (A) Subjects drive a specially instrumented vehicle for approximately 1 h over a 100-km primary highway circuit, accompanied by a licensed driving instructor having access to dual controls. The subject's task is to drive with a steady lateral position between the delineated boundaries of the slower (right) traffic lane, while maintaining a constant speed of 95 km/h. (B) A camera on top of the car continuously registers the lateral position of the car on the road with respect to the left lane delineation. (C) The standard deviation of lateral position (SDLP, in cm) is an index of road-tracking error or “weaving”. SDLP scores increase compared with placebo after the use many sedating drugs including low doses of alcohol.
In this test, subjects operate a specially instrumented vehicle for approximately 1 h over a 100-km (61 mile) primary highway circuit, accompanied by a licensed driving instructor having access to dual controls (brakes and accelerator). The subjects' task is to drive with a steady lateral position between the delineated boundaries of the slower (right) traffic lane, while maintaining a constant speed of 95 km/h (58 mph). Subjects may deviate from those instructions only to pass a slower vehicle, and to leave and re-enter the highway at the turnaround point. During the drive, the vehicle's speed and lateral distance to the left lane line are continuously recorded. These signals are digitized at a rate of 4 Hz and stored on an onboard computer disk file for later preprocessing and analysis. Preprocessing consists of offline visual inspection of all data by trained processors (blinded as to treatment condition) to mark data segments that reveal signal loss or disturbances such as passing maneuvers and turnaround point. The preprocessed dataset is then used to calculate means and variances of lateral position and speed of clean (unmarked) data. The primary outcome variable is SDLP in cm.
Procedure
Within 2 w before the first treatment condition, subjects slept 1 night in the same facilities as during treatment conditions, to overcome possible sleep disturbances associated with sleeping in an unfamiliar environment. In the evening preceding their habituation night, subjects were individually trained to perform the driving test.
On treatment days, subjects arrived at the sleeping facility at approximately 22:00 and their eligibility and compliance with study restrictions was verified by questioning, urine screens for drugs of abuse and pregnancy, breath testing for alcohol, and measurement of vital signs. Four subjects were treated on the same night and tested the following day with 5 min difference between their activities. At 23:15 the first subject ingested a first dose of medication or PBO in the presence of an investigator and retired to bed. At 04:15 or 05:15 the investigator awakened the subject by a telephone call, administered the second dose, and instructed the subject to resume sleeping (Figure 1). At 07:30 the investigator awakened the subjects in the same manner. Following toileting and dress, subjects were provided a standardized light breakfast without caffeine and transported to the highway. The driving test started at approximately 08:15, i.e., 3 or 4 h after MOTN dosing. After completion of the driving test subjects were transported home by study personnel.
Within 10 days after the last treatment, subjects' health and well-being were confirmed by questioning them about adverse events, and by physical examination and laboratory tests (blood chemistry and hematology).
Statistical Analyses
To detect an asymmetry in the distribution of the difference between the drug and PBO SDLP, a Max McNemar test was used. The test examines the difference in the proportions of impaired drivers and improved drivers following drug use using a generalized sign test over all relevant thresholds. Symmetry implies that the probability of improvement over PBO is the same as the probability of impairment. Rejecting the null implies that the two probabilities are unequal. The test is based on the maximum of McNemar's statistics over all possible thresholds of concern.27 The lowest change used for this calculation was 1.5 cm. One particularly important criterion is a change in SDLP greater than ± 2.5 cm, which some consider to be clinically meaningful. This threshold criterion, as well as 2.0 cm and 3.5 cm, were individually examined using a McNemar test for each r. To detect an asymmetry of 0.26 versus 0.05 in proportions of impaired and improved drivers with a power of at least 80% and a type I error risk of 0.05, a sample size of 36 was required. Using a Williams design to achieve balance in treatment orders, a total of 40 subjects was needed.
In this study, criteria for impairment were met if a drug-PBO increase in SDLP fell above the threshold for impairment (e.g., > 2.5 cm) or if the test ride was terminated before scheduled completion due to drowsiness.
In addition, repeated-measures analyses of variance were used to detect differences in mean SDLP scores following drug and PBO treatments. The model used included fixed effects for sequence, period, and treatment, and a random effect for subject within sequence. The model was expanded post hoc to add effects of sex and treatment by sex, to evaluate potential sex differences in treatment effects.
Symmetry analyses were performed using a program written in Mathematica (Wolfram, Champaign, IL) that is freely available.27 Analysis of variance calculations were done by using the SAS statistical program version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
A total of 44 volunteers were screened for this study, with 40 enrolled (20 males, 20 females). All enrolled subjects completed the study between June and September 2010. Mean ± standard deviation (SD) age was 37.3 ± 14.8 y. Thirty-nine subjects were white, and one was mixed race. Mean ± SD body mass index was 23.2 ± 2.4 kg/m2. Mean ± SD body weight was 79 ± 8 kg for males and 65 ± 8 kg for females.
Proportions of Impaired and Improved Drivers
Individual subject and mean changes from PBO in SDLP for males and females in each treatment condition are shown in Figure 3.
Figure 3.
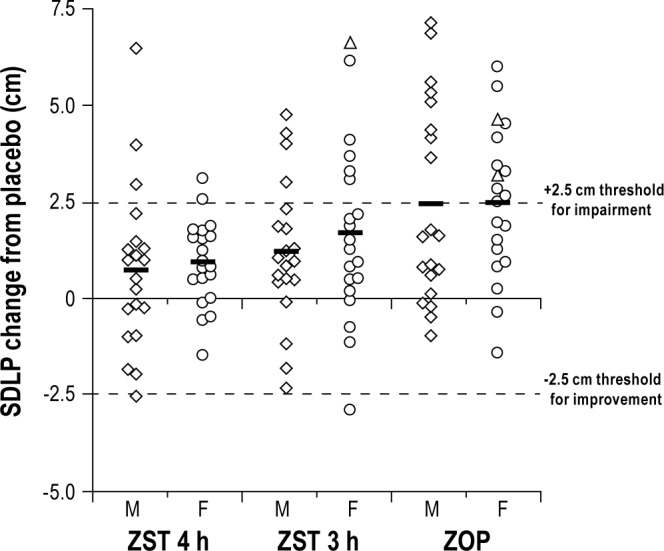
Individual and mean (horizontal lines) drug-placebo changes in driving performance as measured by the standard deviation of lateral position (SDLP). Change scores following administration of zolpidem 3.5 mg sublingual formulation (ZST) 4 h before driving (ZST 4 h), ZST 3 h before driving (ZST 3 h), and zopiclone 7.5 mg (ZOP), are shown separately for males (squares) and females (circles). Dotted lines show thresholds for impaired and improved driving: changes above 2.5 cm reflect impaired drivers, and changes below -2.5 cm reflect improved drivers. SDLP changes of three tests that were terminated prematurely are indicated by triangles.
Two 23-y-old female subjects stopped driving in a total of three driving tests, because the driving instructor judged them too drowsy to safely continue. Both subjects were stopped in ZOP (after 97% and 67% completion of the drive), and one of them was also stopped in ZST 3 h (again after 67% completion). None of the subjects was stopped prematurely in ZST 4 h or PBO. SDLP scores for the prematurely terminated tests were calculated from the data collected until the termination of each ride, and all were increased by more than 2.5 cm compared to their PBO scores (Figure 3).
Symmetry analyses of SDLP changes showed that driving performance was significantly more frequently impaired than improved in ZOP and ZST 3 h, but not in ZST 4 h. Symmetry analyses of SDLP changes showed that driving performance was significantly more frequently impaired than improved in ZOP and ZST 3 h, but not in ZST 4 h. At the third hour, there were 20 subjects whose absolute change from ZST to PBO in SDLPs exceeded 1.5. The Max McNemar test result was 10.88 (P < 0.002.) At the fourth hour, there were 15 subjects whose SDLP exceeded 1.5 and the Max McNemar test result was not significant. Table 1 displays results of tests of symmetry at the specific cut points 2.5, 3.0 and 2.0 cm based on a McNemar test. According to the primary criterion (i.e., a change in SDLP of ≥ 2.5 cm, or test terminated due to drowsiness) 18 subjects (45%; 8 males, 10 females) were impaired in ZOP, whereas none improved (P < 0.0001). In ZST 3 h, 10 subjects (25%; 4 males, 6 females) were impaired, whereas 1 (female) improved (P = 0.0117). In ZST 4 h, 5 subjects were impaired (12.5%; 3 males, 2 females), whereas 1 (male) was improved. This asymmetry was not statistically significant (P = 0.2188).
Table 1.
Symmetry analysis of proportions impaired and improved drivers in each treatment condition, at three thresholds of impairment
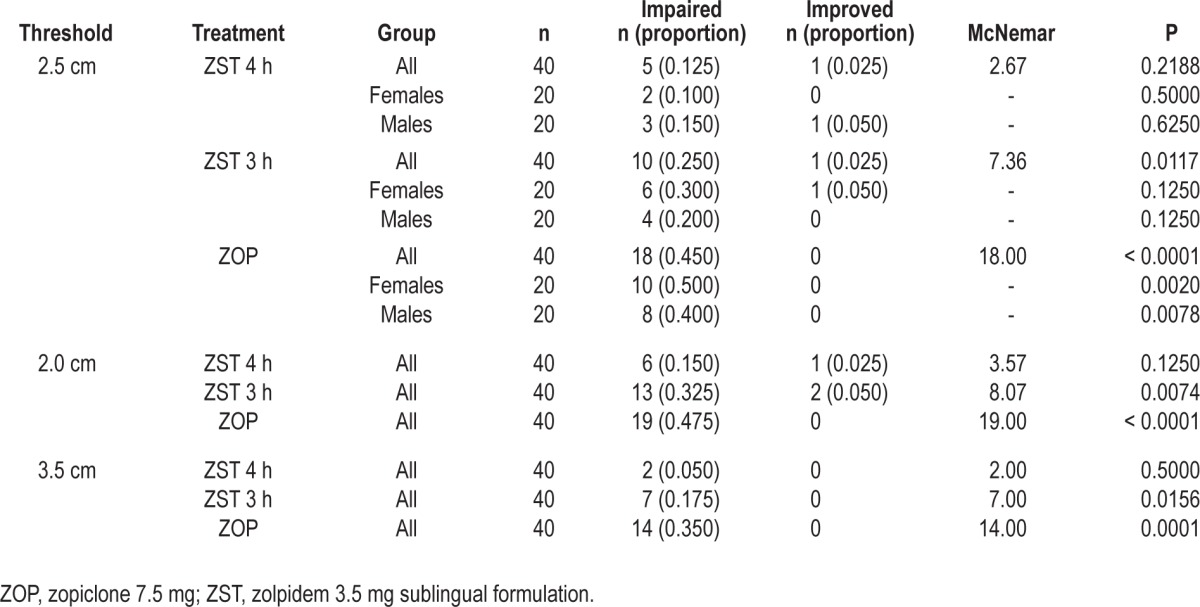
The patterns of treatment effects were comparable within males and females. Fisher exact tests showed that the proportions of impaired and improved drivers after each treatment did not differ between males and females.
Compared to ZOP, 16 subjects drove better in ZST 4 h (40%), whereas 2 drove worse (5%). This asymmetry was significant (P = 0.0013). Driving in ZST 3 h was not significantly better than in ZOP.
Mean Changes in SDLP Scores
Mean ± SD SDLP scores were 15.88 ± 3.14, 16.71 ± 3.34, 17.33 ± 3.57, and 18.34 ± 4.01 in PBO, ZST 4 h, ZST 3 h, and ZOP, respectively (Table 2).
Table 2.
Mean (standard deviation) standard deviation of lateral position scores in each treatment condition and results of analysis of variance of drug-placebo contrasts
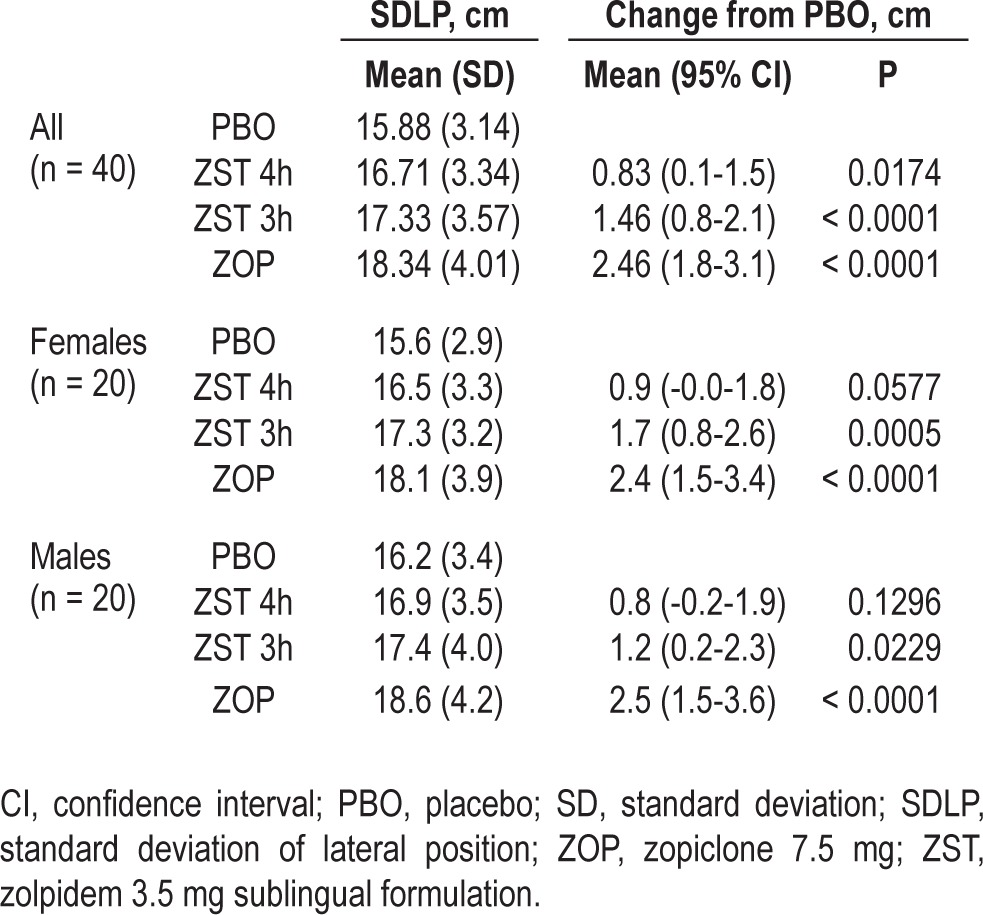
Repeated-measures of analysis of variance showed that the differences between treatments were significant (P < 0.0001). Mean changes from PBO in SDLP in ZST 4 h and ZST 3 h were +0.83 cm (P = 0.0174) and +1.46 cm (P < 0.0001), respectively. Although these changes are statistically significantly different from PBO, the upper limits of the 95% CIs were below the threshold of 2.5 cm. In ZOP, the mean change in SDLP was +2.46 cm (P < 0.0001). As expected, the 95% CI included the criterion for impairment.
Means and changes in SDLP scores were comparable for males and females (Table 2). Post hoc analysis showed no significant effect of sex (P = 0.52) or treatment by sex interaction (P = 0.86).
Safety
A total of 22 adverse events were spontaneously reported by 14 of 40 subjects (35%). After ZST administration, 17.5% of the subjects reported adverse events that were all minor in nature, except one episode of moderate nausea in the ZST 4 h group. The most frequently reported adverse events were minor headache and somnolence. Headache was reported by 7.5% of subjects after administration of ZST, and by 2.5% of subjects after administration of PBO or ZOP. Somnolence was reported by 5.0% of subjects after administration of each active treatment and by 2.5% of subjects after administration of PBO. Fatigue was reported by 5.0% of subjects receiving PBO treatment.
There were no serious adverse events, and no clinically significant changes in physical examination findings or laboratory measures identified during the study. There were no sex differences in safety measures and adverse event reporting.
DISCUSSION
The aim of the current study was to assess the effects of ZST 3.5 mg on driving at 4 h after MOTN dosing (consistent with patient instructions for appropriate use) and 3 h after MOTN dosing (inconsistent with patient instructions). Results showed that when ZST was taken 4 h before driving, there was no statistically significant difference in the proportions of impaired and improved drivers. The mean SDLP at that time was significantly higher than PBO, but the overall increase was small (0.83 cm), and the 95% CI was well below the 2.5 cm threshold for impairment (95% CI, 0.1-1.5 cm). When ZST was taken later in the night, 3 h before driving, a significantly greater proportion of subjects were impaired than improved (25% versus 2.5%). Yet, the mean increase in SDLP was still small (1.46 cm) with a 95% CI below the 2.5 cm threshold for impairment (95% CI, 0.8-2.1 cm). This indicates that although the effects of ZST administered 3 h before driving were statistically significant, they were less severe than those of alcohol at the legal limit for driving, and those following bedtime doses of most intermediate- and long-acting hypnotic drugs.3 Overall, the data support that driving at least 4 h after taking ZST 3.5 mg, consistent with labeling instructions, does not negatively affect driving performance. Driving within 3 h of taking ZST 3.5 mg, however, may result in a negative effect on driving performance. The negative effect on driving performance is not surprising, given the previous data on the duration of action of ZST on a variety of performance tests.19 The results support current dosing guidelines indicating that ZST should only be used when at least 4 h of time in bed are anticipated.
Compared with the effects of MOTN administration of ZST in its conventional dose of 10 mg, the effects of the low-dose buffered formulation are clearly reduced. ZST 10 mg produced mean increases in SDLP at 4 and 5 h after MOTN administration of 3.8 cm and 3.5 cm, respectively,7,15 which is well above the 2.5 cm criterion for impairment, and just below the effects found for alcohol when BACs are 0.08 % (4.1cm).30
Effects of ZOP 7.5 mg clearly demonstrated assay-sensitivity in the current study. It significantly increased mean SDLP by 2.46 cm and was associated with 45% impaired drivers, as defined by a change in SDLP of more than 2.5 cm. The observed mean increase in SDLP is comparable to that found in previous studies using the same driving test, indicating that the subjects in the current study showed normal sensitivity to drug effects.7,10,11,13,31 The proportion of impaired drivers was consistent with mean change. For example, assuming a normal distribution of change, an observed mean of 2.5 cm would be accompanied by about half of the subjects worsening by more than the 2.5-cm threshold for impairment, a smaller proportion falling in the indifference zone and a still smaller proportion improving their driving by more than 2.5 cm.
Overall, ZST 3.5 mg was well tolerated, and there were no sex differences in the types or rates of adverse events. Nonetheless, one female subject had to discontinue the driving test after 67% completion due to excessive drowsiness after use of ZST 3 h before driving. The same subject was also stopped from driving at the same point because of drowsiness after bedtime use of ZOP, suggesting she may have been sensitive to the sedative effects of this class of drugs. One other female subject was stopped from driving because of excessive drowsiness after use of ZOP. Associated changes in SDLP scores relative to PBO all exceeded the primary impairment criterion of 2.5 cm, but absolute SDLP scores of the terminated rides were still within the normal range of PBO conditions (i.e., between 10 cm and 30 cm). These data are in line with the results of symmetry analyses and mean changes in SDLP, showing that driving can be significantly impaired after use of ZOP and ZST 3 h before driving.
In contrast to the lack of sex differences in ZST's effects in the current study, a recent paper reporting a post hoc sex analysis of data from a study by Verster and colleagues showed that driving performance in women was more impaired than in men after MOTN use of ZST 10 mg and 20 mg.15,32 A major difference between these studies is the dose. As sex-related differences in performance are potentially related to differences in drug concentrations, it seems that the differences in concentrations after 3.5 mg may be too small to produce significant differences in performance between men and women.
It is not unusual that driving tests are terminated prematurely. The overall percentage of tests terminated prematurely in the current study (1.9% of a total of 160) is comparable to that found in other studies. A recent review of 47 papers reporting 50 Dutch clinical trials using the same driving test showed that on average approximately 3.1% of the tests are stopped, not only after drug treatment (4.1%) but also after PBO (0.7%).33 Thus, the percentage of tests terminated in the current study is below average. The review concludes that the decision to stop driving is not a good correlate of objective driving performance.
The results of this study are in line with previous findings that ZST 3.5 mg no longer produces clinically relevant sedation or performance impairment at 4 h or more after MOTN dosing.17,18 In a pharmacokinetic/pharmacodynamic study with healthy volunteers by Roth and colleagues, ZST 3.5 mg impaired performance in tests of digit symbol substitution, symbol copying, choice reaction time, and word learning within the first 3 h, but not after that.19 Although these tests are useful for initial screening of relevant impairment, based on the results of this study, it appears that they do not reliably predict more subtle effects on driving performance, such as those found between 3 and 4 h after dosing in the current study.34
Limitations
The use of healthy volunteers instead of patients with insomnia could be seen as a limitation of the current study. An important reason normal volunteers are enrolled is to facilitate comparisons to previous driving studies, which were virtually all conducted with normal volunteers.3,7,10,11,13,30 More importantly, a recent study comparing the effects of ZOP on driving in patients with insomnia and healthy volunteers suggests that healthy volunteers may be more sensitive to the residual effects.8 Thus, studying drug effects in healthy volunteers minimizes the risk of failing to detect clinically relevant impairment associated with use of a drug. To determine the modifying effects of insomnia diagnosis and the interaction as well as other comorbid disorders and concomitant medication, additional data from studies in patient populations are needed. This study intended to determine the impairment potential of the drug alone, as compared with that of other drugs; for example, ZOP 7.5 mg at bedtime, or ZST 10 mg in the MOTN.
Another point for discussion may be the criteria used for clinically relevant impairment in this study. We used a newly developed statistical method to evaluate impairment rates using improvement rates as an internal control over all relevant thresholds as the defining criterion.27 We also focused on a specific criterion based on known average effects of alcohol, the only drug for which widely accepted thresholds in blood concentrations and accident risk are available.30,35,36 Because there is no widely accepted criterion for driving impairment based on any other drug, alcohol is still the most important benchmark. In studies evaluating the residual effects of hypnotic drugs, ZOP 7.5 mg is increasingly used as a reference because its effects are reliable and moderately severe, in the sense that the magnitude of effects on driving is comparable those of alcohol concentrations of 0.05 %, which is the legal limit for driving a car in most countries.37
In conclusion, the results of this study suggest that ZST 3.5 mg has a minimal risk of impairing driving performance in the morning, 4 h or more after MOTN use. When taken 3 to 4 h before driving, the drug may have minor impairing effects, so caution should be exercised at that time. Patients should be instructed accordingly.
DISCLOSURE STATEMENT
This study was financially supported by Transcept Pharmaceuticals, Inc. and Purdue Pharma L.P. Maastricht University received financial support from Transcept Pharmaceuticals, Inc., to conduct this study. Drs. Vermeeren, Vuurman, Van Leeuwen and Ms. Van Oers are employees of Maastricht University. Dr. Leufkens was an employee of Maastricht University at the time this study was conducted, and is currently a full time employee of Philips Research. Dr. Laska is employed at the Nathan Kline Institute for Psychiatric Research and at New York University, School of Medicine. Dr. Laska has acted as a consultant for GlaxoSmithKline, Intec Pharma, Merck & Co., Neuraxon, Novartis, Transcept Pharmaceuticals, Purdue Pharma L.P., and Vanda. Drs. Rico and Steinberg are employees of Transcept Pharmaceuticals, Inc. Dr. Roth has received grants/research support from Apnex Medical, Inc., Aventis Pharmaceuticals, Inc., Cephalon, Inc., GlaxoSmith-Kline plc, Merck & Co., Inc., Neurocrine Biosciences, Inc., Pfizer, Inc., Sanofi U.S., Schering-Plough Corp., Sepracor, Inc., Somaxon Pharmaceuticals, Inc., Somnus Therapeutics, Inc., Syrex Corp., Takeda Pharmaceutical Co., Ltd, Transcept Pharmaceuticals, Inc., Ventus Medical, Inc., Wyeth, LLC, and Xenoport, Inc. He has acted as a consultant for Abbott Laboratories, Acadia Pharmaceuticals, Inc., Acologix, Inc., Acorda Therapeutics, Inc., Actelion Pharmaceuticals Ltd, Addrenex Pharmaceuticals, Inc., Alza Corp., Ancile, Arena Pharmaceuticals, Inc., AstraZeneca plc, Aventis Pharmaceuticals, Inc., Aver Pharmaceuticals Pvt. Ltd, Bayer AG, Bristol-Myers Squibb Company, BTG International Ltd, Cephalon, Inc., Cypress Pharmaceutical, Inc., Dove Pharmaceuticals, Eisai Co., Ltd, Elan Corp., Eli Lilly and Company, Evotec AG, Forest Laboratories, Inc., GlaxoSmithKline plc, Hypnion, Inc., Impax Laboratories, Inc., Intec Pharma Ltd, Intra-Cellular Therapies, Inc., Jazz Pharmaceuticals plc, Johnson & Johnson, Inc., King Pharmaceuticals, Inc., Lundbeck A/S, McNeil Consumer Healthcare, MediciNova, Inc., Merck & Co., Inc., Neurim Pharmaceuticals Ltd, Neurocrine Biosciences, Inc., Neurogen Corp., NovaDel Pharma, Inc., Novartis AG, Ocera Therapeutics, Inc., Orexo AB, Organon Pharmaceuticals, Inc., Otsuka Pharmaceutical Co., Ltd, Prestwick Pharmaceuticals, Inc., Procter & Gamble, Pfizer, Inc., Purdue Pharma L.P., Teva, Hoffman-La Roche, Inc., Sanofi S.A., Schering-Plough Corp., Sepracor, Inc., Servier, Shire plc, Somaxon Pharmaceuticals, Inc., Syrex Corp., Takeda Pharmaceutical Co., Ltd, Transcept Pharmaceuticals, Inc., Vanda Pharmaceuticals, Inc., Ventus Medical, Inc., VivoMetrics, Inc., Wyeth, LLC, Yamanuchi Pharmaceutical Co., Ltd, and Xenoport, Inc. In the past 12 months he has participated in speaking engagements supported by Purdue Pharma L.P. Work was performed at Maastricht University, Maastricht, The Netherlands.
ACKNOWLEDGMENTS
The authors thank Henk Brauers, Jo Gorissen, Hans Sleebe, Bert Adriaens, Irma Brauers, Anique van Dorp, Natalie Valle Guzman, Lizzy Vuurman, ZsaZsa Weerts, Bart Wagemans, Sander Huisman, Iwan de Jong, Francis Palmen, Odile Schoffelen, and Arno Skrabanja (Maastricht University) for their assistance in carrying out this study; Elizabeth Li (Pharmastat) for statistical analyses; and Margaret Moline and Margi Goldstein (Purdue Pharma L.P.) and Tam Vo and Gautam Bijur (Excerpta Medica) for providing editorial assistance with the manuscript.
ABBREVIATIONS
- BAC
blood alcohol concentration
- CI
confidence interval
- h
hour(s)
- km
kilometers
- mph
miles per hour
- MOTN
middle of the night
- PBO
placebo
- SD
standard deviation
- SDLP
standard deviation of lateral position
- y
year(s)
- ZOP
zopiclone
- ZST
zolpidem sublingual formulation
REFERENCES
- 1.Ohayon MM. Nocturnal awakenings and difficulty resuming sleep: their burden in the European general population. J Psychosom Res. 2010;69:565–71. doi: 10.1016/j.jpsychores.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM, Krystal A, Roehrs TA, Roth T, Vitiello MV. Using difficulty resuming sleep to define nocturnal awakenings. Sleep Med. 2010;11:236–41. doi: 10.1016/j.sleep.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vermeeren A. Residual effects of hypnotics: epidemiology and clinical implications. CNS Drugs. 2004;18:297–328. doi: 10.2165/00023210-200418050-00003. [DOI] [PubMed] [Google Scholar]
- 4.DuPont RL, Voas RB, Walsh JM, Shea C, Talpins SK, Neil MM. The need for drugged driving per se laws: a commentary. Traffic Inj Prev. 2012;13:31–42. doi: 10.1080/15389588.2011.632658. [DOI] [PubMed] [Google Scholar]
- 5.Dassanayake T, Michie P, Carter G, Jones A. Effects of benzodiazepines, antidepressants and opioids on driving: a systematic review and meta-analysis of epidemiological and experimental evidence. Drug Saf. 2011;34:125–56. doi: 10.2165/11539050-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Gustavsen I, Al-Sammurraie M, Mørland J, Bramness JG. Impairment related to blood drug concentrations of zopiclone and zolpidem compared to alcohol in apprehended drivers. Accid Anal Prev. 2009;41:462–6. doi: 10.1016/j.aap.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Leufkens TR, Lund JS, Vermeeren A. Highway driving performance and cognitive functioning the morning after bedtime and middle-of-the-night use of gaboxadol, zopiclone and zolpidem. J Sleep Res. 2009;18:387–96. doi: 10.1111/j.1365-2869.2009.00746.x. [DOI] [PubMed] [Google Scholar]
- 8.Leufkens TR, Ramaekers JG, de Weerd AW, Riedel WJ, Vermeeren A. Residual effects of zopiclone 7.5 mg on highway driving performance in insomnia patients and healthy controls: a placebo controlled crossover study. Psychopharmacology (Berl) 2014 Jan 24; doi: 10.1007/s00213-014-3447-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leufkens TR, Vermeeren A. Highway driving in elderly the morning after bedtime use of hypnotics: a comparison between temazepam 20 mg, zopiclone 7.5 mg, and placebo. J Clin Psychopharmacol. 2009;29:432–8. doi: 10.1097/JCP.0b013e3181b57b43. [DOI] [PubMed] [Google Scholar]
- 10.Mets MA, de Vries JM, de Senerpont Domis LM, Volkerts ER, Olivier B, Verster JC. Next-day effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects. Sleep. 2011;34:1327–34. doi: 10.5665/SLEEP.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramaekers JG, Conen S, de Kam PJ, et al. Residual effects of esmirtazapine on actual driving performance: overall findings and an exploratory analysis into the role of CYP2D6 phenotype. Psychopharmacology Berl. 2011;215:321–32. doi: 10.1007/s00213-010-2149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vermeeren A, Danjou PE, O'Hanlon JF. Residual effects of evening and middle-of-the-night administration of zaleplon 10 and 20 mg on memory and actual driving performance. Hum Psychopharmacol Clin Exp. 1998;13(Suppl 2):S98–107. [Google Scholar]
- 13.Vermeeren A, Riedel WJ, van Boxtel MP, Darwish M, Paty I, Patat A. Differential residual effects of zaleplon and zopiclone on actual driving: a comparison with a low dose of alcohol. Sleep. 2002;25:224–31. [PubMed] [Google Scholar]
- 14.Volkerts ER, O'Hanlon JF. Hypnotics' residual effects on driving performance. In: O'Hanlon JF, De Gier JJ, editors. Drugs and driving. Oxford: Taylor Francis; 1986. pp. 123–35. [Google Scholar]
- 15.Verster JC, Volkerts ER, Schreuder AH, et al. Residual effects of middle-of-the-night administration of zaleplon and zolpidem on driving ability, memory functions, and psychomotor performance. J Clin Psychopharmacol. 2002;22:576–83. doi: 10.1097/00004714-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Perdue Pharma LP. Intermezzo - zolpidem tartrate tablet. [Accessed August 27, 2013]. Available from: http://app.purduepharma.com/xmlpublishing/pi.aspx?id=i.
- 17.Roth T, Hull SG, Lankford DA, Rosenberg R, Scharf MB Intermezzo Study Group. Low-dose sublingual zolpidem tartrate is associated with dose-related improvement in sleep onset and duration in insomnia characterized by middle-of-the-night (MOTN) awakenings. Sleep. 2008;31:1277–84. [PMC free article] [PubMed] [Google Scholar]
- 18.Roth T, Krystal A, Steinberg FJ, Singh NN, Moline M. Novel sublingual low-dose zolpidem tablet reduces latency to sleep onset following spontaneous middle-of-the-night awakening in insomnia in a randomized, double-blind, placebo-controlled, outpatient study. Sleep. 2013;36:189–96. doi: 10.5665/sleep.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roth T, Mayleben D, Corser BC, Singh NN. Daytime pharmacodynamic and pharmacokinetic evaluation of low-dose sublingual transmucosal zolpidem hemitartrate. Hum Psychopharmacol. 2008;23:13–20. doi: 10.1002/hup.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenblatt DJ, Harmatz JS, von Moltke LL, et al. Comparative kinetics and dynamics of zaleplon, zolpidem, and placebo. Clin Pharmacol Ther. 1998;64:553–61. doi: 10.1016/S0009-9236(98)90139-4. [DOI] [PubMed] [Google Scholar]
- 21.Greenblatt DJ, Roth T. Zolpidem for insomnia. Expert Opin Pharmacother. 2012;13:879–93. doi: 10.1517/14656566.2012.667074. [DOI] [PubMed] [Google Scholar]
- 22.O'Hanlon JF. Driving performance under the influence of drugs: rationale for, and application of, a new test. Br J Clin Pharmacol. 1984;18:121S–29S. doi: 10.1111/j.1365-2125.1984.tb02590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verster JC, Roth T. Standard Operation Procedures for conducting the on-the-road driving test, and measurement of the Standard Deviation of Lateral Position (SDLP) Int J Gen Med. 2011;4:359–71. doi: 10.2147/IJGM.S19639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Hanlon JF, Ramaekers JG. Antihistamine effects on actual driving performance in a standard test: a summary of Dutch experience, 1989-94. Allergy. 1995;50:234–42. doi: 10.1111/j.1398-9995.1995.tb01140.x. [DOI] [PubMed] [Google Scholar]
- 25.Vuurman EF, Muntjewerff ND, Uiterwijk MM, et al. Effects of mefloquine alone and with alcohol on psychomotor and driving performance. Eur J Clin Pharmacol. 1996;50:475–82. doi: 10.1007/s002280050144. [DOI] [PubMed] [Google Scholar]
- 26.Brookhuis K. How to measure driving ability under the influence of alcohol and drugs, and why. Hum Psychopharmacol Clin Exp. 1998;13:S64–9. [Google Scholar]
- 27.Laska E, Meisner M, Wanderling J. A maximally selected test of symmetry about zero. Stat Med. 2012;31:3178–91. doi: 10.1002/sim.5384. [DOI] [PubMed] [Google Scholar]
- 28.Olubodun JO, Ochs HR, von Moltke LL, et al. Pharmacokinetic properties of zolpidem in elderly and young adults: possible modulation by testosterone in men. Br J Clin Pharmacol. 2003;56:297–304. doi: 10.1046/j.0306-5251.2003.01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenblatt DJ, Harmatz JS, von Moltke LL, et al. Comparative kinetics and response to the benzodiazepine agonists triazolam and zolpidem: evaluation of sex-dependent differences. J Pharmacol Exp Ther. 2000;293:435–43. [PubMed] [Google Scholar]
- 30.Louwerens JW, Gloerich ABM, De Vries G, Brookhuis KA, O'Hanlon JF. International Congress on Alcohol, Drugs and Traffic Safety. Amsterdam: Exerpta Medica; 1987. The relationship between drivers' blood alcohol concentration (BAC) and actual driving performance during high speed travel; pp. 183–6. [Google Scholar]
- 31.Vermeeren A, Vuurman E, Van Oers A, et al. Effects of suvorexant, an orexin receptor antagonist, on next day driving performance in healthy volunteers. Neuropsychopharmacology. 2012;38:S320–1. [Google Scholar]
- 32.Verster JC, Roth T. Gender differences in highway driving performance after administration of sleep medication: a review of the literature. Traf Inj Prev. 2012;13:286–92. doi: 10.1080/15389588.2011.652751. [DOI] [PubMed] [Google Scholar]
- 33.Verster JC, Roth T. The prevalence and nature of stopped on-the-road driving tests and the relationship with objective performance impairment. Accid Anal Prev. 2012;45:498–506. doi: 10.1016/j.aap.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Verster JC, Roth T. Predicting psychopharmacological drug effects on actual driving (SDLP) from psychometric tests measuring driving-related skills. Psychopharmacology. 2012;220:293–301. doi: 10.1007/s00213-011-2484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borkenstein RF, Crowther RF, Shumate RP, Ziel WB, Zylman R. The role of the drinking driver in traffic accidents (The Grand Rapids Study). 2nd ed. Blutalkohol. 1974;11:1–132. [Google Scholar]
- 36.Krüger HP, Vollrath M. The alcohol-related accident risk in Germany: procedure, methods and results. Accid Anal Prev. 2004;36:125–33. doi: 10.1016/s0001-4575(02)00134-3. [DOI] [PubMed] [Google Scholar]
- 37.Verster JC, Spence DW, Shahid A, Pandi-Perumal SR, Roth T. Zopiclone as positive control in studies examining the residual effects of hypnotic drugs on driving ability. Curr Drug Saf. 2011;6:209–18. doi: 10.2174/157488611798280933. [DOI] [PubMed] [Google Scholar]


