Abstract
Study Objectives:
Musculoskeletal pain in humans is often associated with poor sleep quality. We used a model in which mechanical hypersensitivity was induced by injection of acidified saline into muscle to study the impact of musculoskeletal sensitization on sleep of mice.
Design:
A one month pre-clinical study was designed to determine the impact of musculoskeletal sensitization on sleep of C57BL/6J mice.
Methods:
We instrumented mice with telemeters to record the electroencephalogram (EEG) and body temperature. We used an established model of musculoskeletal sensitization in which mechanical hypersensitivity was induced using two unilateral injections of acidified saline (pH 4.0). The injections were given into the gastrocnemius muscle and spaced five days apart. EEG and body temperature recordings started prior to injections (baseline) and continued for three weeks after musculoskeletal sensitization was induced by the second injection. Mechanical hypersensitivity was assessed using von Frey filaments at baseline (before any injections) and on days 1, 3, 7, 14, and 21 after the second injection.
Results:
Mice injected with acidified saline developed bilateral mechanical hypersensitivity at the hind paws as measured by von Frey testing and as compared to control mice and baseline data. Sleep during the light period was fragmented in experimental mice injected with acidified saline, and EEG spectra altered. Musculoskeletal sensitization did not alter the duration of time spent in wakefulness, non-rapid eye movement sleep, or rapid eye movement sleep.
Conclusions:
Musculoskeletal sensitization in this model results in a distinct sleep phenotype in which sleep is fragmented during the light period, but the overall duration of sleep is not changed. This study suggests the consequences of musculoskeletal pain include sleep disruption, an observation that has been made in the clinical literature but has yet to be studied using preclinical models.
Citation:
Sutton BC; Opp MR. Musculoskeletal sensitization and sleep: chronic muscle pain fragments sleep of mice without altering its duration. SLEEP 2014;37(3):505-513.
Keywords: Musculoskeletal sensitization, mechanical hypersensitivity, sleep fragmentation
INTRODUCTION
It is estimated that 1.5 billion people worldwide suffer from moderate to severe chronic pain.1 Individuals suffering chronic pain comprise one of the costliest patient populations, especially in terms of lost work and reduced productivity.2,3 Persons with chronic pain also report some of the lowest quality of life among patients suffering from chronic diseases.4 Poor sleep is another major public health issue, with almost 40% of the US population reporting chronic insufficient sleep, and 50-70 million Americans diagnosed with sleep disorders.5,6 Sleep disorders and chronic pain are often comorbid conditions, and the overall prevalence and economic burden of chronic pain and insufficient sleep make these diseases an important topic of public health research.
Data derived from clinical research supports a bidirectional relationship between sleep and chronic pain.7–9 A variety of chronic pain conditions have comorbid sleep disturbances.10–12 Sleep of patients with chronic pain is characterized by difficulty initiating sleep, maintaining sleep, excessive nighttime awakenings, and feeling unrefreshed after sleeping.7,8,11,13 For example, individuals suffering with chronic low back pain have insomnia rates over 50%, and subjective pain correlates with severity of insomnia.14 Persons with primary insomnia also report chronic pain at rates over 50%, the highest associated comorbidity for insomnia.15 Recent epidemiological research identifies a history of poor sleep quality as a significant risk factor in the development of fibromyalgia.16 Furthermore, experimental disruption or deprivation of sleep reduces pain thresholds.7,17,18 Conversely, extension of the sleep period is sufficient to reduce pain sensitivity, suggesting that sufficient sleep may reduce pain.19 Collectively, these and other data contribute to our understanding of the relationship between poor sleep quality and chronic pain.
Three of the most prevalent types of chronic pain in our society are low back pain, neck pain, and facial pain,20 all of which are musculoskeletal. The most prevalent chronic pain conditions associated with insomnia are arthritis (primarily rheumatoid), spinal pain (including low back pain), and fibromyalgia.21–23 Although preclinical models of osteoarthritis,24–27 sciatic nerve injury,28–31 and inflammatory pain32 have been used to determine the impact of chronic pain on sleep, none of these conditions constitute musculoskeletal pain. To the best of our knowledge, no preclinical models have been used to investigate the effect of chronic musculoskeletal pain on sleep. Changes in rodent sleep in models of osteoarthritis, sciatic nerve injury, and inflammatory pain include increased wakefulness, decreased rapid eye movement (REM) sleep and non-rapid eye movement (NREM) sleep, and an increased latency to sleep onset.24,26,28,32,33 Given the clinical correlations between some musculoskeletal pain conditions and altered sleep, we hypothesized that musculoskeletal sensitization would disrupt sleep of rodents. To test this hypothesis, we quantified sleep of mice before and after musculoskeletal sensitization. We now report that musculoskeletal sensitization fragments sleep of mice and alters some facets of the sleep EEG.
METHODS
Animals
Adult male C57BL/6J mice (22-25 g) were purchased from the Jackson Laboratory (Bar Harbor, ME). All mice were maintained on a 12:12 h light:dark cycle at 27°C with ad libitum access to food and water. All procedures using mice in these studies were approved in advance by the University of Washington Institutional Animal Care and Use Committee (IACUC), in accordance with the US Department of Agriculture Animal Welfare Act and the National Institutes of Health policy on Humane Care and the Use of Laboratory Animals.
Musculoskeletal Sensitization
Musculoskeletal sensitization using acidified saline injections has previously been used to study aspects of the muscle pain associated with chronic pain conditions, including chronic widespread pain and fibromyalgia, in rodents.34,35 Briefly, this protocol involves 2 unilateral injections into the gastrocnemius muscle spaced 5 days apart of either normal (pH 7.2, control) or acidified (pH 4.0) saline. When acidified saline is injected using this protocol, a robust bilateral secondary mechanical hypersensitivity at the hindpaws develops and persists at least 4 weeks.34,35 In each experiment, mice were randomized into groups injected with either normal saline or acidified saline. At the time of injection, mice were briefly anesthetized using isoflurane, a hind leg cleaned using alcohol, and 20 μL of normal or acidified saline injected into the gastrocnemius muscle using a 31g needle. All animals were immediately returned to their home cage and observed by the investigator until fully ambulatory.
Mechanical Hypersensitivity Testing
The von Frey filament test is used to measure sensitivity to a non-noxious punctate pressure stimulus. All habituation and testing took place at light onset and was completed during the first 2 h of the light period. Mice were habituated to a galvanized steel mesh testing platform for a minimum of 60 min for 3 days prior to baseline testing. On testing days, mice were given a minimum of 30 min (or until quiet) to habituate to the testing platform. Calibrated filaments (0.07, 0.45, and 1.45 g pressure deflection) were presented in ascending order to the glabrous skin of the hindpaw until they bowed slightly.36 Hindpaws were alternated until a total of 5 presentations per filament per paw was reached. Testing continued until all 3 filaments had been presented with a minimum of 1-min break between filament presentations. If mice became active, testing was suspended until mice were quiet before continuing. Positive responses were recorded when mice retracted the paw in response to the filament.
Experimental Design and Clinical Health Monitoring
A total of 30 C57BL/6J mice were used in this study. A subset (n = 14) of mice was implanted with telemetry units to record EEG and body temperature, which were used to determine sleep state (see later). Surgically implanted mice were given three weeks of recovery before the study began. All mice, irrespective of whether they were surgically implanted or uninstrumented, were 9-12 weeks at the time von Frey testing began. All mice underwent 3 days of baseline von Frey testing to determine mechanical sensitivity. For mice implanted with telemetry units, 2 days of baseline EEG and body temperature recordings were collected prior to sensitization injections. All mice were twice injected with either normal (n = 14 total; n = 6 instrumented) or acidified saline (n = 16 total; n = 8 instrumented), 5 days apart as described above. Mechanical sensitivity was assessed at baseline (before any injections) and 1, 3, 7, 14, 21 days after the second sensitization injection. EEG and body temperature were recorded from mice instrumented with telemeters for the duration of the protocol.
Daily food consumption, water consumption, and body weight were recorded at light onset throughout the experimental protocol. These measures provided an assessment of the impact of musculoskeletal sensitization on the overall health of the animal.
Surgical Procedures
Mice that were implanted with the telemeters were deeply anesthetized with isoflurane (4% induction, 2% maintenance) and surgically implanted with telemeters (ETA10-F20, Data Sciences International, Minneapolis, MN) to permit recording of the electroencephalogram (EEG), core body temperature (CBT) and activity as previously reported.37,38 Transmitter leads were passed subcutaneously to the base of the skull and attached to stainless steel screws (#80 × 1/8 in., Small Parts, Miami Lakes, FL) placed bilaterally over frontal and parietal cortices. These screws served as EEG recording electrodes. Mice were injected subcutaneously with Penicillin G Procaine (0.1 to 0.2 mL, 300,000 units/mL) immediately after surgery to reduce risk of infection. Perioperative pain management consisted of providing ibuprofen (0.2 mg/ mL) in drinking water for 48 h after surgery and administration of buprenorphine (0.05 mg/kg, subcutaneously) at the time of surgery and for 2 days following surgery, if needed. Lidocaine and triple antibiotic ointment were applied topically at the incision site immediately after surgery. Mice were monitored during recovery from anesthesia until ambulatory and were then transferred to recording cages for recovery and habituation.
Physiological Monitoring and Data Acquisition
Signals from telemeters were fed to an analog converter (DSI ART Analog-8 CM) that converted EEG and temperature signals to voltages using a transmitter-specific calibration factor provided by DSI. The output from the converter was captured by an AD board (model PCI-3033E, National Instruments) that re-digitized the data at 128 Hz with 16-bit precision. Temperature voltages were converted to engineering units by regression using calibration coefficients specific for each transmitter. General cage activity was detected using infrared sensors. All signals (EEG, core body temperature, and cage activity) were stored as binary files until further processing.
During acquisition, the EEG was digitally filtered using Chebyschev filters with 3rd order coefficients into delta (0.5-4.5 Hz) and theta (6.0-9.0) Hz frequency bands. These filtered EEG signals were integrated over 1-s periods and stored as part of the binary file structure. Arousal state designations were made on the basis of visual inspection of the recordings using custom software (ICELUS, M. Opp, University of Michigan) written in LabView for Windows (National Instruments) as previously described.37,38 Briefly, wakefulness (W), NREM (NREM) sleep, or REM (REM) sleep was determined for each 10-s epoch of the recording period based on the EEG, integrated delta and theta frequency components of the EEG, and general cage activity. Any epoch containing either movement artifacts or electrical noise was tagged and excluded from subsequent spectral analyses. The raw, non-integrated EEG signals were processed offline using fast Fourier transforms (FFT) to yield power spectra between 0.5 and 40 Hz in 0.5 Hz frequency bins. These spectra were computed by averaging the 5 consecutive 2-s EEG segments comprising each 10-s epoch. The resulting spectrum was matched to state to provide state-specific spectra. Spectra were normalized as a percentage of total power across all frequencies for specific behavioral states within the 12-h light or dark period.
The extent to which sleep was consolidated or fragmented was determined by evaluating the number of transitions from one arousal state to the next. These determinations were made irrespective of arousal state designation and without the use of arbitrary criteria for sleep architecture parameters. Latency to REM sleep was defined and recorded as the time in minutes from light onset to the first REM sleep bout consisting of a minimum of 2 consecutive epochs (20 s) of REM sleep.
Statistical Analysis
Statistical analyses were performed using SPSS for Windows. All data are presented as mean ± standard error of the mean (SEM). To determine the impact of manipulations across time, analyses were restricted to within-group (normal saline, acidified saline) comparisons for time spent in each behavioral state, core body temperature, food and water consumption, and body weights. These within-group comparisons were made by means of a general linear model for repeated measures using a within subjects factor of time. To determine if there was an effect of intramuscular injections on these parameters, a general linear model for repeated measures with between-subjects factor of treatment (normal saline, acidified saline) was used.
To determine if there was an effect of intramuscular injections on mechanical sensitivity, comparisons were made by evaluating the response incidence ([response per filament / 5 possible responses] × 100) data for each paw (ipsilateral, contralateral to intramuscular injection) from each individual monofilament, as well as a total response incidence ([Total responses per paw / 15 possible responses] × 100). Repeated-measures ANOVA with between-subjects factor of treatment (control, acidified) and a within-subjects factor of time (day of experimental protocol) was used to test ipsilateral and contralateral paw data. An α level of P ≤ 0.05 was accepted for all statistical tests as indicating significant departures between the groups across the testing period.
RESULTS
Bilateral Mechanical Hypersensitivity
Unilateral injections of acidified saline spaced 5 days apart produced mechanical hypersensitivity at the hindpaws relative to control animals injected with normal saline (Figure 1). This hypersensitivity was detected across all 3 filament pressures and manifest as a significant increase in response incidence to von Frey testing that was apparent on day 3 and continued across all 21 protocol days. Total responsiveness to filaments of mechanically sensitized animals was significantly increased for the 3 week testing period when compared with control mice (between groups) and preinjection baseline (within groups).
Figure 1.
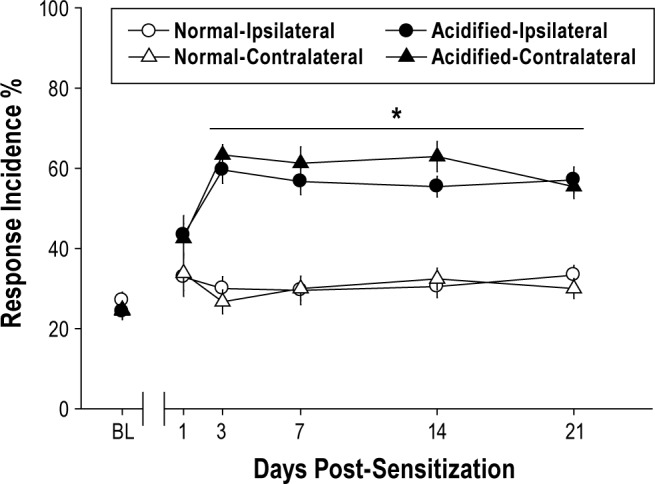
Musculoskeletal sensitization enhances bilateral responses to von Frey testing. Mice injected with acidified saline (n = 16) exhibit mechanical hypersensitivity for at least 21 days, whereas mechanical hypersensitivity does not develop in mice injected with normal pH saline (n = 14). Responsiveness to von Frey filaments are plotted as mean ± SEM total response incidence percent ([total responses / total filament presentations] × 100) per paw. *P ≤ 0.05 vs. normal pH saline injection.
Sleep State Transitions
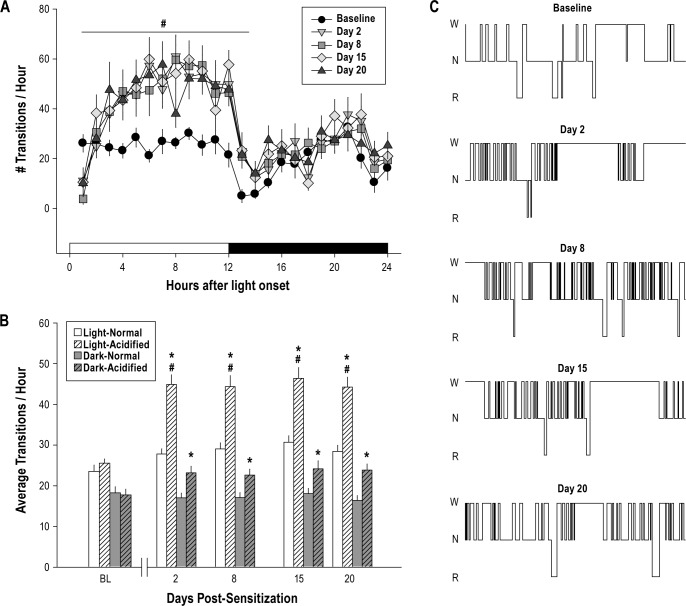
The number of state transitions during the baseline recording period did not significantly differ among mice subsequently randomized into the two treatment groups (Figure 2, Table 1). Within subjects analysis revealed modest, yet statistically significant increases in the number of state transitions for control mice only during the light period across all recording days. However, mice injected with acidified saline and subsequently sensitized exhibited an increase in the number of state transitions during both the light and dark period post-injection. Furthermore, between subjects analysis revealed that experimental mice with musculoskeletal sensitization manifest a greater number of state transitions than control mice at all time points assessed during the protocol (Figure 2, Table 1).
Figure 2.
Sleep is fragmented after musculoskeletal sensitization with acidified saline. (A) The total number of transitions/h is plotted across the 24-h light/ dark period only for animals injected with acidified saline (n = 8). Symbols are the mean ± SEM for pre-injection baseline and for 20 days after mechanical hypersensitivity is induced. Acidified saline injections fragment of sleep during the light period. (B) The average number of transitions/h during the 12-h light or dark period is plotted for pre-injection baseline (BL), and for days 2, 8, 15, and 20 after mechanical hypersensitivity is induced. Values are the mean ± SEM for n = 8 mice. (C) Representative hypnograms from one mouse obtained during pre-injection baseline, and at days 2, 8, 15, and 20 after induction of mechanical hypersensitivity. Hypnograms are from a 1-h recording 10 h after light onset during each of the days depicted. W, Wakefulness; N, NREM; R, REM sleep. #P ≤ 0.05 vs. pre-injection baseline. *P ≤ 0.05 vs. normal pH saline injection.
Table 1.
Sleep duration, core temperature, normalized delta power, and sleep state transitions across the recording period
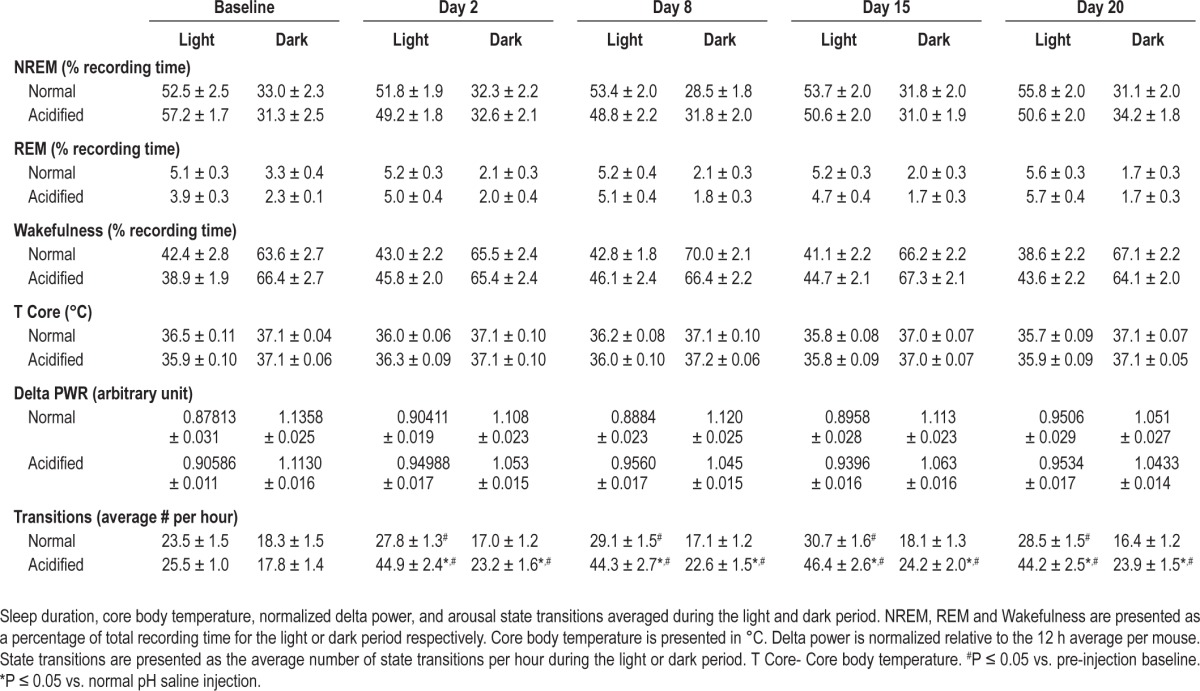
Sleep Duration
The amount of NREM and REM sleep during the baseline recording period did not differ among mice subsequently randomized into the 2 treatment groups (Table 1). Within subjects analyses did not reveal a significant change in either NREM or REM sleep duration during the recording period for either injection group (Table 1). Similarly, between subjects analyses did not reveal a significant impact of musculoskeletal sensitization on NREM or REM sleep time (Table 1). Latency to REM sleep increased significantly for mice injected with acidified saline. The average REM sleep latency increased from 21 min at baseline to 75 min after musculoskeletal sensitization.
NREM Delta Power and Spectral Analysis
EEG spectral characteristics were analyzed from recordings obtained at baseline and days 2, 8, 15, and 20 after the second intramuscular injection. Delta power during NREM is a common measure of sleep intensity,39,40 with NREM delta power increasing during recovery sleep after periods of prolonged wakefulness.41 Because of inter-animal variations in the EEG, all analyses were performed on values normalized relative to the 12 hour average NREM delta power for the light and dark period ([Hourly value / 12 h period average] × 100).42 At pre-injection baseline, normalized delta power during NREM sleep during the 12-h light or dark period did not significantly differ between mice subsequently randomized to injection groups. Within subjects analysis did not reveal a significant change in NREM delta power within injection groups across the recording period. Similarly, between subjects analysis did not reveal significant effects of musculoskeletal sensitization on normalized NREM delta power (Table 1).
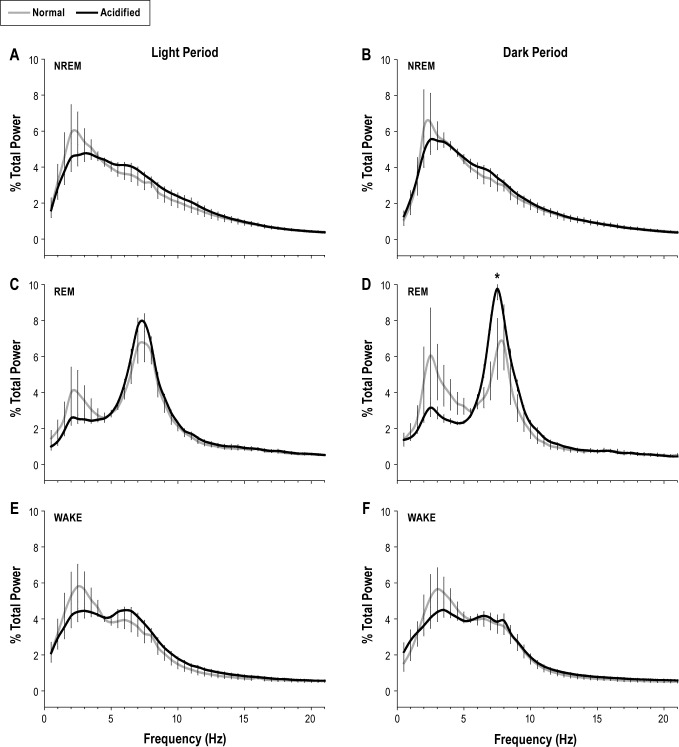
State-specific EEG power spectra were normalized as a percentage of total power across all frequencies for specific behavioral states within the 12-h light or dark period. Statistical analyses were performed on bins in the delta (0.5-4.5 Hz) and theta (6.0-9.0 Hz) frequency bands for NREMS and REMS, respectively. Although statistical significance was not achieved across the frequency bands, there was a significant increase in the peak theta frequency of acidified saline injected mice during dark period REM sleep (Figure 3).
Figure 3.
State-specific electroencephalogram (EEG) power spectra are altered during musculoskeletal hypersensitivity. State-specific EEG power spectra were obtained from mice injected with either normal pH saline (n = 6; gray lines) or acidified pH saline (n = 8; black lines). Data presented were obtained 20 days following musculoskeletal sensitization (or control injections). Spectra were normalized as a percentage of total power within each frequency band during the 12-h light or dark period and are plotted as mean ± SEM for each frequency bin. Statistical analyses were performed on bins comprising the delta frequency band (0.5-4.5 Hz) and the theta frequency band (6.0-9.0 Hz) for NREMS and REMS, respectively. A significant change was detected between the peak theta frequency during the dark period for NREM sleep between injection groups on day 20. *P ≤ 0.05 vs. normal pH saline injection.
Food Consumption, Water Consumption, Body Weight, and Core Body Temperature
Daily food consumption, water consumption, and body weight were not significantly impacted by intramuscular acidified saline injections (data not shown). Repeated-measures analysis did not reveal a significant effect of manipulation (normal saline, acidified saline) on these parameters.
Pre-injection baseline core body temperature did not differ among mice that were subsequently randomized to the injection groups. No significant effect of injection was detected by repeated measures analysis within subjects or between injection groups (Table 1).
DISCUSSION
Approximately 20% of Americans report that pain or physical discomfort disrupts their sleep at least a few nights a week,43 and patients with chronic pain conditions often report sleep disruption as a comorbidity to their pain.44,45 Although three of the most prevalent types of chronic pain in the United States are musculoskeletal; low back pain, neck pain, and facial pain,20 most preclinical studies of pain have focused on neuropathic or inflammatory pain.46,47 Improving sleep can reduce next day pain,19 especially in patients with ongoing musculoskeletal pain.48 Persons with musculoskeletal disorders, including pain, have a lower quality of life as compared with other chronic ongoing health conditions.4 Sleep affects a wide range of homeostatic biological functions such as mood regulation, cardiovascular function, and cognitive functions including decision making, memory, and attention.49 The negative impact of musculoskeletal pain on sleep may in turn influence the collective well-being of the patient more than a chronic pain state independent of sleep disruption.
The novel finding of this study is that musculoskeletal sensitization fragments sleep of mice without altering the total amount of time spent in NREM sleep, REM sleep, or wakefulness. Furthermore, musculoskeletal sensitization does not impact the clinical health of mice as evidenced by measures of body weight, food and water consumption and body temperature. Our observations that acidified saline injections into mice induce bilateral secondary mechanical hypersensitivity replicate findings in the literature,34,35 and suggest this model may be of utility for studies of interactions between sleep and musculoskeletal sensitization.
In this present study, musculoskeletal sensitization did not alter the amount of time spent in NREM or REM sleep. The literature is varied with respect to the extent to which sleep amounts are disrupted during chronic pain.28,50 For example, chronic constriction injury (CCI), in which a surgically implanted suture constricts the sciatic nerve and produces allodynia at the hindpaw, in one study is reported to transiently alter sleep of rats,28 an effect that was most robust during the first 10 days after nerve constriction. Another study using the same model in rats reported no changes to sleep.50 Differences in findings between these studies may be due to the post-injury time course selected for recording. In the first study sleep state was monitored continuously 21 days after surgery,28 whereas the study that saw no change recorded for single days with the first occurring 13 days post-surgery.50 This difference in time course suggests that CCI may have resulted in significant changes in sleep during the first 10 days post-surgery as previously reported, but beginning recordings on day 13 may have missed this significant change.
It is also possible that subpopulations of rodents differ in their susceptibility to chronic pain.51–53 Monassi and colleagues identify 3 distinct phenotypes of responders after CCI; animals that manifest pain with persistent disability, those that exhibit pain with only transient disability, and those that indicate pain, but no disability.51 In these studies, all rats developed sensitivity to mechanical and cold stimuli to the same degree, but exhibited different phenotypic changes in sleep. Rats exhibiting pain with persistent disability spent less time in NREM sleep and increased wakefulness during both the light and dark periods, an effect that persisted for the 8-day follow up period after CCI. Rats with pain and only transient disability spent less time in NREM sleep and increased wakefulness, but only during the light period, and this effect normalized by the end of the 8-day recording period. Sleep was not altered in rats that exhibited pain without disability.51 To investigate a role for astrocytes as mediators of pain and disability after CCI, the periaqueductal gray (PAG) was stained for glial fibrillary acid protein (GFAP), a marker of activated astrocytes. Increased staining for GFAP was detected in the lateral and caudal ventrolateral columns of the PAG in rats exhibiting pain with persistent disability.53 The anatomical specification of this upregulation of GFAP suggests that afferents from both the spinal column and nucleus of the solitary tract may be critical as the ventrolateral PAG is the site of termination. Furthermore, mRNA expression for markers of cell death in the PAG is upregulated in rats with pain and persistent disability.52 Because the PAG is a brain region involved in the regulation of sleep54–56 and pain,54,57 data from these collective studies indicate that the PAG may serve as a critical site of integration for interactions between pain and sleep.
Although the PAG may be functionally implicated in regulating sleep and pain, the PAG has limited direct projections to the spinal cord.58 The PAG does however, have direct projections to the rostral ventral medulla (RVM), which in turn projects to the spinal cord.59 The RVM is involved in pain transmission57,59,60 and is implicated in mediating muscle sensitivity.61–63 Microinjections of local anesthetic61 or NMDA receptor antagonists63 into the RVM after bilateral mechanical hypersensitivity has developed reverses mechanical hypersensitivity. After one intramuscular injection with acidified saline, glycine concentrations in the RVM are reduced.62 Following the second acidified saline injection, but not the first, glutamate concentration increase in the RVM.62 The RVM contributes to the maintenance of hypersensitivity in the musculoskeletal sensitization model through regulation of neurotransmitter release, changes in NMDA receptor expression, and changes in neuronal excitability.62–64 As such, data support the hypothesis that the RVM and PAG may independently or synergistically contribute to the sleep fragmentation and mechanical hypersensitivity associated with musculoskeletal sensitization. Future experiments will test this mechanistic hypothesis.
Sleep fragmentation, characterized by an increased number of transitions between arousal states, is frequently reported in preclinical models of chronic pain. Several studies report changes in sleep of rats using an adjuvant-induced arthritis model.24,27 Sleep of arthritic rats is characterized by increased total number of sleep and wakefulness bouts, increased micro-arousals, decreased NREM and REM sleep duration, and a reduction in sleep efficiency.24,27 Sleep fragmentation has also been recorded in both male and female rats with experimental osteoarthritis characterized by reduced NREM and REM sleep and reduced sleep efficiency.25 Arthritis induced by intra-articular knee injections of uric acid produces lasting increases in wakefulness, reductions in REM sleep, and REM bout numbers of rats.65 Sleep is also fragmented during orofacial pain, a model in which chronic pain is induced by injecting Freund's adjuvant into the masseter muscle. Under these conditions, sleep efficiency is reduced and the amount of time spent in wakefulness is increased.66,67 In mice with experimental neuropathic pain induced by sciatic nerve ligation, NREM sleep is suppressed and wakefulness increased for at least 28 days following surgery.29 The common thread among these studies of chronic pain using different preclinical models is one of fragmented sleep, usually accompanied by a change in sleep duration. Our findings of increased state transitions during musculoskeletal sensitization are consistent with these previous observations and contribute to the growing literature of the manner in which sleep is disrupted during chronic pain.
Fibromyalgia is a chronic condition of unknown etiology characterized by widespread musculoskeletal pain and sleep disruption.68 Among chronic pain conditions, fibromyalgia is unique because unrefreshing sleep is a diagnostic factor.69,70 Patients often complain of non-restorative sleep, insomnia, early morning awakenings, and overall poor sleep quality.71–73 The pain that is experienced by fibromyalgia patients correlates with quality of sleep, such that diminished subjective sleep quality is associated with enhanced pain.74,75 Changes in the EEG of patients with fibromyalgia are characterized by an intrusion of alpha waves into the NREM sleep that corresponds with next day pain,72,76 although recent studies do not replicate these findings.77 Our study demonstrates changes in theta frequency components of the EEG spectra during REM sleep that persist for at least 20 days post sensitization. At present, the functional significance of altered EEG spectra during musculoskeletal sensitization in this model remains to be determined.
The economic costs and personal impact of chronic pain and sleep disruption on quality of life underscore the need for additional treatment options. Clinical surveys identify that subjectively restorative sleep reduces next day pain, especially in patients with musculoskeletal pain.48 Conversely, reduction of daytime pain does not predict subsequent restorative sleep,48 and a lack of restorative sleep could further exacerbate pain. These relationships between sleep and pain suggest a “vicious cycle” that perhaps may be broken by focusing on manipulation of sleep, not pain, as a critical target for intervention. In patients with chronic pain and sleep disturbance, it may be possible to alleviate or reduce pain by effective interventions to improve sleep quality using either targeted pharmacological treatments, behavioral treatments, or a combined approach. Indeed, recent studies demonstrate that cognitive behavioral therapy to treat insomnia in patients with fibromyalgia and other chronic pain conditions also is effective in reducing pain.78,79
Our data demonstrate that musculoskeletal sensitization using acidified saline injections fragments sleep of mice without reducing amounts of NREM or REM sleep. Food and water intake, as well as body weight, are not altered during musculoskeletal sensitization in this model. Collectively, our data support findings in the clinical literature that musculo-skeletal pain fragments sleep. Our present results are an initial attempt to determine the extent to which sleep is disrupted during musculoskeletal sensitization. These results demonstrate a relationship between musculoskeletal sensitization and sleep, yet do not provide knowledge about mechanisms underlying these interactions. The similarity between the patient reported experience of sleep disruption during musculoskeletal pain and sleep fragmentation of mice during musculoskeletal sensitization provides a framework to begin investigating the mechanisms underlying relationships between musculoskeletal sensitivity and sleep.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported in part by the University of Michigan Department of Anesthesiology (MRO) and the University of Washington Department of Anesthesiology & Pain Medicine (MRO). The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The technical assistance of Ms. Jill Priestley and Ms. Amrita George is acknowledged and greatly appreciated.
REFERENCES
- 1.Jacobs T. No pain, no gain? Nat Biotechnol. 2005;23:934. doi: 10.1038/nbt0805-934. [DOI] [PubMed] [Google Scholar]
- 2.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8:8–20. doi: 10.1016/j.spinee.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Phillips CJ, Harper C. The economics associated with persistent pain. Curr Opin Support Palliat Care. 2011;5:127–30. doi: 10.1097/SPC.0b013e3283458fa9. [DOI] [PubMed] [Google Scholar]
- 4.Sprangers MA, de Regt EB, Andries F, et al. Which chronic conditions are associated with better or poorer quality of life? J Clin Epidemiol. 2000;53:895–907. doi: 10.1016/s0895-4356(00)00204-3. [DOI] [PubMed] [Google Scholar]
- 5.Effect of short sleep duration on daily activities--United States, 2005-2008. Morb Mortal Wkly Rep. 2011;60:239–42. [PubMed] [Google Scholar]
- 6.National Sleep Foundation. Washington, DC: National Sleep Foundation, ed; 2005. 2005 Sleep in America poll: summary of findings. [Google Scholar]
- 7.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–69. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Moldofsky H. Sleep and pain. Sleep Med Rev. 2001;5:385–96. doi: 10.1053/smrv.2001.0179. [DOI] [PubMed] [Google Scholar]
- 9.Lautenbacher S. Pain, sleeping problems and their many relatives. Pain. 2012;153:1138. doi: 10.1016/j.pain.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain Med. 2004;5(Suppl 1):S9–S27. doi: 10.1111/j.1526-4637.2004.04019.x. [DOI] [PubMed] [Google Scholar]
- 11.Parish JM. Sleep-related problems in common medical conditions. Chest. 2009;135:563–72. doi: 10.1378/chest.08-0934. [DOI] [PubMed] [Google Scholar]
- 12.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–8. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 13.Roehrs T, Roth T. Sleep and pain: interaction of two vital functions. Semin Neurol. 2005;25:106–16. doi: 10.1055/s-2005-867079. [DOI] [PubMed] [Google Scholar]
- 14.Tang NK, Wright KJ, Salkovskis PM. Prevalence and correlates of clinical insomnia co-occurring with chronic back pain. J Sleep Res. 2007;16:85–95. doi: 10.1111/j.1365-2869.2007.00571.x. [DOI] [PubMed] [Google Scholar]
- 15.Dikeos D, Georgantopoulos G. Medical comorbidity of sleep disorders. Curr Opin Psychiatry. 2011;24:346–54. doi: 10.1097/YCO.0b013e3283473375. [DOI] [PubMed] [Google Scholar]
- 16.Mork PJ, Nilsen TI. Sleep problems and risk of fibromyalgia: Longitudinal data from the Norwegian HUNT-study. Arthritis Rheum. 2011;64:281–4. doi: 10.1002/art.33346. [DOI] [PubMed] [Google Scholar]
- 17.Tiede W, Magerl W, Baumgartner U, Durrer B, Ehlert U, Treede RD. Sleep restriction attenuates amplitudes and attentional modulation of pain-related evoked potentials, but augments pain ratings in healthy volunteers. Pain. 2010;148:36–42. doi: 10.1016/j.pain.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 18.Chhangani BS, Roehrs TA, Harris EJ, et al. Pain sensitivity in sleepy pain-free normals. Sleep. 2009;32:1011–7. [PMC free article] [PubMed] [Google Scholar]
- 19.Roehrs TA, Harris E, Randall S, Roth T. Pain sensitivity and recovery from mild chronic sleep loss. Sleep. 2012;35:1667–72. doi: 10.5665/sleep.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Center for Health Statistics. Hyattsville, MD: National Center for Health Statistics; 2006. Chartbook on Trends in the Health of Americans, Special Feature: Pain. [Google Scholar]
- 21.Sivertsen B, Krokstad S, Overland S, Mykletun A. The epidemiology of insomnia: associations with physical and mental health. The HUNT-2 study. J Psychosom Res. 2009;67:109–16. doi: 10.1016/j.jpsychores.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Gureje O, Kola L, Ademola A, Olley BO. Profile, comorbidity and impact of insomnia in the Ibadan study of ageing. Int J Geriatr Psychiatry. 2009;24:686–93. doi: 10.1002/gps.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ancoli-Israel S. The impact and prevalence of chronic insomnia and other sleep disturbances associated with chronic illness. Am J Manag Care. 2006;12:S221–9. [PubMed] [Google Scholar]
- 24.Landis CA, Robinson CR, Levine JD. Sleep fragmentation in the arthritic rat. Pain. 1988;34:93–9. doi: 10.1016/0304-3959(88)90186-8. [DOI] [PubMed] [Google Scholar]
- 25.Silva A, Araujo P, Zager A, Tufik S, Andersen ML. Sex differences in sleep pattern of rats in an experimental model of osteoarthritis. Eur J Pain. 2011;15:545–53. doi: 10.1016/j.ejpain.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Silva A, Andersen ML, Tufik S. Sleep pattern in an experimental model of osteoarthritis. Pain. 2008;140:446–55. doi: 10.1016/j.pain.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 27.Landis CA, Levine JD, Robinson CR. Decreased slow-wave and paradoxical sleep in a rat chronic pain model. Sleep. 1989;12:167–77. doi: 10.1093/sleep/12.2.167. [DOI] [PubMed] [Google Scholar]
- 28.Andersen ML, Tufik S. Sleep patterns over 21-day period in rats with chronic constriction of sciatic nerve. Brain Res. 2003;984:84–92. doi: 10.1016/s0006-8993(03)03095-6. [DOI] [PubMed] [Google Scholar]
- 29.Narita M, Niikura K, Nanjo-Niikura K, et al. Sleep disturbances in a neuropathic pain-like condition in the mouse are associated with altered GABAergic transmission in the cingulate cortex. Pain. 2011;152:1358–72. doi: 10.1016/j.pain.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Takemura Y, Yamashita A, Horiuchi H, et al. Effects of gabapentin on brain hyperactivity related to pain and sleep disturbance under a neuropathic pain-like state using fMRI and brain wave analysis. Synapse. 2011;65:668–76. doi: 10.1002/syn.20898. [DOI] [PubMed] [Google Scholar]
- 31.Torigoe K, Nakahara K, Rahmadi M, et al. Usefulness of olanzapine as an adjunct to opioid treatment and for the treatment of neuropathic pain. Anesthesiology. 2012;116:159–69. doi: 10.1097/ALN.0b013e31823c7e56. [DOI] [PubMed] [Google Scholar]
- 32.Carli G, Montesano A, Rapezzi S, Paluffi G. Differential effects of persistent nociceptive stimulation on sleep stages. Behav Brain Res. 1987;26:89–98. doi: 10.1016/0166-4328(87)90158-6. [DOI] [PubMed] [Google Scholar]
- 33.Andersen ML, Tufik S. Altered sleep and behavioral patterns of arthritic rats. Sleep Res Online. 2000;3:161–7. [PubMed] [Google Scholar]
- 34.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Sharma NK, Ryals JM, Liu H, Liu W, Wright DE. Acidic saline-induced primary and secondary mechanical hyperalgesia in mice. J Pain. 2009;10:1231–41. doi: 10.1016/j.jpain.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith SB, Crager SE, Mogil JS. Paclitaxel-induced neuropathic hypersensitivity in mice: responses in 10 inbred mouse strains. Life Sci. 2004;74:2593–604. doi: 10.1016/j.lfs.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Olivadoti MD, Opp MR. Effects of i.c.v. administration of interleukin-1 on sleep and body temperature of interleukin-6-deficient mice. Neuroscience. 2008;153:338–48. doi: 10.1016/j.neuroscience.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrow JD, Opp MR. Sleep-wake behavior and responses of interleukin-6-deficient mice to sleep deprivation. Brain Behav Immun. 2005;19:28–39. doi: 10.1016/j.bbi.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 40.Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557–68. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 41.Davis CJ, Clinton JM, Jewett KA, Zielinski MR, Krueger JM. Delta wave power: an independent sleep phenotype or epiphenomenon? J Clin Sleep Med. 2011;7:S16–8. doi: 10.5664/JCSM.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franken P, Malafosse A, Tafti M. Genetic variation in EEG activity during sleep in inbred mice. Am J Physiol. 1998;275:R1127–37. doi: 10.1152/ajpregu.1998.275.4.R1127. [DOI] [PubMed] [Google Scholar]
- 43.National Sleep Foundation. Sleep in America Poll. 2000 [Google Scholar]
- 44.Roizenblatt M, Rosa Neto NS, Tufik S, Roizenblatt S. Pain-related diseases and sleep disorders. Braz J Med Biol Res. 2012;45:792–8. doi: 10.1590/S0100-879X2012007500110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dominick CH, Blyth FM, Nicholas MK. Unpacking the burden: understanding the relationships between chronic pain and comorbidity in the general population. Pain. 2012;153:293–304. doi: 10.1016/j.pain.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 46.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- 47.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–94. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 48.Davies KA, Macfarlane GJ, Nicholl BI, et al. Restorative sleep predicts the resolution of chronic widespread pain: results from the EPIFUND study. Rheumatology. 2008;47:1809–13. doi: 10.1093/rheumatology/ken389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kontinen VK, Ahnaou A, Drinkenburg WH, Meert TF. Sleep and EEG patterns in the chronic constriction injury model of neuropathic pain. Physiol Behav. 2003;78:241–6. doi: 10.1016/s0031-9384(02)00966-6. [DOI] [PubMed] [Google Scholar]
- 51.Monassi CR, Bandler R, Keay KA. A subpopulation of rats show social and sleep-waking changes typical of chronic neuropathic pain following peripheral nerve injury. Eur J Neurosci. 2003;17:1907–20. doi: 10.1046/j.1460-9568.2003.02627.x. [DOI] [PubMed] [Google Scholar]
- 52.Mor D, Bembrick AL, Austin PJ, Keay KA. Evidence for cellular injury in the midbrain of rats following chronic constriction injury of the sciatic nerve. J Chem Neuroanat. 2011;41:158–69. doi: 10.1016/j.jchemneu.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Mor D, Bembrick AL, Austin PJ, et al. Anatomically specific patterns of glial activation in the periaqueductal gray of the sub-population of rats showing pain and disability following chronic constriction injury of the sciatic nerve. Neuroscience. 2010;166:1167–84. doi: 10.1016/j.neuroscience.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 54.Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–25. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luppi PH, Clement O, Sapin E, et al. Brainstem mechanisms of paradoxical (REM) sleep generation. Pflugers Arch. 2012;463:43–52. doi: 10.1007/s00424-011-1054-y. [DOI] [PubMed] [Google Scholar]
- 56.Mason P. Deconstructing endogenous pain modulations. J Neurophysiol. 2005;94:1659–63. doi: 10.1152/jn.00249.2005. [DOI] [PubMed] [Google Scholar]
- 57.Mason P. Ventromedial medulla: pain modulation and beyond. J Comp Neurol. 2005;493:2–8. doi: 10.1002/cne.20751. [DOI] [PubMed] [Google Scholar]
- 58.Sandkuhler J, Gebhart GF. Relative contributions of the nucleus raphe magnus and adjacent medullary reticular formation to the inhibition by stimulation in the periaqueductal gray of a spinal nociceptive reflex in the pentobarbital-anesthetized rat. Brain Res. 1984;305:77–87. doi: 10.1016/0006-8993(84)91121-1. [DOI] [PubMed] [Google Scholar]
- 59.Fields HL, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J Neurophysiol. 1995;74:1742–59. doi: 10.1152/jn.1995.74.4.1742. [DOI] [PubMed] [Google Scholar]
- 60.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Ann Rev Neurosci. 1984;7:309–38. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 61.Tillu DV, Gebhart GF, Sluka KA. Descending facilitatory pathways from the RVM initiate and maintain bilateral hyperalgesia after muscle insult. Pain. 2008;136:331–9. doi: 10.1016/j.pain.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Radhakrishnan R, Sluka KA. Increased glutamate and decreased glycine release in the rostral ventromedial medulla during induction of a pre-clinical model of chronic widespread muscle pain. Neurosci Lett. 2009;457:141–5. doi: 10.1016/j.neulet.2009.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Da Silva LF, Desantana JM, Sluka KA. Activation of NMDA receptors in the brainstem, rostral ventromedial medulla, and nucleus reticularis gigantocellularis mediates mechanical hyperalgesia produced by repeated intramuscular injections of acidic saline in rats. J Pain. 2010;11:378–87. doi: 10.1016/j.jpain.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Da Silva LF, Walder RY, Davidson BL, Wilson SP, Sluka KA. Changes in expression of NMDA-NR1 receptor subunits in the rostral ventromedial medulla modulate pain behaviors. Pain. 2010;151:155–61. doi: 10.1016/j.pain.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guevara-Lopez U, Ayala-Guerrero F, Covarrubias-Gomez A, Lopez-Munoz FJ, Torres-Gonzalez R. Effect of acute gouty arthritis on sleep patterns: a preclinical study. Eur J Pain. 2009;13:146–53. doi: 10.1016/j.ejpain.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 66.Schutz TC, Andersen ML, Tufik S. Sleep alterations in an experimental orofacial pain model in rats. Brain Res. 2003;993:164–71. doi: 10.1016/j.brainres.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 67.Schutz TC, Andersen ML, Tufik S. Influence of temporomandibular joint pain on sleep patterns: role of nitric oxide. J Dent Res. 2004;83:693–7. doi: 10.1177/154405910408300907. [DOI] [PubMed] [Google Scholar]
- 68.Annemans L, Le Lay K, Taieb C. Societal and patient burden of fibromyalgia syndrome. Pharmacoeconomics. 2009;27:547–59. doi: 10.2165/11313650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 69.Mease PJ, Clauw DJ, Arnold LM, et al. Fibromyalgia syndrome. J Rheumatol. 2005;32:2270–7. [PubMed] [Google Scholar]
- 70.Ablin J, Neumann L, Buskila D. Pathogenesis of fibromyalgia: a review. Joint Bone Spine. 2008;75:273–9. doi: 10.1016/j.jbspin.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 71.Harding SM. Sleep in fibromyalgia patients: subjective and objective findings. Am J Med Sci. 1998;315:367–76. doi: 10.1097/00000441-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Roizenblatt S, Moldofsky H, Benedito-Silva AA, Tufik S. Alpha sleep characteristics in fibromyalgia. Arthritis Rheum. 2001;44:222–30. doi: 10.1002/1529-0131(200101)44:1<222::AID-ANR29>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 73.Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord. 2007;8:27. doi: 10.1186/1471-2474-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Theadom A, Cropley M, Humphrey KL. Exploring the role of sleep and coping in quality of life in fibromyalgia. J Psychosom Res. 2007;62:145–51. doi: 10.1016/j.jpsychores.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 75.Affleck G, Urrows S, Tennen H, Higgins P, Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68:363–8. doi: 10.1016/s0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- 76.Moldofsky H, Lue FA. The relationship of alpha and delta EEG frequencies to pain and mood in ‘fibrositis’ patients treated with chlorpromazine and L-tryptophan. Electroencephalogr Clin Neurophysiol. 1980;50:71–80. doi: 10.1016/0013-4694(80)90324-7. [DOI] [PubMed] [Google Scholar]
- 77.Besteiro Gonzalez JL, Suarez Fernandez TV, Arboleya Rodriguez L, Muniz J, Lemos Giraldez S, Alvarez Fernandez A. Sleep architecture in patients with fibromyalgia. Psicothema. 2011;23:368–73. [PubMed] [Google Scholar]
- 78.Jungquist CR, Tra Y, Smith MT, et al. The durability of cognitive behavioral therapy for insomnia in patients with chronic pain. Sleep Disord. 2012;2012:679648. doi: 10.1155/2012/679648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martinez MP, Miro E, Sanchez AI, et al. Cognitive-behavioral therapy for insomnia and sleep hygiene in fibromyalgia: a randomized controlled trial. J Behav Med. 2013 Jun 7; doi: 10.1007/s10865-013-9520-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]