Abstract
Study Objectives:
Sleep deprivation, or sleep disruption, enhances pain in human subjects. Chronic musculoskeletal pain is prevalent in our society, and constitutes a tremendous public health burden. Although preclinical models of neuropathic and inflammatory pain demonstrate effects on sleep, few studies focus on musculoskeletal pain. We reported elsewhere in this issue of SLEEP that musculoskeletal sensitization alters sleep of mice. In this study we hypothesize that sleep fragmentation during the development of musculoskeletal sensitization will exacerbate subsequent pain responses and alter sleep-wake behavior of mice.
Design:
This is a preclinical study using C57BL/6J mice to determine the effect on behavioral outcomes of sleep fragmentation combined with musculoskeletal sensitization.
Methods:
Musculoskeletal sensitization, a model of chronic muscle pain, was induced using two unilateral injections of acidified saline (pH 4.0) into the gastrocnemius muscle, spaced 5 days apart. Musculoskeletal sensitization manifests as mechanical hypersensitivity determined by von Frey filament testing at the hindpaws. Sleep fragmentation took place during the consecutive 12-h light periods of the 5 days between intramuscular injections. Electroencephalogram (EEG) and body temperature were recorded from some mice at baseline and for 3 weeks after musculoskeletal sensitization. Mechanical hypersensitivity was determined at preinjection baseline and on days 1, 3, 7, 14, and 21 after sensitization. Two additional experiments were conducted to determine the independent effects of sleep fragmentation or musculoskeletal sensitization on mechanical hypersensitivity.
Results:
Five days of sleep fragmentation alone did not induce mechanical hypersensitivity, whereas sleep fragmentation combined with musculoskeletal sensitization resulted in prolonged and exacerbated mechanical hypersensitivity. Sleep fragmentation combined with musculoskeletal sensitization had an effect on subsequent sleep of mice as demonstrated by increased numbers of sleep-wake state transitions during the light and dark periods; changes in nonrapid eye movement (NREM) sleep, rapid eye movement sleep, and wakefulness; and altered delta power during NREM sleep. These effects persisted for at least 3 weeks postsensitization.
Conclusions:
Our data demonstrate that sleep fragmentation combined with musculoskeletal sensitization exacerbates the physiological and behavioral responses of mice to musculoskeletal sensitization, including mechanical hypersensitivity and sleep-wake behavior. These data contribute to increasing literature demonstrating bidirectional relationships between sleep and pain. The prevalence and incidence of insufficient sleep and pathologies characterized by chronic musculoskeletal pain are increasing in the United States. These demographic data underscore the need for research focused on insufficient sleep and chronic pain so that the quality of life for the millions of individuals with these conditions may be improved.
Citation:
Sutton BC; Opp MR. Sleep fragmentation exacerbates mechanical hypersensitivity and alters subsequent sleep-wake behavior in a mouse model of musculoskeletal sensitization. SLEEP 2014;37(3):515-524.
Keywords: Mice, pain, rodent, sleep restriction, von Frey
INTRODUCTION
Sleep loss negatively affects homeostatic functions including, but not limited to metabolism, cognition, emotional regulation, immune function, cardiovascular function, and pain.1–6 In the United States, approximately 30% of the adult population reports insufficient sleep7 and approximately 70 million Americans have a diagnosed sleep disorder.7 Sleep disorders, including insomnia, narcolepsy, and sleep apnea, fragment or restrict sleep.8–10 Experimental deprivation or restriction of sleep in humans and rodents enhances pain.11–16 Subjectively sleepy persons have reduced pain thresholds in comparison with well-rested individuals,17 and chronic pain is the most frequently associated co-morbidity with primary insomnia.18 Epidemiological studies identify subjectively poor sleep quality as an independent risk factor for the development of chronic pain conditions, especially those characterized by musculoskeletal pain.19,20
Musculoskeletal pain is prevalent in our society, with low back pain, neck pain, and facial pain21 constituting a major public health burden. Arthritis (primarily rheumatoid), spinal pain (including low back pain), and fibromyalgia are the most prevalent chronic pain conditions associated with insomnia.20,22,23 Data derived from preclinical studies of rodents support clinical findings that sleep loss reduces pain threshold.15,16,24 In a rodent model of musculoskeletal pain, musculoskeletal sensitization induces long-lasting mechanical hypersensitivity characterized by increased responsiveness to mechanical stimuli.25–27 We report in this issue27 that the sleep of mice in which musculo-skeletal sensitization has been induced is fragmented. In this present study, we hypothesize that sleep fragmentation during the period when musculoskeletal sensitization develops will exacerbate the effects of musculoskeletal sensitization. To test this hypothesis, we fragmented sleep of mice, induced musculo-skeletal sensitization, and determined the effect on subsequent sleep-wake behavior and mechanical hypersensitivity. We now report that sleep fragmentation combined with musculoskeletal sensitization exacerbates for prolonged periods mechanical hypersensitivity and alters multiple facets of mouse sleep.
EXPERIMENTAL PROCEDURES
Animals
Adult male C57BL/6J mice (4-6 weeks of age; 25 g) were used in this study. All mice were purchased from Jackson Laboratory (Bar Harbor, ME), and maintained on a 12:12 h light:dark cycle at 27°C with ad libitum access to food and water. All procedures using mice in these studies were approved in advance by the University of Washington Institutional Animal Care and Use Committee (IACUC), in accordance with the US Department of Agriculture Animal Welfare Act and the National Institutes of Health policy on Humane Care and the Use of Laboratory Animals.
The clinical health of mice was monitored daily at light onset and consisted of measures of food consumption, water consumption, and body weight. These data were collected throughout the surgical recovery period and for the duration of the protocols.
Musculoskeletal Sensitization
Musculoskeletal sensitization was induced by two unilateral injections of acidified saline into the gastrocnemius muscle. The injections were spaced 5 days apart and consisted of either normal (pH 7.2; control) or acidified (pH 4.0) saline. Acidified saline injections in this protocol produce a robust bilateral secondary mechanical hypersensitivity at the hindpaws that lasts at least 4 weeks.25–27 At the time of injection, mice were briefly anesthetized using isoflurane, a hind leg cleaned using alcohol, and 20 μL of normal or acidified saline injected into the gastrocnemius muscle using a 31-g needle. All animals were immediately returned to their home cage and observed by the investigator until fully ambulatory.
Mechanical Hypersensitivity Testing
The von Frey filament test measures sensitivity to a non-noxious punctate pressure stimulus using calibrated monofilaments. Determination of mechanical hypersensitivity was done as previously reported.27 Briefly, mice were habituated to the testing procedure and the galvanized steel mesh testing platform for a minimum of 60 min each day for 3 days prior to obtaining baseline values. On testing days, mice were placed on the testing platform for a minimum of 30 min (or until quiet). Calibrated filaments (0.07, 0.45, and 1.45 g pressure deflection) were then presented in ascending order to the glabrous skin of the hindpaws until they bowed slightly.27,28 Hindpaws were alternated until a total of five presentations per filament per paw were reached. Each of the three filaments was presented with a minimum 1-min break between. Positive responses were recorded when mice retracted the paw in response to the filament pressure. If mice became active, testing was suspended until they were quiet. All testing was done during the first 2 h of the light period.
Sleep Fragmentation
All animals undergoing sleep fragmentation were placed into the sleep disruption devices 1 day prior to the start of the sleep fragmentation protocol. The sleep disruption device consists of a circular Plexiglas chamber divided to form two compartments.29 The floor of the chamber is a motorized disc that rotates for specific durations as selected by the investigator.
Individual animals were placed into separate compartments prior to device habituation. Device habituation consisted of rotation of the disc for 8 sec once every 30 min during one 12-h light period of the 12:12 light:dark cycle. No disc rotation occurred during the dark period, during which mice were left undisturbed. Intramuscular injections and the beginning of sleep fragmentation began at light onset the day after habituation. Sleep was fragmented for 5 days by disc rotations that lasted for 8 sec and occurred every 30 sec, on average, during the 12-h light periods. During the 12-h dark periods, the disc did not rotate and mice were free to behave normally. The direction of disc rotation and exact inter-rotation interval were computer randomized to prevent behavioral adaptation of the mice to the rotations. Each disc rotation was greater than 180° to ensure the mice had to move to avoid bumping into the center divider of the chamber. We have demonstrated that this method of fragmenting sleep of mice is effective in protocols lasting up to 9 days.29
Surgical Procedures
Mice from which recordings of the electroencephalogram (EEG) were to be obtained were deeply anesthetized with isoflurane (4% induction, 2% maintenance) and surgically implanted with telemeters (ETA10-F20, Data Sciences International, Minneapolis, MN). As previously described,27,30,31 transmitters were implanted in the peritoneum and leads were passed subcutaneously to the skull and attached to stainless steel screws (#80 × 1/8 inch, Small Parts, Miami Lakes, FL) placed bilaterally over frontal and parietal cortices. These screws served as EEG recording electrodes. Mice were injected subcutaneously with penicillin G procaine (0.1 to 0.2 mL, 300,000 units/mL) immediately after surgery to reduce risk of infection. Perioperative pain management consisted of ibuprofen in drinking water (0.2 mg/mL; beginning 24 h before surgery and continuing for 48 h after surgery) and administration of buprenorphine (0.05 mg/kg, subcutaneously) at the time of surgery and for 2 days following surgery, if needed. Lidocaine and triple antibiotic ointment were applied topically at the incision site immediately after surgery. Mice were monitored until ambulatory and then transferred to recording cages for recovery and acclimation.
Data Acquisition
Signals from telemeters were fed to an analog converter (Data Sciences International ART Analog-8 CM) that converted EEG and temperature signals to voltages using transmitter-specific calibration factors provided by DSI. The output from the converter was captured by an AD board (model PCI-3033E, National Instruments, Austin, TX) that redigitized the data at 128 Hz with 16-bit precision. Temperature voltages were converted by regression using calibration coefficients specific for each transmitter. General activity in the cage was detected using infrared sensors (BioBserve, GmbH, Bonn, Germany). Movements detected by the sensors were converted to a voltage output, the magnitude of which was directly related to the magnitude of movements detected. All signals (EEG, core body temperature, and cage activity) were stored as binary files until further processing.
During acquisition, the EEG was digitally filtered using Chebyschev filters with third-order coefficients into delta (0.5-4.5 Hz) and theta (6.0 - 9.0 Hz) frequency bands. These filtered EEG signals were integrated over 1-sec periods and stored as part of the binary file structure. Arousal state designations were made with 10-sec resolution on the basis of visual inspection of the recordings using custom software (ICELUS, M. Opp, University of Washington, Seattle, WA) written in LabView for Windows (National Instruments). Arousal state was assigned for each 10-sec interval on the basis of the EEG, body movements, and integrated delta and theta frequency values using previously published criteria.27,30,32 Any epoch during which the EEG contained either movement artifacts or electrical noise was tagged and excluded from subsequent spectral analyses. The raw, non-integrated EEG signals were processed offline using fast Fourier transforms to yield power spectra between 0.5 and 40 Hz in 0.5-Hz frequency bins. These spectra were computed by averaging the five consecutive 2-sec EEG segments comprising each 10-sec epoch. The resulting spectrum was matched to state to provide state-specific spectra. Our primary focus in this study was power in the delta frequency band during nonrapid eye movement (NREM) sleep. These values for delta power during NREM sleep were obtained by summing the values of all 0.5 Hz frequency bins from 0.5-4.5 Hz.
The extent to which spontaneous sleep was consolidated or disrupted was determined by evaluating the number of transitions from one arousal state to the next. These determinations were made as previously described27,30,32 irrespective of arousal state designation and without the use of arbitrary criteria for sleep architecture parameters.
Determination of the Effect of Sleep Fragmentation on Mechanical Hypersensitivity
Three groups of mice (n = 56 total) were used in this study to determine the combined effects of sleep fragmentation and musculoskeletal sensitization on outcome measures of interest. Mice in group 1 (n = 12) were used to determine the effect of 5 days of sleep fragmentation per se on mechanical hypersensitivity. These animals did not receive intramuscular injections. All mice underwent 3 days of habituation to the von Frey testing platform and 3 days of baseline von Frey testing (described earlier). Mice were then habituated to the sleep disruption device, after which they were subjected to sleep fragmentation during the 12-h light period for 5 consecutive days. After the 5-day sleep fragmentation protocol, mice were housed singly under standard conditions. Von Frey testing was performed at baseline (BL), on the third day of sleep fragmentation, and days 1, 3, and 7 post-fragmentation.
Whereas mice in group 1 were used to determine the effect of sleep fragmentation on mechanical hypersensitivity, mice in group 2 (n = 22) were used to determine the effect of musculo-skeletal sensitization without sleep fragmentation on mechanical hypersensitivity. The sleep disruption device was not used in these mice, and they were housed singly in standard caging throughout the experimental period. Mice underwent habituation and baseline von Frey testing to determine mechanical hypersensitivity prior to experimental manipulations. Mice were subsequently randomized to a musculoskeletal sensitization group [n = 10 normal saline (control animals) or n = 12 acidified saline], with intramuscular injections administered 5 days apart. Mechanical hypersensitivity was assessed on days 1, 3, 7, 14, and 21 following sensitization.
We report in this issue27 results of an experiment that demonstrated the effect of musculoskeletal sensitization on sleep of mice. Mice in that study were allowed spontaneous behavior during the sensitization period. The purpose of the current experiment was to determine the effect of sleep fragmentation during the musculoskeletal sensitization period on mechanical hyper-sensitivity and subsequent sleep-wake behavior. Twenty-two mice (group 3) were used in this experiment, a subset of which (n = 16) was instrumented to allow determination of sleep-wake behavior (Figure 1). These 16 mice were implanted with telemeters to record EEG and core body temperature as described earlier. After recovery, baseline recordings were obtained from mice in which telemetry units had been implanted for 2 days prior to experimental manipulation. All mice were habituated and underwent baseline von Frey testing for mechanical hyper-sensitivity. For all mice used in this experiment (instrumented, uninstrumented), sleep fragmentation started after the first intramuscular injection at light onset and ended at light onset 5 days later when the second sensitization injection was given (Figure 1). All mice were removed from the sleep disruption device and returned to single- housed standard caging at the end of the 5-day sleep fragmentation period. Mechanical hypersensitivity was measured on days 1, 3, 7, 14, and 21 postsensitization. Recordings were obtained from mice instrumented with telemeters for 22 days after sensitization. Two instrumented mice were excluded from data analysis due to poor EEG signal quality, reducing the final sample size of instrumented mice to n = 14 (seven mice per injection group).
Figure 1.
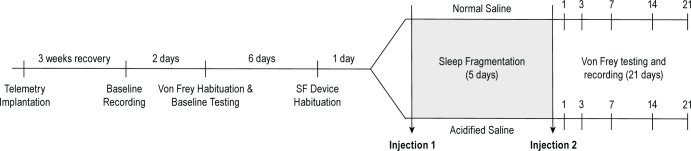
Experimental protocol to determine the effect of sleep fragmentation (SF) in combination with musculoskeletal sensitization on mechanical hypersensitivity and subsequent sleep-wake behavior of mice. Mice in group 3 (n = 22) were used to determine the effect of sleep fragmentation combined with musculoskeletal sensitization on mechanical hypersensitivity. These mice were subjected to sleep fragmentation during the light period of the 5-day interval between the first and second intramuscular injections. A subset of mice (n = 16) was used to determine the effect of sleep fragmentation combined with musculoskeletal sensitization on subsequent sleep-wake behavior. These n = 16 mice were implanted with telemeters, and allowed 3 weeks of recovery. Baseline EEG and body temperature recordings were then obtained for 2 days. All mice (instrumented, uninstrumented) were habituated to the von Frey testing platform and underwent baseline testing, which was then followed by 1 day of habituation to the sleep disruption device. Mice then were randomized into an acidified or normal saline injection group (n = 11 per injection; n = 8 with telemeters per injection group). The first injection with acidified or normal saline was given at light onset, which was followed by sleep fragmentation for 5 consecutive light periods before the second injection was given. Testing with von Frey filaments took place on postsensitization days 1, 3, 7, 14, and 21.
Statistical Analysis
Statistical analyses were performed using SPSS for Windows. All data are presented as mean ± standard error of the mean. The effects of musculoskeletal sensitization, sleep fragmentation, or a combined treatment on von Frey response incidence, sleep/wake behavior, and clinical measures were evaluated by mixed-effects analysis of variance (ANOVA) with a between-subjects factor of treatment (acidified saline, normal saline) and a within-subjects factor of time (day of the experimental protocol). Response incidence values were calculated for each paw (ipsilateral, contralateral to injection muscle) as a total response incidence ([total responses per paw / 15 total possible responses] × 100). The effect of sleep fragmentation versus undisturbed sleep on mechanical hypersensitivity was determined using a two-way ANOVA with a between-subjects factor of treatment (fragmented sleep versus undisturbed sleep) and a within-subjects factor of time (baseline, experimental day). An alpha level of P ≤ 0.05 was accepted for all statistical tests as indicating significant departures between the groups across the testing period.
RESULTS
Mechanical Hypersensitivity
For mice in groups 1, 2, and 3, the response incidence of the ipsilateral and contralateral paws did not significantly differ. Data from mice in group 1 demonstrated that sleep fragmentation by itself for 5 days had no significant effect on mechanical hypersensitivity (Figure 2). As such, sleep fragmentation by this protocol did not independently induce mechanical hypersensitivity. As previously published, unilateral injections of acidified saline 5 days apart produced bilateral mechanical hypersensitivity at the hindpaws (group 2; Figure 3A).25–27 Mechanical hypersensitivity after musculoskeletal sensitization lasted at least 21 days, which was the duration of von Frey testing in this study.
Figure 2.
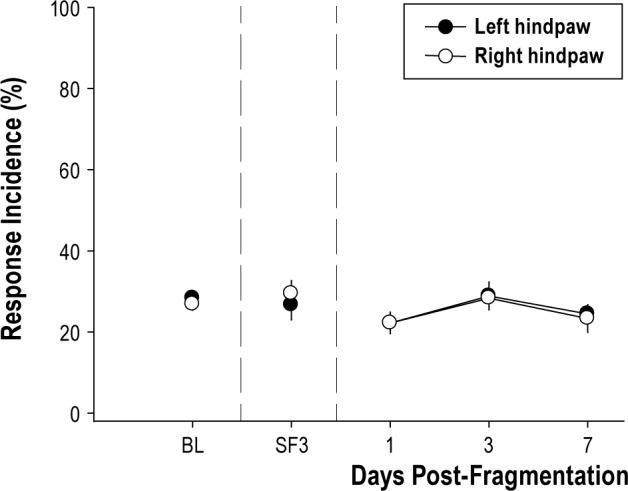
Sleep fragmentation by itself does not induce mechanical hypersensitivity. Mice in group 1 (n = 12) that had sleep fragmented for 5 consecutive light periods (days) did not develop mechanical hypersensitivity. Response incidence to von Frey filament presentation did differ among baseline (BL), sleep fragmentation day 3 (SF3), or postfragmentation days 1, 3, and 7 in either the left or right hindpaw [F(1,22) = 0.001, P = 0.973]. Responses to von Frey filaments are plotted as mean ± standard error of the mean percent of total response incidence ([total responses / total filament presentations] × 100) per paw.
Figure 3.
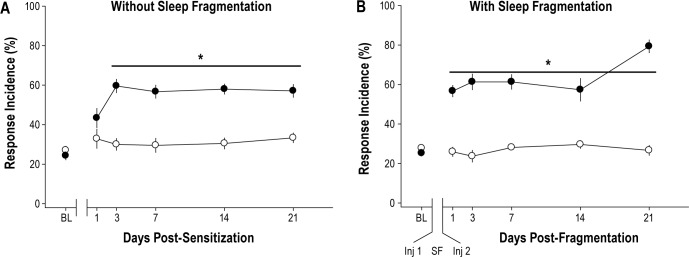
Musculoskeletal sensitization with or without sleep fragmentation induces bilateral mechanical hypersensitivity. (A) Mechanical hypersensitivity in mice was induced by two injections of acidified saline spaced 5 days apart. Mechanical hypersensitivity manifests as increased response incidence to von Frey filaments on postsensitization days 3 through 21 [F(1,20) = 22.633, P < 0.01]. Mice subsequently randomized to injection groups did not differ at preinjection baseline [F(1,20) = 1.099, P = 0.307] or on day 1 postinjection [F(1,20) = 0.129, P = 0.723] (n = 12 acidified saline, n = 10 normal saline). (B) Mice in which sleep was fragmented during the musculoskeletal sensitization period developed mechanical hypersensitivity that was apparent on day 1 postinjection and lasted for at least 21 days [F(1,17) = 129.998, P < 0.001] (n = 10 acidified saline, n = 9 normal saline). Responses to von Frey filaments are plotted as mean ± standard error of the mean percent of total response incidence ([total responses / total filament presentations] x 100) per paw for the ipsilateral paw. Asterisk, P ≤ 0.05 versus.normal saline. BL, baseline; Inj, injection; SF, sleep fragmentation. Closed circle denotes acidified saline injection; open circle denotes normal saline injection.
Effect of Sleep Fragmentation Combined With Musculoskeletal Sensitization on Mechanical Hypersensitivity and Subsequent Sleep-Wake Behavior
Whereas control mice (normal saline injections) in group 3 that were subjected to sleep fragmentation did not develop mechanical hypersensitivity, mice in which musculoskeletal sensitization had been induced by injections of acidified saline exhibited mechanical hypersensitivity on the first post-sensitization day (Figure 3B). Mechanical hypersensitivity in mice subjected to the combined manipulations of sleep fragmentation and musculoskeletal sensitization persisted for 21 days post-sensitization (Figure 3B). The observation that mechanical hypersensitivity was apparent on the first postsensitization day in mice subjected to sleep fragmentation during the musculoskeletal sensitization period was unexpected. Our previous study27 and data obtained in this present study from mice in group 2 demonstrated that mechanical hypersensitivity does not manifest fully in this model until the third postsensitization day. Because the only factor that differed in this experiment was sleep fragmentation during the sensitization period, we compared response incidence values obtained from mice in group 2 that had undisturbed sleep in their home cages during the sensitization period with those from mice in group 3 that had sleep fragmented during the sensitization period. Direct comparison of the effect of undisturbed sleep or fragmented sleep on mechanical hypersensitivity is presented in Figure 4. Response incidence values obtained from animals injected with normal saline did not differ at any time irrespective of whether or not they had been subjected to sleep fragmentation. Furthermore, response incidence values did not differ from preinjection baseline values, indicating that being housed on the sleep disruption device and being subjected to sleep fragmentation per se did not induce mechanical hypersensitivity. However, mice in which sleep was fragmented during the period of musculoskeletal sensitization developed mechanical hypersensitivity that was of greater magnitude than that of mice allowed undisturbed sleep (Figure 4). This increased mechanical hypersensitivity observed in mice subjected to the combination of sleep fragmentation and musculoskeletal sensitization was apparent on the first postsensitization day and on post-sensitization day 21 (Figure 4).
Figure 4.
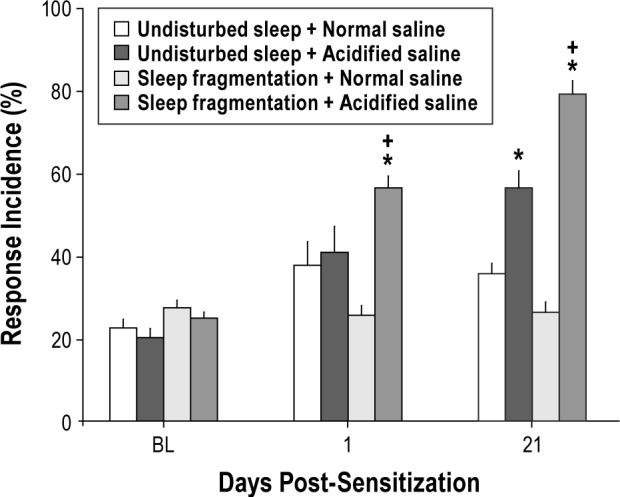
Sleep fragmentation combined with musculoskeletal sensitization exacerbates mechanical hypersensitivity. Comparisons were made between mice allowed undisturbed sleep during the musculoskeletal sensitization period (group 2) and mice in which sleep was fragmented during the sensitization period (group 3). Response incidence values did not differ among manipulation groups at baseline (BL). Mechanical hypersensitivity did not develop in mice injected with normal saline, irrespective of whether sleep was fragmented. Mice subjected to sleep fragmentation during the musculoskeletal sensitization period exhibited greater mechanical hypersensitivity on day 1 [F(1,20) = 4.427, P = 0.048] and day 21 [F(1,20) = 17.540, P < 0.001] postsensitization than did mice that were sensitized without sleep fragmentation. Responses to von Frey filaments are plotted as mean ± standard error of the mean percent of total response incidence ([total responses / total filament presentations] × 100) per paw for the leg ipsilateral to the injection site. Plus sign, P < 0.05 versus undisturbed sleep + acidified saline. Asterisk, P < 0.05 versus normal saline of the same sleep manipulation.
In addition to its effect on mechanical hypersensitivity, the combination of sleep fragmentation and musculoskeletal sensitization had dramatic and long-lasting effects on subsequent sleep-wake behavior (Figures 5 and 6). Before any manipulations, mice used in group 3 exhibited normal diurnal distributions of sleep-wake behavior during baseline recording periods, with increased time spent in NREM and rapid eye movement (REM) sleep during the light period and increased time spent in wakefulness during the dark period (data not shown). To examine the effect of experimental manipulations, data obtained from mice among treatment groups were normalized to the preinjection baseline measurements for the 12-h light and dark periods and are expressed as the percent change from baseline ([postmanipulation value / baseline value] × 100). Because von Frey testing is disruptive to spontaneous sleep and occurred early in the light period of postsensitization days 1, 3, 7, 14, and 21, sleep-wake behavior was determined from recordings that were obtained on post-sensitization days 2, 8, 15, and 22.
Figure 5.
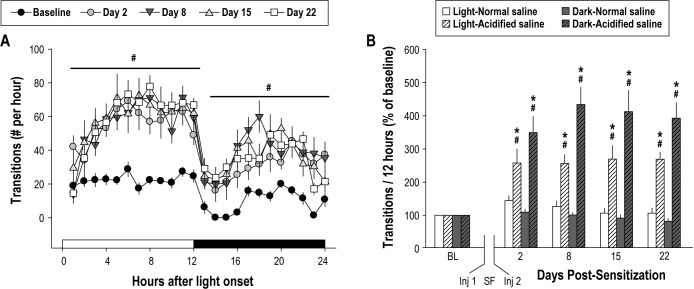
The combination of sleep fragmentation and musculoskeletal sensitization disrupts subsequent sleep for prolonged periods. (A) The average number of sleep-wake state transitions per hour across the 24-h light:dark period is plotted for preinjection baseline and postmanipulation days 2, 8, 15, and 22. All mice were subjected to sleep fragmentation (SF) with musculoskeletal sensitization (acidified saline). Sleep fragmentation combined with musculoskeletal sensitization significantly increased state transitions during the light [F(1,12) = 30.348, P < 0.001] and dark [F(1, 12) = 20.479, P = 0.001] periods. (B) The percent change from baseline (BL) for the 12-h light and the 12-h dark period is plotted for postsensitization days 2, 8, 15, and 22. On all postmanipulation days, mice with musculoskeletal sensitization (acidified saline) had significantly more sleep-wake state transitions than mice without musculoskeletal sensitization (normal saline), an effect apparent during the light period [F(1,12) = 20.782, P = 0.001] and during the dark [F(1,12) = 45.219, P < 0.001] periods. Values are the mean ± standard error of the mean for n = 7 mice per injection group. Number sign, P ≤ 0.05 versus preinjection baseline; asterisk, P ≤ 0.05 versus normal saline.
Figure 6.
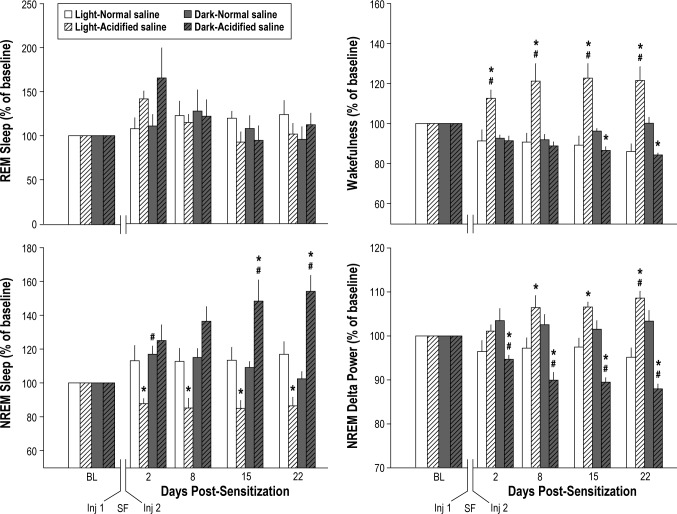
The combination of sleep fragmentation and musculoskeletal sensitization alters the duration and quality of subsequent sleep. The effect of sleep fragmentation (SF) during the musculoskeletal sensitization period on rapid eye movements (REM) sleep, nonrapid eye movement (NREM) sleep, wakefulness, and delta power during NREM sleep is presented as percent change from baseline (BL) values. Values are the mean ± standard error of the mean for n = 7 mice per injection group. Although there was a trend for increased REM sleep on the second post-sensitization day, no effect of treatment was revealed for REM sleep during the light period [F(1,12) = 0.525, P = 0.482] or dark period [F(1,12) = 0.254, P = 0.245]. NREM sleep of mice subjected to the combination of sleep fragmentation and musculoskeletal sensitization was decreased during the light period across all postmanipulation days [F(1,12) = 10.509, P = 0.007], and increased during the dark period [F(1,12) = 10.658, P = 0.007] on postmanipulation days 15 and 22. Wakefulness during the light period of mice in which sleep fragmentation was combined with musculoskeletal sensitization increased during the entire postsensitization period [F(1,12) = 16.903, P = 0.001]. Sensitized mice had significantly less wakefulness during the dark period [F(1,12) = 19.155, P = 0.001] on post-manipulation days 15 and 22. NREM delta power in mice subjected to sleep fragmentation and musculoskeletal sensitization significantly increased during light periods [F(1,12) = 13.555, P = 0.003] and decreased during dark periods [F(1,12) = 28.153, P < 0.001]. For all panels: number sign, P ≤ 0.05 versus premanipulation baseline; asterisk, P ≤ 0.05 versus normal pH saline injections. Inj, injection.
Following sleep fragmentation combined with musculoskeletal sensitization, sensitized mice had a significant increase in sleep-wake state transitions. Increased sleep-wake state transitions were apparent during the light and during the dark periods, and differed statistically from preinjection baseline values and from control mice injected with normal saline (Figures 5A and 5B). These effects were robust, and persisted for the duration of the 22-day postsensitization period evaluated in this study.
NREM sleep and wakefulness were altered during the post-manipulation period in mice that had been subjected to sleep fragmentation during musculoskeletal sensitization (acidified saline injections; Figure 6). The amount of time spent in REM sleep during the postmanipulation period did not differ among conditions, although there was a trend toward increased REM sleep during the light and dark periods on postsensitization day 2 (Figure 6). The combination of sleep fragmentation and musculoskeletal sensitization had differential effects on NREM sleep of mice during the post-manipulation period. NREM sleep of sensitized mice was reduced during the light period across all recording days evaluated, and increased during the dark period on postmanipulation days 15 and 22 (Figure 6). By comparison, mice injected with normal saline and subjected to sleep fragmentation had a modest increase in NREM sleep that was restricted to the dark period of postmanipulation day 2 (Figure 6). Wakefulness was significantly increased in sensitized mice during the light period on all postmanipulation recording days (Figure 6). Sensitized mice also had a significant decrease in wakefulness during the dark period on postmanipulation days 15 and 22. There were no significant changes in REM sleep or wakefulness of mice injected with normal saline (Figure 6).
Because of interanimal variations in properties of the recorded EEG, analyses of NREM delta power were performed on values normalized to the 24-h average for each animal ([hourly value / 24 h average] × 100).33 These values were then expressed as the percent change from baseline ([post-sensitization 12-h normalized value / baseline 12-h normalized value] × 100). The combination of sleep fragmentation and musculo-skeletal sensitization increased NREM delta power during the light period and decreased NREM delta power during the dark period. These effects were most apparent on postmanipulation days 8, 15, and 22 (Figure 6). There were no changes in NREM delta power of mice injected with normal saline during the musculoskeletal sensitization period.
Sleep fragmentation combined with musculoskeletal sensitization did not significantly alter daily food consumption, water consumption, or body weight. Repeated-measures ANOVA did not reveal a significant group or time effect on these clinical parameters (data not shown).
DISCUSSION
Results of this study demonstrate that disrupting sleep of mice during the light periods of the inter-injection interval required to induce musculoskeletal sensitization exacerbates mechanical hypersensitivity and alters subsequent sleep-wake behavior. The combined effects of sleep fragmentation and musculoskeletal sensitization on sleep-wake behavior include increases in the number of sleep-wake state transitions, alterations in NREM sleep and wakefulness, and in delta power during NREM sleep. Our data suggest that exacerbated mechanical hypersensitivity and changes in sleep-wake behavior under the conditions of this study are the result of a synergistic effect of sleep fragmentation combined with musculoskeletal sensitization. Importantly, these data also demonstrate that in this preclinical model, sleep fragmentation exacerbates pain as manifested by prolonged induction of mechanical hypersensitivity.
Data from this study demonstrate that sleep fragmentation combined with musculoskeletal sensitization increases the number of sleep-wake state transitions of mice during the light and dark periods for at least 3 weeks following sensitization. We report elsewhere in this issue27 that musculoskeletal sensitization by itself increases state transitions of sensitized mice during the light period, but not the dark period. Increased numbers of state changes reflect poor sleep quality, and have been reported in several preclinical pain studies, including those of neuropathic and arthritic pain.34–36 Collectively, results of our other study27 and these new data suggest that sleep fragmentation combined with musculoskeletal sensitization exacerbates effects on sleep quality relative to responses to either manipulation alone. These preclinical results contribute to increasing literature demonstrating that musculoskeletal sensitization fragments sleep. Clinically, fibromyalgia is a chronic pain condition of unknown etiology that is characterized by fragmented sleep and musculoskeletal pain.37,38 Sodium oxybate, a medication that consolidates sleep, improves subjective pain ratings in fibromyalgia patients.39,40 Consolidation of sleep may improve pain symptoms, especially in patients with ongoing musculo-skeletal pain.41 Additional studies are necessary to determine if sleep consolidation in this preclinical model would ameliorate pain symptoms associated with musculoskeletal sensitization.
Musculoskeletal sensitization by itself, i.e., without concurrent sleep fragmentation, does not alter the amount of time mice spend in NREM sleep, REM sleep, or wakefulness, or change delta power during NREM sleep.27 A novel finding of this study is that when sleep fragmentation is combined with musculoskeletal sensitization, each of these parameters is altered. Furthermore, these effects are prolonged, and persist for at least 3 weeks. NREM sleep of mice subjected to sleep fragmentation combined with musculoskeletal sensitization is increased during the dark period and reduced during the light period (this study). Reductions in NREM sleep are reported in other preclinical pain models, including nerve constriction injury,36,42 osteoarthritis,35 and nerve ligation.43 In the current study, changes in NREM sleep are mirrored by increased wakefulness during the light period and reduced wakefulness during the dark period. These changes in NREM sleep and wakefulness suggest insufficient sleep during the light period, which is compensated by a NREM sleep rebound during the dark period. However, delta power during NREM sleep is increased during the light period and reduced during the dark period. Although NREM sleep duration and delta power during NREM sleep may change in parallel, there is ample literature demonstrating dissociation between these two parameters under a variety of conditions.44 Our data demonstrate that in this model of musculoskeletal sensitization not only are changes in NREM sleep duration and delta power during NREM sleep dissociated, but the relationships between NREM sleep duration and delta power during NREM sleep are very complex. Ample literature demonstrates that NREM delta power generally increases with duration of prior wakefulness.44 Therefore, increased NREM delta power during the dark period is one anticipated consequence of insufficient sleep during the light period. This is not the case for data obtained from mice in this study subjected to sleep fragmentation during the musculoskeletal sensitization period. The precise mechanisms underlying the reciprocal changes in NREM sleep and delta power during NREM sleep in this model remain to be elucidated.
REM sleep deprivation of humans or rodents enhances pain across sensory modalities, including thermal, mechanical, chemical, and electrical stimuli.2,15,45 Conversely, REM sleep is reduced in animals subjected to some preclinical pain models, such as gouty arthritis,46 diabetic neuropathy,47 or orofacial pain.48 Observations such as these suggest a link between REM sleep and pain symptoms such that reduced REM sleep enhances pain, and/or enhanced pain reduces REM sleep. Data obtained in this study demonstrate that sleep fragmentation by itself, i.e., in the absence of musculoskeletal sensitization, does not induce mechanical hypersensitivity. Although our sleep fragmentation method does not dramatically alter NREM sleep, REM sleep is essentially abolished during periods when the disc is rotating.29 Our finding that sleep fragmentation by this method does not induce mechanical hypersensitivity may be important as studies of humans and rodents that use total sleep deprivation,12,49 REM sleep deprivation,11,15,16 or sleep disruption50,51 report increases in pain symptoms using other outcome measures. Ongoing studies aim to understand the effect of sleep disruption by this method on multiple aspects of rodent physiology and behavior, including pain symptoms. The initial study using this method and protocol to disrupt sleep of mice suggests that 5 days of REM sleep loss during the light period may not have the same effect on pain symptoms in otherwise healthy rodents as reported in some studies that used other approaches to eliminate or disrupt sleep, or in other pain models.
Musculoskeletal sensitization does not damage peripheral tissue, and mechanical hypersensitivity in this model is mediated by changes in the central nervous system.52,53 Sleep fragmentation combined with musculoskeletal sensitization enhances mechanical hypersensitivity to a greater extent than that elicited by musculoskeletal sensitization alone, i.e., there is an exacerbated response. Our data indicate that the combination of sleep fragmentation and musculoskeletal sensitization induces mechanical hypersensitivity at least 2 days earlier than when musculoskeletal sensitization occurs in mice allowed undisturbed sleep. In addition, the exacerbated mechanical hypersensitivity is apparent 21 days after sensitization. One possible explanation for the exacerbated increase in mechanical hypersensitivity 3 weeks after sensitization is the change in sleep that occurs during this period. In human subjects, sleepiness increases subjective pain and lowers thresholds for evoked pain responses.17,54 Three weeks after sensitization, sleep-wake behavior of mice during the light period is altered such that NREM sleep duration is reduced, there is more wakefulness, and the number of sleep-wake state transitions is increased. These changes in sleep of mice indicate sleep of poor quality that fundamentally differs from sleep during baseline conditions prior to musculoskeletal sensitization. Although the manipulations used in this study induce complex changes in NREM delta power and NREM sleep duration, these data suggest that in mice poor quality of sleep contributes to pain perception. Within this context, musculoskeletal sensitization combined with sleep fragmentation may model aspects of the relationship between sleep and pain reported in human subjects.19,55,56
Sleep deprivation, disruption, and fragmentation all can contribute to a systemic proinflammatory state.57–59 Circulating proinflammatory cytokines increase under conditions of disrupted sleep in rodents and human subjects.58,60,61 Sleep fragmentation may thus contribute to an inflammatory state that alters outcomes of musculoskeletal sensitization. Unpublished data from our laboratory demonstrate that sleep fragmentation of mice during the light period by the method used in this study increases proinflammatory cytokines in plasma and discrete brain regions. Sleep fragmentation by this method also enhances the febrile response of mice to an intraperitoneal lipopolysaccharide (LPS) injection.29 LPS, an endotoxin found in the membrane of gram-negative bacteria, induces a systemic inflammatory response. Inflammation may play an important role in this model because replacing the first intramuscular injection of acidified saline with a systemic injection of LPS can induce musculoskeletal sensitization.62 An LPS injection into rats that precedes intramuscular injection of acidified saline by 5 days is sufficient to induce mechanical hypersensitivity that is similar in magnitude to that of animals subjected to two intramuscular injections of acidified saline.62 Ongoing studies in our laboratory focus on the role of inflammatory mediators in this model of sleep fragmentation combined with musculoskeletal sensitization.
One unanticipated finding of our study is the magnitude of the effects on mechanical hypersensitivity of sleep fragmentation combined with musculoskeletal sensitization. The findings that effects of sleep fragmentation combined with musculo-skeletal sensitization on mechanical hypersensitivity are earlier in onset and of greater magnitude may be clinically important within the context of the transition from acute pain after initial injury to that of a chronic persistent pain state. The transition to chronic pain is the subject of active investigation by many, yet mechanisms mediating this transition are not fully understood. Although it was not the intent of the current study to determine whether sleep fragmentation is a contributing factor to the chronic pain transition, our data suggest the intriguing possibility that this may be the case. Mechanical hypersensitivity without sleep fragmentation persists for at least 3 to 6 weeks.27,63,64 The data presented in this manuscript demonstrate an enhancement of mechanical hypersensitivity on day 21 relative to mice with undisturbed sleep. Because our protocol ended after 3 weeks, we do not know how long the synergistic effects of sleep fragmentation on musculoskeletal sensitization persist. Because the magnitude of the combined effect is greater at day 21 than at earlier time points, it may be that pain symptoms in these animals persist for even longer periods than previously reported in the literature. Such findings would suggest that sleep disruption may be a contributing factor in the complex events necessary for the transition from acute to chronic pain. Additional studies are necessary to determine if this is indeed the case, and whether this model of musculoskeletal sensitization combined with sleep fragmentation is of utility in determining mechanisms underlying the transition from acute to chronic pain.27,54,65–69
In conclusion, our data demonstrate that in this model, sleep fragmentation combined with musculoskeletal sensitization induces prolonged effects on mechanical hypersensitivity and sleep-wake behavior of mice. These effects result from synergistic interactions between sleep fragmentation and musculoskeletal sensitization, and do not result from sleep fragmentation or from musculoskeletal sensitization per se. Given the prevalence and increasing incidence of insufficient sleep in the United States, the relationship between chronic pain and sleep will continue to be a prominent public health issue. Additional preclinical, translational, and clinical investigations are needed so that the quality of life for the millions of individuals suffering these conditions may be improved.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by the University of Washington Department of Anesthesiology and Pain Medicine (MRO), and by the Rackham Graduate School of the University of Michigan (BCS). The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr. Tom Gardiner and Dr. Linda Toth, Southern Illinois University School of Medicine, who designed and developed the sleep disruption devices and controlling software.
REFERENCES
- 1.Effect of short sleep duration on daily activities--United States, 2005-2008. MMWR Morb Mortal Wkly Rep. 2011;60:239–42. [PubMed] [Google Scholar]
- 2.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–69. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanlon EC, Van Cauter E. Quantification of sleep behavior and of its impact on the cross-talk between the brain and peripheral metabolism. Proc Natl Acad Sci U S A. 2011;108(Suppl 3):15609–16. doi: 10.1073/pnas.1101338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Opp MR. Sleep and psychoneuroimmunology. Immunol Allergy Clin North Am. 2009;29:295–307. doi: 10.1016/j.iac.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Sleep Foundation. Washington, DC: National Sleep Foundation, ed; 2000. 2000 Sleep in America poll: summary of findings. [Google Scholar]
- 8.Tafti M, Villemin E, Carlander B, Besset A, Billiard M. Sleep in human narcolepsy revisited with special reference to prior wakefulness duration. Sleep. 1992;15:344–51. doi: 10.1093/sleep/15.4.344. [DOI] [PubMed] [Google Scholar]
- 9.Zorick F, Roehrs T, Wittig R, Lamphere J, Sicklesteel J, Roth T. Sleep-wake abnormalities in narcolepsy. Sleep. 1986;9:189–93. doi: 10.1093/sleep/9.1.189. [DOI] [PubMed] [Google Scholar]
- 10.Roth T, Roehrs T. Insomnia: epidemiology, characteristics, and consequences. Clin Cornerstone. 2003;5:5–15. doi: 10.1016/s1098-3597(03)90031-7. [DOI] [PubMed] [Google Scholar]
- 11.Daya VG, Bentley AJ. Perception of experimental pain is reduced after provoked waking from rapid eye movement sleep. J Sleep Res. 2010;19:317–22. doi: 10.1111/j.1365-2869.2009.00784.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 13.Kundermann B, Spernal J, Huber MT, Krieg JC, Lautenbacher S. Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosom Med. 2004;66:932–7. doi: 10.1097/01.psy.0000145912.24553.c0. [DOI] [PubMed] [Google Scholar]
- 14.Lentz MJ, Landis CA, Rothermel J, Shaver JL. Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. J Rheumatol. 1999;26:1586–92. [PubMed] [Google Scholar]
- 15.Hakki Onen S, Alloui A, Jourdan D, Eschalier A, Dubray C. Effects of rapid eye movement (REM) sleep deprivation on pain sensitivity in the rat. Brain Res. 2001;900:261–7. doi: 10.1016/s0006-8993(01)02320-4. [DOI] [PubMed] [Google Scholar]
- 16.Hicks RA, Moore JD, Findley P, Hirshfield C, Humphrey V. REM sleep deprivation and pain thresholds in rats. Percept Mot Skills. 1978;47:848–50. doi: 10.2466/pms.1978.47.3.848. [DOI] [PubMed] [Google Scholar]
- 17.Chhangani BS, Roehrs TA, Harris EJ, et al. Pain sensitivity in sleepy pain-free normals. Sleep. 2009;32:1011–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–8. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 19.Mork PJ, Nilsen TI. Sleep problems and risk of fibromyalgia: Longitudinal data from the Norwegian HUNT-study. Arthritis Rheum. 2011;64:281–4. doi: 10.1002/art.33346. [DOI] [PubMed] [Google Scholar]
- 20.Sivertsen B, Krokstad S, Overland S, Mykletun A. The epidemiology of insomnia: associations with physical and mental health. The HUNT-2 study. J Psychosom Res. 2009;67:109–16. doi: 10.1016/j.jpsychores.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 21.National Center for Health Statistics. Chartbook on Trends in the Health of Americans, Special Feature: Pain. In, 2006. [PubMed]
- 22.Gureje O, Kola L, Ademola A, Olley BO. Profile, comorbidity and impact of insomnia in the Ibadan study of ageing. Int J Geriatr Psychiatry. 2009;24:686–93. doi: 10.1002/gps.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ancoli-Israel S. The impact and prevalence of chronic insomnia and other sleep disturbances associated with chronic illness. Am J Manag Care. 2006;12:S221–9. [PubMed] [Google Scholar]
- 24.Nascimento DC, Andersen ML, Hipolide DC, Nobrega JN, Tufik S. Pain hypersensitivity induced by paradoxical sleep deprivation is not due to altered binding to brain mu-opioid receptors. Behav Brain Res. 2007;178:216–20. doi: 10.1016/j.bbr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Sharma NK, Ryals JM, Liu H, Liu W, Wright DE. Acidic saline-induced primary and secondary mechanical hyperalgesia in mice. J Pain. 2009;10:1231–41. doi: 10.1016/j.jpain.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutton BC, Opp MR. Musculoskeletal sensitization and sleep: chronic muscle pain fragments sleep of mice without altering its duration. Sleep. 2014;37:505–13. doi: 10.5665/sleep.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith SB, Crager SE, Mogil JS. Paclitaxel-induced neuropathic hypersensitivity in mice: responses in 10 inbred mouse strains. Life Sci. 2004;74:2593–604. doi: 10.1016/j.lfs.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Ringgold KM, Barf RP, George A, Sutton BC, Opp MR. Prolonged sleep fragmentation of mice exacerbates febrile responses to lipopolysaccharide. J Neurosci Methods. 2013;219:104–12. doi: 10.1016/j.jneumeth.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olivadoti MD, Weinberg JB, Toth LA, Opp MR. Sleep and fatigue in mice infected with murine gammaherpesvirus 68. Brain Behav Immun. 2011;25:696–705. doi: 10.1016/j.bbi.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrow JD, Opp MR. Sleep-wake behavior and responses of interleukin-6-deficient mice to sleep deprivation. Brain Behav Immun. 2005;19:28–39. doi: 10.1016/j.bbi.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Olivadoti MD, Opp MR. Effects of i.c.v. administration of interleukin-1 on sleep and body temperature of interleukin-6-deficient mice. Neuroscience. 2008;153:338–48. doi: 10.1016/j.neuroscience.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franken P, Malafosse A, Tafti M. Genetic variation in EEG activity during sleep in inbred mice. Am J Physiol. 1998;275:R1127–37. doi: 10.1152/ajpregu.1998.275.4.R1127. [DOI] [PubMed] [Google Scholar]
- 34.Landis CA, Levine JD, Robinson CR. Decreased slow-wave and paradoxical sleep in a rat chronic pain model. Sleep. 1989;12:167–77. doi: 10.1093/sleep/12.2.167. [DOI] [PubMed] [Google Scholar]
- 35.Silva A, Andersen ML, Tufik S. Sleep pattern in an experimental model of osteoarthritis. Pain. 2008;140:446–55. doi: 10.1016/j.pain.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 36.Andersen ML, Tufik S. Sleep patterns over 21-day period in rats with chronic constriction of sciatic nerve. Brain Res. 2003;984:84–92. doi: 10.1016/s0006-8993(03)03095-6. [DOI] [PubMed] [Google Scholar]
- 37.Ablin J, Neumann L, Buskila D. Pathogenesis of fibromyalgia - A review. Joint Bone Spine. 2008;75:273–9. doi: 10.1016/j.jbspin.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Mease PJ, Clauw DJ, Arnold LM, et al. Fibromyalgia syndrome. J Rheumatol. 2005;32:2270–7. [PubMed] [Google Scholar]
- 39.Russell IJ, Perkins AT, Michalek JE. Sodium oxybate relieves pain and improves function in fibromyalgia syndrome: a randomized, double-blind, placebo-controlled, multicenter clinical trial. Arthritis Rheum. 2009;60:299–309. doi: 10.1002/art.24142. [DOI] [PubMed] [Google Scholar]
- 40.Staud R. Sodium oxybate for the treatment of fibromyalgia. Expert Opin Pharmacother. 2011;12:1789–98. doi: 10.1517/14656566.2011.589836. [DOI] [PubMed] [Google Scholar]
- 41.Davies KA, Macfarlane GJ, Nicholl BI, et al. Restorative sleep predicts the resolution of chronic widespread pain: results from the EPIFUND study. Rheumatology (Oxford) 2008;47:1809–13. doi: 10.1093/rheumatology/ken389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monassi CR, Bandler R, Keay KA. A subpopulation of rats show social and sleep-waking changes typical of chronic neuropathic pain following peripheral nerve injury. Eur J Neurosci. 2003;17:1907–20. doi: 10.1046/j.1460-9568.2003.02627.x. [DOI] [PubMed] [Google Scholar]
- 43.Narita M, Niikura K, Nanjo-Niikura K, et al. Sleep disturbances in a neuropathic pain-like condition in the mouse are associated with altered GABAergic transmission in the cingulate cortex. Pain. 2011;152:1358–72. doi: 10.1016/j.pain.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 44.Davis CJ, Clinton JM, Jewett KA, Zielinski MR, Krueger JM. Delta wave power: an independent sleep phenotype or epiphenomenon? J Clin Sleep Med. 2011;7:S16–8. doi: 10.5664/JCSM.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hicks RA, Coleman DD, Ferrante F, Sahatjian M, Hawkins J. Pain thresholds in rats during recovery from REM sleep deprivation. Percept Mot Skills. 1979;48:687–90. doi: 10.2466/pms.1979.48.3.687. [DOI] [PubMed] [Google Scholar]
- 46.Guevara-Lopez U, Ayala-Guerrero F, Covarrubias-Gomez A, Lopez-Munoz FJ, Torres-Gonzalez R. Effect of acute gouty arthritis on sleep patterns: a preclinical study. Eur J Pain. 2009;13:146–53. doi: 10.1016/j.ejpain.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Kapas L, Payne L, Obal F, Jr., Opp M, Johannsen L, Krueger JM. Sleep in diabetic rats: effects of interleukin 1. Am J Physiol. 1991;260:R995–9. doi: 10.1152/ajpregu.1991.260.5.R995. [DOI] [PubMed] [Google Scholar]
- 48.Schutz TC, Andersen ML, Tufik S. Sleep alterations in an experimental orofacial pain model in rats. Brain Res. 2003;993:164–71. doi: 10.1016/j.brainres.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Kundermann B, Krieg JC, Schreiber W, Lautenbacher S. The effect of sleep deprivation on pain. Pain Res Manag. 2004;9:25–32. doi: 10.1155/2004/949187. [DOI] [PubMed] [Google Scholar]
- 50.Goodin BR, Smith MT, Quinn NB, King CD, McGuire L. Poor sleep quality and exaggerated salivary cortisol reactivity to the cold pressor task predict greater acute pain severity in a non-clinical sample. Biol Psychol. 2012;91:36–41. doi: 10.1016/j.biopsycho.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 52.Da Silva LF, Walder RY, Davidson BL, Wilson SP, Sluka KA. Changes in expression of NMDA-NR1 receptor subunits in the rostral ventromedial medulla modulate pain behaviors. Pain. 2010;151:155–61. doi: 10.1016/j.pain.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gautam M, Benson CJ, Ranier JD, Light AR, Sluka KA. ASICs Do Not Play a Role in Maintaining Hyperalgesia Induced by Repeated Intramuscular Acid Injections. Pain Res Treat. 2012;2012:817347. doi: 10.1155/2012/817347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roehrs TA, Harris E, Randall S, Roth T. Pain sensitivity and recovery from mild chronic sleep loss. Sleep. 2012;35:1667–72. doi: 10.5665/sleep.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moldofsky H. Sleep, neuroimmune and neuroendocrine functions in fibromyalgia and chronic fatigue syndrome. Adv Neuroimmunol. 1995;5:39–56. doi: 10.1016/0960-5428(94)00048-s. [DOI] [PubMed] [Google Scholar]
- 56.Roehrs T, Roth T. Sleep and pain: interaction of two vital functions. Semin Neurol. 2005;25:106–16. doi: 10.1055/s-2005-867079. [DOI] [PubMed] [Google Scholar]
- 57.Dinges DF, Douglas SD, Hamarman S, Zaugg L, Kapoor S. Sleep deprivation and human immune function. Adv Neuroimmunol. 1995;5:97–110. doi: 10.1016/0960-5428(95)00002-j. [DOI] [PubMed] [Google Scholar]
- 58.Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–4. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu J, Chen Z, Gorczynski CP, et al. Sleep-deprived mice show altered cytokine production manifest by perturbations in serum IL-1ra, TNFa, and IL-6 levels. Brain Behav Immun. 2003;17:498–504. doi: 10.1016/j.bbi.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Shearer WT, Reuben JM, Mullington JM, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–70. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 61.Yehuda S, Sredni B, Carasso RL, Kenigsbuch-Sredni D. REM sleep deprivation in rats results in inflammation and interleukin-17 elevation. J Interferon Cytokine Res. 2009;29:393–8. doi: 10.1089/jir.2008.0080. [DOI] [PubMed] [Google Scholar]
- 62.Jasper LL, MacNeil BJ. Diverse sensory inputs permit priming in the acidic saline model of hyperalgesia. Eur J Pain. 2012;16:966–73. doi: 10.1002/j.1532-2149.2011.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sluka KA, Rohlwing JJ, Bussey RA, Eikenberry SA, Wilken JM. Chronic muscle pain induced by repeated acid Injection is reversed by spinally administered mu- and delta-, but not kappa-, opioid receptor agonists. J Pharmacol Exp Ther. 2002;302:1146–50. doi: 10.1124/jpet.102.033167. [DOI] [PubMed] [Google Scholar]
- 64.Ledeboer A, Mahoney JH, Milligan ED, Martin D, Maier SF, Watkins LR. Spinal cord glia and interleukin-1 do not appear to mediate persistent allodynia induced by intramuscular acidic saline in rats. J Pain. 2006;7:757–67. doi: 10.1016/j.jpain.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 65.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–8. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang H, Zhang H, Dougherty PM. Dynamic effects of TNF-alpha on synaptic transmission in mice over time following sciatic nerve chronic constriction injury. J Neurophysiol. 2013;110:1663–71. doi: 10.1152/jn.01088.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tiede W, Magerl W, Baumgartner U, Durrer B, Ehlert U, Treede RD. Sleep restriction attenuates amplitudes and attentional modulation of pain-related evoked potentials, but augments pain ratings in healthy volunteers. Pain. 2009 doi: 10.1016/j.pain.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 68.Edwards RR, Almeida DM, Klick B, Haythornthwaite JA, Smith MT. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202–7. doi: 10.1016/j.pain.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dimsdale JE, Norman D, DeJardin D, Wallace MS. The effect of opioids on sleep architecture. J Clin Sleep Med. 2007;3:33–6. [PubMed] [Google Scholar]