Abstract
Study Objectives:
Strong clinical and preclinical evidence suggests that acute ethanol promotes sleep. However, very little is known about how and where ethanol acts to promote sleep. We hypothesized that ethanol may induce sleep by increasing extracellular levels of adenosine and inhibiting orexin neurons in the perifornical hypothalamus.
Design:
Experiments 1 and 2: Within-Subject Design; Experiment 3: Between-Subject Design.
Setting:
N/A.
Patients or Participants:
N/A.
Interventions:
N/A.
Measurements and Results:
Using adult male Sprague-Dawley rats as our animal model, we performed three experiments to test our hypothesis. Our first experiment examined the effect of A1 receptor blockade in the orexinergic perifornical hypothalamus on sleep- promoting effects of ethanol. Bilateral microinjection of the selective A1 receptor antagonist 1,3-dipropyl-8-phenylxanthine (500 μM; 250 nL/side) into orexinergic perifornical hypothalamus significantly reduced nonrapid eye movement sleep with a concomitant increase in wakefulness, suggesting that blockade of adenosine A1 receptor attenuates ethanol-induced sleep promotion. Our second experiment examined adenosine release in the orexinergic perifornical hypothalamus during local ethanol infusion. Local infusion of pharmacologically relevant doses of ethanol significantly and dose-dependently increased adenosine release. Our final experiment used c-Fos immunohistochemistry to examine the effects of ethanol on the activation of orexin neurons. Acute ethanol exposure significantly reduced the number of orexin neurons containing c-Fos, suggesting an inhibition of orexin neurons after ethanol intake.
Conclusions:
Based on our results, we believe that ethanol promotes sleep by increasing adenosine in the orexinergic perifornical hypothalamus, resulting in A1 receptor-mediated inhibition of orexin neurons.
Citation:
Sharma R; Sahota P; Thakkar MM. Role of adenosine and the orexinergic perifornical hypothalamus in sleep-promoting effects of ethanol. SLEEP 2014;37(3):525-533.
Keywords: Adenosine, ethanol, microdialysis, orexin, sleep
INTRODUCTION
Alcoholism is a chronic ailment and a third leading cause of preventable death in the United States.1 Sleep disruptions are among the most severe and protracted symptoms associated with initial development of alcohol addiction, its maintenance, and relapse after withdrawal.2–4 In addition, sleep disorder associated with alcoholism have profound socioeconomic costs that exceeds $18 billion annually.5,6 Although the effects of ethanol on human sleep were first described almost 70 years ago, little is known about how and where ethanol acts in the brain to alter sleep-wakefulness.7
It was almost 30 years ago when Dar and colleagues first demonstrated a functional relationship between ethanol and the sleep factor, adenosine (AD).8 Since then, strong and consistent evidence has been gathered, suggesting that AD is responsible for mediating several cellular and behavioral effects of ethanol including ethanol drinking, motor incoordination, and sleep promotion.9–15 Acute ethanol inhibits AD uptake via equilibrative nucleoside transporter type 1 (ENT1) to increase extracellular AD. Chronic ethanol down regulates ENT1 expression.16,17 ENT1 null mice show increased ethanol consumption and reduced hypnotic response to ethanol.18
The orexin zone of perifornical hypothalamus (PFH) contains several neuronal subtypes including the orexin-containing neurons. However, to a significant degree, the wake-promoting function of the PFH is attributed to orexin neurons that are exclusively localized in the PFH. Strong evidence suggests that PFH orexin-containing neurons are critical for the maintenance of wakefulness. Deficiency or loss of orexinergic neuro-transmission reduces wakefulness and causes narcolepsy.19–24 In contrast, activation of orexin neurons or orexinergic transmission exerts wake-promoting effects. In fact, orexin exerts excitatory influence on almost all wake-promoting systems in the brain.25–35 Furthermore, orexin neurons exhibit a wake-on/ sleep-off discharge pattern.36–39
Consistent evidence exists to suggest that AD may regulate sleep via orexin neurons. For example, orexin neurons express AD A1 receptor (A1R).40 Both in vivo and in vitro studies suggest that AD inhibits orexin neurons via A1R41,42. Activation of A1R in the PFH promotes sleep, whereas blockade of A1R promotes arousal and attenuates recovery sleep following sleep deprivation.43,44
To evaluate whether ethanol-induced sleep promotion is mediated by AD via A1R and involves inhibition of orexin neurons, we performed three experiments: Our first experiment examined whether blockade of A1R in PFH attenuates ethanol-induced sleep promotion. Our second experiment examined the effect of ethanol, locally administered into the PFH, on AD release. Our third experiment determined the effect of ethanol exposure on c-Fos expression in orexin neurons.
MATERIALS AND METHODS
Adult male Sprague-Dawley rats (250-350 g; Charles River, Wilmington, MA) were housed in Harry S. Truman vivarium under standard 12:12 h light-dark cycle, with ambient temperature and ad libitum access to food and water. All experiments were performed according to the Association for Assessment and Accreditation of Laboratory Animal Care policies and Guide for the Care and Use of Laboratory Animals. All protocols were approved by local committees.
Experiment 1: To examine ethanol-induced sleep promotion in rats pretreated with a selective A1R antagonist into the PFH.
Surgery
Using standard surgical procedure and under inhalation anesthesia,45 rats were implanted with electrodes for electrographic recording of electroencephalogram (EEG) and electromyogram (EMG) to determine sleep-wakefulness. Intracerebral guide cannulas (22 gauge; Plastics One, Roanoke, VA, USA) were also implanted bilaterally at a 90° angle above the target site in the PFH.44 The target coordinates for the tip of the injector cannulas were: AP -3.3, ML ± 1.5, DV -8.5 [relative to bregma46]. Flunixin (1.5 mg/kg), administered subcutaneously, was used as a postsurgical analgesic.
Experimental protocol
All experiments were conducted in a sound-attenuated chamber with food and water available ad libitum. After 2 days of postoperative recovery, rats were tethered to a lightweight recording cable and habituated to recording setup for at least 5 days. In addition, during the last 3 days, each rat was also habituated to intragastric (ig) administration as described: The rat was disconnected from the recording cable, swaddled in a towel, and the length of the stainless steel curved animal feeding needle (length = 3 inches; Popper and Sons, Hyde Park, NY) to be inserted (from the oral cavity to the end of the caudal rib) was measured. Next, the feeding needle was attached to a syringe and placed into the right lateral side of the oral cavity and gently inserted into the esophagus. As soon as the desired length was inserted, sterile water (10 mL/kg) was slowly injected. On completion, the animal was reconnected to the recording cable and the experiment was begun.
On day 1 of the experiment, sterile water (10 mL/kg; ig) was administered at dark onset and a 12-h (entire dark period) electrographic recording of baseline sleep-wakefulness was performed by amplifying EEG and EMG signals with the Neurodata Amplifier System (Model 15, Astromed Inc., West Warwick, RI, USA) followed by digitization and acquisition (128 Hz/channel) by Icelus data acquisition software (build 071503, authored by Mark Opp, University of Michigan, Ann Arbor, MI, USA) and stored in a Dell desktop computer as described previously.44,45 Acquired data were visually scored offline in 10-sec epochs as (1) wake, which included both active and quiet waking; (2) Non-rapid eye movement (NREM) sleep, and (3) Rapid eye movement (REM) sleep. Latency to NREM sleep and REM sleep were also calculated. Latency to NREM sleep was defined as the amount of time between ethanol administration and first non-interrupted 60-sec bout of NREM sleep. Latency to REM sleep was defined the amount of time between ethanol administration and first noninterrupted 30-sec bout of REM sleep.10,44
On day 2, 30 min before dark onset, bilateral vehicle micro-injections were performed in the PFH as follows: the rat was disconnected and swaddled in a towel. The stylus was removed and the injector cannula [connected to a 0.5 μL Hamilton syringe (Hamilton Company, Reno, NV)] was inserted into the guide cannula. After waiting for approximately 1 min, the plunger was slowly pushed to complete the injection within 1 min. The injector cannula was kept in place for 1 min before retracting. The same protocol was repeated on the other side after 1 min. Subsequently, at dark onset, ethanol [35% (v/v in water); 3 g/kg, ig] was administered. The rat was reconnected and sleep-wakefulness recordings were initiated and continued for 12 h until the end of the dark period.
Animals were left undisturbed (with continuous sleep-wakefulness recordings, but no treatment) for the next 3 days. On day 6, 30 min before dark onset, the animals were bilaterally microinjected with 500 μM (250 nl/side) of A1R antagonist, 1,3-dipropyl-8-phenylxanthine (DPX; Tocris Biosciences Ellis-ville, Missouri) followed by ethanol administration (35%; 3 g/ kg, ig) and initiation of sleep recording that lasted for 12 h.
On completion, rats were euthanized under deep phenobarbital anesthesia and perfused transcardially with 200 mL of 0.9% saline followed by perfusion of 200 mL 10% formalin (Sigma-Aldrich Co. LLC, St Louis, MO). The brains were removed, blocked, and postfixed in the same fixative overnight and stored in 20% sucrose at 4°C until equilibrated. The brains were blocked to isolate the hypothalamic region and sectioned into 40-μm coronal sections with freezing microtome.10,44,45 The sections were serially collected in three separate wells containing 0.1 mM phosphate buffered saline (PBS; pH 7.4) and one out of every three section was processed for orexin immunohistochemistry as described later in experiment 3.
Statistical analysis
One-way repeated measures analysis of variance (ANOVA; Graphpad Prism, San Diego, CA, USA) followed by the Newman-Keuls post hoc test, was performed to examine the effect of A1R blockade on ethanol-induced sleep promotion.
Experiment 2: Effects of local ethanol perfusion on AD release in the PFH.
Surgery
Using standard surgical procedure and under inhalation anesthesia, rats were surgically implanted with a unilateral guide cannula (CMA, Stockholm, Sweden) in the PFH (stereotaxic coordinates as described for experiment 1). After 5 days of postoperative recovery, microdialysis probe was inserted through the guide cannula into the PFH and artificial cerebrospinal fluid [aCSF; NaCl 147 mM, KCl 3 mM, CaCl2 1.2 mM, MgCl2 1.0 mM, pH 7.2) was perfused at a flow rate = 0.7 μL/min.
Experimental protocol
The experiment was begun at dark onset after allowing 12-16 h for probe insertion recovery. In addition, 4 × 20 min (14 μL/sample) pre-ethanol baseline samples were collected. Subsequently, 30-, 100-, and 300-mM doses of ethanol were perfused. Each dose of ethanol was perfused for 80 min and 4 × 20 min samples were collected. Finally, aCSF was perfused and 4 × 20 post-ethanol samples were collected. Samples were stored in ice until analyzed. The flow rate was maintained at 0.7 μL/min during the entire experiment. On completion, probes were removed and processed for in vitro recovery.47
AD separation and quantification was achieved by high-performance liquid chromatography (HPLC) coupled with an ultraviolet (UV) detector.16,47–50 In brief, 10 μL of microdialysis sample was injected into the HPLC. The mobile phase contained 8 mM NaH2PO4 and 8% methanol (pH = 4; flow rate = 80 μL/min). AD was separated out with a microbore column (1 × 100 mm; MF-8949; BASi, West Lafayette, IN) and detected by a UV detector (wavelength = 258 nm; Model SPD20, Shimadzu, Columbia, MD, USA). Chromatogram data were acquired and analyzed by PowerChrom 280 system (eDAQ Inc, Colorado Springs, CO, USA). AD peak in the sample was identified and quantified by comparing its retention time and area under the peak to pure known amounts of external AD standards (Sigma-Aldrich Co. LLC).
On completion, rats were euthanized as described previously. One series of sections was used to perform orexin immunohistochemistry. A second series was stained with cresyl violet to assess any potential neuronal damage due to ethanol perfusion.47
Statistical analysis
One-way repeated-measures ANOVA (Graphpad Prism) followed by the Newman-Keuls post hoc test was performed to examine the effect of local ethanol infusion on AD release in the PFH. Linear regression was used to assess dose dependency of ethanol on AD release.
Experiment 3: To examine whether acute ethanol exposure inhibits orexin.
Experimental protocol
Each animal was habituated to ig administration for 3 days as described previously. On completion, the animals were then divided in two groups: control and ethanol. The controls received sterile water (10 mL/kg; ig) at dark onset, whereas ethanol (35%; 3 g / kg; ig) was administered to the ethanol group. Rats were left undisturbed for 2 h. Subsequently, the rats were euthanized, brains blocked, and sectioned (as described previously for experiment 1) and processed for c-Fos and orexin immunohistochemistry: All washings were performed three times (10 min/ washing) in PBS. The entire procedure was performed at room temperature. Day 1: Sections were washed and incubated for 20 min in PBS containing 0.3% hydrogen peroxide and 20% methanol. After washing, sections were incubated for 2 h in blocking solution [containing PBS, 3% normal donkey serum (Jackson ImmunoResearch, West Grove, PA, USA) and 0.3% Triton X-100 (Sigma-Aldrich Co. LLC)]. Next, sections were incubated overnight in blocking solution containing primary, rabbit anti-c-Fos, antibody (1:5000; Calbiochem, Rockland, MA, USA). Day 2: Sections were washed and incubated in biotinylated donkey antirabbit secondary antibody (1:400, Jackson ImmunoRe-search) for 2 h followed by washing and incubation in Avidin Biotin Complex (ABC; Vector, Burlingame, CA, USA) for 1 h. This was followed by washing and incubation in 0.05% 3, 3'-diaminobenzidine (DAB) solution containing nickel chloride (0.05%) and hydrogen peroxide (0.015%) until desired intensity of black color was confirmed under a microscope.
Next, the sections were washed and processed for orexin immunohistochemistry by incubating overnight in blocking solution containing primary goat anti-orexin-A antibody (1:600; Millipore, Billerica, MA, USA). Day 3: The sections were washed and incubated in biotinylated donkey antigoat secondary antibody (1:1000, Jackson ImmunoResearch) for 1 h followed by washing and incubation in ABC for 1 h. Subsequently, the sections were washed and incubated in 0.05% DAB solution containing hydrogen peroxide (0.015%) until desired intensity of brown color was confirmed under a microscope. Upon completion, the sections were mounted on subbed slides, processed, and coverslipped. Control immunohistochemistry was performed by eliminating the primary antibodies.
For cell counting, four sections containing maximal number of orexin neuron, from each animal of the control and ethanol groups were selected and used for the quantification of c-Fos immunoreactivity (IR) by a blinded investigator. Counting was performed under Leica DM 4000 microscope (magnification 40× + 10× for eyepiece) with Adobe Photoshop CS3 software counting tool as described previously.10 All single labeled orexin +ve neurons (brown cytoplasm), single labeled c-Fos +ve neurons (black nucleus) along with double labeled orexin and c-Fos +ve neurons (brown cytoplasm with dark black nucleus) were counted. The percentage of orexin +ve neurons with nuclear c-Fos IR was calculated for each animal and used for statistical analysis.
Statistical analysis
Two-tailed unpaired t-test was used (Graphpad Prism) to examine the effect of acute ethanol exposure on c-Fos expression in orexin and nonorexin neurons.
RESULTS
Experiment 1: Microinjection of A1R antagonist, DPX, in the PFH blocks sleep-promoting effects of ethanol.
Localization of the drug infusion sites
A representative photomicrograph depicting the bilateral injection sites in the orexinergic PFH is described in Figure 1A. All microinjection sites were in the orexinergic zone of PFH. A representation of all bilateral injections sites (n = 7) in one coronal schematic [adapted from Figure 33 in reference 46] is shown in Figure 1B.
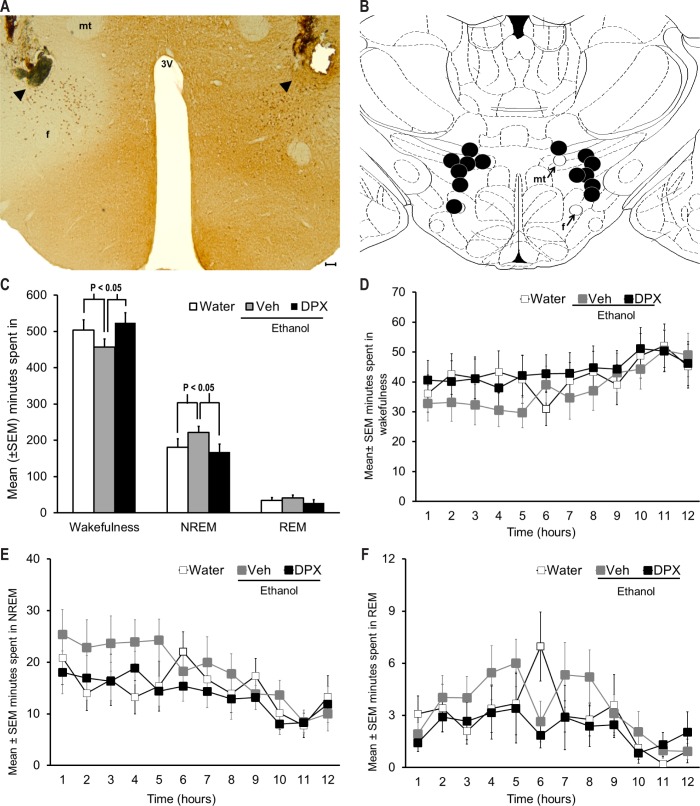
Figure 1.
Effect of A1 receptor blockade in the PFH on sleep promotion following acute ethanol intake. (A) Representative photomicrograph depicting bilateral microinjection sites (arrowhead) in the midst of orexin neurons in the PFH. (B) A coronal schematic describing anatomical localization of histologically identified sites of 1,3-dipropyl-8-phenylxanthine (DPX) microinjection (black circles) in the PFH. All microinjection sites were located between AP -3.2 and -3.6 and are mapped onto one coronal brain section [AP = -3.3, adapted from Figure 33 in Paxinos and Watson, 1998]. f = fornix; mt = mammillothalamic tract; 3V = third ventricle. For identification of other structures, see Figure 33 in reference 46. Calibration bar = 50 μm. (C) Acute ethanol exposure produced a robust sleep promotion as evident by a significant increase in non-rapid eye movement (NREM) sleep (P < 0.05; Neuman-Keuls post hoc test; n = 7) coupled with a reduction in wakefulness (P < 0.05; Neuman-Keuls post hoc test) during 12 h of dark period post-ethanol. However, bilateral infusion of A1R antagonist, DPX, into the PFH blocked ethanol induced NREM sleep promotion (P < 0.05; Neuman-Keuls post hoc test) and promoted wakefulness (P < 0.05; Neuman-Keuls post hoc test). Rapid eye movement (REM) sleep remained unchanged (see text for details). (D-F) depicts the hourly profile of wakefulness, NREM sleep, and REM sleep during 12 h of dark period, across treatment. See text for details.
Effect of A1R antagonist in the PFH on ethanol-induced changes in sleep-wakefulness
Repeated-measures ANOVA indicated a significant main effect of ethanol on NREM sleep [F = 6.4; df (total) = 20; P < 0.05] and wakefulness [F = 8.8; df (total) = 20; P < 0.01], whereas a non-significant, trend level, effect was observed on REM sleep [F = 3.2; df (total) = 20; P = 0.07] (Figure 1C). The amount of time spent in minutes in all three states across treatments (Table 1) along with the temporal distribution of wakefulness (Figure 1D), NREM sleep (Figure 1E), and REM sleep (Figure 1F) is also described.
Table 1.
Mean ± SEM min spent in sleep-wakefulness and NREM and REM latencies (in min) across treatment
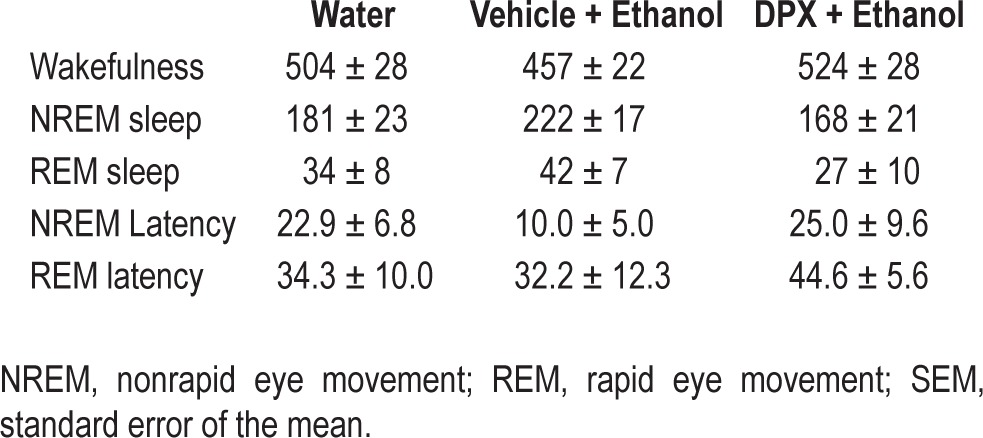
When NREM sleep during baseline was compared with NREM sleep after PFH vehicle pretreatment and intragastric ethanol administration, significant (P < 0.05; Neuman-Keuls test) increase in NREM sleep with a concomitant (P < 0.05; Neuman-Keuls test) reduction in wakefulness was observed confirming a robust NREM sleep promoting effect of acute ethanol.
Bilateral microinjections of the selective A1R antagonist DPX into the PFH followed by ethanol administration produced a significant (P < 0.05; Neuman-Keuls test) reduction in NREM sleep with a concomitant, significant (P < 0.05; Neuman-Keuls test) increase in wakefulness when compared to vehicle pretreatment followed by ethanol administration.
Although there was a decrease in NREM sleep latency following ethanol administration, it did not reach significance. Latency to REM sleep remained unaffected (Table 1).
These results demonstrate that infusion of A1R antagonist into the PFH blocks sleep promotion after acute ethanol intake.
Experiment 2: Local ethanol perfusion in PFH increases extracellular AD.
We started with a total of 10 rats. Three had probe malfunctions and had to be excluded. One rat removed the implant during the experiment. The data from six rats are presented.
Localization of the microdialysis sampling sites
A representative photomicrograph showing a lesion caused by microdialysis probe, surrounded by orexinergic neurons, is shown in Figure 2A. Nissl (cresyl violet) staining revealed that local ethanol perfusion did not cause generalized neurotoxicity because the damage was restricted to the region proximate to the tip of the probe (Figure 2B). Figure 2C describes probe site localization for all six animals in one coronal schematic [n = 6, adapted from Figure 33 in reference 46]. All microdialysis probe tips were localized in midst of orexin neurons in the PFH between AP levels -3.2 and -3.6.
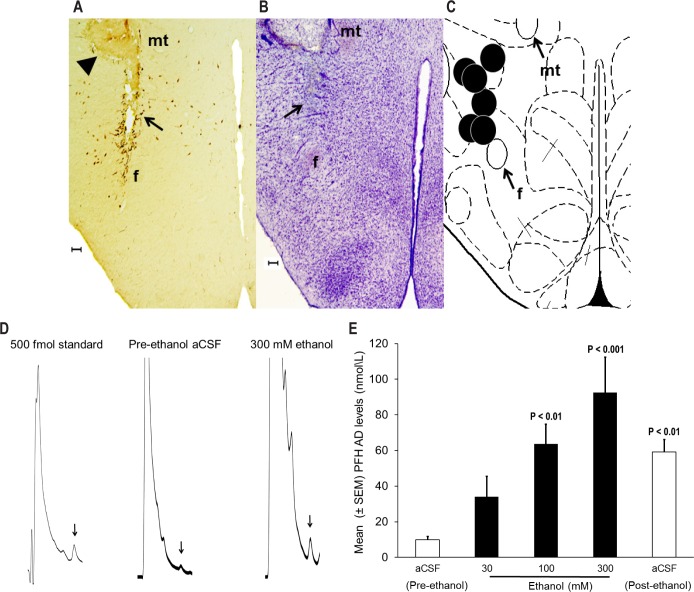
Figure 2.
Effect of local ethanol perfusion on adenosine release in the PFH. (A) Representative photomicrograph depicting lesions caused by microdialysis guide cannula (arrowhead) and probe implantation (black arrow) in the midst of orexin neurons. (B) There was no generalized neurotoxicity as evident from cresyl violet staining and intact neurons are clearly visible surrounding the probe target site. (C) All probe sites (black circles) were located between AP -3.2 to -3.6 mm from the bregma and are mapped onto one side of the coronal schematic brain section at the level of AP -3.3 [adapted from Figure 33 in Paxinos and Watson, 1998]. f = fornix; mt = mammillothalamic tract. For identification of other structures see Figure 33 in Paxinos and Watson, 1998. Calibration bar = 100 μm. (D) Standard (500 fmol) adenosine (AD) peak (arrow); AD peak (arrow) from a sample (10 μL) collected from the PFH during pre-ethanol aCSF and 300 ethanol perfusion (from rat #127). (E) Local ethanol perfusion significantly [F = 9.6; df (total) = 29; P < 0.001; One-way repeated-measures analysis of variance] and dose-dependently (R2 = 0.9; P < 0.05) increased AD release in the orexinergic PFH. See text for details.
Sensitivity and viability of AD assay
Our AD assay was very sensitive (detection limit = 500 fmol) as evident from a well-resolved and easily detectable peak of pure AD standard (10 μL; 50 mol/L), with signal-to-noise ratio > 2:1 (Figure 2D). In addition, AD peak in a 10-μL sample collected from the PFH during pre-ethanol aCSF and during 300 mM ethanol perfusion is also described in Figure 2D.
Effect of local ethanol perfusion on AD release
Local perfusion of ethanol in the PFH significantly [F = 9.6; df (total) = 29; P < 0.001; one-way repeated-measures ANOVA] and dose dependently (R2 = 0.9; P < 0.05) increased extracellular AD. Post hoc analysis (Newman-Keuls test) indicated a significant increase with 100 (P < 0.01) and 300 (P < 0.001) mM ethanol. Although the levels of AD were higher (P < 0.05) during post-ethanol aCSF perfusion, they were in a steady decline (Figure 2E).
In vitro recovery
The average (± standard error of the mean [SEM]) in vitro recovery of six probes = 8.03 (± 0.6%), suggesting that the effective ethanol concentration outside the probe will be lowered to approximately 2.4, 8, and 24 mM, which is the pharmacological relevant range.47,51
Experiment 3: Ethanol inhibits orexin neurons.
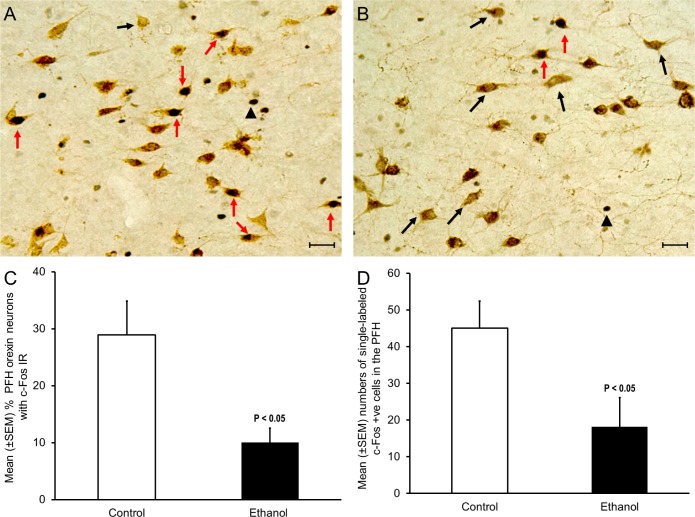
Ethanol significantly (P < 0.05; unpaired t-test, t = 2.94, df = 6) reduced the number of orexin +ve neurons with c-Fos IR. Out of a total of 1,287 orexin neurons in controls (n = 4; mean ± SEM = 322 ± 76), 29% were c-Fos +ve (n = 4; Figure 3A; red arrows). In contrast, of a total of 1,327 orexin neurons in ethanol exposed animals (n = 4; mean ± SEM = 332 ± 73), 10% displayed c-Fos IR (n = 4; Figure 3B; red arrows). Graphical representation of our data is described in Figure 3C.
Figure 3.
Effect of acute ethanol exposure on c-Fos expression in orexin neurons. (A) A representative photomicrograph describing orexin neurons with c-Fos immunoreactivity (IR) in controls. (B) A representative photomicrograph describing orexin neurons with c-Fos IR in the ethanol group. Red arrow = double labeled orexin and c-Fos +ve neuons; black arrow = single labeled orexin neurons; black arrowhead = single labeled c-Fos +ve cells. Calibration bar = 30 um. (C) The percentage of orexin neurons with c-Fos IR was significantly (P < 0.05) reduced after acute ethanol administration (n = 4; see text for details). (D) Ethanol administration significantly (P < 0.05) reduced the number of single-labeled c-Fos IR neurons in the orexinergic zone of perifornical hypothalamus.
Single-labeled c-Fos IR in the PFH was also significantly (P < 0.05; unpaired t-test, t = 2.47, df = 6) reduced after ethanol treatment suggesting an overall reduction in the PFH neuronal activity following ethanol administration (Figure 3D).
DISCUSSION
This is the first study implicating an interaction of ethanol and orexin neurons via AD in the sleep-promoting effects of ethanol. Our results demonstrate that (1) blockade of A1R in the PFH blocks sleep promotion following acute ethanol exposure; (2) local infusion of ethanol dose-dependently increases extra-cellular AD in the PFH; and (3) acute ethanol exposure inhibits wake-promoting orexin neurons. Based on these results, we believe that acute ethanol intake will increase extracellular AD. Increased AD will act via A1R to inhibit orexin neurons, resulting in sleep promotion.
We performed all three experiments at dark onset because orexin neurons are maximally active at dark onset. At light onset, orexin neurons are minimally active, which makes it difficult to monitor ethanol induced inhibition of orexin neurons. Extracellular levels of AD are highest at light onset and may not increase further after ethanol (ceiling effect). In contrast, AD levels are lowest when the animal wakes up at dark onset. In order to be consistent across experiments (see discussion below for microdialysis experiment), all experiments were performed during the dark period when the rats are maximally awake.
In experiments 1 and 3, we used the 3 g/kg dose of ethanol because this dose of ethanol has been previously used by our laboratory and produces a robust increase in NREM sleep.10 We did not measure blood alcohol levels because collection of blood requires animal handling, which may cause pain and stress the animal. This is likely to affect the animal's sleep. However, as described in our previous study, the 3 g/kg dose of ethanol produces blood ethanol level of approximately 135 g/dL.10
Our first experiment was designed to (1) verify the sleep-promoting effect of acute ethanol exposure and (2) examine whether blockade of A1R in the PFH will block sleep promotion following ethanol exposure. We used a within-subject design because it offers several advantages, including an increase in statistical power without increasing animal numbers and a reduction in error variance associated with individual differences. Furthermore, nonspecific effects of microinjections are better controlled by performing vehicle injections in the same animal. We used DPX, the selective A1 receptor antagonist, because it blocks A1R in the PFH and promotes wakefulness, both during spontaneous bouts and during recovery sleep following sleep deprivation.44 Interestingly, in contrast to our previous study where bilateral DPX infusion into the PFH significantly increased wakefulness, in this study we found that bilateral PFH DPX pretreatment followed by ethanol did not induce a significant increase in wakefulness; rather, it blocked the effect of ethanol-induced sleep and normalize sleep-wakefulness, further supporting our hypothesis that ethanol-induced sleep promotion may be mediated via A1R in the PFH.
Our results blockade of A1R in the PFH, by DPX, followed by acute ethanol, attenuates the sleep-promoting effect of ethanol and the sleep-wakefulness values returned to baseline, suggest that sleep-promoting effects of ethanol are mediated by A1R activation in the PFH.
There is compelling evidence suggesting that AD, via A1R, interacts with orexin neurons to promote sleep.43,44 However, for ethanol to promote sleep via AD-A1R-orexin interaction, ethanol should increase extracellular levels of AD in the PFH. Thus, our second experiment focused on examining whether ethanol increases extracellular AD in the PFH. Frequent sample collections along with syringe changes (for perfusion of different doses of ethanol) can disturb the animal and cause partial sleep deprivation. Sleep deprivation may alter extra-cellular AD levels, especially because orexin has been implicated in sleep homeostasis.44 Thus, to avoid this confound, we conducted the experiment during the first 5 h after dark onset when the rats are maximally awake and unlikely to be sleep deprived. Ethanol was delivered locally, via reverse microdialysis, to confirm a direct effect of ethanol on AD release in the PFH. Reverse microdialysis, whereby microdialysis probe is used to locally deliver drugs in specific brain region is a well-established drug delivery technique and has been effectively used previously to deliver ethanol locally into selected brain region and measure neurotransmitter release without causing any neurotoxicity.47,51 We confirmed the absence of neurotoxicity by orexin immunochemistry and cresyl violet staining, which showed continued presence of orexin neurons without any evidence of general toxicity. In vitro probe recovery studies confirmed pharmacological relevancy of ethanol doses used in this report.47,51
Methodology used for detection and measurement of AD has been verified by our laboratory and others.16,47,48,50 Our results suggest that ethanol significantly and dose-dependently increased extracellular AD in the PFH with the highest levels observed during a 300 mM dose of ethanol. Although AD levels during 80 min of post-ethanol aCSF perfusion were higher than pre-ethanol baseline, they were lower than AD levels during the preceding ethanol dose (300 mM), suggesting that the AD levels were in a steady decline following cessation of ethanol infusion.
Does ethanol exposure inhibit orexin neurons? At dark onset, the animals display maximal activation of orexin neurons.52 However, as demonstrated by our third experiment, ethanol significantly reduced the number of orexin neurons with c-Fos IR, suggesting that acute ethanol intake inhibits orexin neurons. Because orexin neurons are wake-promoting, inhibition of orexin neurons by ethanol is likely to promote sleep. In fact, although the PFH contains several neuronal subtypes including orexin, melanin concentrating hormone (MCH), gamma- aminobutyric acid, and glutamate, most neurons in the PFH are wakefulness-active. Thus, it is not surprising that there is a significant reduction in c-Fos expression in the PFH after ethanol administration. Furthermore, because MCH neurons are sleep-promoting53, they may not be affected by ethanol-induced AD increase. In addition, it is yet unknown whether MCH neurons express A1R.
In summary, we have described three experiments in this report: The results of our first experiment demonstrates that ethanol-induced sleep promotion is blocked by local infusion of A1R antagonist in the PFH, suggesting that ethanol-induced sleep promotion may be involved in the activation of A1R in the PFH. The results of our second experiment suggest that pharmacologically relevant doses of ethanol locally infused in the PFH dose-dependently increase extracellular AD. Results from the third experiment suggest that acute ethanol intake inhibits orexin neurons. Based on these results, we believe that acute ethanol intake increases extracellular AD. Increased AD, in the PFH, acts on A1R to inhibit orexin neurons. Inhibition of orexin neurons is likely to promote sleep. Thus, ethanol-induced sleep promotion may be mediated by adenosinergic inhibition of orexin neurons in the PFH.
Finally, ethanol has a profound effect on sleep. In human subjects, while acute ethanol intake promotes sleep, recovering alcoholics suffer from severe insomnia along with alteration in the sleep architecture.54,55 Orexinergic neurons are intimately linked to sleep-wakefulness. Do alcoholics have impaired AD-orexin interaction, which may be responsible for sleep disruptions observed in alcoholics? Indeed, there is in vitro and in vivo evidence suggesting that chronic ethanol exposure may impair AD transport.16,17
In conclusion, our study is the first to demonstrate that the adenosinergic mechanisms interacting with orexin neurons in the PFH may play a pivotal role in mediating sleep promotion following acute ethanol intake.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by the Harry S. Truman Memorial Veterans Hospital, and funded by research grants (AA020334 & AA0174720) from National Institute of Alcohol Abuse and Alcoholism. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Harry S. Truman Memorial Veterans' Hospital for their support, Larry Propp for administrative support, Lon Dixon, VMO for animal care, Rachel Alt, Samuel Engemann, Shafi Lodhi, and Katherine Walsh for their help with data analysis.
ABBREVIATIONS
- aCSF
artificial cerebrospinal fluid
- AD
adenosine
- A1R
adenosine A1 receptor
- DPX
1,3-Dipropyl-8-phenylxanthine
- DAB
3, 3'-diaminobenzidine
- EEG
electroencephalogram
- EMG
electromyogram
- ig
intragastric
- IR
immunoreactivity
- NREM
non-rapid eye movement
- PFH
orexinergic zone of perifornical hypothalamus
- REM
rapid eye movement
REFERENCES
- 1.Gunzerath L, Hewitt BG, Li TK, Warren KR. Alcohol research: past, present, and future. Ann N Y Acad Sci. 2011;1216:1–23. doi: 10.1111/j.1749-6632.2010.05832.x. [DOI] [PubMed] [Google Scholar]
- 2.Bernd F, Susanne S, Hornyak M, Gann H, Riemann D. Sleep electroencephalographic spectral power after withdrawal from alcohol in alcohol-dependent patients. Alcohol Clin Exp Res. 2007;31:19–27. doi: 10.1111/j.1530-0277.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- 3.Brower KJ, Perron BE. Sleep disturbance as a universal risk factor for relapse in addictions to psychoactive substances. Med Hypotheses. 2010;74:928–33. doi: 10.1016/j.mehy.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong MM, Brower K, Fitzgerald HE, Zucker RA. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcohol Clin Exp Res. 2004;28:578–87. doi: 10.1097/01.alc.0000121651.75952.39. [DOI] [PubMed] [Google Scholar]
- 5.Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41:516–24. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 6.Brower KJ. Alcohol's effects on sleep in alcoholics. Alcohol Res Health. 2001;25:110–25. [PMC free article] [PubMed] [Google Scholar]
- 7.Kleitman N. Sleep and wakefulness. Chicago: The University of Chicago Press; 1963. [Google Scholar]
- 8.Dar MS, Mustafa SJ, Wooles WR. Possible role of adenosine in the CNS effects of ethanol. Life Sci. 1983;33:1363–74. doi: 10.1016/0024-3205(83)90819-6. [DOI] [PubMed] [Google Scholar]
- 9.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 10.Thakkar MM, Engemann SC, Sharma R, Sahota P. Role of wake-promoting basal forebrain and adenosinergic mechanisms in sleep-promoting effects of ethanol. Alcohol Clin Exp Res. 2010;34:997–1005. doi: 10.1111/j.1530-0277.2010.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asatryan L, Nam HW, Lee MR, et al. Implication of the purinergic system in alcohol use disorders. Alcohol Clin Exp Res. 2011;35:584–94. doi: 10.1111/j.1530-0277.2010.01379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batista LC, Prediger RD, Morato GS, Takahashi RN. Blockade of adenosine and dopamine receptors inhibits the development of rapid tolerance to ethanol in mice. Psychopharmacology (Berl) 2005;181:714–21. doi: 10.1007/s00213-005-0014-7. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan GB, Bharmal NH, Leite-Morris KA, Adams WR. Role of adenosine A1 and A2A receptors in the alcohol withdrawal syndrome. Alcohol. 1999;19:157–62. doi: 10.1016/s0741-8329(99)00033-6. [DOI] [PubMed] [Google Scholar]
- 14.Prediger RD, da Silva GE, Batista LC, Bittencourt AL, Takahashi RN. Activation of adenosine A1 receptors reduces anxiety-like behavior during acute ethanol withdrawal (hangover) in mice. Neuropsychopharmacology. 2006;31:2210–20. doi: 10.1038/sj.npp.1301001. [DOI] [PubMed] [Google Scholar]
- 15.Nam HW, Hinton DJ, Kang NY, et al. Adenosine transporter ENT1 regulates the acquisition of goal-directed behavior and ethanol drinking through A2A receptor in the dorsomedial striatum. J Neurosci. 2013;33:4329–38. doi: 10.1523/JNEUROSCI.3094-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma R, Engemann S, Sahota P, Thakkar MM. Role of adenosine and wake-promoting basal forebrain in insomnia and associated sleep disruptions caused by ethanol dependence. J Neurochem. 2010;115:782–94. doi: 10.1111/j.1471-4159.2010.06980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagy LE, Diamond I, Casso DJ, Franklin C, Gordon AS. Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J Biol Chem. 1990;265:1946–51. [PubMed] [Google Scholar]
- 18.Choi DS, Cascini MG, Mailliard W, et al. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci. 2004;7:855–61. doi: 10.1038/nn1288. [DOI] [PubMed] [Google Scholar]
- 19.Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene [see comments] Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 20.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 21.Thakkar MM, Ramesh V, Cape EG, Winston S, Strecker RE, McCarley RW. REM sleep enhancement and behavioral cataplexy following orexin (hypocretin)-II receptor antisense perfusion in the pontine reticular formation. Sleep Res Online. 1999;2:112–20. [PubMed] [Google Scholar]
- 22.Gerashchenko D, Kohls MD, Greco M, et al. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci. 2001;21:7273–83. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L, Thakkar MM, Winston S, Bolortuya Y, Basheer R, McCarley RW. REM sleep changes in rats induced by siRNA-mediated orexin knockdown. Eur J Neurosci. 2006;24:2039–48. doi: 10.1111/j.1460-9568.2006.05058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagan JJ, Leslie RA, Patel S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–6. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eggermann E, Serafin M, Bayer L, et al. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–81. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- 27.Korotkova TM, Eriksson KS, Haas HL, Brown RE. Selective excitation of GABAergic neurons in the substantia nigra of the rat by orexin/ hypocretin in vitro. Regul Pept. 2002;104:83–9. doi: 10.1016/s0167-0115(01)00323-8. [DOI] [PubMed] [Google Scholar]
- 28.Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tao R, Ma Z, McKenna JT, et al. Differential effect of orexins (hypocretins) on serotonin release in the dorsal and median raphe nuclei of freely behaving rats. Neuroscience. 2006;141:1101–5. doi: 10.1016/j.neuroscience.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 30.Thakkar MM, Ramesh V, Strecker RE, McCarley RW. Microdialysis perfusion of orexin-A in the basal forebrain increases wakefulness in freely behaving rats. Arch Ital Biol. 2001;139:313–28. [PubMed] [Google Scholar]
- 31.Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/ hypocretin, histamine and noradrenaline) J Neurosci. 2002;22:8850–9. doi: 10.1523/JNEUROSCI.22-20-08850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamanaka A, Tsujino N, Funahashi H, et al. Orexins activate histaminergic neurons via the orexin 2 receptor. Biochem Biophys Res Commun. 2002;290:1237–45. doi: 10.1006/bbrc.2001.6318. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi K, Koyama Y, Kayama Y, Yamamoto M. Effects of orexin on the laterodorsal tegmental neurones. Psychiatry Clin Neurosci. 2002;56:335–6. doi: 10.1046/j.1440-1819.2002.00967.x. [DOI] [PubMed] [Google Scholar]
- 34.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, De LL. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–4. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Methippara MM, Alam MN, Szymusiak R, McGinty D. Effects of lateral preoptic area application of orexin-A on sleep- wakefulness. Neuroreport. 2000;11:3423–6. doi: 10.1097/00001756-200011090-00004. [DOI] [PubMed] [Google Scholar]
- 36.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–98. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and nonorexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153:860–70. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 38.Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J Physiol. 2002;538:619–31. doi: 10.1113/jphysiol.2001.012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–20. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thakkar MM, Winston S, McCarley RW. Orexin neurons of the hypothalamus express adenosine A1 receptors. Brain Res. 2002;944:190–4. doi: 10.1016/s0006-8993(02)02873-1. [DOI] [PubMed] [Google Scholar]
- 41.Liu ZW, Gao XB. Adenosine inhibits activity of hypocretin/orexin neurons by the A1 receptor in the lateral hypothalamus: a possible sleep-promoting effect. J Neurophysiol. 2007;97:837–48. doi: 10.1152/jn.00873.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rai S, Kumar S, Alam MA, Szymusiak R, McGinty D, Alam MN. A1 receptor mediated adenosinergic regulation of perifornical-lateral hypothalamic area neurons in freely behaving rats. Neuroscience. 2010;167:40–8. doi: 10.1016/j.neuroscience.2010.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alam MN, Kumar S, Rai S, Methippara M, Szymusiak R, McGinty D. Role of adenosine A(1) receptor in the perifornical-lateral hypothalamic area in sleep-wake regulation in rats. Brain Res. 2009;1304:96–104. doi: 10.1016/j.brainres.2009.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thakkar MM, Engemann SC, Walsh KM, Sahota PK. Adenosine and the homeostatic control of sleep: Effects of A1 receptor blockade in the perifornical lateral hypothalamus on sleep-wakefulness. Neuroscience. 2008;153:875–80. doi: 10.1016/j.neuroscience.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 45.Thakkar MM, Winston S, McCarley RW. Effect of microdialysis perfusion of 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridine-3-ol in the perifornical hypothalamus on sleep-wakefulness: role of delta-subunit containing extrasynaptic GABAA receptors. Neuroscience. 2008;153:551–5. doi: 10.1016/j.neuroscience.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Boston: Academic Press; 1998. [Google Scholar]
- 47.Sharma R, Engemann SC, Sahota P, Thakkar MM. Effects of ethanol on extracellular levels of adenosine in the basal forebrain: an in vivo microdialysis study in freely behaving rats. Alcohol Clin Exp Res. 2010;34:813–8. doi: 10.1111/j.1530-0277.2010.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–8. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murillo-Rodriguez E, Blanco-Centurion C, Gerashchenko D, Salin-Pascual RJ, Shiromani PJ. The diurnal rhythm of adenosine levels in the basal forebrain of young and old rats. Neuroscience. 2004;123:361–70. doi: 10.1016/j.neuroscience.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Blanco-Centurion C, Xu M, Murillo-Rodriguez E, et al. Adenosine and sleep homeostasis in the Basal forebrain. J Neurosci. 2006;26:8092–100. doi: 10.1523/JNEUROSCI.2181-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ericson M, Molander A, Lof E, Engel JA, Soderpalm B. Ethanol elevates accumbal dopamine levels via indirect activation of ventral tegmental nicotinic acetylcholine receptors. Eur J Pharmacol. 2003;467:85–93. doi: 10.1016/s0014-2999(03)01564-4. [DOI] [PubMed] [Google Scholar]
- 52.Estabrooke IV, McCarthy MT, Ko E, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–62. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konadhode RR, Pelluru D, Blanco-Centurion C, et al. Optogenetic Stimulation of MCH Neurons Increases Sleep. J Neurosci. 2013;33:10257–63. doi: 10.1523/JNEUROSCI.1225-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brower KJ, Aldrich MS, Robinson EA, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry. 2001;158:399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gillin JC, Smith TL, Irwin M, Butters N, DeModena A, Schuckit M. Increased pressure for rapid eye movement sleep at time of hospital admission predicts relapse in nondepressed patients with primary alcoholism at 3-month follow-up. Arch Gen Psychiatry. 1994;51:189–97. doi: 10.1001/archpsyc.1994.03950030025003. [DOI] [PubMed] [Google Scholar]