Abstract
Study Objectives:
Permanent night-shift workers may develop shift-work disorder (SWD). In the current study, we evaluated neurophysiological and behavioral indices of distractibility across times prior to the night shift (T1), during night hours (T2), and after acute sleep deprivation (T3) in permanent hospital night workers with and without SWD.
Methods:
Ten asymptomatic night workers (NW) and 18 NW with SWD participated in a 25-h sleep deprivation study. Circadian phase was evaluated by dim-light salivary melatonin onset (DLMO). Objective sleepiness was evaluated using the Multiple Sleep Latency Test (MSLT). Electrophysiological distractibility was evaluated by brain event-related potentials (ERP), whereas behavioral distractibility was evaluated by performance on a visual task in an auditory-visual distraction paradigm.
Statistical analyses:
Comparisons of ERP results were performed by repeated-measures analysis of variance, and t-tests were used where appropriate. A Mann-Whitney U test was used for comparison of variables (MLST, Stanford Sleepiness Scale, and DLMO) that deviated from normal.
Results:
First, in the SWD group, the reorienting negativity ERP amplitude was significantly attenuated compared to that in the NW group. Second, the SWD group had shorter MSLT during night shift hours (4.8 ± 4.9 min) compared to that in NW (7.8 ± 3.7 min; U = 47; z = -2.1; P < 0.03). Third, NW with SWD had a DLMO at 20:27 ± 5.0 h, whereas healthy NW had a DLMO at 05:00 ± 3.4 h (U = 43.5; z = -2.22, P < 0.03). Finally, acute sleep deprivation impaired behavioral performance and the P3a ERP in both groups.
Conclusions:
Our results demonstrate specific deficits in neurophysiological activity in the attentional domain among the shift-work disorder group relative to night workers.
Citation:
Gumenyuk V; Howard R; Roth T; Korzyukov O; Drake CL. Sleep loss, circadian mismatch, and abnormalities in reorienting of attention in night workers with shift work disorder. SLEEP 2014;37(3):545-556.
Keywords: Shift work disorder, circadian phase, distractibility, ERPs
INTRODUCTION
Nearly 20% of the labor force worldwide, working in industries such as protective services, transportation, health care, and food preparation, have work hours that take place during the night, and thus are often exposed to the negative effects of shift work on health and safety.1–5 Night-shift work hours, which generally fall between 21:00 and 08:00, are associated with chronic shortening of sleep time (< 7 h per 24 h), which leads to increased sleepiness and deficits in function. Alterations in circadian timing that produce a mismatch between internal biological timing and the sleep-wake schedule also contribute to excessive sleepiness, impaired function, and disturbed sleep in night-shift workers.
As sleep-wake function is modulated by a circadian process and a homeostatic process,6,7 and deficits in cognitive function arise via an interaction between these two processes.8,9 For example, in well-controlled laboratory studies in healthy volunteers, cognitive function was shown to rapidly decay when circadian alertness is lowest and sleep pressure is elevated,10 even when participants have been awake for less than 16 h.6,11 In a recent study, Lo et al.12 found that subjective alertness and sustained attention were most affected by an interaction between sleep deprivation (partial and total) and circadian phase. Consistent with these results, studies on regular night workers as well as day workers asked to acutely work at night showed a rapid increase in impairment in attentional functions during night hours compared to daytime hours, measured by performance decrements and slowed response time.8,13–21 Santhi and colleagues8 also demonstrated an interaction between circadian trough and acute sleep deprivation on visual selective attention and subjective alertness during the first night of simulated shift work.
Many shift workers have a misaligned circadian rhythm with respect to their night work schedule.22,23 These night workers are at higher risk of developing shift work disorder (SWD), which is characterized by excessive sleepiness and/or sleep disturbances associated with workers' shift work schedule.24–27 However, some night workers are able to adjust their circadian rhythm to night work.28–31
The daily rhythm of melatonin secretion has been shown to be a reliable marker of an individual's circadian rhythm,32 and dim-light melatonin onset (DLMO) can be used as an indicator of circadian phase position.33 In middle-aged day-active participants, DLMO has been found to be 21:31 ± 55 min,34 and 21:41 ± 29.6 min.22 Interestingly, some night workers successfully adjust their circadian phase to accommodate their night work schedule, and manage to stay free of sleep-wake symptoms (DLMO occurs later than in day workers: 04:42 ± 3.25 h).31 Thus, the time of their biological night is delayed in the direction of daytime, when night workers normally sleep. In contrast, night workers who have not adjusted their circadian rhythm (DLMO = 20:42 ± 2.21 h) often experience the negative effects of the interaction between circadian misalignment and increased homeostatic pressure during their work shift.
In the current study, we address the differences in distractibility just before a night shift, during the night shift, and following a period of acute sleep deprivation between well-adjusted, healthy night workers (NW) and poorly adjusted NW in whom shift work disorder (SWD) was diagnosed. Generally, the effect of SWD on underlying neurophysiological processes related to attention has remained largely ignored in studies of shift workers. Understanding the neuronal mechanism of attentional deficits in SWD will offer insight into the mechanisms of occupational errors, accidents, and injuries that often occur during night hours.
According to well-established research on auditory distractibility, an auditory-visual distraction paradigm35–42 was used to identify normal and abnormal processing of an auditory distractor during the performance of a visual discrimination task. Normally, auditory distraction causes an increased response time to visual stimuli following a distracting sound compared to visual stimuli following a nondistracting sound.35 The accuracy of performance on the main (visual) task may also decrease as a result of a distracting sound momentarily capturing attention away from the primary task, whereas nondistracting sounds have a minimal distracting effect on behavioral performance.
Electrophysiologically, the brain responses to novel sounds, collectively referred to as the distraction potential, are characterized by three distinctive event-related brain potentials (ERPs).38 These brain responses are neuronal processes in the auditory modality that underlie neurophysiological distractibility and precede a behavioral response (e.g., pressing a button) in a visual distraction task. The first is the N1-enhancement, which reflects the process of change-detection, occurring at a latency of 100-150 ms; the second is the P3a, which reflects the orientation of attention to the distracting stimulus, occurring at latency of 200-350 ms; finally, the reorienting negativity (RON), which reflects the reorientation of attention back to the main task after the momentary distraction, occurs at a latency of 400-600 ms after the onset of the distracting sound.38,43 Each of these neuronal processes represents the timing and path of processing the distracting auditory information in the brain and, at the functional level, underlie how dynamic shifts of attention occur when triggered by a distracting sound.
Research on sleep and circadian-related deficits in attention is varied. However, the effect of those factors is important to know because abnormalities in distractibility may be responsible for a substantial number of accidents. Although that may relate to all workers, it is likely that abnormal distractibility in night workers, especially those with SWD, may be underpinned by a deficiency in neurophysiological processing caused by sleep and circadian disturbances, relative to night workers who are well adjusted to night work. We can also expect that the effect of a potential interaction between sleep loss and lack of circadian adjustment observed in night workers with SWD will contribute to a deficit in processing of distracting information during the night shift.
The aim of the current study was to evaluate the differences between night workers without SWD and night workers with SWD across multiple parameters: circadian rhythm, habitual sleep, sleepiness and distractibility prior to work-shift, during the work shift, and after acute sleep deprivation.
METHODS
Participants
Participants were recruited through flyers and an online newsletter in the Henry Ford Health System. Ten healthy NW (6 females, mean age: 34.9 ± 7.2 y) and 18 NW with SWD (11 females, mean age: 36.0 ± 5.7 y) participated in the study. All subjects were currently working nights (starting between 19:00-22:00 and ending between 06:30-07:30) for at least three consecutive shifts per week for at least 6 mo. All subjects were right handed, with normal hearing and vision, and free of psychiatric and neurological conditions (see Table 1 for detailed demographic and general characteristics of participants).
Table 1.
Demographic, work and clinic characteristics of study sample
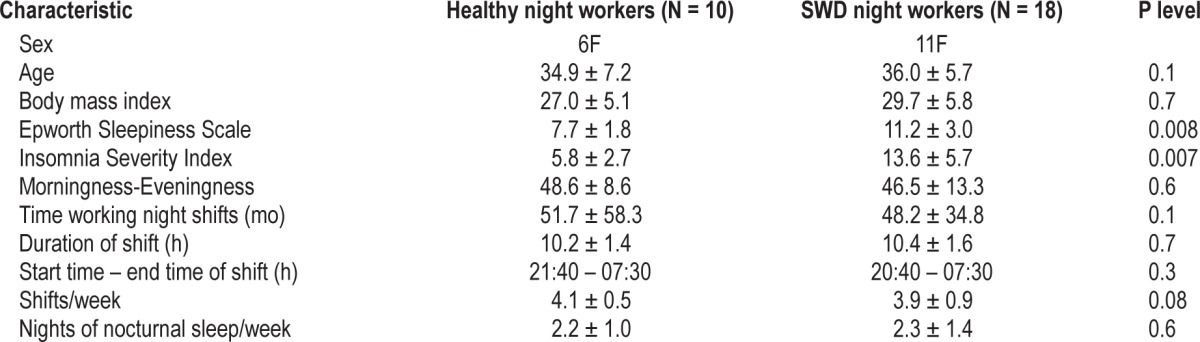
All participants were required to pass a health screen, including physical examination, an interview with a clinical psychologist, and several questionnaires: morningness-eveningness,44 Epworth Sleepiness Scale (ESS),45 Insomnia Severity Index (ISI),46 Profile of Mood States questionnaire (POMS),47 and the Berlin questionnaire to screen out sleep apnea.48 All participants were free of medications for a minimum of 2 weeks prior to the study. Exclusion criteria for participants included any sleep disorder other than SWD, head injury, hearing problems, alcohol and substance abuse, smoking, and caffeine use > 300 mg/day.
For the SWD group, the inclusion criteria for the study were: excessive sleepiness (ESS ≥ 10) and/or insomnia (ISI ≥ 11) in addition to insomnia criteria determined by Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition (DSM-IV). All subjects with SWD were diagnosed based on the results of an interview with a board-certified sleep clinician. In the NW group, participants could not have insomnia symptoms (ISI < 10) or an ESS score outside the normal range (ESS < 10; Table 1).
All participants were asked to maintain their habitual sleep schedule during the day of the study and have at least 3 consecutive work nights prior to the study. All participants were asked to record their sleep habits related to working and nonworking days in a sleep diary for 2 weeks prior to the laboratory study.
It should be noted that the sample in the current study was independent of the sample in our previous study.31
Procedures
Figure 1 illustrates the laboratory study protocol. All participants were admitted to the Henry Ford Sleep Disorders and Research Center at 16:00, where they remained for 25 h in a dimly lit (< 15 lux), sound-attenuated private suite with personal bedroom and bathroom facilities. Subjects were aware of clock time. Continuous video and audio monitoring of the bedroom was conducted throughout the study. Participants were not allowed to sleep during the entire study and had to remain out of bed, except for Multiple Sleep Latency Test (MSLT) nap trials (see next section). Technicians monitored the subjects throughout the study and prevented subjects from falling asleep by talking to them or asking them to leisurely walk around the room (∼1 m2). These walks were not more than 2 min long, and occurred at least 20 min before giving a saliva sample or an MSLT nap. For each saliva collection, all participants were seated in a comfortable chair until an adequate sample was produced. When subjects were not completing MSLT nap trials, they were allowed to sit in a comfortable chair and watch movies, drink water or juice, and eat food that was provided for them. Ten min prior to saliva collection, no food or drink was allowed and subjects were asked to rinse their mouth using clean water prior to collection.
Figure 1.
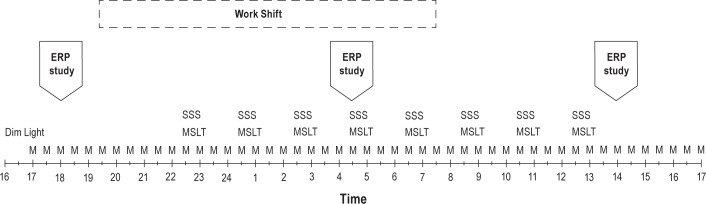
An illustration of the laboratory study protocol. M, sample for melatonin assay; SSS, Stanford Sleepiness Scale; MSLT, Multiple Sleep Latency Test; ERP, event-related brain potential study.
This protocol was approved by the institutional review board committee of Henry Ford Health System. Prior to taking part in the study, all participants were informed about the nature of the study and provided written consent. Participants were compensated for their participation.
Circadian Phase Assessment
Saliva samples were collected at 30-min intervals using a Salivette tube (Sarstedt AG & Co Nümbrecht, Germany) with a cotton insert. Prior to the study, participants were instructed about the amount of saliva needed for each collection. Each sample was weighed to ensure a minimum of 1 mL of saliva before it was centrifuged. A total of 49 frozen salivary melatonin samples per participant were sent to SolidPhase, Inc. (Portland, Maine, USA) for radioimmunoassay.
The threshold for DLMO was calculated for each individual's melatonin profile as the average of the five lowest continuous concentrations of melatonin during the phase assessment, plus 15% of the average of the five highest continuous concentrations of melatonin across the 49 samples. DLMO was defined as the time that the amplitude of the fitted LOWESS curve for melatonin concentration rose and remained above the individual's threshold for at least 1 h. The DLMOoff was the time at which LOWESS curve amplitude fell and remained below the individual's threshold for at least 2 h.31,49
Assessment of Objective and Subjective Sleepiness
Objective sleepiness was assessed using the MSLT, with eight nap sessions taking place every 2 h from 22:30 to 12:30, following standard research protocol50 (see Figure 1). Prior to each MSLT, the electrodes were routinely checked and replaced if necessary. The five MSLT naps related to work-shift hours (22:30-06:30) and last three related to acute sleep deprivation (08:30-12:30) were averaged and the means were compared between groups to evaluate objective sleepiness.
Subjective sleepiness was assessed by the Stanford Sleepiness Scale (SSS). Participants completed the SSS prior to each MSLT test (see Figure 1). The five scores of SSS (22:30-06:30) and the last three (08:30-12:30) were averaged separately and compared between groups.
Assessment of Distraction by Event-Related Brain Potential Stimuli
Participants completed three EEG/ERP sessions during the study (18:00 (T1), 04:30 (T2), and 14:00 (T3)). Each session was approximately 1 h long, and included three 6-min trials of the auditory-visual distraction task. The first ERP session (T1) evaluated neurophysiological and behavioral measures of distractibility during prework or baseline hours. The second ERP session (T2) tested the neurophysiological and behavioral changes occurring during the working hours. The final session (T3) evaluated effect of acute sleep deprivation (see Figure 1). There were additional neurophysiological assessments performed, results of which will be reported elsewhere. During the task all participants were presented with two categories of visual, equiprobable stimuli (i.e., numbers and letters; see Figure 2). Each visual stimulus was preceded by a task-irrelevant auditory stimulus that was either a pure tone (serving as a nondistracting event) or novel sound (serving as a distracting event). The interval (onset-to-onset) between auditory and visual stimuli was 300 ms.
Figure 2.
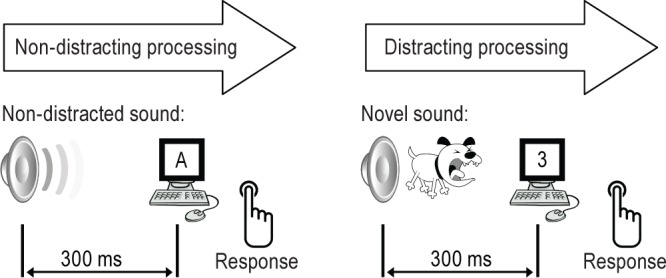
An illustration of the auditory-visual distraction paradigm. Auditory: non-distracted sounds were frequently presented pure tones intermixed with 20%- novel/distracted (environmental) sounds. Visual task consisted of 50% of letters and 50% of numbers randomly occurred on the computer screen every 1.2 sec.
Nondistracting pure tones (800 Hz, 200 ms duration) were presented frequently with a probability of P = 0.8. Distracting sounds were novel, unique, environmental sounds (such as a telephone ringing, baby crying, dog barking, etc.) with 200 ms duration, including rise/fall times of 10 ms). These novel sounds were presented randomly with a probability of P = 0.2. Each trial included 70 novel sounds drawn from a library of 200 in order to maintain the novelty of the distracting sounds. The result was that each novel sound was used only once in each trial. All auditory stimuli were presented binaurally at an intensity of 75 dB SPL via internal earphones.
The visual stimuli were white capital letters and digits (same size font) displayed randomly against a black background in the center of a computer monitor for 200 ms. The distance between the computer screen and the participant's eyes was approximately 1.5 m. Each participant was instructed to fix their gaze on the center of the screen and, as accurately as possible, press one button with their right thumb when they saw digits and another button with their left thumb when they saw letters. Instructions were also given to ignore auditory stimuli, and to avoid excessive blinking and body movements.
During this task, participants were seated in a comfortable armchair in the same sound-attenuated and light-illuminated room used throughout the study. Prior to the first recording at T1, each participant underwent a brief training session consisting of a short practice trial that included visual stimuli only. The trials were then started after the participant was familiar with the requirements of the visual task.
Electrophysiological Recordings
For each session, EEG was recorded using a 32-channel EEG cap (10-20 system, Easy Cap, Gilching, Germany) and an ASA-EEG system (ANT, Netherlands). In addition, an electrooculogram (EOG) was recorded using two electrodes attached to the left and right canthus of each eye, and two electrodes attached below and above the left eye. All impedances were kept < 10 kΩ during each session. A band-pass filter was set from 0.1 to 100 Hz, and the sampling rate was 1024 Hz.
EEG data were analyzed using Brain Vision Analyzer software (Brain Products GmbH, Gilching, Germany). ERP data were segmented separately for distracting and non-distracting stimuli, and each segment was started 100 ms prior to stimulus onset and continued for 800 ms after the stimulus onset. A bandpass filter ranging from 0.1 to 30 Hz was applied to segmented data. Segments in which the EEG exceeded ± 75μV were excluded from the average. ERPs in response to distracting and nondistracting stimuli were averaged separately. On average, at least 90 distracting and 140 nondistracting stimuli were included for each subject's grand average for each session. Baseline correction (100 ms prestimulus interval) was applied to the averaged data.
Behavioral and ERP Data Analyses
A correct response within a 1,000-ms interval after visual stimulus onset was considered as “correct”. An incorrect button press during this interval was classified as an “error,” and a response beyond the 1000 ms interval or no response was classified as a “miss.” Mean response time (RT), correct, error, and miss rates were calculated separately for distracting and nondistracting stimuli.
For each participant, the obligatory auditory N1 was identified as the largest negative waveform falling within a latency window of 80-100 ms. N1 peak amplitude was measured at the Cz electrode in ERPs to the distracting and nondistracting sounds for each EEG session.
The distraction potential was computed as a difference waveform by subtracting the ERPs elicited to nondistracting trials from those elicited by distracting trials60. Taking the grand average of these individual difference waveforms allows for identification of distraction-related brain responses: N1-enhancement, P3a, and RON. Mean amplitude for N1 enhancement was measured within a 120-150 ms latency window at the Cz electrode.
The P3a is a biphasic brain response that consists of an early (e) and late (l) phase.35 The mean amplitude of the eP3a was measured within a 220-250 ms latency window, and the lP3a was measured within a 260-320 ms latency window for each participant. Mean amplitude of the RON component was measured within a 420-470 ms latency window.
Statistical Analyses
Due to the deviations from normality in several variables (MSLT, DLMO), group comparisons of demographics, clinical characteristics, sleep diary data, MSLT, and circadian phase differences (DLMO) were performed using nonparametric Mann-Whitney U tests.
The performance and ERP data were normally distributed. To evaluate whether distracting sounds preceding visual stimuli caused a behavioral distraction in each group, RT, correct, error, and miss rates obtained at T1 were compared to those following non-distracting sounds in each group using t-tests for dependent samples.
The between-group comparison of mean RT, correct, error, and miss rates related to distracting and nondistracting stimuli were compared across T1, T2, and T3 by repeated- measures analysis of variance (ANOVA) with group (NW versus SWD) as the between and time (T1 versus T2 versus T3) as the within factors.
For the ERP analyses, the obligatory N1 peak amplitude to the distracting and non-distracting sounds measured at the Cz electrode was similarly compared using two-way repeated-measures ANOVAs with group (NW versus SWD) and time (T1 versus T2 versus T3) as factors.
The grand average of difference waves for each group was visually examined. Based on the grand-averaged data, each subcomponent of the distraction potential (N1-enhancement, eP3a, lP3a, and RON) was examined for the largest amplitude value. Electrodes with the largest amplitude of each subcomponent were selected for mean amplitude measurements and then compared using three-way repeated-measures ANOVAs with group (NW versus SWD), time (T1 versus T2 versus T3), and electrodes (C3, Cz, C4 for N1-enhancement, F3, Fz, F4, C3, Cz, and C4 for e/lP3a and Fc3, Fcz, and Fc4 for the RON) as factors.
Newman-Keuls post hoc tests were used to further specify the significance of the main effects and interactions revealed by ANOVA analyses.
Because there were no significant differences between groups on behavioral response prior to shift, during shift, or following acute sleep deprivation nor a between-group difference in RON after acute sleep deprivation, a post hoc correlational analysis was performed only on the relation between habitual total sleep time (TST)/24 h (daytime sleep) and baseline (T1) RON amplitude across both groups.
RESULTS
Table 1 presents the participant's characteristics obtained prior to the laboratory study.
Habitual Sleep
Table 2 summarizes the 2-week sleep diary data collected prior to the study for each group. Bed and wake time for daytime sleep were similar between groups 09:20 ± 1.1h and 15:40 ± 1.30h [NW] versus 09:40 ± 1.30 h and 15:09 ± 2.0 h; P = 0.6 for bedtime and P = 0.3 for wake time comparisons between groups.
Table 2.
Two-week sleep diary data obtained prior to the study (means ± SD)
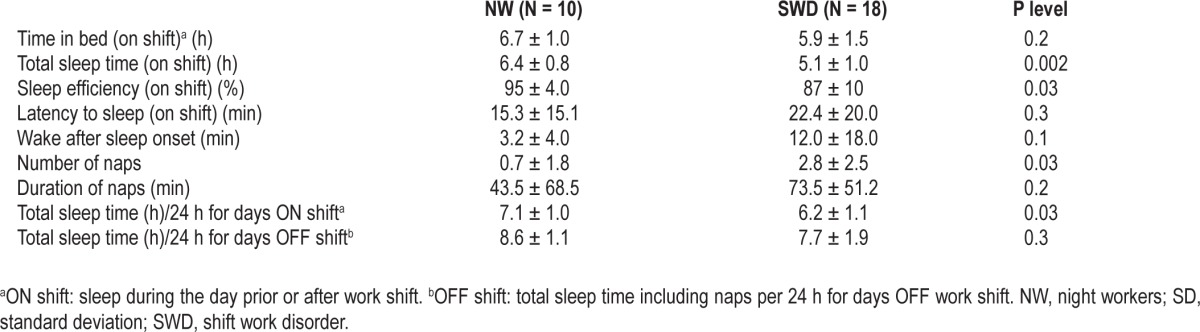
The NW group had a longer TST, higher sleep efficiency, and number of naps than night workers with SWD (see Table 2) across the 2 weeks of the diary. In addition, the NW group had relatively longer TST per 24-h period on nonworking days than workers with SWD; however, this difference was not significant (see Table 2, bottom).
For daytime sleep immediately prior to the laboratory study, participants from both groups showed similar arise times 14:10 ± 1.6 h [NW] versus 14:50 ± 1.8 h [SWD], P = 0.8.
Circadian Phase
The mean (± standard error of the mean [SEM]) of averaged LOWESS curves based on the melatonin profile for each group is presented in Figure 3.
Figure 3.
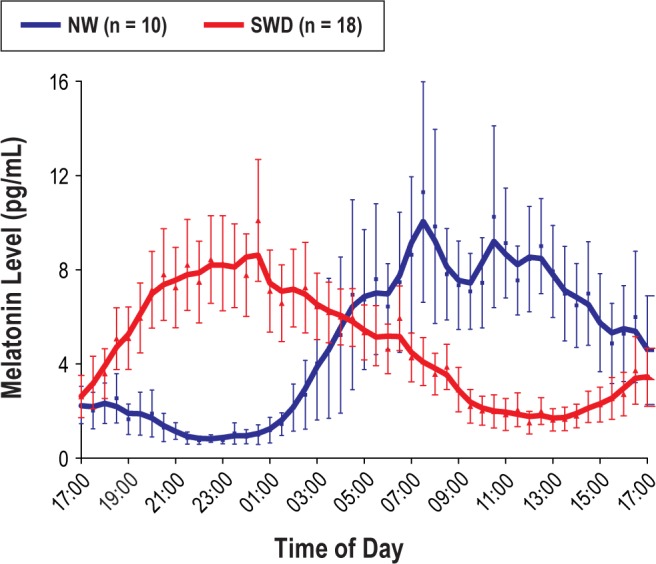
Group averaged LOWESS fit curves of salivary melatonin (means and SEM) for asymptomatic night workers (NW) and shift work disorder (SWD) groups. Time of day is a clock time when saliva ample was collected for each participant.
The timing of DLMO among the NW group was significantly delayed (mean ± standard deviation [SD] 05:00 ± 3.4 h) relative to DLMO in the SWD group (20:27 ± 5 h) (U = 43.5; z = -2.22, P < 0.03). Similarly, DLMOoff was significantly later in NW group (16:00 ± 3.1 h) compared to DLMOoff in the SWD group (08:00 ± 4.4 h) (U = 28; z = 2.5; P < 0.01).
Sleepiness
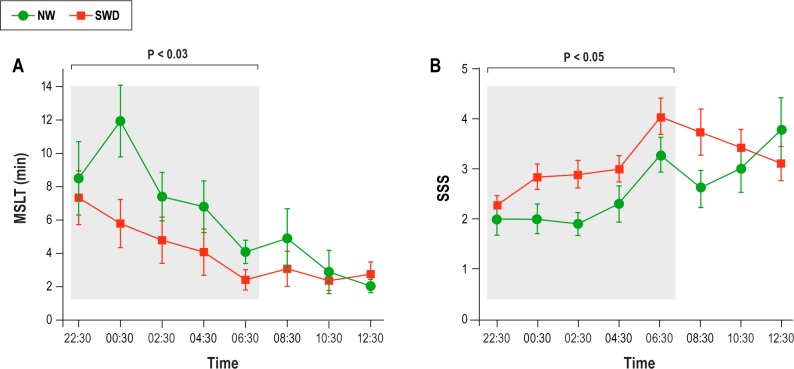
Figure 4, panel A, shows the mean ± SEM of MSLT for two groups. Mean MSLT latency across five trials (22:30-06:30) was significantly shorter in the SWD group (4.8 ± 4.9 min) than in the NW group (7.8 ± 3.7 min; U = 47; z = -2.1; P < 0.03). Mean MSLT latency across three trials following acute sleep deprivation (08:03-12:30) showed similar latency to sleep in both groups (2.6 ± 2.8 [SWD] versus 3.3 ± 2.5 [NW], P = 0.1).
Figure 4.
(A) Mean sleep latency in min for each single sleep test for each group. The means of five tests recorded between 22:30 and 06:30 was significantly different between groups (see Results). (B) Means of Stanford Sleepiness Scale scores for each group. The bars represent the errors for each measure. MSLT, Multiple Sleep Latency Test; NW, night workers; SSS, Stanford Sleepiness Scale; SWD, shift work disorder.
Figure 4, panel B, shows the mean ± SEM of SSS for the two groups across time ranging from 22:30 to 12:30 h. Statistical comparison revealed that night workers with SWD were subjectively sleepier than the NW group between 22:30 and 06:30 h (3.0 ± 0.7 [SWD] versus 2.1 ± 0.5 [NW], U = 57, z = 1.8; P = 0.05), whereas following acute sleep deprivation (08:30-12:30) both groups were not different in subjective sleepiness (3.4 ± 0.3 [SWD] versus 3.1 ± 0.6 [NW] P > 0.5) (see Figure 4, panel B).
Indices of Behavioral and Neurophysiological Distraction
Behavioral
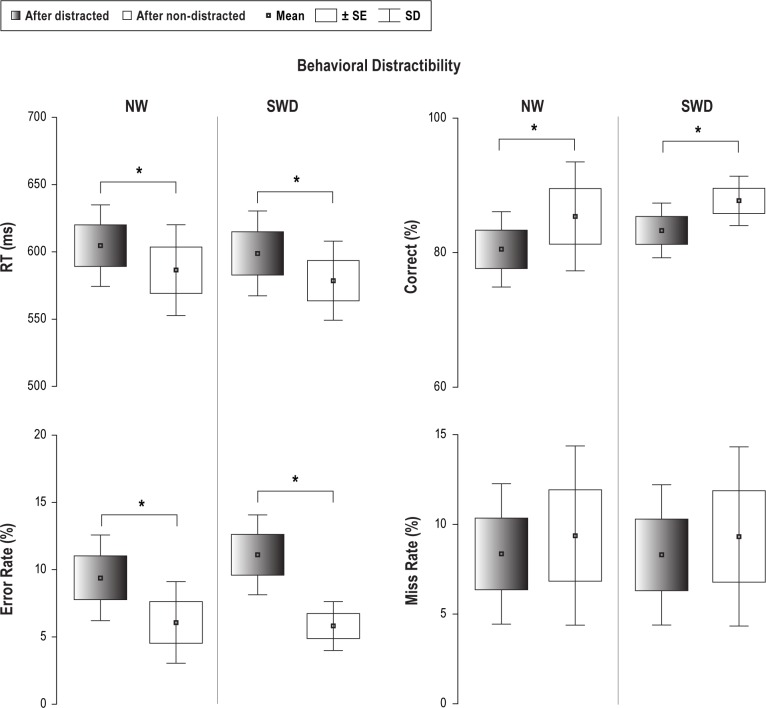
Figure 5 displays results of behavioral distractibility for both groups at T1. The effectiveness of the distraction paradigm was supported as distracting sounds presented prior to visual stimuli significantly prolonged RT in both groups relative to RT associated with nondistracting sounds (t = 5.06; P < 0.001 [NW]; t = 3.9; P < 0.002 [SWD]). The rate of correct responses to visual stimuli following distracting sounds was lower in both groups relative to nondistracting sounds (t = -3.9; P < 0.003 [NW]; t = -5.4; P < 0.001 [SWD]). Similarly, the error rate was higher in both groups after being distracted (t = 2.8; P < 0.01 [NW]; t = 2.4; P < 0.03 [SWD]). The miss rate was not significantly different between distracting and nondistracting sounds for either group.
Figure 5.
Behavioral data (means, SD and SEM) obtained in T1 session (18:00) shows similar distraction effect caused by distracted sounds with respect to non-distracted sounds in reaction time (RT), accuracy (correct rate) and errors in both groups. NW, night workers; SD, standard deviation; SEM, standard error of the mean; SWD, shift work disorder. *Indicates significance in P-values (see Results).
Reaction time in the between-group comparison showed no significant difference across T1, T2, and T3 in response to a visual task following distracting and nondistracting sounds.
There was a significant main effect of time in accuracy (F(2,50) = 8.206; P < 0.001), showing that correct responses to the visual stimuli following nondistracting sounds were decreased from 86% (± 2) in T1 to 76% (± 4), P < 0.001 in T2 and in T3 to 73% (± 4) (P = 0.08).
The error rate was significantly higher for non-distracting sounds from T1- 5.9% (± 1) to 9.4% (± 3) in T2 and 12.4% (± 4) in T3 (F(2,50) = 8.9; P < 0.002).
Both groups had more misses with prolonged wakefulness. Thus, in T1 the miss rate was 8.6% (± 2) following nondistracting sounds and 9% (± 1.3) followed distracting sounds, whereas in T2 there were 13% ± 3% and 14.8 % ± 3%. In T3 it was increased to 16% (± 2) and 18% (± 5) (F(2.50) = 4.76; P < 0.02; F(2,50) = 6.09; P < 0.004, respectively).
There were no other significant main effects or interactions observed.
Table 3 presents a summary of the statistical results. A time-of-day effect as well as the effect of acute sleep deprivation in behavioral distraction was similar between the groups.
Event-Related Brain Potentials
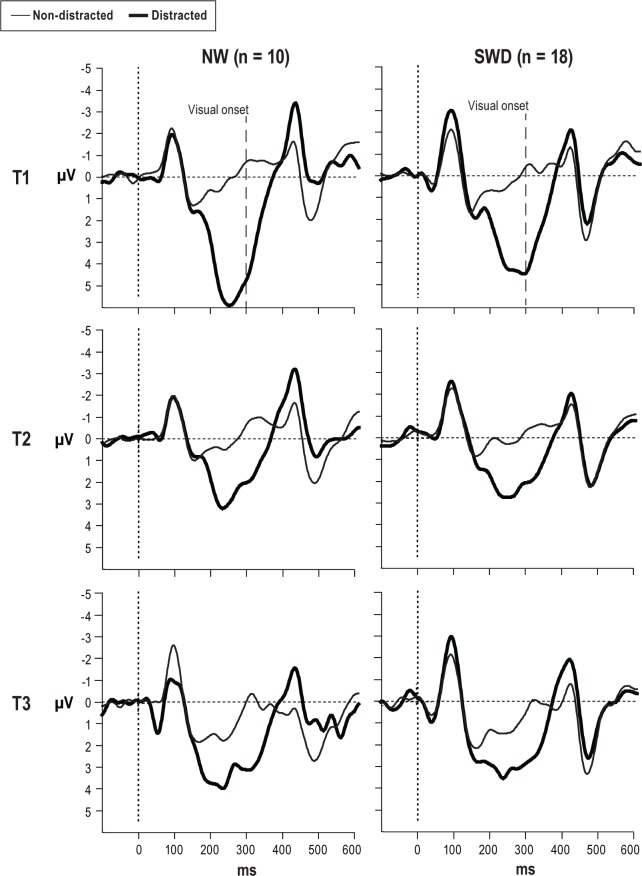
The ERPs to nondistracting and distracting sounds in both groups for all three times are presented in Figure 6. The peak amplitude of the obligatory auditory N1 elicited by distracting and nondistracting sounds was not significantly different between groups at T1, T2, or T3. However, in each group the amplitude of N1 to distracting sound was larger than N1 to nondistracting, frequently repeating sounds.
Figure 6.
Event-related brain potentials elicited to nondistracted and distracted stimuli at Fcz electrode for both groups in T1, T2, and T3 sessions. The visual stimuli onset, depicted by the dashed line, was at 300 ms. NW, night workers; SWD, shift work disorder.
Distraction Potential
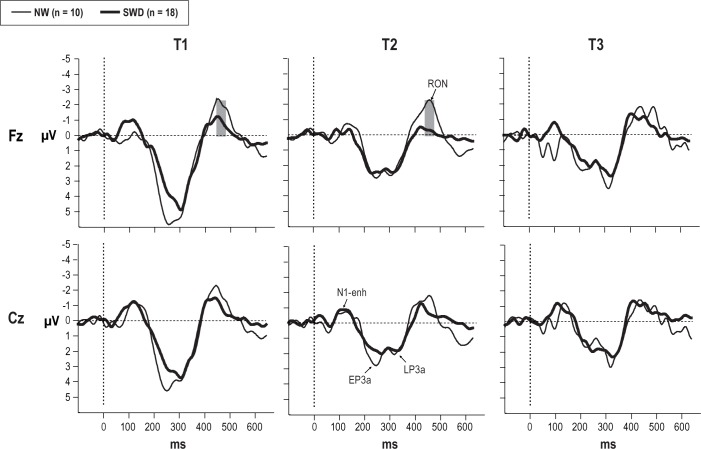
Figure 7 presents the distraction potential in both groups for each of the three sessions. The first subcomponent of the distraction potential, N1-enhancement, was not significantly different between groups and between sessions (Figure 7).
Figure 7.
The difference waveforms at Fz and Cz electrodes obtained from both groups in T1, T2 and T3 sessions. The gray highlighted the latency range for significant differences between groups in RON component. NW, night workers; RON, reorienting negativity; SWD, shift work disorder.
The subsequent P3a component was elicited by distracting sounds with two typical phases: eP3a and lP3a in both groups for all sessions. ANOVA analyses of the eP3a amplitude showed a significant main effect of electrode location (F(2,46) = 80.251; P < 0.001). Follow-up post hoc comparisons indicated that eP3a amplitude was larger over central electrodes than over frontal locations. There was a significant interaction between time and electrode locations (F(4,92) = 3.64; P < 0.008), and post hoc tests confirmed that the amplitude of the eP3a over frontal electrodes was significantly smaller at T2 and T3 relative to that at T1 (all groups combined). This analysis also revealed that eP3a amplitude was not significantly different between central locations or between sessions. There were no other significant interactions observed.
For the lP3a amplitude, a main effect of electrode location (F(2,48) = 64.226; P < 0.001) was found and subsequent post hoc tests confirmed the observation that amplitude of lP3a was greater at Fz than at Cz (P < 0.004). The main effect of time showed a significant decrease in lP3a amplitude at T2 and T3 across groups (F(2,52) = 44.94; P < 0.001).
There was a significant two-way interaction for electrode location × time (F(10,260) = 7.986; P < 0.0002; Figure 8), and post hoc tests showed that the amplitude of the lP3a at frontal (F3, Fz) and central (Cz) locations was reduced at T2 and T3 relative to that at T1. There were no other significant interactions observed.
Figure 8.
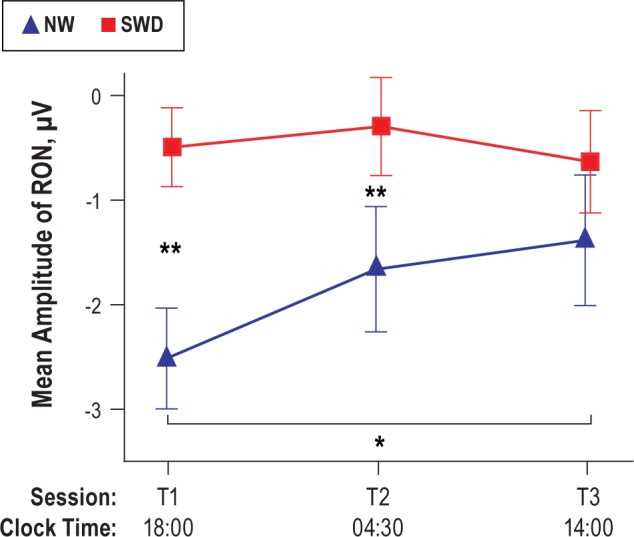
Two-way interaction (group × time) shows significant attenuation of the RON amplitude in SWD group with respect to NW group in T1 and T2 sessions. In T3, the RON was similar between two groups across three frontal electrodes. NW, night workers; RON, reorienting negativity; SWD, shift work disorder. *P < 0.03, **P < 0.004.
Following the P3a complex, a frontal-central negative-polarity waveform falling within the 420-520 ms interval was identified as the RON component in both groups (see Figure 7). The NW group had a significantly larger amplitude of RON than the SWD group (main effect of group: (F(1,26) = 21.03; P < 0.001)). A significant main effect was also found for time (F(2.52) = 3.71; P < 0.04), with a reduction in RON amplitude at T2 and T3 relative to that in T1. Figure 8 illustrates the results of a two-way interaction for group × time for RON amplitude (F(2,52) = 2.77; P < 0.05). As seen in Figure 8, RON amplitude decreased significantly at T3 with respect to T1 (P < 0.03) in the NW group. At T1 and T2, the SWD group showed significantly lower amplitude of the RON than in NW group (P < 0.004). However, after 25 h of wakefulness (T3), both groups showed similar RON amplitude. There were no other significant interactions observed.
Correlation Analysis
TST per 24-h period related to working nights was negatively correlated with RON amplitude measured at Fz in T1 (r = -0.45, P < 0.01). Thus, night workers with shorter TST/24 h had lower RON amplitude than workers with longer TST/24 h at Fz in T1.
DISCUSSION
This study identified a circadian misalignment in the SWD group (DLMO ∼20:00) as compared to the NW group (∼05:00). The total sleep time per 24 h for nonworking days was not significantly different between groups in our study (Table 2); however, the NW group showed a tendency to sleep more during nonworking days (by 0.9 h). Additionally, the SWD group slept significantly less by approximately 1 h (TST/24 h) during working days across 2 weeks than did the NW group. This ability to sleep longer during the day is potentially a consequence of the phase-delayed circadian rhythm observed in asymptomatic night workers, and may contribute to the observed neurophysiological deficits.
The current study clearly demonstrates that night workers in whom SWD has been diagnosed had reduced frontal brain activity associated with the reorienting process in comparison with asymptomatic night workers. Results of correlation analysis indicated that this deficit may be related to sleep loss, reflected by short habitual sleep time (< 6 h), specifically daytime sleep.
Previous studies involving sleep deprivation have demonstrated that sleep loss affects the cognitive functions that rely on frontal lobe activity.31,51–59 Neurophysiologically, the frontal lobe has been shown to be an essential region in the generation of activity underlying the distraction potential.60–62 Therefore, application of an auditory-distraction task in neurophysiological studies of night workers may add to the literature by providing evidence for normal and abnormal variation in frontal lobe activity in night workers.
In clinical research, abnormal neuronal activation underlying the distraction potential has been demonstrated in patients with sleep and/or circadian disturbances such as chronic alcoholism,42 obstructive sleep apnea (OSA),63 and schizophrenia.41 Interestingly, the P3a ERP component was shown to be consistently affected (either decreased or increased relative to the P3a in healthy matched controls) across all three studies evaluating the distractibility in patients, whereas N1 enhancement caused by distracting sounds was similar between patients and control subjects. Finally, the RON amplitude was shown to be significantly reduced in patients who have neurophysiological impairments in the attentional domain (e.g., schizophrenia, alcoholism) with respect to healthy participants. From these studies, it is not clear whether sleep and/or circadian disturbances are responsible, at least in part, for observed deficiencies in the orienting and reorienting of attention during the distraction process.
In the current study we found that acute sleep loss (T3) did not affect the brain activity underlying sound detection, as measured by N1 enhancement. There were no group differences in this first subcomponent of the distraction potential. These results suggest that symptomatic and asymptomatic night workers show similar activation patterns in neuronal generators underlying auditory N1 components, predominantly in the auditory cortex.64–66 And yet, these results are consistent with the results from other studies reviewed above, and showing no differences in N1 enhancement between controls and patients with sleep disturbances (e.g., OSA,63 alcoholism,42 and schizophrenia,41 using similar experimental tasks.
Although we did not find significant differences in eP3a and lP3a between groups, there was a significant reduction of the early and late phase of the P3a in T2 and T3 relative to T1 over frontal central locations. That can be explained by a sensitivity of the P3a amplitude to the acute sleep loss experienced by both groups as part of the protocol. In the literature, there is evidence demonstrating that sleep loss attenuates the activity of the frontal lobe when measured by EEG,67 by ERPs,68 and by brain imaging.69–72 Clinical impairments in the involuntary attentional network were demonstrated across several patient populations with sleep disturbances and chronically accumulated sleep debt, showing a consistent reduction of P3a amplitude in the patient group with respect to the control group.63,73
In our study, we did not find significant differences between groups in behavioral measures of distractibility prior and during night working hours. In addition, symptomatic and asymptomatic night workers responded similarly to acute sleep deprivation. The significant difference between groups found in the RON amplitude may be explained by an increased sensitivity of neurophysiological measures, compared with behavioral responses. Two independent studies using a similar paradigm on patients with OSA and alcoholics did not find significant differences between groups in behavioral performance (reaction time and accuracy); however, neurophysiological deficits in auditory distractibility preceding button response were clearly demonstrated in both studies in patient's groups relative to controls.
A recent study of simulated shift work evaluated the interaction of chronic sleep debt and circadian phase on sustained attentional performance.12 The investigators found that the deficit in sustained attention was greater during early morning hours than during the daytime or evening in participants with chronic sleep deprivation in comparison with participants who received adequate sleep (5.7 h/7 nights versus 8.56 h/7 nights).12 In our study, we found that the neuronal network responsible for the reorienting of the attentional process during distraction is most affected in night workers with sleep-wake symptoms who are poorly adjusted to night work, compared to asymptomatic night workers who are able to delay their circadian phase.
Overall, our results show that reorienting of attention is impaired in night workers with SWD. This brain component is more sensitive to habitual sleep loss than either detection (N1) or orienting (P3a) processes. These results also demonstrate specific neurophysiological deficits in the attentional domain in poorly adjusted night workers (who do not delay their circadian phase) relative to well-adjusted night workers (who manage to delay their circadian phase).
DISCLOSURE STATEMENT
This was not an industry supported study. This study is supported by grant # 1K01OH009996-02 to Dr. Gumenyuk from the National Institute of Occupational Safety and Health. Dr. Roth has received research support from Cephalon, Marck, and Transcept; has consulted for Abbott, Accadia, AstraZenca, Aventis, AVER, Bayer, BMS, Cypress, Ferrer, Glaxo Smith-Kline, Impax, Intec, Jazz, Johnson & Johnson, Merck, Neurocrine, Novartis, Proctor & Gamble, Pfizer, Purdue, Shine, Somaxon, and Transcept; and participated in speaking engagements for Purdue. Dr. Drake has received research support from Merck and Teva; has participated in speaking engagements for Teva, Purdue, and Jazz; and has consulted for Teva. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank all participants for their great contribution to that research. We thank Ms Laura Spear, RPSGT, Mr Mike Gable, RPSGT, Ms Jennifer Philport, RPSGT, Ms Anne Rojas, RPSGT, Mr Dave McCanon, RPSGT, Mr Michael Middleton, RPSGT, and Ms Julie Day, RPSGT for their professional assistance in MSLT recordings and collection of saliva samples.
REFERENCES
- 1.Purnell MT, Feyer AM, Herbison GP. The impact of a nap opportunity during the night shift on the performance and alertness of 12-h shift workers. J Sleep Res. 2002;11:219–27. doi: 10.1046/j.1365-2869.2002.00309.x. [DOI] [PubMed] [Google Scholar]
- 2.Lockley SW, Cronin JW, Evans EE, et al. Effect of reducing interns' weekly work hours on sleep and attentional failures. N Engl J Med. 2004;351:1829–37. doi: 10.1056/NEJMoa041404. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki K, Ohida T, Kaneita Y, et al. Mental health status, shift work, and occupational accidents among hospital nurses in Japan. J Occup Health. 2004;46:448–54. doi: 10.1539/joh.46.448. [DOI] [PubMed] [Google Scholar]
- 4.Dula DJ, Dula NL, Hamrick C, Wood GC. The effect of working serial night shifts on the cognitive functioning of emergency physicians. Ann Emerg Med. 2001;38:152–5. doi: 10.1067/mem.2001.116024. [DOI] [PubMed] [Google Scholar]
- 5.Folkard S, Tucker P. Shift work, safety and productivity. Occup Med (Lond) 2003;53:95–101. doi: 10.1093/occmed/kqg047. [DOI] [PubMed] [Google Scholar]
- 6.Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–7. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 7.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 8.Santhi N, Horowitz TS, Duffy JF, Czeisler CA. Acute sleep deprivation and circadian misalignment associated with transition onto the first night of work impairs visual selective attention. PLoS One. 2007;2:e1233. doi: 10.1371/journal.pone.0001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akerstedt T. Shift work and disturbed sleep/wakefulness. Occup Med (Lond) 2003;53:89–94. doi: 10.1093/occmed/kqg046. [DOI] [PubMed] [Google Scholar]
- 10.Jewett ME, Dijk DJ, Kronauer RE, Dinges DF. Dose-response relationship between sleep duration and human psychomotor vigilance and subjective alertness. Sleep. 1999;22:171–9. doi: 10.1093/sleep/22.2.171. [DOI] [PubMed] [Google Scholar]
- 11.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–63. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 12.Lo JC, Groeger JA, Santhi N, et al. Effects of partial and acute total sleep deprivation on performance across cognitive domains, individuals and circadian phase. PLoS One. 2012;7:e45987. doi: 10.1371/journal.pone.0045987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santhi N, Aeschbach D, Horowitz TS, Czeisler CA. The impact of sleep timing and bright light exposure on attentional impairment during night work. J Biol Rhythms. 2008;23:341–52. doi: 10.1177/0748730408319863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamond N, Dorrian J, Roach GD, et al. The impact of a week of simulated night work on sleep, circadian phase, and performance. Occup Environ Med. 2003;60:e13. doi: 10.1136/oem.60.11.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh JK, Muehlbach MJ, Humm TM, Dickins QS, Sugerman JL, Schweitzer PK. Effect of caffeine on physiological sleep tendency and ability to sustain wakefulness at night. Psychopharmacology. 1990;101:271–3. doi: 10.1007/BF02244139. [DOI] [PubMed] [Google Scholar]
- 16.Folkard S, Totterdell P, Minors D, Waterhouse J. Dissecting circadian performance rhythms: implications for shiftwork. Ergonomics. 1993;36:283–8. doi: 10.1080/00140139308967883. [DOI] [PubMed] [Google Scholar]
- 17.Kelly SM, Rosekind MR, Dinges DF, et al. Flight controller alertness and performance during spaceflight shiftwork operations. Hum Perf Extrem Environ. 1998;3:100–6. [PubMed] [Google Scholar]
- 18.Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Complete or partial circadian re-entrainment improves performance, alertness, and mood during night-shift work. Sleep. 2004;27:1077–87. doi: 10.1093/sleep/27.6.1077. [DOI] [PubMed] [Google Scholar]
- 19.Signal TL, Gander PH, Anderson H, Brash S. Scheduled napping as a countermeasure to sleepiness in air traffic controllers. J Sleep Res. 2009;18:11–9. doi: 10.1111/j.1365-2869.2008.00702.x. [DOI] [PubMed] [Google Scholar]
- 20.Naughton PA, Aggarwal R, Wang TT, et al. Skills training after night shift work enables acquisition of endovascular technical skills on a virtual reality simulator. J Vasc Surg. 2011;53:858–66. doi: 10.1016/j.jvs.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Chapdelaine S, Paquet J, Dumont M. Effects of partial circadian adjustments on sleep and vigilance quality during simulated night work. J Sleep Res. 2012;21:380–9. doi: 10.1111/j.1365-2869.2012.00998.x. [DOI] [PubMed] [Google Scholar]
- 22.Roden M, Koller M, Pirich K, Vierhapper H, Waldhauser F. The circadian melatonin and cortisol secretion pattern in permanent night shift workers. Am J Physiol. 1993;265:R261–7. doi: 10.1152/ajpregu.1993.265.1.R261. [DOI] [PubMed] [Google Scholar]
- 23.Madokoro S, Nakagawa H, Misaki K, Ihara H, Ito T, Isaki K. Nocturnal melatonin profiles before and one year after beginning shift-work. Psychiatry Clin Neurosci. 1997;51:17–22. doi: 10.1111/j.1440-1819.1997.tb02360.x. [DOI] [PubMed] [Google Scholar]
- 24.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–62. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 25.Waage S, Moen BE, Pallesen S, et al. Shift work disorder among oil rig workers in the North Sea. Sleep. 2009;32:558–65. doi: 10.1093/sleep/32.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Milia L, Waage S, Pallesen S, Bjorvatn B. Shift work disorder in a random population sample--prevalence and comorbidities. PLoS One. 2013;8:e55306. doi: 10.1371/journal.pone.0055306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright KP, Jr., Bogan RK, Wyatt JK. Shift work and the assessment and management of shift work disorder (SWD) Sleep Med Rev. 2013;17:41–54. doi: 10.1016/j.smrv.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Quera-Salva MA, Defrance R, Claustrat B, De Lattre J, Guilleminault C. Rapid shift in sleep time and acrophase of melatonin secretion in short shift work schedule. Sleep. 1996;19:539–43. [PubMed] [Google Scholar]
- 29.Weibel L, Spiegel K, Gronfier C, Follenius M, Brandenberger G. Twenty-four-hour melatonin and core body temperature rhythms: their adaptation in night workers. Am J Physiol. 1997;272:R948–54. doi: 10.1152/ajpregu.1997.272.3.R948. [DOI] [PubMed] [Google Scholar]
- 30.Boivin DB, James FO. Circadian adaptation to night-shift work by judicious light and darkness exposure. J Biol Rhythms. 2002;17:556–67. doi: 10.1177/0748730402238238. [DOI] [PubMed] [Google Scholar]
- 31.Gumenyuk V, Roth T, Drake CL. Circadian phase, sleepiness, and light exposure assessment in night workers with and without shift work disorder. Chronobiol Int. 2012;29:928–36. doi: 10.3109/07420528.2012.699356. [DOI] [PubMed] [Google Scholar]
- 32.Revell VL, Kim H, Tseng CY, Crowley SJ, Eastman CI. Circadian phase determined from melatonin profiles is reproducible after 1 wk in subjects who sleep later on weekends. J Pineal Res. 2005;39:195–200. doi: 10.1111/j.1600-079X.2005.00236.x. [DOI] [PubMed] [Google Scholar]
- 33.Lewy AJ, Sack RL. The dim light melatonin onset as a marker for circadian phase position. Chronobiol Int. 1989;6:93–102. doi: 10.3109/07420528909059144. [DOI] [PubMed] [Google Scholar]
- 34.Sack RL, Blood ML, Lewy AJ. Melatonin rhythms in night shift workers. Sleep. 1992;15:434–41. doi: 10.1093/sleep/15.5.434. [DOI] [PubMed] [Google Scholar]
- 35.Escera C, Alho K, Schroger E, Winkler I. Involuntary attention and distractibility as evaluated with event-related brain potentials. Audiol Neurootol. 2000;5:151–66. doi: 10.1159/000013877. [DOI] [PubMed] [Google Scholar]
- 36.Escera C, Yago E, Alho K. Electrical responses reveal the temporal dynamics of brain events during involuntary attention switching. Eur J Neurosci. 2001;14:877–83. doi: 10.1046/j.0953-816x.2001.01707.x. [DOI] [PubMed] [Google Scholar]
- 37.Escera C, Corral MJ, Yago E. An electrophysiological and behavioral investigation of involuntary attention towards auditory frequency, duration and intensity changes. Brain Res Cogn Brain Res. 2002;14:325–32. doi: 10.1016/s0926-6410(02)00135-0. [DOI] [PubMed] [Google Scholar]
- 38.Escera C, Yago E, Corral MJ, Corbera S, Nunez MI. Attention capture by auditory significant stimuli: semantic analysis follows attention switching. Eur J Neurosci. 2003;18:2408–12. doi: 10.1046/j.1460-9568.2003.02937.x. [DOI] [PubMed] [Google Scholar]
- 39.Gumenyuk V, Korzyukov O, Alho K, Escera C, Naatanen R. Effects of auditory distraction on electrophysiological brain activity and performance in children aged 8-13 years. Psychophysiology. 2004;41:30–6. doi: 10.1111/1469-8986.00123. [DOI] [PubMed] [Google Scholar]
- 40.Gumenyuk V, Korzyukov O, Escera C, et al. Electrophysiological evidence of enhanced distractibility in ADHD children. Neurosci Lett. 2005;374:212–7. doi: 10.1016/j.neulet.2004.10.081. [DOI] [PubMed] [Google Scholar]
- 41.Cortinas M, Corral MJ, Garrido G, Garolera M, Pajares M, Escera C. Reduced novelty-P3 associated with increased behavioral distractibility in schizophrenia. Biol Psychol. 2008;78:253–60. doi: 10.1016/j.biopsycho.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 42.Polo MD, Escera C, Yago E, Alho K, Gual A, Grau C. Electrophysiological evidence of abnormal activation of the cerebral network of involuntary attention in alcoholism. Clin Neurophysiol. 2003;114:134–46. doi: 10.1016/s1388-2457(02)00336-x. [DOI] [PubMed] [Google Scholar]
- 43.Schroger E, Wolff C. Attentional orienting and reorienting is indicated by human event-related brain potentials. Neuroreport. 1998;9:3355–8. doi: 10.1097/00001756-199810260-00003. [DOI] [PubMed] [Google Scholar]
- 44.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 45.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5–11. doi: 10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 46.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 47.McNair DM, Lorr M, Droppleman LF. San Diego, CA: EdITS/Educational and Industrial Testing Service; 1992. Profile of mood states. (Revised) [Google Scholar]
- 48.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 49.Lee C, Smith MR, Eastman CI. A compromise phase position for permanent night shift workers: circadian phase after two night shifts with scheduled sleep and light/dark exposure. Chronobiol Int. 2006;23:859–75. doi: 10.1080/07420520600827160. [DOI] [PubMed] [Google Scholar]
- 50.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–24. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 51.Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–29. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 52.Lim J, Wu WC, Wang J, Detre JA, Dinges DF, Rao H. Imaging brain fatigue from sustained mental workload: an ASL perfusion study of the time-on-task effect. Neuroimage. 2010;49:3426–35. doi: 10.1016/j.neuroimage.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tucker AM, Whitney P, Belenky G, Hinson JM, Van Dongen HP. Effects of sleep deprivation on dissociated components of executive functioning. Sleep. 2010;33:47–57. doi: 10.1093/sleep/33.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Groeger JA, Viola AU, Lo JC, von Schantz M, Archer SN, Dijk DJ. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. Sleep. 2008;31:1159–67. [PMC free article] [PubMed] [Google Scholar]
- 55.Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–7. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- 56.Braun AR, Varga M, Stager S, et al. Altered patterns of cerebral activity during speech and language production in developmental stuttering. An H2(15)O positron emission tomography study. Brain. 1997;120:761–84. doi: 10.1093/brain/120.5.761. [DOI] [PubMed] [Google Scholar]
- 57.Gumenyuk V, Roth T, Korzyukov O, et al. Shift work sleep disorder is associated with an attenuated brain response of sensory memory and an increased brain response to novelty: an ERP study. Sleep. 2010;33:703–13. doi: 10.1093/sleep/33.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Babajani-Feremi A, Gumenyuk V, Roth T, Drake CL, Soltanian-Zadeh H. Connectivity analysis of novelty process in habitual short sleepers. Neuroimage. 2012;63:1001–10. doi: 10.1016/j.neuroimage.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 59.Horne JA. Human sleep, sleep loss and behaviour. Implications for the prefrontal cortex and psychiatric disorder. Br J Psychiatry. 1993;162:413–9. doi: 10.1192/bjp.162.3.413. [DOI] [PubMed] [Google Scholar]
- 60.Escera C, Alho K, Winkler I, Naatanen R. Neural mechanisms of involuntary attention to acoustic novelty and change. J Cogn Neurosci. 1998;10:590–604. doi: 10.1162/089892998562997. [DOI] [PubMed] [Google Scholar]
- 61.Baudena P, Halgren E, Heit G, Clarke JM. Intracerebral potentials to rare target and distractor auditory and visual stimuli. III. Frontal cortex. Electroencephalogr Clin Neurophysiol. 1995;94:251–64. doi: 10.1016/0013-4694(95)98476-o. [DOI] [PubMed] [Google Scholar]
- 62.Chao LL, Knight RT. Prefrontal deficits in attention and inhibitory control with aging. Cereb Cortex. 1997;7:63–9. doi: 10.1093/cercor/7.1.63. [DOI] [PubMed] [Google Scholar]
- 63.Gosselin N, Mathieu A, Mazza S, Petit D, Malo J, Montplaisir J. Attentional deficits in patients with obstructive sleep apnea syndrome: an event-related potential study. Clin Neurophysiol. 2006;117:2228–35. doi: 10.1016/j.clinph.2006.07.130. [DOI] [PubMed] [Google Scholar]
- 64.Csepe V, Karmos G, Molnar M. Evoked potential correlates of stimulus deviance during wakefulness and sleep in cat--animal model of mismatch negativity. Electroencephalogr Clin Neurophysiol. 1987;66:571–8. doi: 10.1016/0013-4694(87)90103-9. [DOI] [PubMed] [Google Scholar]
- 65.Javitt DC, Schroeder CE, Steinschneider M, Arezzo JC, Vaughan HG., Jr Demonstration of mismatch negativity in the monkey. Electroencephalogr Clin Neurophysiol. 1992;83:87–90. doi: 10.1016/0013-4694(92)90137-7. [DOI] [PubMed] [Google Scholar]
- 66.Halgren E, Baudena P, Clarke JM, et al. Intracerebral potentials to rare target and distractor auditory and visual stimuli. II. Medial, lateral and posterior temporal lobe. Electroencephalogr Clin Neurophysiol. 1995;94:229–50. doi: 10.1016/0013-4694(95)98475-n. [DOI] [PubMed] [Google Scholar]
- 67.Cote KA, Milner CE, Osip SL, Baker ML, Cuthbert BP. Physiological arousal and attention during a week of continuous sleep restriction. Physiol Behav. 2008;95:353–64. doi: 10.1016/j.physbeh.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 68.Gosselin A, De Koninck J, Campbell KB. Total sleep deprivation and novelty processing: implications for frontal lobe functioning. Clin Neurophysiol. 2005;116:211–22. doi: 10.1016/j.clinph.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 69.Jones K, Harrison Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Med Rev. 2001;5:463–75. doi: 10.1053/smrv.2001.0203. [DOI] [PubMed] [Google Scholar]
- 70.Choo WC, Lee WW, Venkatraman V, Sheu FS, Chee MW. Dissociation of cortical regions modulated by both working memory load and sleep deprivation and by sleep deprivation alone. Neuroimage. 2005;25:579–87. doi: 10.1016/j.neuroimage.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 71.Drummond SP, Brown GG, Stricker JL, Buxton RB, Wong EC, Gillin JC. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport. 1999;10:3745–8. doi: 10.1097/00001756-199912160-00004. [DOI] [PubMed] [Google Scholar]
- 72.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 73.Jaaskelainen IP, Schroger E, Naatanen R. Electrophysiological indices of acute effects of ethanol on involuntary attention shifting. Psychopharmacology (Berl) 1999;141:16–21. doi: 10.1007/s002130050801. [DOI] [PubMed] [Google Scholar]