Abstract
Study Objectives:
Rapid eye movement (REM)-induced hypotonia of the major upper airway dilating muscle (genioglossus) potentially contributes to the worsening of obstructive sleep apnea that occurs during this stage. No prior human single motor unit (SMU) study of genioglossus has examined this possibility to our knowledge. We hypothesized that genioglossus SMUs would reduce their activity during stable breathing in both tonic and phasic REM compared to stage N2 sleep. Further, we hypothesized that hypopneas occurring in REM would be associated with coincident reductions in genioglossus SMU activity.
Design:
The activity of genioglossus SMUs was studied in (1) neighboring epochs of stage N2, and tonic and phasic REM; and (2) during hypopneas occurring in REM.
Setting:
Sleep laboratory.
Participants:
29 subjects (38 ± 13 y) (17 male).
Intervention:
Natural sleep, including REM sleep and REM hypopneas.
Measurement and Results:
Subjects slept overnight with genioglossus fine-wire intramuscular electrodes and full polysomnography. Forty-two SMUs firing during one or more of stage N2, tonic REM, or phasic REM were sorted. Twenty inspiratory phasic (IP), 17 inspiratory tonic (IT), and five expiratory tonic (ET) SMUs were characterized. Fewer units were active during phasic REM (23) compared to tonic REM (30) and stage N2 (33). During phasic REM sleep, genioglossus IP and IT SMUs discharged at slower rates and for shorter durations than during stage N2. For example, the SMU peak frequency during phasic REM 5.7 ± 6.6 Hz (mean ± standard deviation) was less than both tonic REM 12.3 ± 9.7 Hz and stage N2 16.1 ± 10.0 Hz (P < 0.001). The peak firing frequencies of IP/IT SMUs decreased from the last breath before to the first breath of a REM hypopnea (11.8 ± 10.9 Hz versus 5.7 ± 9.4 Hz; P = 0.001)
Conclusion:
Genioglossus single motor unit activity is significantly reduced in REM sleep, particularly phasic REM. Single motor unit activity decreases abruptly at the onset of REM hypopneas.
Citation:
McSharry DG; Saboisky JP; DeYoung P; Jordan AS; Trinder J; Smales E; Hess L; Chamberlin NL; Malhotra A. Physiological mechanisms of upper airway hypotonia during REM sleep. SLEEP 2014;37(3):561-569.
Keywords: Obstructive sleep apnea, rapid eye movement sleep, upper airway hypotonia, genioglossus, single motor unit
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is common1 and has serious sequelae such as road traffic accidents and cardiovascular disease;2–5 yet, its pathophysiology remains incompletely understood. Due to poor adherence or variable efficacy with the standard treatments, further mechanistic work is required to determine new potential treatment strategies of OSAS.6,7 The severity of OSA typically worsens during rapid eye movement (REM) sleep with respiratory events being more common, longer, and associated with more hypoxemia than during stage N2 sleep.8 Moreover, cardiac ischemia and pulmonary hypertension can occur in susceptible individuals during REM.9,10 Understanding the physiological mechanisms underlying the worsening of OSAS during REM is therefore vital and could lead to new therapeutic approaches.
During nonrapid eye movement (NREM) sleep, the major upper airway dilator muscle (genioglossus) shows increased activity in stable breathing compared to periods of cyclical breathing when obstructive apneas occur,11 suggesting that upper airway dilator muscles are both necessary and sufficient to stabilize the upper airway. Given the stabilizing properties of the genioglossus,11,12 it is reasonable to predict that diminished genioglossus activity during REM compared to stage N2 would account for the worsening of OSA during REM. Sauerland and Harper showed that multiunit genioglossus activity is reduced during REM.13 The physiological mechanisms for this genioglossus hypotonia during REM are not known. Furthermore, no studies to date have compared genioglossus single motor unit (SMU) activity in REM compared to stage N2 in humans or experimental animals.
One multiunit electromyographic (EMG) genioglossus study showed progressively worsening activity going from stage N2 to tonic REM to phasic REM.14 The exact physiological mechanisms underlying this reduced genioglossus activity are unknown, and another multiunit EMG study did not show increased activity during normal breathing between REM and NREM sleep.15 Thus, the existing multiunit literature has not fully explained the role of the genioglossus (or other factors) in the worsening of OSAS seen in REM. Therefore, through the analysis of human genioglossus SMUs, we sought to address the effects of REM sleep on the motor output of the genioglossus muscle. We examined genioglossus activity during stable breathing and during episodes of hypopneas or obstructive apneas in REM sleep. We hypothesized that SMU activity would be reduced during periods of stable breathing in REM compared to similar periods in stage N2 sleep, thus providing physiological mechanisms for upper airway vulnerability during REM. In addition, we hypothesized that hypoglossal motor output would decrease at the onset of hypopneas in REM sleep.
METHODS
Study Subjects
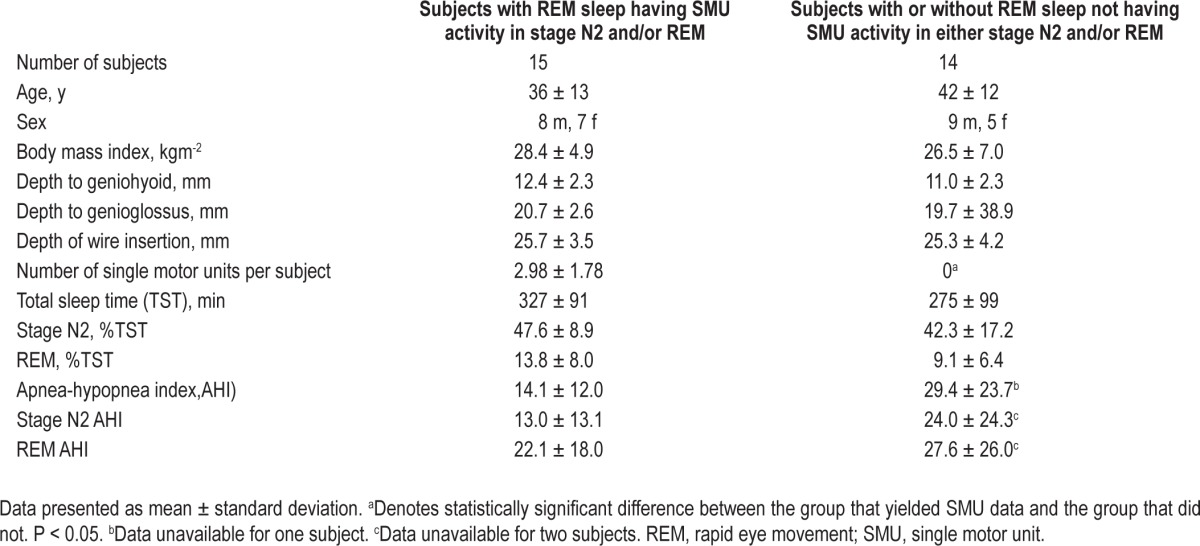
Ethics approval was granted by the Partners' Institutional Review Board (IRB). Exclusion criteria were: active cardio-respiratory disease, diabetes mellitus or other endocrine disorders, myopathy, pregnancy, neurological illness, or medications that could alter neuromuscular function (e.g., selective serotonin reuptake inhibitors or benzodiazepines). Twenty-nine human volunteers were recruited from advertisements in a local newspaper, website, and our clinical sleep clinic. Baseline demographic information for the 15 subjects who yielded appropriate data (i.e., sortable motor units during one or both of REM and stage N2) is provided in Table 1 (along with those who did not yield sortable units).
Table 1.
Demographics and polysomnography details for the subjects who yielded single motor unit data and those who did not
Materials and Procedures
Subjects arrived at our Center for Clinical Investigation 3 h before their usual bedtime. All subjects gave written informed consent. Medical assessment and physical examination was carried out by a physician. Pregnancy was excluded with a urinary human chorionic gonadotropin test in all women of childbearing capacity. Topical local anesthetic cream (lidocaine 2.5% / prilo-caine 2.5%, E. Fougera & Co., Melville, NY, USA) was applied to the skin posterior to the genial tubercle of the mandible for 30 min prior to fine-wire insertion. Ultrasonography with electronic calipers was performed in the sagittal and coronal planes to define the anatomy to ensure fine-wire electrode placement in the genioglossus (12L high-frequency linear array transducer, Vivid i GE Healthcare, Chalfont St. Giles, Bucks, UK).16
Subjects were instrumented with three electroencephalograms (F3-A2, C3-A2, O2-A1), submental EMG, left and right electrooculograms, and an electrocardiogram with surface electrodes for the duration of their overnight sleep. Subjects were fitted with a nasal mask connected to a pneumotachograph (Hans Rudolph, Shawnee, KS, USA) with differential pressure transducer for measurement of airflow and calculation of ventilation. End-tidal CO2 (ETCO2) (Vacumed, Ventura, CA, USA) was monitored from one nostril and arterial oxygen saturation (SaO2) measured with finger pulse oximetry. Respiratory effort bands (Protech ZRIP Effort Sensor, Respironics, Mukilteo, WA, USA) were placed on the chest (midsternum) and abdomen (umbilicus).
The final part of the instrumentation was conducted as follows. The local anesthetic cream was removed with sterile alcohol wipes. The subject was asked to lie flat and supine on the bed with one pillow. Fine-wire electrode insertion was conducted similar to the technique of Eastwood et al.16 A cross was drawn in the midline 10mm posterior to the genial tubercle of the mandible.16 Three 27-G needles (Franklin Lakes, Becton Dickinson, NJ, USA) were used to guide the three fine-wire electrodes into the genioglossus. Each of these guide needles was immediately removed once the fine-wire electrodes were placed in situ. The fine-wire electrodes were custom made stainless steel Teflon coated wires (0.076-mm bare, 0.14-0 mm coated, A-M Systems, Inc., Carlsborg, WA) hooked over the bevel of each needle with 0.5 mm of insulation removed. The hooked portion was approximately 3-4 mm long. The needles were inserted 90° to the skin surface to a depth determined by the ultrasound. The first needle was inserted 3-5 mm from the center of the marked cross anterolaterally. The second and third needles respectively were inserted 3-5 mm from the center of the cross posterolaterally to the left and right of the midline. The fine-wire electrodes were referenced to a surface electrode placed over the mandible (MEDI-TRACE 100 series, Kendall Healthcare, Mansfield, MA) and grounded to a large electrode placed over the right scapula (1180, 3M Health Care, St. Paul, MN). Finally, the subject's mouth was taped shut to encourage nasal breathing, enabling accurate measurement of flow measured with the pneumotachograph and ETCO2 measured via nasal probe. If the subject was reluctant to have his or her mouth taped shut, then the study proceeded without mouth tape. The subject was then given the opportunity to sleep for up to 8 h with the fine-wires electrodes in place. All subjects were instructed to sleep supine. When a subject turned on their side during sleep, he or she was awakened after 5 min in this position to facilitate their return to supine sleeping. Genioglossus EMG signals were filtered (10 Hz to 3KHz) and amplified (×1000-20,000) (Model 15, Grass Technologies, Warwick, RI, USA). All signals were recorded on a Spike 2 data acquisition system (Spike2 version 7.03 with 1401 interface, Cambridge Electronic Design, Cambridge, UK). Genioglossus EMG signals were recorded at 25 kHz; electroencephalography, electrooculography, electrocardiography, and submental EMG at 500 Hz; ETCO2, airflow and tidal volume at 125 Hz, and SaO2 at 25 Hz. All data were stored on a computer for offline analysis.
Data Analysis
Sleep Staging and Identification of SMUs
All sleep periods were staged and scored in accordance with the American Academy of Sleep Medicine criteria17,18 by a registered polysomnographic technician with airflow used in place of nasal pressure/thermistor. All sleep staging and scoring was performed while blinded to the three genioglossus EMG channels. After the formal scoring of the sleep study, one author (DMcS) chose the areas of data for analysis based on a priori defined criteria. The segments of data used for analysis were chosen from (1) stage N2 -REM (or vice versa) transitions and (2) hypopneas or apneas as follows.
(1) Data from each subject were stored in 1-h files. Each file was opened. In files that contained REM sleep, SMU activity was sought in either REM or stage N2. REM was further classified as tonic or phasic (when rapid eye movements occur). A full epoch (30 sec) of SMU data was thoroughly sorted (each discharge of every motor unit was fully characterized) in each of stage N2 and tonic and phasic REM. Data were not analyzed if they occurred during or less than 10 sec after an apnea, hypopnea or arousal. Snoring or flow limitation that did not meet criteria for an hypopnea18 did not preclude inclusion. The epochs were chosen as follows: if stage N2 occurred first then the penultimate epoch of stage N2 before REM was sorted. In this instance, the tonic REM epoch for analysis was chosen as the second epoch of REM so as to avoid potentially analyzing stage N2 because the transition is often ill defined. If phasic REM occurred in the second epoch or if an arousal, apnea, or hypopnea occurred, the first available uncomplicated 30-sec segment of tonic REM after this was analyzed. The phasic REM was chosen as the first 30 sec of phasic REM after stage N2. Ideally it was 30 sec of consecutive breaths but if this was not available (due to neighboring arousals, apneas, or hypopneas or usually not enough consecutive phasic REM), then subsequent phasic REM breaths were analyzed to ensure the equivalent of an epoch was scrutinized. Figure 1 shows an example of how we chose our data. This procedure was followed in reverse for occasions when REM occurred first. The middle three breaths of the analyzed data segments in stage N2, phasic REM, and tonic REM were further characterized by measuring detailed respiratory and SMU characteristics. If the unit was not present in a given stage, then the relevant frequency was scored as zero. If subjects breathed through their mouth, then the exact times that the SMU fired with respect to the respiratory cycle could not be calculated. The validity of the SMU frequency characteristics was unaffected by mouth breathing.
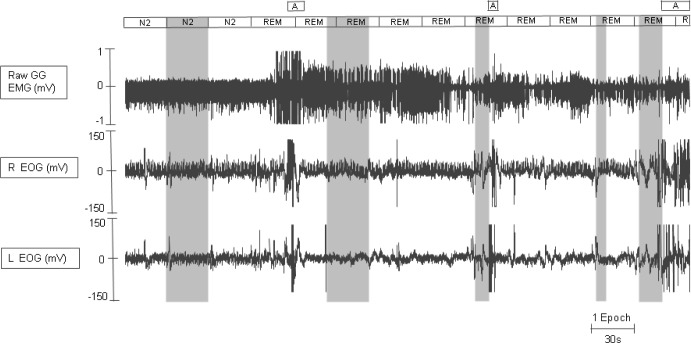
Figure 1.
The genioglossus EMG data chosen for analysis in this example was chosen as follows. As per our protocol, stage N2 data were analyzed from the penultimate epoch before REM (first shaded area going from left to right). The existence of an arousal (A) at the end of the first and beginning of the second tonic REM epoch meant we could not simply chose the second tonic REM epoch to analyze. Therefore as per protocol, tonic REM data was analyzed from > 10 seconds after this arousal (A). A full 30 sec (i.e., one epoch) of consecutive tonic REM was analyzed (see second shaded area). Finally, the phasic REM used for analysis required three separate periods of data so as to make up a 30-sec epoch. The phasic REM segements chosen had sustained rapid eye movements (see final three shaded areas coinciding with REMs). A, arousal; EMG, electromyography;. EOG, electrooculography; GG, genioglossus.
(2) The SMU activity before, during, and after hypopneas and obstructive apneas was characterized as follows: SMU activity was sorted and measured for the last two breaths before, the first two breaths of, the last two breaths of, and the two breaths after the hypopnea/apnea. If the unit did not fire during a given breath, then the relevant frequency was scored as zero.
Sorting and Classification of SMUs
Sorting is the process by which each discharge of a SMU is extracted from the raw EMG signal. The SMUs were extracted from the raw EMG signal offline using a Spike-triggered threshold (Spike 2 version 7.03 software, Cambridge Electronic Design, Cambridge, England). Spike templates were identified on the basis of the size and detailed morphology of the motor units. Every single discharge of each motor unit was manually inspected to confirm that it was classified correctly. Manual inspection was facilitated by a computer script (courtesy of the Gandevia Laboratory, Randwick, Sydney, Australia). Instantaneous discharge frequency plots were derived from the time of discharge of the unit.
The activity of each motor unit was classified based on its pattern of discharge during the respiratory cycle; determined from the volume signal.19,20 Motor units that fired throughout the respiratory cycle were classified as tonic. Motor units that fired for part of the respiratory cycle were classified as phasic. Motor units were further visually subclassified as inspiratory, expiratory, or other depending on when they fired at their peak discharge frequency. Following the visual classification of the different types of motor units, cross-correlation between volume and instantaneous firing frequency (smoothed over 200 ms) were calculated between the signals on a breath-by-breath basis. The volume signal was derived from the flow signal using a script. Figure 2 shows the instantaneous discharge frequency and the volume signal. The strength of the correlation between these measures [linear coefficient of determination (r2)] and the timing of the maximal value of this coefficient, the lag time, were calculated. Time zero is the reference point and represents the end of inspiration. Inspiratory units have a peak r2 before the end of inspiration (negative lag times), and expiratory units have a peak r2 after the end of inspiration during expiration (positive lag times). Like the other timing characteristics, the lag times were not calculated for the mouth breathers because they would likely be inaccurate and therefore potentially misleading.
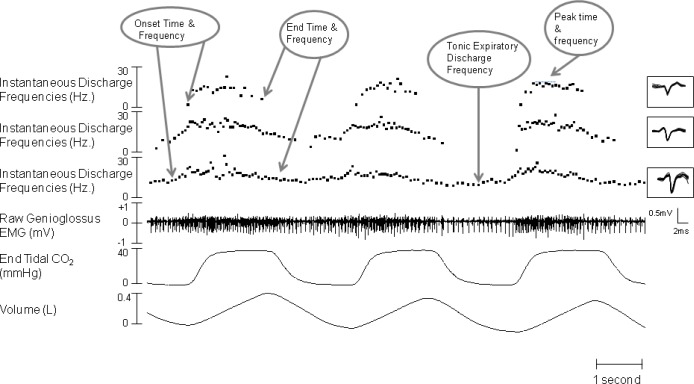
Figure 2.
Example of raw data from stage N2. This figure shows the instantaneous discharge frequency plots of the three SMUs, which were sorted and extracted from the raw genioglossus EMG signals. The overlaid detailed morphologies for each SMU are seen in the inset. The baseline tonic frequency is measured as the tonic expiratory discharge frequency averaged over 500 ms. The onset time of inspiratory tonic units refers to the time that the units start firing faster than the baseline/tonic expiratory discharge frequency. The end time of inspiratory tonic units refers to the time that the units resume firing at the baseline/tonic expiratory discharge frequency.
Measurement of Respiratory Variables and Discharge Properties of SMUs
The respiratory variables analyzed [inspiratory time (TI, s), tidal volume (liter), and ETCO2 (mmHg)] were measured over the three breaths each of stage N2, tonic REM, and phasic REM. Mean values were calculated for each of the three sleep stages yielding three data points. The instantaneous discharge frequency plots of the sorted motor unit were used to identify the discharge behavior (including discharge frequency and discharge duration). All motor unit characteristics reported were calculated from the instantaneous discharge frequency plots using Spike 2 (Spike2 version 7.03, Cambridge Electronic Design) and a custom-developed script (courtesy of the Gandevia Laboratory, Randwick, Sydney, Australia). An example of instantaneous discharge frequency plots are given in Figure 2. Discharge timing was expressed relative to the integrated flow signal and referenced to the start of inspiration. The discharge timing was calculated as a percentage of the inspiratory time (%TI). Mean motor unit discharge characteristics were measured over the three continuous breaths and averaged in stage N2, tonic REM, and phasic REM, yielding three data points that allowed comparisons between the sleep stages.
For inspiratory phasic units, the onset discharge time was measured at the first motor unit discharge for each breath and the end time was measured at the last discharge in each breath (Figure 2). For inspiratory tonic units, onset time was taken visually from when the discharge frequency first increased above the tonic levels and the end time when the discharge frequency first returned to the tonic level (Figure 1). Onset discharge frequency for inspiratory phasic units was calculated from the first interspike interval for each breath (Figure 2). The tonic level was the baseline discharge frequency of the inspiratory tonic unit. This tonic level is the tonic expiratory discharge frequency which is measured and averaged over 500 ms (Figure 2). Peak discharge frequencies were derived from the peak of the instantaneous frequency with a running average for each breath (smoothed over 200 ms; see Figure 2). The mean frequency was calculated over 200 ms.
Statistical Analysis
All results of the SMUs were compared between stage N2 and tonic REM and phasic REM by one-way repeated-measures analysis of variance using SigmaPlot (version 11) statistical software (SigmaPlot, San Jose, CA, USA). If the normality test failed, then the alternative requisite statistical test, E.G. Friedman Repeated Measures Analysis of Variance on Ranks, was performed. The number of units active in each of the three stages was statistically analyzed by chi-square analysis. When comparing data between males and females, t tests were used. When the normality test failed, then the Mann-Whitney rank-sum test was performed. The REM-related hypopnea data were analyzed using Wilcoxon signed rank tests. Data are displayed and expressed as mean ± standard deviation (SD) unless otherwise specified. Significance was P < 0.05.
RESULTS
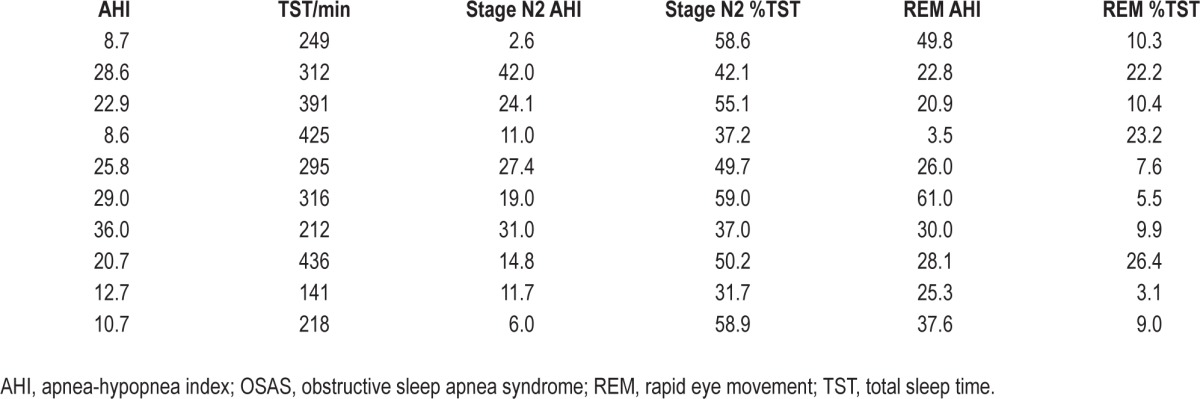
Fifteen of the 29 study subjects yielded sortable SMUs in either REM or stage N2 or both stages. The demographic and polysomnographic details of these 15 subjects and the other 14 for comparative purposes are given in Table 1. Forty-two SMUs firing during one or another of stage N2, tonic REM, or phasic REM were sorted from these 15 individuals. Twenty inspiratory phasic (IP), 17 inspiratory tonic (IT), and 5 expiratory tonic (ET) SMUs were characterized. Fewer units (23) were active during phasic REM compared to tonic REM (30) and stage N2 (33) (P > 0.05, NS). These data are presented graphically in Figure 3. Ten of the 15 subjects had OSAS and their polysomnography findings are presented in Table 2. The mean ± SD number of SMUs per subject was 2.8 ± 1.7. One subject contributed six SMUs. One subject contributed five SMUs. Four subjects each contributed four SMUs. One subject contributed three SMUs. Four subjects each contributed two SMUs. Four subjects each contributed one SMU.
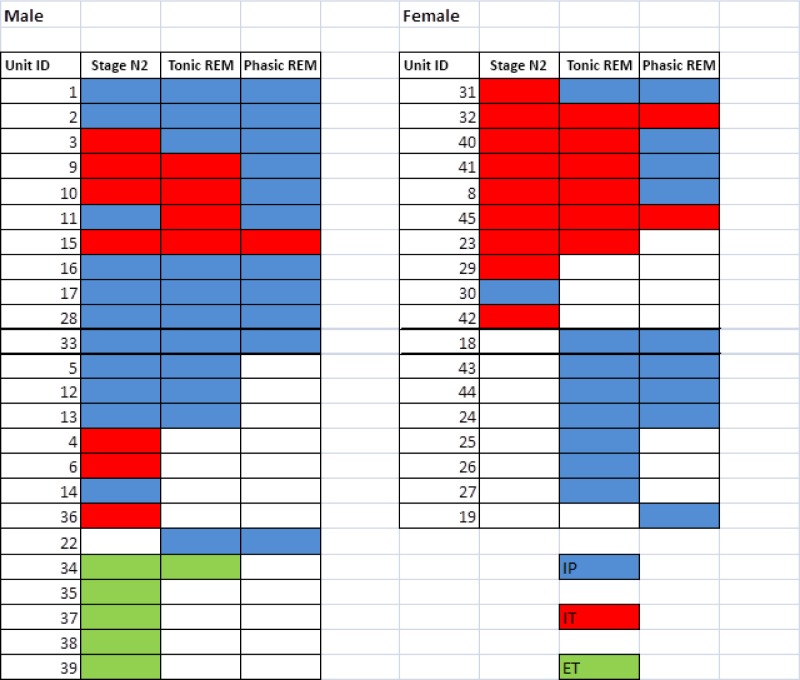
Figure 3.
A global outline of the 42 single motor units showing the stage(s) in which they were active. IP, inspiratory phasic; IT, inspiratory tonic; ET, expiratory tonic.
Table 2.
Apnea-hypopnea indices in the various sleep stages in the 10 subjects with obstructive sleep apnea syndrome
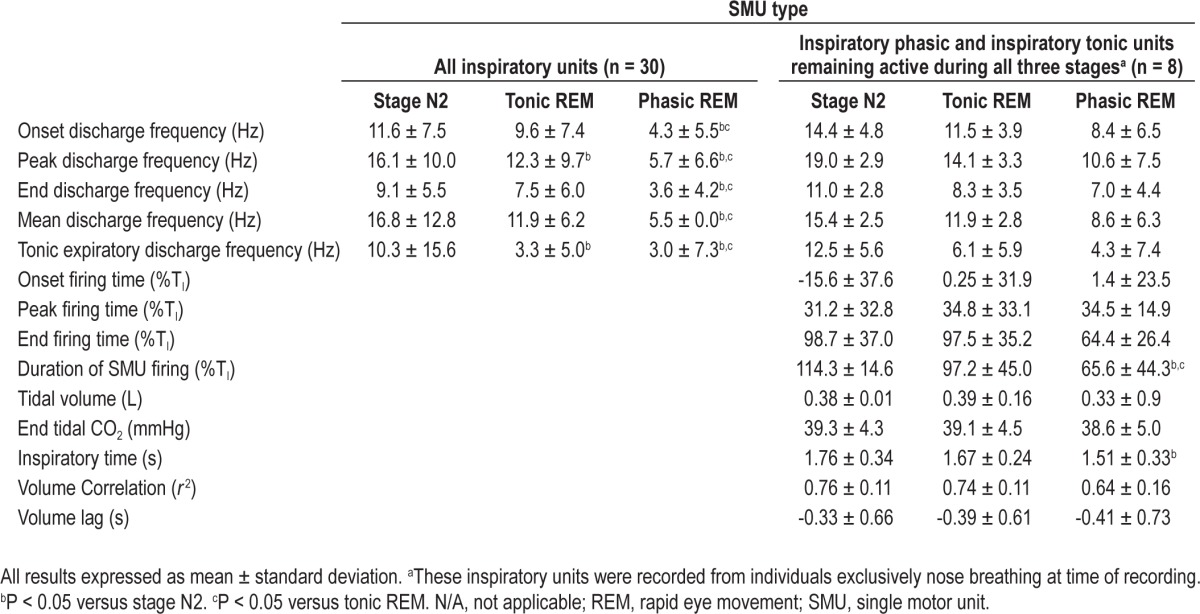
Thirty of the 37 inspiratory units (IP and IT) were fully measured. Of these 30 units, 17 were from subjects who breathed exclusively through their noses. The other seven inspiratory units were not fully measured either because the EMG signals were multiunit in part or if the unit's discharge was not sustained. Pooling these 30 measured inspiratory units together, the onset, peak, end, and mean discharge frequencies were significantly lower in phasic REM compared to both tonic REM and stage N2 (P < 0.05). The tonic expiratory discharge frequency was significantly lower in phasic REM compared to stage N2 but not compared to tonic REM. These data are presented in Table 3. Eight inspiratory units that remained active during the three stages from subjects who breathed exclusively through their noses showed a shorter duration of discharge during phasic REM compared to both tonic REM and stage N2 (65.6 ± 44.3 %TI versus 97.2 ± 45.0 %TI versus 114.3 ± 14.6 %TI; P < 0.05). These data are shown in Table 3.
Table 3.
Effect of sleep stages on single motor unit activity
In order to determine the relationship of SMU behavior with respect to obstructive apneas and hypopneas, 14 different SMUs were studied during 15 hypopneas. Twelve of these 14 SMUs were IP units and two were IT units. Genioglossus SMUs had greatly reduced activity at the onset of REM-related hypopneas. The peak discharge frequencies of inspiratory SMUs decreased from the last breath before a hypopnea to the first breath of that hypopnea (11.8 ± 10.9 Hz versus 5.7 ± 9.4 Hz; P = 0.001). At the end of the hypopnea the peak discharge frequencies of inspiratory SMUs increased from the last breath of the hypopnea to the first breath after the hypopnea (4.9 ± 7.5 Hz versus 9.9 ± 8.3 Hz; P = 0.003).
Seven of the 15 subjects who provided sortable SMUs were female. During both tonic and phasic REM, the SMUs behaved similarly in men and women both in terms of overall activity (Figure 3) and firing frequencies. For example, during phasic REM the peak frequencies in males were 5.9 ± 6.9 Hz compared to 5.4 ± 6.2 Hz in females (P = 0.93). During stage N2, the peak frequencies were higher in males than females (19.2 ± 8.8 Hz versus 11.4 ± 10.3; P = 0.04).
DISCUSSION
Overall these data show diminished genioglossus activity during REM (particularly phasic REM) as evidenced by fewer SMUs being active, reduced discharge frequencies, and shorter firing durations. These state-dependent changes likely account for the clinically important hypotonia and the worsening of OSAS during REM sleep. Further support for this idea comes from our discovery of the direct and, we hypothesize, causal relationship between a drop in genioglossus SMU activity coincident with the onset of the REM hypopnea/obstructive apnea (Figure 4).
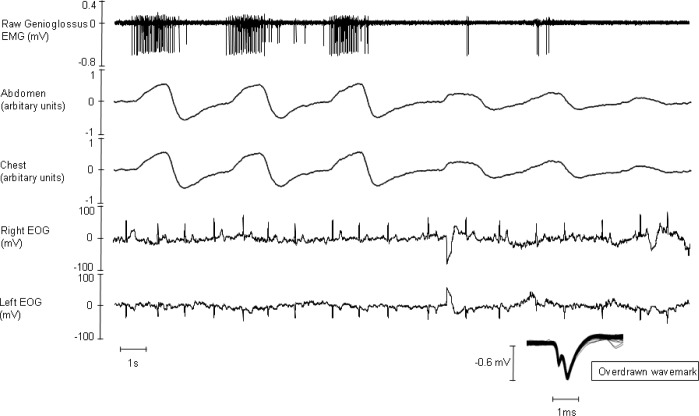
Figure 4.
Raw data from a single subject encapsulate the key findings of our study. Our study shows that the activity of the genioglossus is greatly diminished in phasic rapid eye movement (REM). Also, during REM-related hypopneas genioglossus (GG), single motor unit (SMU) activity decreases and is coincident with the onset of the hypopnea. In this example, REM heralds an immediate reduction in GG SMU activity and with it a hypopnea.
Our findings are novel and are important because understanding the neurochemistry of these units through animal experiments may lead to new therapeutic targets in OSAS and because REM sleep is a potentially hazardous time for some people.21,22 Increased cardiac ischemia and pulmonary hypertension9,10 can occur more commonly during REM than other sleep stages in susceptible individuals. Figure 4 presents raw data from one of our subjects and illustrates all of our key findings succinctly. In this example, REM heralds a precipitous drop in genioglossus SMU activity and with it a hypopnea. Our findings are consistent with those of our multiunit study (conducted with participants receiving nasal continuous positive airway pressure), which showed that genioglossus activity is reduced from stable NREM, to tonic REM to phasic REM sleep.14 A potential limitation of our study was that we had to exclude the timing characteristics (such as onset firing time) in the subjects who breathed through their mouth. The exact timing of SMU activity with respect to inspiration cannot be definitively measured in mouth breathers because the pneumotachographs are attached to their noses.
Genioglossus SMU techniques are being increasingly used in upper airway physiology studies, providing insight into the neural output of the hypoglossal motor nucleus. No study had compared genioglossus SMU activity in stage N2 to REM prior to our current study. Wilkinson et al. showed that there was a marked derecruitment of inspiratory SMUs at sleep onset, but expiratory and tonic unit were unaffected by the state transition.23 These authors speculated that upper airway patency during sleep was dependent on the stiffening properties of expiratory and tonic units. We fully defined five expiratory units that were inactive in phasic REM when the upper airway is at its most vulnerable. Our findings, albeit from a small number of units, are consistent with those of Wilkinson et al. Other studies using SMU techniques have shown that upper airway dilating muscles have a preference for recruitment/derecruitment rather than rate coding particularly in response to rising ETCO2.24,25 Our study showed that in REM-reduced numbers of active units, discharge frequencies and discharge durations all are important physiological mechanisms of hypotonia in this sleep stage. Therefore, behavioral state and respiratory stimuli have qualitatively different effects.
REM-related sleep disordered breathing is common in women.26 There were no significant differences between women and men with respect to body mass index, apnea-hypopnea index, or age. We noted no significant differences in SMU activity between women and men during REM sleep, although the peak discharge frequency was higher in men during stage N2. Therefore, a factor other than diminished genioglossus activity is likely to be responsible for the female preponderance of REM-related apneas. However, we acknowledge that our study was underpowered for subgroup analyses, emphasizing the need for further research.
The cause of the reduced motor output of the genioglossus we have shown particularly in phasic REM is unclear. No significant differences were seen in ETCO2 between sleep stages. Moreover, changes in ETCO2 are unlikely to account for the observed changes in firing rate. More likely, state-dependent changes in inputs to genioglossus motoneurons,27 such as the recently described flip-flop switch (which is composed of gamma-aminobutyric acidergic and glutaminergic neurons), suppress hypoglossal motor nucleus output21 coincident with REM sleep. Disfacilitation due to loss of excitatory inputs is a favored hypothesis.27 However, Grace et al. recently showed that genioglossal hypotonia in rats is most likely mediated by REM-related inhibition by acetylcholine acting on G-protein-coupled inwardly rectifying potassium channels.22 Neither the source of this cholinergic input nor the nature of interactions between cholinergic, noradrenergic, and other excitatory neurochemicals is understood. Our physiological findings explaining decreased genioglossus activity during REM may help to sort this out.
In summary, we have shown reduced activity of genioglossus inspiratory and expiratory SMUs during REM. This hypotonia likely causes the OSAS as further evidenced by the significant decrease in firing frequencies coincident with the start of REM hypopneas. Defining the neurochemistry of these SMUs in animal models could yield new therapeutic targets.
DISCLOSURE STATEMENT
This was not an industry supported study. The following grants provided support for this study: Dr. Malhotra is PI on AHA 0840159N, NIH R01 HL090897, NIH K24 HL 093218, NIH 1 P01 HL 095491 (Overall PI: Saper, Brigham PI: Malhotra), NIH R01HL110350, NIH UM1HL108724 (Overall PIs: Talmor/Loring, Brigham PI: Malhotra), NIH R01- AG035117, NIH R01 HL085188. The Harvard Catalyst is funded by UL1 RR 025758-01. David McSharry was PI on AHA 11POST5660004. Dr. Malhotra has received consulting and/or research income from Philips Respironics, Pfizer, SHC, SGS, Apnex, Apnicure, but had no personal outside income following May 2012. Dr. Jordan consulted for Apnex Medical from 2009-2010. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the research subjects and the nursing staff of the Brigham and Women's Hospital Center for Clinical Investigation for their help during the study nights. We acknowledge Andrea Carusona and Alison Foster for recruiting study subjects, Lisa Campana for writing computer scripts, Ellen Young for administrative support, and Jessie Bakker for statistical advice.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 3.McNicholas WT, Bonsigore MR. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–78. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 4.Mulgrew AT, Nasvadi G, Butt A, et al. Risk and severity of motor vehicle crashes in patients with obstructive sleep apnoea/hypopnoea. Thorax. 2008;63:536–41. doi: 10.1136/thx.2007.085464. [DOI] [PubMed] [Google Scholar]
- 5.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 6.Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15:365–9. doi: 10.1155/2008/534372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lojander J, Maasilta P, Partinen M, Brander PE, Salmi T, Lehtonen H. Nasal-CPAP, surgery, and conservative management for treatment of obstructive sleep apnea syndrome. A randomized study. Chest. 1996;110:114–9. doi: 10.1378/chest.110.1.114. [DOI] [PubMed] [Google Scholar]
- 8.Findley LJ, Wilhoit SC, Suratt PM. Apnea duration and hypoxemia during REM sleep in patients with obstructive sleep apnea. Chest. 1985;87:432–6. doi: 10.1378/chest.87.4.432. [DOI] [PubMed] [Google Scholar]
- 9.Schafer H, Koehler U, Ploch T, Peter JH. Sleep-related myocardial ischemia and sleep structure in patients with obstructive sleep apnea and coronary heart disease. Chest. 1997;111:387–93. doi: 10.1378/chest.111.2.387. [DOI] [PubMed] [Google Scholar]
- 10.Niijima M, Kimura H, Edo H, et al. Manifestation of pulmonary hypertension during REM sleep in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1999;159:1766–72. doi: 10.1164/ajrccm.159.6.9808064. [DOI] [PubMed] [Google Scholar]
- 11.Jordan AS, White DP, Lo YL, et al. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep. 2009;32:361–8. doi: 10.1093/sleep/32.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng S, Butler JE, Gandevia SC, Bilston LE. Movement of the tongue during normal breathing in awake healthy humans. J Physiol. 2008;586:4283–94. doi: 10.1113/jphysiol.2008.156430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol. 1976;51:160–70. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- 14.Eckert DJ, Malhotra A, Lo YL, White DP, Jordan AS. The influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest. 2009;135:957–64. doi: 10.1378/chest.08-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okabe S, Hida W, Kikuchi Y, Taguchi O, Takishima T, Shirato K. Upper airway muscle activity during REM and non-REM sleep of patients with obstructive apnea. Chest. 1994;106:767–73. doi: 10.1378/chest.106.3.767. [DOI] [PubMed] [Google Scholar]
- 16.Eastwood PR, Allison GT, Shepherd KL, Szollosi I, Hillman DR. Heterogeneous activity of the human genioglossus muscle assessed by multiple bipolar fine-wire electrodes. J Appl Physiol. 2003;94:1849–58. doi: 10.1152/japplphysiol.01017.2002. [DOI] [PubMed] [Google Scholar]
- 17.Rechtschaffen A, Kales A. Washington: US Government Printing Office, Public Health Service; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 18.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 19.Saboisky JP, Butler JE, Fogel RB, et al. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol. 2006;95:2213–21. doi: 10.1152/jn.00940.2005. [DOI] [PubMed] [Google Scholar]
- 20.Saboisky JP, Butler JE, McKenzie DK, et al. Neural drive to human genioglossus in obstructive sleep apnoea. J Physiol. 2007;585:135–46. doi: 10.1113/jphysiol.2007.139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–94. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 22.Grace KP, Hughes SW, Horner RL. Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am J Respir Crit Care Med. 2013;187:311–9. doi: 10.1164/rccm.201209-1654OC. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson V, Malhotra A, Nicholas CL, et al. Discharge patterns of human genioglossus motor units during sleep onset. Sleep. 2008;31:525–33. doi: 10.1093/sleep/31.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson PA, Bailey EF. Tonically discharging genioglossus motor units show no evidence of rate coding with hypercapnia. J Neurophysiol. 2010;103:1315–21. doi: 10.1152/jn.00686.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholas CL, Bei B, Worsnop C, et al. Motor unit recruitment in human genioglossus muscle in response to hypercapnia. Sleep. 2010;33:1529–38. [PMC free article] [PubMed] [Google Scholar]
- 26.Conwell W, Patel B, Doeing D, et al. Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath. 2012;16:519–26. doi: 10.1007/s11325-011-0537-6. [DOI] [PubMed] [Google Scholar]
- 27.Horner RL. The tongue and its control by sleep state-dependent modulators. Arch Ital Biol. 2011;149:406–25. doi: 10.4449/aib.v149i4.1256. [DOI] [PubMed] [Google Scholar]