Abstract
Background:
Pediatric obstructive sleep apnea (OSA) is associated with neurocognitive deficits. However, the neural substrates underlying such deficits remain unknown.
Methods:
To examine executive control and emotional processing in OSA, 10 children age 7 to 11 y with polysomnographically diagnosed OSA and 7 age- and sex-matched controls underwent a color-word Stroop task and an empathy task consisting of dynamic visual scenarios depicting interpersonal harm or neutral actions in a magnetic resonance imaging (MRI) scanner. Functional MRI data were processed using MATLAB 7.12 with SPM8 for region of interest (ROI) analyses, and a general linear model was used with regressors for each trial type in each task.
Results:
For the Stroop task, accuracy was similar in the two groups, with no differences in the effect of incongruency on success rates. OSA showed greater neural activity than controls in eight ROI clusters for incongruent versus congruent trials (P < 0.001). Within the a priori ROIs, the anterior cingulate cortex was significantly different between groups (P < 0.05). For perceiving harm versus neutral actions, ROI analysis revealed a significant correlation between apnea-hypopnea index and left amygdala activity in harm versus neutral actions (r = -0.71, P < 0.05).
Conclusions:
These results provide the first functional MRI evidence that cognitive and empathetic processing is influenced by obstructive sleep apnea (OSA) in children. Children with OSA show greater neural recruitment of regions implicated in cognitive control, conflict monitoring, and attentional allocation in order to perform at the same level as children without OSA. When viewing empathy-eliciting scenarios, the severity of OSA predicted less sensitivity to harm in the left amygdala.
Citation:
Kheirandish-Gozal L; Yoder K; Kulkarni R; Gozal D; Decety J. Preliminary functional mri neural correlates of executive functioning and empathy in children with obstructive sleep apnea. SLEEP 2014;37(3):587-592.
Keywords: Pediatric sleep apnea, executive function, empathy, fMRI
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) in children is a highly prevalent condition in which intermittent collapse of the upper airway during sleep leads to recurrent hypoxic events, intermittent elevations in carbon dioxide levels, sleep fragmentation, and reduced sleep efficiency.1 Children with OSAS exhibit increased risk for altered executive function and mood disturbances, particularly anhedonia, potentially resulting in maladaptive daytime behaviors and poor school performance.2–6 In the context of cognitive deficits in pediatric OSAS, changes in brain metabolic function and systemic inflammatory responses have been identified as determinants of such adverse outcomes.7,8 However, despite theoretical assumptions and animal-based evidence suggestive of structural and functional deficits within specific brain regions in pediatric OSAS,9,10 the neural correlates of behavioral and cognitive problems in children with OSAS remain unknown.
Studies using functional MRI (fMRI) in adults with OSAS have shown that alterations in working memory are associated with decreased activation in specific brain regions associated with such tasks, particularly in the prefrontal cortex,11–13 suggesting the presence of significant neural injury that may be potentially reversible with treatment.14–17 However, additional studies have indicated the presence of increased, rather than decreased, activation of neural regions during memory or attention-related tasks,18–21 which could reflect the need for increased recruitment of more extensive brain networks to perform specifically susceptible tasks, either adequately or even suboptimally.
Although we are unaware of any fMRI studies in children with OSAS, preliminary evidence derived from geodesic-evoked potential arrays suggests expanded regional recruitment within the prefrontal cortex among children with OSAS during performance of an oddball attention task.22 Therefore, we hypothesized that children with OSAS who display otherwise cognitively preserved cognitive performances in standardized cognitive tests would display enhanced fMRI activation patterns during an executive function task. In addition, given that sleep deficits or sleep disorders may also affect emotional processing and mood,23,24 we also sought to examine potential differences in functional neural activation in response to empathy-eliciting scenarios, a function that is commonly affected in depression and other mood disorders, using a task that has been previously validated with normally developing children.
PATIENTS AND METHODS
Participants
Children being evaluated for suspected OSAS in the pediatric sleep medicine center were invited to participate. Ageand sex-matched nonsnoring controls were recruited from the community as part of a National Institutes of Health-supported project (HL065270). The enrolled sample was representative of the general population living in neighborhoods on the south side of Chicago, and socioeconomic status, sex, and ethnicity were matched between the two groups. Parental informed consent and child assent were obtained. The study was approved by the Institutional Review Board (protocol # 11-0280-CR002).
Inclusion criteria for the OSAS group were: suspected OSAS at physician visit and confirmation of OSAS by polysomnography (PSG) and age 7 to 11 y, such as to enable improved participation in fMRI procedures. Inclusion criteria for controls included absence of OSAS or snoring on screening questionnaire and normal PSG. Exclusion criteria for both groups included preexisting medical, behavioral, or learning disorders indicated by screening questionnaires; medications within 3 days of protocol; body mass index over 95th percentile; failure to pass hearing or vision screening; failure to pass IQ and behavioral screening tests (Peabody Picture Vocabulary Test III [PPVT-III] and “at-risk” behavioral scores on the Child Behavior Checklist [CBCL]; see next paragraphs).
Overnight PSG
All children underwent full, in-laboratory PSG by study-certified technicians according to a standardized protocol25 and following American Academy of Sleep Medicine (AASM) guidelines.26 Scoring was performed according to the AASM pediatric criteria, by certified technologists. The obstructive apnea-hypopnea index (AHI) was defined as the sum of all obstructive apneas plus hypopneas associated with a 50% reduction in airflow and either a > 3% desaturation or electroencephalographic (EEG) arousal, divided by hours of total sleep time. The diagnosis of children with OSAS was defined by the presence of an obstructive AHI ≥ 2/h of total sleep time (hTST) and a nadir oxyhemoglobin saturation < 92% or a respiratory arousal index > 2/rTST. Control children had AHI < 1/hTST and no oxygen desaturations events during sleep.
Peabody Picture Vocabulary Test-III
The PPVT-III27 was used as an individually administered IQ-screening tool with high construct validity in which children select one of four pictures that best correspond to the word the tester read. Raw scores are transformed to standard scores, based around a mean of 100 and a standard deviation of 15. Correlations between PPVT-III scores and Wechsler Intelligence Scale for Children-Third Edition range around 0.82 to 0.92.28
Child Behavior Checklist-Revised
The CBCL screened for behavior disorders by parental report of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) symptoms using the forms designed for children age 6-18 y.29
Mock Scanner Training
Before entering MRI scanning, participants were trained on the tasks in a mock scanner. All participants were asked to lie down in the mock scanner and were exposed to recordings of scanner noise. After they felt comfortable, participants were introduced to the color-word Stroop task and practiced until they could correctly respond to six consecutive trials of mixed congruent and incongruent stimuli. After successful training on the Stroop task, participants were shown three examples each of the harm and action scenes. Participants were also acquainted with the empathy task.
Stroop Task
The color-word Stroop task30 consisted of presenting three color words (“red,” “green,” “blue”) with letters in red, green, or blue font. Participants were shown a word and asked to indicate the color of the letters by pressing one of three buttons on a button box. Words were displayed at the center of the screen on a white background. In an event-related design, congruent and incongruent trials were presented randomly. Half of all trials were incongruent. All word stimuli remained on the screen for 2.2 sec and were followed by a fixation cross with a jittered duration (2.13 ± 0.68 sec). While in the scanner, participants were shown 96 color-word combinations in two separate runs (264 sec per run). Stimuli for both tasks were presented using E-Prime software (Psychology Software Tools, Inc., Sharpsburg, PA) and viewed on a back-projection screen.
Empathy Task
For the empathy task, participants viewed 60 dynamic visual scenarios. Stimuli were a subset of those used in previous fMRI studies with children.31,32 Thirty of the scenes depicted interpersonal harm (harm) and 30 scenes showed neutral actions that did not involve harm (neutral actions). Both harm and control scenarios involved two individuals interacting. Participants were instructed to lie still and pay attention to the people in the scenes. Each scene lasted 2.2 sec and consisted of three successive digital color photographs (720 × 405 pixels) that were presented to imply motion (1000, 200, and 1000 ms). Stimuli of the same class were presented in blocks. In each block, six scenes were interspersed with a jittered fixation cross (2.2 ± 2.7 sec). Participants viewed each scene twice across four runs (212 sec per run). Within each run, five task blocks were presented in pseudorandom order, interspersed with rest blocks of 11 sec each.
MRI Scanning
Participants were scanned with a 3T Philips Achieva Quasar scanner (Philips, Eindhoven, The Netherlands) at the University of Chicago Brain Research Imaging Center. For functional runs, contiguous axial slices of 4-mm thickness were acquired using a single-shot Echo-Planar Imaging (EPI) sequence (repetition time [TR] = 2000 ms; echo time [TE] = 25 ms; flip angle = 77o; field of view [FOV] = 224 mm × 224 mm; matrix = 64 × 64; 3.5 mm × 3.5 mm × 4 mm voxel size). A T1 -weighted three-dimensional turbo field echo- Magnetization Prepared RApid Gradient Echo (TFE/MP-RAGE) sequence (TR = 8.1 ms; TE = 3.7 ms; matrix = 224 × 224; 1-mm isotropic voxels) was used to acquire high resolution anatomical images.
MRI Processing
MRIs were processed using MATLAB 7.12 (Mathworks Inc., Sherborn, MA) with a statistical parametric map (SPM8, Wellcome Department of Imaging Neuroscience, London, UK). The ArtRepair toolbox33 was used to identify and repair slices containing spikes. These repaired EPI were then realigned to the mean functional image, normalized to the EPI template, and smoothed with a gaussian smoothing kernel set to 8 mm full-width half maximum. Following high-pass filtering (128-sec cutoff), event onsets—either Stroop trial or task block— were modeled with a boxcar function and convolved with the canonical heterocyclic response frequency (HRF). Individual volumes containing large movements were marked as bad, interpolated, and de-weighted in the first-level SPM. For the Stroop task, a general linear model (GLM) was used with regressors for each trial type (incongruent/congruent). Movement parameters from realignment were included as nuisance repressors. For the empathy task, the GLM included three regressors for block type (rest/neutral actions/harm). As before, the planned contrast harm versus neutral actions was thresholded at P < 0.001 with a cluster extent of 10 voxels. A second-level random effects model was used to assess between-group differences. The contrast incongruent versus congruent, across and between groups, was thresholded at P < 0.001 with a cluster extent of 10 contiguous voxels. All statistical analyses were carried out with MATLAB.
For region of interest (ROI) analysis, peak voxels were extracted with 6-mm-diameter spheres centered at the Montreal Neurological Institute (MNI) coordinates taken from previous literature. ROIs were analyzed using rfxplot toolbox for SPM8.34 ROI coordinates for the Stroop task were taken from a meta-analysis of 26 functional neuroimaging studies of Stroop tasks, which included the anterior cingulate cortex (ACC: 2, 16, 41), inferior frontal junction (IFJ: -44, 6, 34), and inferior parietal lobule (IPL: -36, -52, 44).32 For each ROI, the neurohemo-dynamic activity during congruent trials was subtracted from that during incongruent trials for each participant. An unpaired two-sample t-test was used to compare these difference scores between groups.
The six ROI coordinates for the empathy task were taken from a recent study using the same stimuli in typically developing children32 and included the right amygdala (22, 4, -16), the left amygdala (-22, 4, -16), right insula (44, 0, -6), left insula (-44, 0, -6), right anterior midcingulate cortex (10, 20, 32), left anterior midcingulate cortex (-10, 20, 32), right ventromedial prefrontal cortex (10, 50, -6), left ventromedial prefrontal cortex (-2, 58, -8), right inferior frontal gyrus (44, 32, 4), and left inferior frontal gyrus (-44, 32, 4). Peak activity for harm versus neural action within each ROI was then correlated against individual participants' AHI, the latter being log transformed because it was not normally distributed (LogAHI).
RESULTS
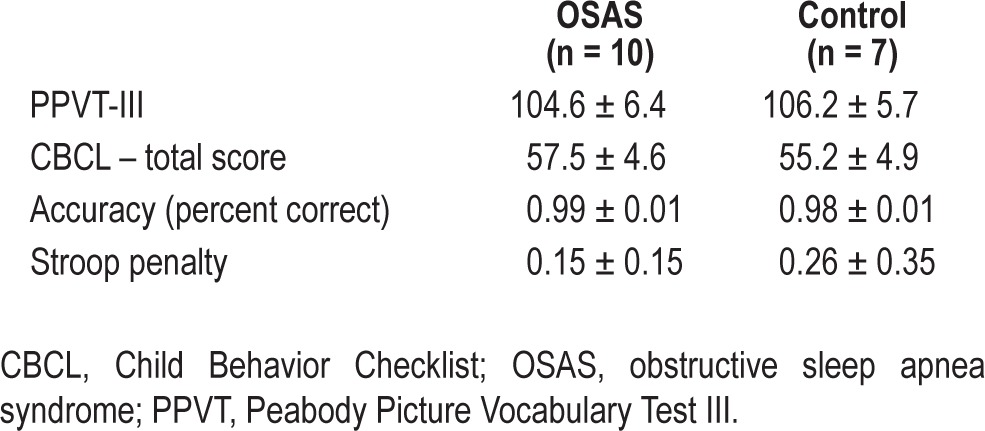
Nineteen children were originally included; however, two were excluded from the final data analyses due to either movement artifacts or phobic reaction to the MRI scanner. No families declined to participate in the use of the fMRI procedures, which were well tolerated by all. Thus, the final sample included 10 children (five females), age 7 to 11 y, with a polysomnographic diagnosis of OSA and seven matched control children (Table 1). All children had PPVT-III and CBCL scores within the normal range (Table 2).
Table 1.
Demographic and polysomnographic characteristics of children with obstructive sleep apnea syndrome and matched controls
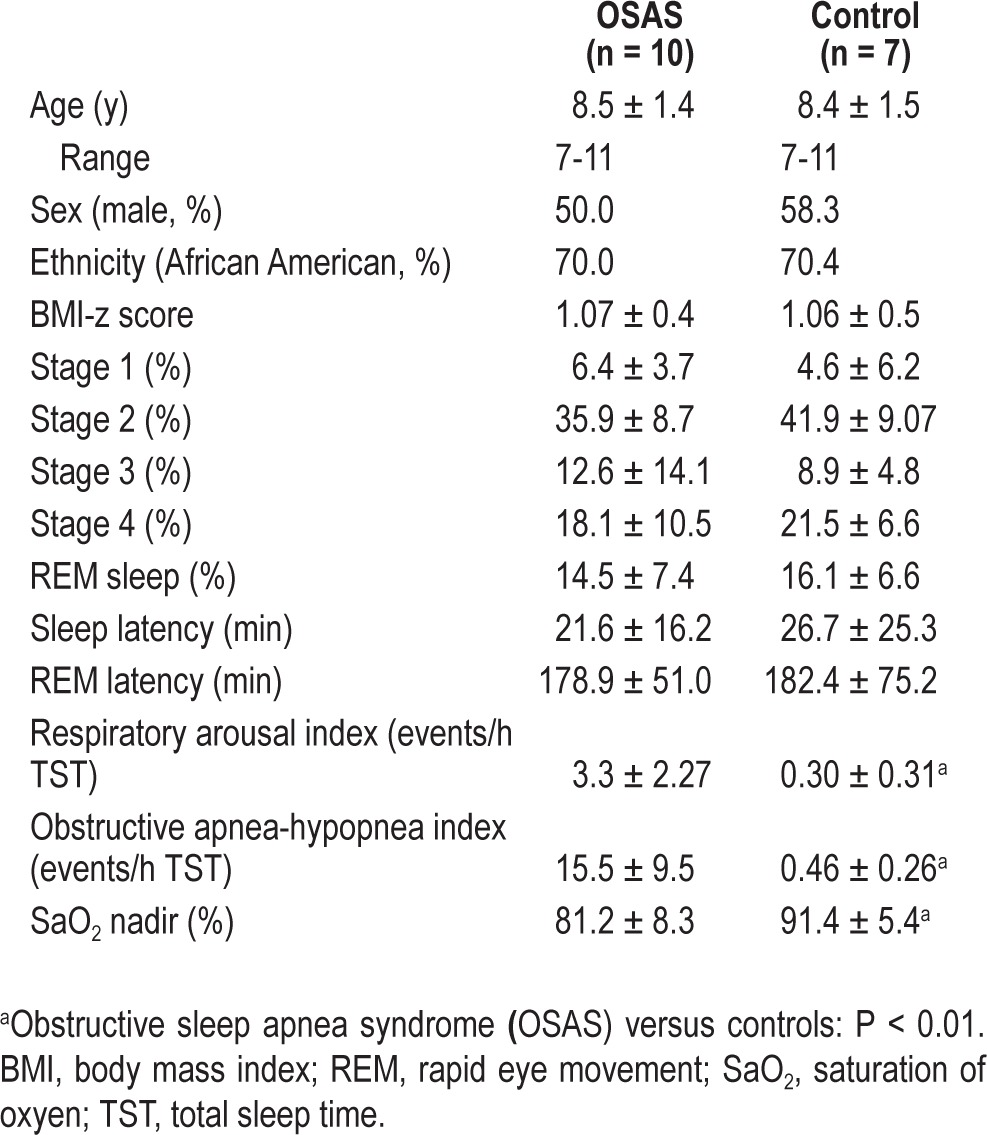
Table 2.
Behavioral results from PPVT-III, CBCL, and Stroop task
Functional MRI Data During the Stroop Task
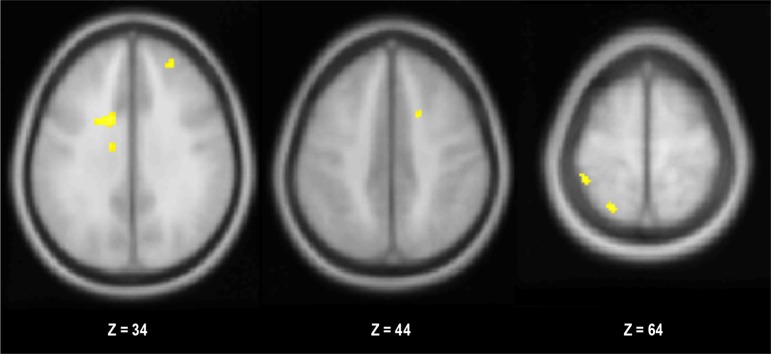
Accuracy was not significantly different between groups (P > 0.39; Table 2). Also, there was no difference in the effect of incongruence on success rate between groups (P > 0.42; Table 2). When groups were collapsed, there were no clusters that showed significant effects of trial type (incongruent versus congruent and its reverse). However, the children with OSA showed greater neurohemodynamic response than controls in eight clusters (Figure 1; Table S1, supplemental material). Within the a priori ROIs, the activity in the ACC was significantly different between the groups (P < 0.05). The OSA group showed greater ACC activity during incongruent trials, whereas the control group demonstrated the reverse pattern. Response in the inferior frontal gyrus and inferior parietal lobule did not show significant differences between groups for incongruent versus congruent (P > 0.36 for both).
Figure 1.
Clusters showing significantly greater activity in the OSAS group for incongruent versus congruent trials during the Stroop test (P < 0.001).
Functional MRI Data During Empathy Task
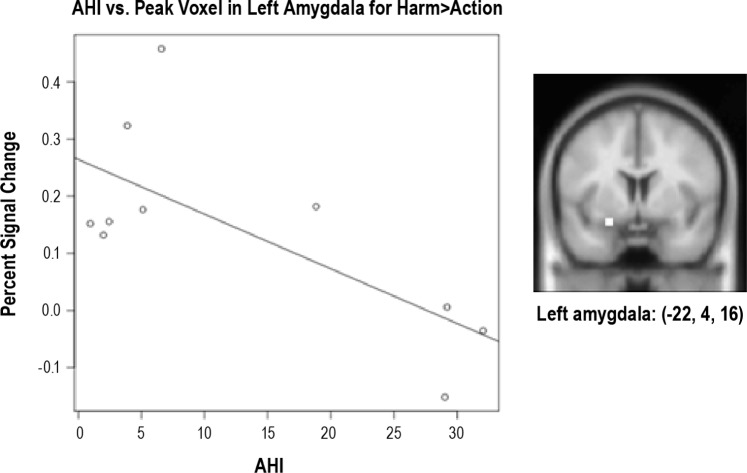
When perceiving harmful actions was contrasted to neutral actions, there was one significant cluster in middle occipital lobe (-42, 72, 14). When LogAHI was entered as a covariate, no significant clusters remained above the significant threshold except for left amygdala. Indeed, ROI analysis revealed a significant correlation between obstructive AHI and left amygdala activity in harm versus neutral actions (r = -0.71, P < 0.05; Figure 2; Table S2, supplemental material). LogOAHI was not significantly related to activity in any other ROI.
Figure 2.
Apnea-hypopnea index (AHI) plotted against percent functional MRI signal change in left amygdala (shown in the picture) for harmful actions versus neutral actions (r = -0.71, P < 0.05) during the empathy task.
DISCUSSION
This is the first study in otherwise cognitively preserved children with OSAS examining neural activation patterns in response to an executive function task and an empathy task with fMRI. The major findings during the attentional executive task indicated significantly more extensive neural recruitment during the Stroop task in children with OSAS. In addition, reduced neural recruitment in previously identified brain regions associated with empathy emerged.
Substantial attention has been paid in the past two decades to the neurocognitive consequences of OSAS in children,2–8,35 and to potential mechanisms underlying such morbidities.2–8,36–39 However, not all children with OSAS exhibit cognitive deficits, suggesting that both genetic and environmental factors are operationally involved.40 It remains unclear however, whether differences in brain activity patterns exist among children with OSAS without neurocognitive deficits and healthy children. This study provides initial insights into this issue, and confirms our previous findings using geodesic evoked potential arrays, whereby satisfactory performance during a standardized executive task, such as the Stroop task, is associated with expanded neural activation within brain functional networks subserving such task.22
During the Stroop task, children with OSAS exhibited greater difference in activity between incongruent and congruent trials. Greater activity was identified in regions implicated in cognitive control, conflict monitoring, and attentional allocation. The a priori ROI analysis further revealed group differences in the response of anterior cingulate cortex, with the OSAS group showing greater recruitment during congruent trials, while the control subjects showed increased activity during incongruent trials. Given that the groups did not significantly differ on task performance, these results suggest that the children with OSAS either required greater neural recruitment, or were using alternative strategies, or some combination of both. It will be of interest to examine whether children with similar OSAS severity but who differ in their cognitive performance will also exhibit divergent neural activation maps during standardized cognitive tasks such as Stroop.7 Furthermore, the reversibility of such alterations with effective treatment will have to be thoroughly investigated.41 Notwithstanding such considerations, the robust statistical differences in brain region recruitment identified by the fMRI procedures during the Stroop task among the two groups, and the opposite effects of OSAS on empathy task-induced brain region activation patterns provides compelling evidence that differential neural activity processing is present in pediatric OSAS during tasks, even when global performance as assessed during standardized neuropsychological testing by experienced child psychologists does not detect any discernible differences.
In the empathy task, a task that has never been included in the assessment of children with OSAs, a condition exists that has been associated with mood perturbations, particularly depressive traits.23 Because hypoactive responses occur in the amygdala in depressed children,42 we reasoned that the fMRI responses to an empathy assessment paradigm would potentially uncover thus far unknown alterations in neural processing differences within this brain region. Indeed, the obstructive AHI predicted neural responses in left amygdala, a key region known to be involved in the processing of social stimuli, especially responsive to salient negative information.42–44 Previous studies with normally developing children have reported activation of the amygdala in response to the perception of intentional harm to others.31,32 This suggests that increased respiratory disturbance during sleep may be related to reduced sensitivity in the amygdala. However, because this task used a passive-viewing paradigm, as dictated by the fMRI environment, it is possible that children with sleep apnea were engaging in a different evaluative process when viewing the scenes.
Some methodological issues pertaining to the current study deserve comment. First, the sample size enrolled in the current study was relatively small. However, the observed differences reported herein were of sufficient robustness to pass the stringent statistical criteria set forth by the multivariate analyses and post hoc tests applied to the data. Therefore, the major concern emanating from such small sample size involves the possibility that other brain regions that did not emerge as statistically significant in the current study may emerge as differentially activated in future studies with larger samples in the context of a potential β error. Second, this is the first study in children with OSAS using fMRI activation approaches. The two groups were carefully matched such as to exclude neurocognitive dysfunction, and thus enable identification of subtle differences in regional brain activation that are potentially relevant to the daytime functioning of these children in clinical settings. In this context, we should caution, however, that screening for overall intellectual abilities using the PPVT-III may not be sufficiently sensitive to detect subtle differences. Although all children were tested immediately after a night of sleep in the laboratory, i.e., their sleep duration preceding their assessment in the magnet was known and similar, we cannot ascertain whether their long-term sleeping schedules were similar as well.45 We are only aware of one other study ever published in children using MRI approaches; a nuclear magnetic resonance spectroscopy study (i.e., without functional correlates) enrolled 31 children (19 with OSAS and 12 controls).38 We should further emphasize that the results reported in this manuscript are of substantial relevance considering the “negative” results recently reported on neurocognitive functioning outcomes by the first randomized controlled trial on pediatric OSAS and adenotonsillectomy.36
Overall, these results provide the first fMRI evidence that cognitive and empathetic processing is influenced by OSAS, even when executive functioning is preserved. Children with sleep apnea showed greater of regions implicated in cognitive control, conflict monitoring, and attentional allocation in order to perform at the same level as children without sleep apnea. When viewing ecologically valid interactions, apnea-hypopnea predicted less sensitivity to harm in the left amygdala. Thus, sleep apnea appears to influence neural recruitment across a range of brain activities.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. David Gozal is supported by National Institutes of Health grants HL-086662, HL-65270 and HL107160. The authors have indicated no financial conflicts of interest. There was no off-label use of any drug in the current study.
SUPPLEMENTAL MATERIAL
Significant clusters for obstructive sleep apnea > HC on incongruent versus congruent trials
Correlations between apnea-hypopnea index and percent functional MRI signal change in regions of interest when participants viewed intentional harm versus neutral actions
REFERENCES
- 1.Tauman R, Gozal D. Adenotonsillar hypertrophy and obstructive sleep apnea in children. Expert Rev Respir Med. 2011;5:425–40. doi: 10.1586/ers.11.7. [DOI] [PubMed] [Google Scholar]
- 2.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102:616–20. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- 3.Gozal D, Pope D. Snoring during early childhood and academic performance at ages 13-14 years. Pediatrics. 2001;107:1394–9. doi: 10.1542/peds.107.6.1394. [DOI] [PubMed] [Google Scholar]
- 4.Kheirandish L, Gozal D. Neurocognitive dysfunction in children with sleep disorders. Devel Sci. 2006;9:388–99. doi: 10.1111/j.1467-7687.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 5.Crabtree VM, Varni JW, Gozal D. Health-related quality of life and depressive symptoms in children with suspected sleep-disordered breathing. Sleep. 2004;27:1131–8. doi: 10.1093/sleep/27.6.1131. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien LM, Mervis CB, Holbrook CR, et al. Neurobehavioral correlates of sleep-disordered breathing in children. J Sleep Res. 2004;13:165–72. doi: 10.1111/j.1365-2869.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 7.Gozal D, McLaughlin Crabtree V, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med. 2007;176:188–93. doi: 10.1164/rccm.200610-1519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halbower AC, Degaonkar M, Barker PB, et al. Childhood obstructive sleep apnea associates with neuropsychological deficits and neuronal brain injury. PLoS Med. 2006;3:e301. doi: 10.1371/journal.pmed.0030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beebe D, Gozal D. Obstructive sleep apnea and the prefrontal cortex: Towards a comprehensive model linking nocturnal upper airway dysfunction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 10.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001;21:2442–50. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas RJ, Rosen BR, Stern CE, Weiss JW, Kwong KK. Functional imaging of working memory in obstructive sleep-disordered breathing. J Appl Physiol. 2005;98:2226–34. doi: 10.1152/japplphysiol.01225.2004. [DOI] [PubMed] [Google Scholar]
- 12.Ayalon L, Ancoli-Israel S, Aka AA, McKenna BS, Drummond SP. Relationship between obstructive sleep apnea severity and brain activation during a sustained attention task. Sleep. 2009;32:373–81. doi: 10.1093/sleep/32.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayalon L, Ancoli-Israel S, Drummond SP. Obstructive sleep apnea and age: a double insult to brain function? Am J Respir Crit Care Med. 2010;182:413–9. doi: 10.1164/rccm.200912-1805OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar R, Chavez AS, Macey PM, Woo MA, Yan-Go FL, Harper RM. Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea. J Neurosci Res. 2012;90:2043–52. doi: 10.1002/jnr.23083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macey PM, Kumar R, Yan-Go FL, Woo MA, Harper RM. Sex differences in white matter alterations accompanying obstructive sleep apnea. Sleep. 2012;35:1603–13. doi: 10.5665/sleep.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cross RL, Kumar R, Macey PM, et al. Neural alterations and depressive symptoms in obstructive sleep apnea patients. Sleep. 2008;31:1103–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Canessa N, Castronovo V, Cappa SF, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011;183:1419–26. doi: 10.1164/rccm.201005-0693OC. [DOI] [PubMed] [Google Scholar]
- 18.Ayalon L, Ancoli-Israel S, Klemfuss Z, Shalauta MD, Drummond SP. Increased brain activation during verbal learning in obstructive sleep apnea. Neuroimage. 2006;31:1817–25. doi: 10.1016/j.neuroimage.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 19.Archbold KH, Borghesani PR, Mahurin RK, Kapur VK, Landis CA. Neural activation patterns during working memory tasks and OSA disease severity: preliminary findings. J Clin Sleep Med. 2009;15:21–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Castronovo V, Canessa N, Strambi LF, et al. Brain activation changes before and after PAP treatment in obstructive sleep apnea. Sleep. 2009;32:1161–72. doi: 10.1093/sleep/32.9.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prilipko O, Huynh N, Schwartz S, et al. The effects of cpap treatment on task positive and default mode networks in obstructive sleep apnea patients: An fMRI study. PLoS One. 2012;7:e47433. doi: 10.1371/journal.pone.0047433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes ME, Gozal D, Molfese DL. Attention in children with obstructive sleep apnoea: an event-related potentials study. Sleep Med. 2012;13:368–77. doi: 10.1016/j.sleep.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crabtree VM, Varni JW, Gozal D. Health-related quality of life and depressive symptoms in children with suspected sleep-disordered breathing. Sleep. 2004;27:1131–8. doi: 10.1093/sleep/27.6.1131. [DOI] [PubMed] [Google Scholar]
- 24.Rosen IM, Gimotty PA, Shea JA, Bellini LM. Evolution of sleep quantity, sleep deprivation, mood disturbances, empathy, and burnout among interns. Acad Med. 2006;81:82–5. doi: 10.1097/00001888-200601000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117:741–53. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 26.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF. American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events. [Google Scholar]
- 27.Dunn L, Dunn L. Examiner's manual for the PPVT-III Peabody Picture Vocabulary Test. third edition. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 28.Slate JR. Two investigations of the validity of the WISC-III. Psychol Rep. 1995;76:299–306. doi: 10.2466/pr0.1995.76.1.299. [DOI] [PubMed] [Google Scholar]
- 29.Achenbach T. Burlington, VT: University of Vermont, Department of Psychology; 1991. Integrative guide to the 1991 CBCL/4-18, YSR, and TRF Profiles. [Google Scholar]
- 30.Stroop JR. Studies of interference in serial verbal reactions. J Exper Psychol. 1935;18:643–62. [Google Scholar]
- 31.Decety J, Michalska KJ, Kinzler KD. The contribution of emotion and cognition to moral sensitivity: a neurodevelopmental study. Cereb Cortex. 2012;22:209–20. doi: 10.1093/cercor/bhr111. [DOI] [PubMed] [Google Scholar]
- 32.Michalska KJ, Kinzler KD, Decety J. Age-related sex differences in explicit measures of empathy do not predict brain responses across childhood and adolescence. Dev Cognit Neurosci. 2013;3:22–32. doi: 10.1016/j.dcn.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazaika Pl, Hoeft F, Glover GH, Reiss AL. ArtRepair Software: Methods and software for fMRI analysis for clinical subjects. http://cibsr.stanford.edu/tools/human-brain-project/artrepair-software.html.
- 34.Gläscher J. Visualization of group inference data in functional neuroimaging. Neuroinformatics. 2009;7:73–82. doi: 10.1007/s12021-008-9042-x. [DOI] [PubMed] [Google Scholar]
- 35.Laird AR, McMillan KM, Lancaster JL, et al. A comparison of label-based review and ALE meta-analysis in the Stroop task. Hum Brain Mapp. 2005;25:6–21. doi: 10.1002/hbm.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–76. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gozal D, Kheirandish-Gozal L. Neurocognitive and behavioral morbidity in children with sleep disorders. Curr Opin Pulm Dis. 2007;13:505–9. doi: 10.1097/MCP.0b013e3282ef6880. [DOI] [PubMed] [Google Scholar]
- 38.Montgomery-Downs HE, Gozal D. Sleep-associated respiratory disorders and their psychobehavioral consequences in children. Handb Clin Neurol. 2011;98:489–99. doi: 10.1016/B978-0-444-52006-7.00032-0. [DOI] [PubMed] [Google Scholar]
- 39.Kheirandish L, Gozal D, Pequignot JM, Pequignot J, Row BW. Intermittent hypoxia during development induces long-term alterations in working spatial memory, monoamine turnover, and dendritic branching in rat frontal cortex. Pediatr Res. 2005;58:594–9. doi: 10.1203/01.pdr.0000176915.19287.e2. [DOI] [PubMed] [Google Scholar]
- 40.Gozal D, Khalyfa A, Sans Capdevila O, Kheirandish-Gozal L, Khalyfa AA, Kim J. Cognitive function in pre-pubertal children with obstructive sleep apnea: a modifying role for NADPH Oxidase p22 subunit gene polymorphisms? Antioxid Redox Signal. 2012;16:171–7. doi: 10.1089/ars.2011.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gozal D. The intermittent hypoxia attending severe obstructive sleep apnoea does lead to alterations in brain structure and function. J Physiol. 2013;591:379–81. doi: 10.1113/jphysiol.2012.241216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaffrey MS, Barch DM, Singer J, Shenoy R, Luby JL. Disrupted amygdala reactivity in depressed 4- to 6-year-old children. J Am Acad Child Adolesc Psychiatry. 2013;52:737–46. doi: 10.1016/j.jaac.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neta M, Kelley WM, Whalen PJ. Neural responses to ambiguity involve domain-general and domain-specific emotion processing systems. J Cogn Neurosci. 2013;25:547–57. doi: 10.1162/jocn_a_00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suratt PM, Barth JT, Diamond R, D'Andrea L, Nikova M, Perriello VA, Jr, Carskadon MA, Rembold C. Reduced time in bed and obstructive sleep-disordered breathing in children are associated with cognitive impairment. Pediatrics. 2007;119:320–9. doi: 10.1542/peds.2006-1969. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Significant clusters for obstructive sleep apnea > HC on incongruent versus congruent trials
Correlations between apnea-hypopnea index and percent functional MRI signal change in regions of interest when participants viewed intentional harm versus neutral actions