Abstract
Study Objectives:
Poor sleep in childhood is associated with increased obesity risk, possibly by affecting appetite-regulating hormones such as leptin. We examined short- and long-term sleep duration and quality in relation to leptin in two US pediatric cohorts.
Design:
Analysis of data from two prospective cohort studies.
Setting:
Population-based. Adolescent polysomnography assessments performed in a clinical research unit.
Patients or Participants:
Children in Project Viva (n = 655) and adolescents in the Cleveland Children's Sleep & Health Study (n = 502).
Interventions:
N/A.
Measurements and Results:
In Project Viva, mothers reported average child sleep duration annually from infancy through age 7, and we measured leptin at ages 3 and 7. In the Cleveland Children's Sleep & Health Study, we collected self-reported sleep duration, polysomnography-derived measures of sleep quality, and fasting leptin at ages 16-19. In sex-stratified linear regression analyses adjusted for sociodemographic characteristics and adiposity, chronic curtailed sleep was associated with lower leptin at age 7 in girls; a one-unit decrease in sleep score was associated with a 0.08 decrease in log leptin (95%CI: 0.01,0.15). The association was stronger in girls with greater adiposity (P = 0.01). Among adolescents, shorter sleep was associated with lower leptin in males; each one-hour decrease in sleep duration was associated with a 0.06 decrease in log leptin (95%CI: 0.00, 0.11). Sleep duration was not associated with leptin at other ages. Sleep quality indices were not associated with leptin.
Conclusions:
Our results suggest possible age-specific sexual dimorphism in the influence of sleep on leptin, which may partly explain inconsistencies in the literature.
Citation:
Boeke CE; Storfer-Isser A; Redline S; Taveras EM. Childhood sleep duration and quality in relation to leptin concentration in two cohort studies. SLEEP 2014;37(3):613-620.
Keywords: Sleep, leptin, obesity, youth
INTRODUCTION
Given the increasing prevalence of childhood obesity in recent decades, it is important to identify modifiable risk factors in early life. Short sleep has been identified as a risk factor for obesity in children as well as adults.1–4 Although the mechanisms by which poor sleep increases obesity risk are unclear, it is possible that poor sleep impacts glucose regulation, inflammatory markers, and appetite regulating hormones.5 Leptin is a satiety hormone of particular interest as it appreciably influences each of these systems.6 It has been speculated that curtailed sleep duration may contribute to leptin dysregulation, which in turn adversely impacts energy balance through insulin-glucose regulation, inflammatory processes, and energy homeostasis.7 Leptin exposure typically reduces energy intake and increases energy expenditure, leading to weight loss. However, reduced sensitivity to the appetite-regulating effects of leptin, or leptin resistance, may occur, whereby the body does not respond to leptin's signaling mechanisms6,8; in such cases, leptin may be associated with weight gain or no change in weight.
Studies of sleep duration in relation to leptin in adults have yielded mixed findings.9 Laboratory studies of healthy young men have found that sleep restriction was associated with lower leptin10,11 or had no association with leptin,12,13 while several studies in women14,15 have reported an inverse association between sleep duration and leptin. Findings from observational studies that included adult males and females are also inconsistent, whereby shorter sleep duration was associated with lower leptin,16,17 higher leptin7,18,19 or was not associated with leptin.20 Although the inconsistencies among these studies may partly relate to differences in experimental procedures such as ad libitum eating or controlled caloric intake, it is possible that differences in subject characteristics, including adiposity, age and sex, as well as differences in leptin sensitivity across study populations contribute to these discordant findings.9
Less is known about the relation of sleep duration and leptin in pediatric as compared to adult populations. A study of youth aged 6-19 years found that shorter weekday sleep duration was associated with higher leptin among girls but not boys,21 while no association was found between sleep duration and leptin in a study of 13- to 17-year-old adolescents.22 Still less is known about how sleep quality affects leptin in youth. A self-reported measure of sleep quality and self-reported sleep duration were not significantly related to leptin among 14- to 18-year-old adolescent females.23
In the current study, we utilized data from two relatively large cohort studies that together provided both cross-sectional and prospective information on sleep duration in relation to leptin in pre-pubertal (infancy to age 7) and post-pubertal (age 16 to 19 years) youth. Additionally, we assessed leptin in relation to indices of sleep architecture obtained by overnight polysomnography in the postpubertal cohort.
METHODS
Study Populations
We utilized data from two cohorts that jointly provided information during childhood and adolescence, with one cohort including infants and pre-pubertal children (Project Viva) and the other including late adolescents (Cleveland Children's Sleep and Health Study). Project Viva is a prospective cohort study of pre- and perinatal influences on maternal, fetal, and child health outcomes and the Cleveland Children's Sleep and Health Study (Cleveland Cohort) is a community-based urban cohort study designed to evaluate sleep measures and health outcomes in children born full-term and preterm. From 1999-2002, Project Viva recruited pregnant women at in-person visits in the first trimester of pregnancy from 8 obstetric offices of Harvard Vanguard Medical Associations, a multisite group practice in eastern Massachusetts.24 Mother-child in-person visits occurred at 6 months, 3 years, and 7 years. Annually, participants completed questionnaires, and throughout investigators obtained other data from medical records. The number of live births to study participants was 2,128. Of the 2,128 mother-child pairs, 702 children had blood measurements at age 7 and of those, 655 had at least one sleep measurement. Institutional review boards of Harvard Pilgrim Health Care, Brigham and Women's Hospital, and Beth Israel Deaconess Medical Center approved the study protocols and all mothers provided written informed consent.
The Cleveland Children's Sleep and Health Study (Cleveland Cohort), recruited children who had been born at three hospitals in the Cleveland area between 1988 and 1993. As previously described,25 all 907 children that participated in the middle childhood examination at ages 8-11 years were eligible to participate in a late adolescent examination conducted approximately 8 years later (at age 16-19 years). Of the 517 adolescents that were studied at this examination, 502 had leptin data and were included in these analyses; blood samples were not collected at the middle childhood examination and therefore leptin was not available for that time point. The protocol for the late adolescent examination was approved by University Hospitals of Cleveland Institutional Review Board. Informed written consent was obtained from participants aged 18 and older; for participants under age 18 years, the adolescent's legal guardian provided informed written consent, and the adolescent provided assent.
Field and Laboratory Procedures – Project Viva
Mothers reported child average sleep duration in a 24-h period at 6 months and annually from 1 through 7 years of age on questionnaires. At 6 months postpartum, the questionnaire asked: (1) “In the past month, on average, for how long does your baby nap during the morning,” (2) “In the past month, on average, for how long does your baby nap during the afternoon,” and (3) “In the past month, on average, how many hours does your baby sleep during the night.” Subsequent questionnaires asked on average how many hours the child slept in a usual 24-h period in the past month. At 2, 3, and 4 years, the response categories were < 9 h, 9 h/day, 10 h/day, 11 h/day, 12 h/day, 13 h/day, and ≥ 14 h/day. At 1, 5, and 6 years, mothers wrote in the number of hours. At 7 years, mothers wrote in the number of hours on weekdays and weekend days separately. To calculate an age-specific weighted average of sleep duration from ages 6 months to 2 years, we created a cumulative sleep duration score via a sum that was weighted by the interval of time between the data collection of all 3 data points and divided the sum by 2. To estimate adequate sleep duration over time, we created a sleep duration score by taking the sum of the following measures: 1 point for sleep 12+ h at age 6 months to 2 years, 0 points for < 12 h; 2 points for 11+ h at age 3-5 years, 1 point for 10-11 h, 0 points for < 10 h; 2 points for 10+ h at age 6-7 years, 1 point for 9-10 h, 0 points for < 9 h.
At ages 3 and 7 years, the children provided non-fasting, venous whole blood samples. Research assistants immediately refrigerated plasma samples after collection and transferred them to a storage laboratory within 24 hours, where samples were spun and blood components were separated into aliquots for storage in liquid nitrogen. Plasma leptin was measured using a radioimmunoassay (Linco Research Inc, St Charles, Missouri, US).26
To assess child adiposity, weight at birth was obtained from hospital records. At 3 and 7 years, trained research assistants measured child height with a calibrated stadiometer (Shorr Productions, Olney, Maryland, US),27 weight with a calibrated scale (age 3: Seca model 881, Seca Corp, Hanover, Maryland, US; age 7: Tanita model TBF-300A, Tanita Corporation of America, Inc., Arlington Heights, Illinois, US), and subscapular and triceps skinfold thicknesses with Holtain calipers (Holtain Ltd, Crosswell, Wales). At age 7, research assistants measured child dual energy X-ray absorptiometry (DXA) total body fat mass using a Hologic model Discovery A scanner (Hologic, Bedford, Massachusetts, US); a single investigator (CEB) checked all scans and defined body regions for analysis with QDR version 12.6 software. We calculated birth weight-for-gestational age z-score using national reference data from the United States Natality datasets.28 Body mass index (BMI) was calculated by dividing weight in kilograms by height in meters squared, and age-sex-adjusted BMI z-scores and percentiles using the 2000 Centers for Disease Control and Prevention growth charts.29,30 Using questionnaires and interviews designed specifically for this study, we asked mothers about their education, household income, and child's race/ethnicity, television watching, and physical activity.8,24 Preterm labor was defined as birth at less than 37 weeks.
Field and Laboratory Procedures – The Cleveland Cohort
Overnight polysomnography (PSG), physiological, and anthropometric assessments were performed with a standardized protocol at a dedicated clinical research unit when the adolescent was free from acute illness.25,31 Adolescents and their primary caregivers also completed demographic, sleep, and health questionnaires during the examination, which began at approximately 17:00 and ended the following day at 11:00. All research staff (nurses, nutritionists and research assistants) received training, followed a detailed written protocol, and were supervised by a lead nutritionist and the principal investigator (Dr. Redline).
Full-channel overnight polysomnography was performed over a single recording night (E-Series [Compumedics Ltd, Abbotsford, Victoria, Australia]). As previously described,32 sleep stages and arousal were scored using standard criteria.33,34 The obstructive apnea-hypopnea index (OAHI) was summarized as the total number of obstructive apneas and hypopneas associated with ≥ 3% desaturations per hour of sleep. Other PSG-based summary measures of sleep architecture included sleep efficiency (percentage of the sleep period spent asleep), number of arousals per hour (arousal index), and percentage of sleep time in stage N3 (slow wave sleep) and REM sleep. Daytime sleepiness, an additional measure of sleep quality, was assessed with a modified version of the Epworth Sleepiness Scale.35 Finally, we calculated weekday sleep duration using adolescent-reported usual weekday bedtime and wake time.
At approximately 07:00 the morning after the PSG and an overnight fast, venipuncture was performed by trained and certified research nurses. Blood was immediately processed and aliquots of EDTA plasma were frozen until assayed in batch at the University of Vermont Clinical Biochemistry Research Laboratory. Leptin was assayed using Millipore Bio-Rad Luminex Flow Cytometry assay (interassay CV: 1.8 to 11.3%).
Trained research nutritionists used a rigid stadiometer (Holtain, Pembrokeshire, UK) and a digital scale (Health-o-meter, Shelton, Connecticut) to measure height and weight, respectively. BMI and BMI z-score were calculated using the methodology described previously for Project Viva. Total body fat (including percentage of fat free body mass) was estimated by calculating resistance/reactance using bioelectrical impedance analysis (Quantum X; accuracies +1 ohm), and BMI, using Cyprus 1.2 Body-Composition Analysis software (Clinton Twp., Michigan, US) and pediatric prediction equations.36 The adolescent's primary caregiver completed questionnaires specifically designed for this study to ascertain highest level of education, annual household income, and the adolescent's race/ethnicity.
Statistical Analysis
Subject characteristics, sleep measures, and leptin were summarized using descriptive statistics. The distribution of leptin was right-skewed and was transformed using the natural logarithm to achieve approximate normality. As there is evidence to suggest that the association of sleep duration and leptin may vary by sex,19,21,37 we used sex-stratified multivariable linear regression analyses to evaluate the relation of sleep duration and quality to log leptin.
In Project Viva, we assessed the following relationships: (a) mean child sleep duration from 6 months to 2 years and leptin at age 3 years; (b) sleep duration and leptin at ages 3 and 7 years (cross-sectional associations); (c) sleep duration score from infancy through age 7 years and leptin at age 7 years; and (d) change in sleep duration from ages 3 to 7 years and change in leptin from ages 3 to 7 years. Using a multiple imputation algorithm in SAS statistical analysis software, we created 50 multiply imputed datasets to fill in any missing data on the 2,128 children in the cohort. We limited the analysis to the 655 individuals with sleep and leptin data available as described previously. In the Cleveland cohort, data from the late adolescent examination was used to examine the cross-sectional association of leptin with each PSG summary measure as well as self-reported sleep duration and daytime sleepiness. Complete case analyses were used as less than 1% of the data were missing.
Analyses of data from both cohorts included fitting sexstratified linear regression models adjusted for a priori potential confounders: age, race/ethnicity, income, parental education, and preterm status (Cleveland Cohort only). Furthermore, as leptin is produced by adipose tissue and is highly correlated with adiposity, we additionally adjusted for both BMI z-score and body fat to assess the relation between sleep and leptin independent of adiposity. In Project Viva, we ran sensitivity analyses in which we adjusted for TV watching and physical activity, but adjusting for these additional variables did not substantially change our results so we did not include these variables in our final models. In the Cleveland Cohort, we ran sensitivity analyses excluding those with obstructive sleep apnea (OAHI3 ≥ 5).
Given that leptin is produced by adipose cells, and individuals with more body fat may have reduced leptin sensitivity, we assessed whether the association between sleep and leptin varied by adiposity in exploratory analyses. The two-way interaction between the sleep exposure and adiposity was examined by adding a sleep × BMI or sleep × body fat interaction term to the regression model. If the interaction term was significant (P < 0.05), we also assessed the associations by BMI percentile and body fat categories including BMI < 5th, 5th to < 85th, and ≥ 85th percentile and body fat at the ∼20th, ∼50th and ∼80th percentiles based on age- and sex-specific normative values from the U.S. population.38 Results were summarized using point estimates and 95% confidence intervals. All analyses were conducted using SAS version 9.2 or higher (SAS Institute, Cary, North Carolina).
RESULTS
Subject characteristics for the two cohorts are displayed in Table 1. In each cohort, approximately half of the sample was male, about one-third was non-Caucasian, and the proportion of obese participants was between 10.2% (7-year-old males) and 22.9% (17-year-old males). In Project Viva, mean (SD) sleep duration in infancy (6 months to 2 years) and at 7 years were 12.2 (0.9) and 9.8 (0.7) h/day, respectively. The median value of leptin was 1.4 ng/mL at age 3 and 3.1 ng/mL at age 7; leptin was higher in females than males. Participants in the Cleveland cohort were 17.7 (0.4) years old on average and reported sleeping 7.9 (1.7) h on average on weeknights. Findings from overnight PSG indicated that values for sleep architecture were consistent with normative data on adolescents [41]; 3% of adolescents had obstructive sleep apnea (OAHI ≥ 5) and 11% had mild sleep apnea (OAHI 2-4.9). The median value of leptin was 9.6 ng/mL, and the median value was higher in females (16.4) than males (2.9).
Table 1.
Participant characteristics by cohort and child sex

Prepubertal Children (Project Viva)
The results of the regression analyses for males and females are shown in Table 2. In univariate analyses, sleep duration was not associated with leptin at either time period. In analyses adjusted for potential confounders, neither infant sleep duration nor sleep duration at age 3 years were associated with leptin at age 3 in boys or girls. No relation was observed between change in sleep duration between ages 3-7 years and change in leptin during that time period. Findings were also null in cross-sectional analyses of the relation of sleep duration and leptin at age 7 years for both sexes. However, in multivariable-adjusted models, low cumulative sleep duration scores, indicative of chronic sleep curtailment in infancy and childhood, were associated with lower leptin at age 7 years among females only. A one-unit decrease in cumulative sleep duration score was associated with a 0.084 decrease in log leptin (95% CI: 0.014, 0.154). BMI z-score and fat mass index most strongly confounded this association; adjusting for these variables revealed a positive association between sleep duration score and age 7 leptin that was not apparent in univariate analysis. Adjusting for race and education further strengthened the positive association. There was a significant interaction between cumulative sleep duration score and child fat mass index in girls (P = 0.01). The positive association between sleep duration and leptin was stronger in girls with greater adiposity; among overweight or obese girls (n = 94), a one-unit decrease in cumulative sleep duration sleep score was associated with a 0.156 decrease in log leptin (95% CI: 0.011, 0.302). Cumulative sleep duration was not associated with leptin at age 7 in boys.
Table 2.
Sleep duration and quality in relation to the adjusted change in plasma leptin concentration
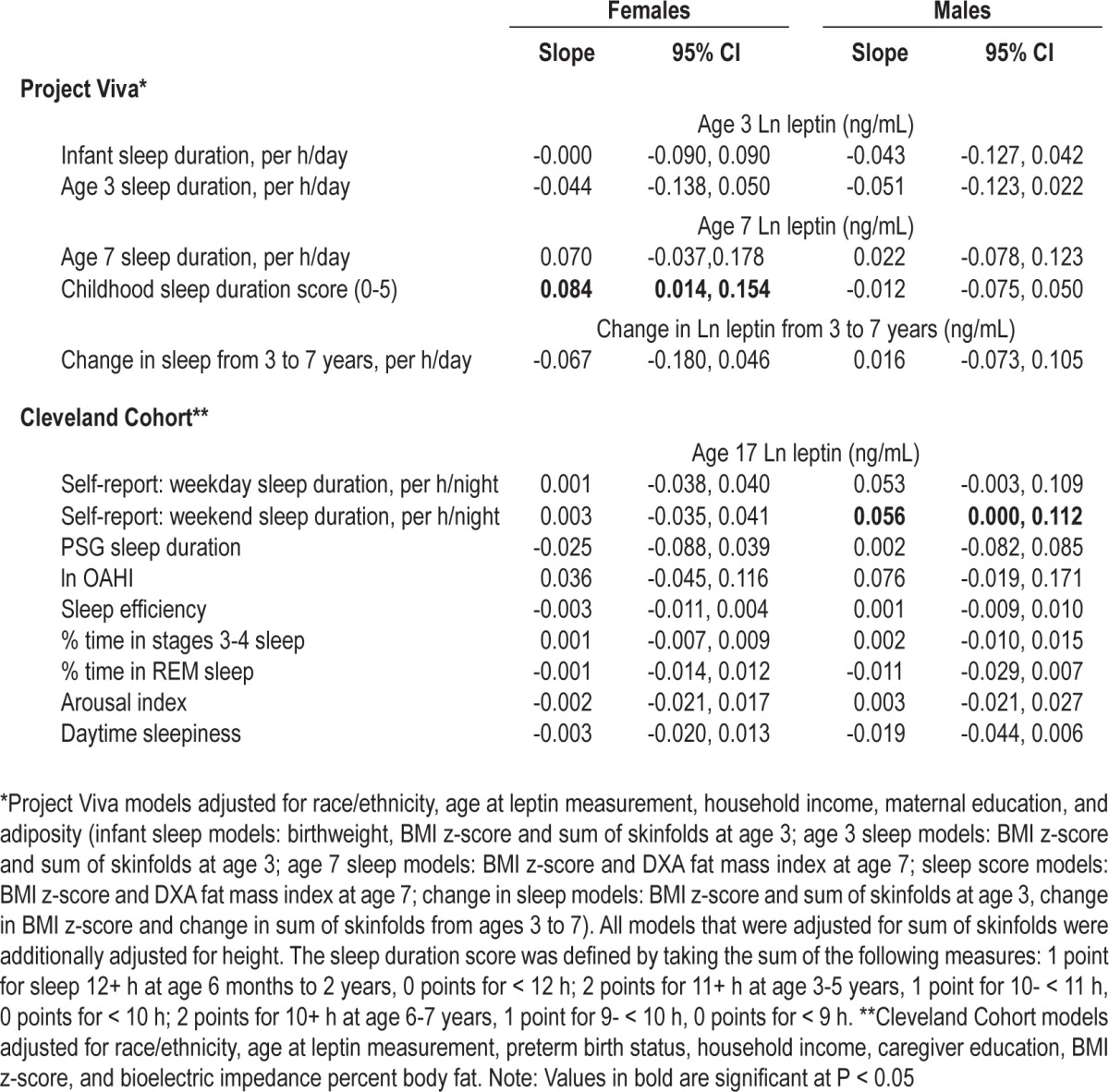
Postpubertal Children (Cleveland Cohort)
Among adolescent males, shorter weekday and weekend sleep duration were associated with lower leptin; each one-hour decrease in weekend sleep duration was associated with a 0.056 decrease in log leptin (95% CI: 0.000, 0.112), with a similar association observed for weekday sleep duration (Table 2). Among adolescent females, sleep duration was not associated with leptin. None of the PSG-based sleep quality measures or subjective daytime sleepiness were related to leptin in adolescent males or females. In exploratory analyses, in females only, there was a significant interaction between body fat and sleep efficiency in relation to leptin (P = 0.01). Post hoc analyses revealed an inverse association between sleep efficiency and leptin among adolescent females with low (28%) body fat, whereby a 10% increase in sleep efficiency was associated with a 0.090 decrease in log leptin (95% CI: -0.178, -0.006; P = 0.04); the association between sleep efficiency and leptin among females with high (40%) body fat was positive but of lower magnitude and was not statistically significant (β = 0.097, 95% CI: -0.025, 0.220, P = 0.12). No association was observed for among females with average (34%) body fat (β = 0.003, 95% CI: -0.075, 0.080; P = 0.95). We observed a similar pattern of results in sensitivity analyses that excluded adolescents with obstructive sleep apnea (OAHI3 ≥ 5).
DISCUSSION
In two large pediatric populations, we observed sex- and age-dependent associations between sleep and leptin. Chronic sleep curtailment in infancy and childhood was associated with lower leptin at age 7 years only in females, especially those with greater adiposity. However, sleep duration at other periods examined was not associated with leptin at ages 3 or 7. In cross-sectional analyses, shorter sleep duration was associated with lower leptin in postpubertal males. Measures of sleep architecture were not associated with leptin among adolescent males or females. Our finding of a 0.08 difference in log leptin concentration is equivalent to a ∼1.1 ng/mL increase in leptin for each one unit increase in child sleep duration score (7-year-old girls) or one hour increase in daily sleep (adolescent males). Taken together, our results suggest that there is a modest positive association between sleep duration and leptin in some subgroups of children and adolescents, and associations likely differ by sex and age, and possibly by level of adiposity.
Our findings extend the limited body of literature on sleep and leptin in youth,21–23 as, to our knowledge, only three prior studies have assessed this relation. The Kiel Obesity Prevention study reported an inverse association of sleep duration and leptin among girls but not boys.21 It is important to note that this study included both children and adolescents aged 6.1-19.9 years in their analyses, which may have obscured age-dependent associations. The AFINOS study of adolescents aged 13-17 years reported no association of sleep duration with leptin and did not find that BMI modified this association, although the small sample size likely limited the statistical power to detect effect modification.22 In a cross-sectional study of 62 lean and 64 obese 14- to 18-year-old Saudi girls, neither self-reported sleep duration nor sleep quality were associated with fasting leptin,23 which is consistent with findings from our study, although again the sample size was relatively small. However, our finding that shorter sleep duration was associated with lower leptin in adolescent males is consistent with the results of laboratory studies of young adult males10,11,39 and several cohort studies.16,17
It is possible that leptin levels may differ in response to chronic as compared to acute sleep deprivation. This could explain why no association was observed in most prior observational cross-sectional studies, while we observed an association with our sleep score variable in Project Viva. Additionally, the discordant results across these previous studies may be partially explained by differences in participant age, sex and adiposity and/or methodological differences (i.e., different amounts of measurement error in the sleep variable in different populations). Our study was unique in that we examined cumulative habitual sleep duration across infancy and early childhood in relation to leptin at age 7 years. This cumulative exposure is likely to have less measurement error than the age-specific sleep exposures because it is less affected by short-term variability in sleep duration. The fact that we observed a stronger association of child 7-year leptin with cumulative sleep duration score than with sleep duration at age 7 may be a result of a more precise measure of chronic sleep curtailment or differential associations of contemporaneous and longer-term sleep curtailment with leptin.
There are many factors that may explain differences in the association of sleep duration with leptin across childhood/ adolescence and in boys and girls, including changes in body size and shape, changes in sleep duration and quality from childhood to adolescence, pubertal changes in leptin concentration, and sensitivity to leptin. In childhood, leptin tends to be slightly higher in girls than boys, and leptin levels rise gradually with age. In girls, leptin levels continue to rise through puberty, whereas, in boys there is a decrease in leptin during puberty.40 The sexual dimorphism in leptin concentrations continues into adulthood; leptin is several-fold higher in women compared to men, and differences in body fat do not fully explain this difference.41 Leptin is likely one of several hormonal factors that allow puberty to begin, rather than a primary stimulant of the pubertal process.42 In addition, since the actions of leptin are primarily through hypothalamic receptors influencing energy balance, it is possible that age and/or pubertal factors influence the central actions of leptin on appetite regulation.
There is previous evidence to suggest that the association of sleep measures and appetite-regulating hormones varies by sex.9 Previous studies found that short sleep duration was associated with higher leptin only among girls,21 and was more strongly associated with elevated leptin among women compared to men.19 Additionally, St-Onge and colleagues37 reported that short sleep duration was associated with increased total ghrelin concentrations only in men, and glucogen-like peptide 1 (GLP-1) was decreased in the short sleep duration condition only in women, although in this study leptin did not differ by sleep duration in men or women.37
Our findings that curtailed sleep was associated with decreased leptin in some age-sex-specific groups may suggest that chronic sleep deprivation could be related to weight gain through reduced leptin concentration, at least among those who are leptin-sensitive. However, we cannot exclude the possibility that the participants in our study are not leptin sensitive. There is some evidence to suggest that children/adolescents in this age range may have some level of leptin resistance. In Project Viva, we found that higher leptin concentration at age 3 was associated with greater weight gain from ages 3 to 7 and greater adiposity at age 7.8 Similarly, higher basal leptin concentrations were associated with greater gains in BMI z-score 2-3 years later among obese girls age 7-18 years.43 The findings from these prior studies suggest that even among youth, the body may not respond appropriately to leptin. If our population is leptin-resistant, it is hard to interpret what an association between sleep and leptin means. Unfortunately, leptin sensitivity is not measurable at this time. More research on leptin sensitivity/resistance is needed to fully interpret our findings.
Measures of sleep architecture and sleep disturbances, including the apnea hypopnea index, sleep efficiency, arousal index, sleep architecture and self-reported daytime sleepiness were not associated with leptin in adolescent males or females. However, in exploratory analyses, we found a negative association of sleep efficiency and leptin among lean adolescent females and a positive (albeit non-significant) association of sleep efficiency and leptin among heavy adolescent females. Since high sleep efficiency in the laboratory may reflect the influence of sleep homeostatic pressure in chronically sleep-deprived adolescents, the findings in lean adolescents is consistent with an effect of chronic sleep insufficiency on leptin levels.
Our analysis, which to our knowledge is the first to look at short- and long-term child sleep duration and sleep quality during adolescence in relation to leptin, has a number of strengths. Using data from two pediatric cohort studies, we were able to examine sleep in relation to leptin during important developmental periods: early childhood and late adolescence. In the younger cohort, we prospectively collected detailed information on sleep habits, including bedtime and wake time, every year during early childhood. Thus we were able to assess the short-term association between sleep and leptin by looking at the cross-sectional relation between the variables collected, as well as long-term average sleep duration in the first 7 years of life with leptin in early childhood. In adolescents, we had objective measures of sleep quality from overnight PSG. Additionally, sex- and age-stratified analyses adjusted for multiple measures of adiposity as well as other confounders enabled us to examine the independent association of sleep and leptin. However, our results need to be interpreted in light of several limitations. Our findings are observational and thus do not have a direct causal interpretation. We used parent- or adolescent-reported sleep duration, which has more measurement error than objective measures of sleep duration and could influence the findings towards the null. Additionally, our results are not adjusted for multiple comparisons as we had a priori reasons to examine each association; however, this increases the chance of type 1 error and thus these associations require further examination in other populations. It is possible that our significant findings in our main analysis and particularly our exploratory analyses are due to chance. Leptin sensitivity, which cannot be measured, could potentially moderate the relation of sleep duration and leptin levels along with downstream effects of leptin on weight and other appetite-regulating hormones, glucose regulation and inflammation.
In two large prospective cohort studies, we found a modest prospective positive association between childhood sleep duration and leptin at age 7 years in females, particularly those with greater adiposity, and a positive cross-sectional association between sleep duration and leptin in adolescent males. Further research is needed to explore the relation of sleep and leptin during the peripubertal period as well as changes in leptin sensitivity and potential variation in leptin resistance during childhood and adolescence. Additionally, it would be interesting to examine how sleep and leptin relate to sex hormones and metabolism in youth. Our results underscore the potential role of age and sex differences in sleep physiology and hormone regulation as well as differences in leptin production in over-weight and obese children.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by NIH grants: NIH HL07567, HL60957, UL1-RR024989 and 1U54CA116867. Caroline Boeke was supported by T32 CA 09001. Susan Redline's employer, Brigham and Women's Hospital, received CPAP equipment from Philips Respironics and ResMed Inc for use in NIH studies. Brigham and Women's Hospital received a grant from ResMed Foundation to supplement a NIH study. Brigham and Women's Hospital received support from ResMed Inc for help in designing a clinical trial. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge and thank Matthew Gillman and Sheryl Rifas-Shiman, as well as the participants and staff of Project Viva. We are grateful for the participation and support of the adolescents and families in the Cleveland Children's Sleep and Health Cohort. We also are indebted to the expert assistance of our research assistants Joan Aylor, Kathryn Clark, Jennifer Frame, and Heather Rodgers.
REFERENCES
- 1.Taveras EM, Rifas-Shiman SL, Oken E, Gunderson EP, Gillman MW. Short sleep duration in infancy and risk of childhood overweight. Arch Pediatr Adolesc Med. 2008;162:305–11. doi: 10.1001/archpedi.162.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell JF, Zimmerman FJ. Shortened nighttime sleep duration in early life and subsequent childhood obesity. Arch Pediatr Adolesc Med. 2010;164:840–5. doi: 10.1001/archpediatrics.2010.143. [DOI] [PubMed] [Google Scholar]
- 3.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landhuis CE, Poulton R, Welch D, Hancox RJ. Childhood sleep time and long-term risk for obesity: a 32-year prospective birth cohort study. Pediatrics. 2008;122:955–60. doi: 10.1542/peds.2007-3521. [DOI] [PubMed] [Google Scholar]
- 5.Killick R, Banks S, Liu PY. Implications of sleep restriction and recovery on metabolic outcomes. J Clin Endocrinol Metab. 2012;97:3876–90. doi: 10.1210/jc.2012-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lago R, Gomez R, Lago F, Gomez-Reino J, Gualillo O. Leptin beyond body weight regulation--current concepts concerning its role in immune function and inflammation. Cell Immunol. 2008;252:139–45. doi: 10.1016/j.cellimm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Hayes AL, Xu F, Babineau D, Patel SR. Sleep duration and circulating adipokine levels. Sleep. 2011;34:147–52. doi: 10.1093/sleep/34.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boeke CE, Mantzoros CS, Hughes MD, et al. Differential associations of leptin with adiposity across early childhood. Obesity (Silver Spring) 2013;21:1430–7. doi: 10.1002/oby.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St-Onge MP. The role of sleep duration in the regulation of energy balance: effects on energy intakes and expenditure. J Clin Sleep Med. 2013;9:73–80. doi: 10.5664/jcsm.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 11.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 12.Schmid SM, Hallschmid M, Jauch-Chara K, Born J, Schultes B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res. 2008;17:331–4. doi: 10.1111/j.1365-2869.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 13.Benedict C, Hallschmid M, Lassen A, et al. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr. 2011;93:1229–36. doi: 10.3945/ajcn.110.006460. [DOI] [PubMed] [Google Scholar]
- 14.Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99:651–6. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 15.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts. 2008;1:266–73. doi: 10.1159/000158874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaput JP, Despres JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity (Silver Spring) 2007;15:253–61. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 18.Pejovic S, Vgontzas AN, Basta M, et al. Leptin and hunger levels in young healthy adults after one night of sleep loss. J Sleep Res. 2010;19:552–8. doi: 10.1111/j.1365-2869.2010.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simpson NS, Banks S, Dinges DF. Sleep restriction is associated with increased morning plasma leptin concentrations, especially in women. Biol Res Nurs. 2010;12:47–53. doi: 10.1177/1099800410366301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hitze B, Bosy-Westphal A, Bielfeldt F, et al. Determinants and impact of sleep duration in children and adolescents: data of the Kiel Obesity Prevention Study. Eur J Clin Nutr. 2009;63:739–46. doi: 10.1038/ejcn.2008.41. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Gomez D, Eisenmann JC, Gomez-Martinez S, et al. Sleep duration and emerging cardiometabolic risk markers in adolescents. The AFINOS study. Sleep Med. 2011;12:997–1002. doi: 10.1016/j.sleep.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Al-Disi D, Al-Daghri N, Khanam L, et al. Subjective sleep duration and quality influence diet composition and circulating adipocytokines and ghrelin levels in teen-age girls. Endocr J. 2010;57:915–23. doi: 10.1507/endocrj.k10e-145. [DOI] [PubMed] [Google Scholar]
- 24.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144:240–5. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 25.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Association of short and long sleep durations with insulin sensitivity in adolescents. J Pediatr. 2011;158:617–23. doi: 10.1016/j.jpeds.2010.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantzoros CS, Liolios AD, Tritos NA, et al. Circulating insulin concentrations, smoking, and alcohol intake are important independent predictors of leptin in young healthy men. Obes Res. 1998;6:179–86. doi: 10.1002/j.1550-8528.1998.tb00335.x. [DOI] [PubMed] [Google Scholar]
- 27.Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, Gillman MW. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics. 2009;123:682–9. doi: 10.1542/peds.2008-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 30.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 31.Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, Redline S. The association of sleep duration with adolescents' fat and carbohydrate consumption. Sleep. 2010;33:1201–9. doi: 10.1093/sleep/33.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson NL, Kirchner HL, Rosen CL, et al. Sleep estimation using wrist actigraphy in adolescents with and without sleep disordered breathing: a comparison of three data modes. Sleep. 2007;30:899–905. doi: 10.1093/sleep/30.7.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 34.Rechtschaffen A, Kales AA. Bethesda, MD: U.S. Department of Health, Education, and Welfare; 1968. A manual of standardized terminology,techniques, and scoring system for sleep stages of human subjects. [Google Scholar]
- 35.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 36.Houtkooper LB, Going SB, Lohman TG, Roche AF, Van Loan M. Bioelectrical impedance estimation of fat-free body mass in children and youth: a cross-validation study. J Appl Physiol. 1992;72:366–73. doi: 10.1152/jappl.1992.72.1.366. [DOI] [PubMed] [Google Scholar]
- 37.St-Onge MP, O'Keeffe M, Roberts AL, RoyChoudhury A, Laferrere B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep. 2012;35:1503–10. doi: 10.5665/sleep.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogden CL, Li Y, Freedman DS, Borrud LG, Flegal KM. Smoothed percentage body fat percentiles for U.S. children and adolescents, 1999-2004. Natl Health Stat Report. 2011;9:1–7. [PubMed] [Google Scholar]
- 39.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 40.Apter DAN. The role of leptin in female adolescence. Ann N Y Acad Sci. 2003;997:64–76. doi: 10.1196/annals.1290.008. [DOI] [PubMed] [Google Scholar]
- 41.Rosenbaum M, Pietrobelli A, Vasselli JR, Heymsfield SB, Leibel RL. Sexual dimorphism in circulating leptin concentrations is not accounted for by differences in adipose tissue distribution. Int J Obes Relat Metab Disord. 2001;25:1365–71. doi: 10.1038/sj.ijo.0801730. [DOI] [PubMed] [Google Scholar]
- 42.Elias CF. Leptin action in pubertal development: recent advances and unanswered questions. Trends Endocrinol Metab. 2012;23:9–15. doi: 10.1016/j.tem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savoye M, Dziura J, Castle J, DiPietro L, Tamborlane WV, Caprio S. Importance of plasma leptin in predicting future weight gain in obese children: a two-and-a-half-year longitudinal study. Int J Obes Relat Metab Disord. 2002;26:942–6. doi: 10.1038/sj.ijo.0802018. [DOI] [PubMed] [Google Scholar]


