Abstract
Introduction:
The purpose of this study was to examine the extent in which concurrent use of khat and tobacco was associated with decrements in working memory. We also tested whether cardiovascular activity during a cognitive task was related to performance outcomes, as research has shown linkages between maladaptive physiological adjustments and cognitive functions.
Methods:
Seventy-four concurrent users of khat and tobacco, 49 khat-only users, and 52 nonusers (M age ± SD: 23.8±4.8) completed a laboratory session including 10min of a mental arithmetic challenge task. Systolic blood pressure (SBP) and diastolic blood pressure and heart rate were collected throughout the task. Analyses of variance and correlational analyses were conducted.
Results:
We found that concurrent users evidenced the lowest number of correct responses on the math task among these 3 groups (ps < .05). Concurrent users also showed fewer number of attempts than khat-only users (ps < .01) and lower accuracy rate than nonusers (ps < .05). The performance of khat-only users and nonusers were comparable on these 3 measures (p > .76). Overall blood pressure levels were lower in concurrent users than in nonusers (p < .05). Correlational analysis found that lower SBP during the math task was associated with fewer number of correct responses and attempts and lower accuracy rate on the task (ps < .05). Multiple regression analysis controlling for gender found that concurrent use predicted math performance (ps < .05). Reported duration and frequency of khat and tobacco use and nicotine dependence predicted performance measures (ps < .05).
Conclusions:
These findings suggest potential linkages between concurrent use of khat and tobacco and impaired working memory.
INTRODUCTION
Identifying psychological and biological determinants associated with substance use may contribute to the development of effective interventions and treatment programs, which may reduce health care and economic burdens. This may be particularly the case among developing countries where polysubstance use is not uncommon (al’Absi & Grabowski, 2012). In East African and Middle Eastern countries, khat (Catha edulis) is a widely accepted substance used in socialization as well as in traditional and religious ceremonies (Cox & Rampes, 2003). Khat is a tree that grows in these regions, and oral consumption is the most common form of use (Cox & Rampes, 2003). Its main constituent is cathinone, and the chemical structure is similar to that of amphetamine (Feyissa & Kelly, 2008). A typical khat session lasts approximately 5hr (Nakajima et al., 2013), and users report euphoria, friendliness, and increased flow of ideas while chewing (Balint, Falkay, & Balint, 2009; Cox & Rampes, 2003). These symptoms are followed by negative symptoms, such as anxiety, irritability, and insomnia.
Khat use is often accompanied by tobacco use (Ayana & Mekonen, 2004; Belew, Kebede, Kassaye, & Enquoselassie, 2000; Griffiths, 1998; Kassim, Islam, & Croucher, 2011; Nakajima et al., 2013). Epidemiological data indicate greater prevalence of smoking among khat users as compared with nonusers (Ali et al., 2011). In our recent work (Nakajima, al’Absi, Dokam, Alsoofi, & Khalil, 2012), reported hours of khat chewing per session were longer in concurrent users of khat and tobacco than in khat-only users, suggesting that the amount of khat used may be greater among smokers than nonsmokers. A growing body of evidence indicates that tobacco use is associated with cognitive impairment. Our focus for addressing the effect of concurrent tobacco use was prompted by several studies reporting that chronic smokers demonstrated poorer performance than nonsmokers on measures of auditory–verbal learning and/or verbal memory (Hill, Nilsson, Nyberg, & Backman, 2003; Nooyens, van Gelder, & Verschuren, 2008; Paul et al., 2006; Sabia, Marmot, Dufouil, & Singh-Manoux, 2008; Starr, Deary, Fox, & Whalley, 2007) and poorer performance on measures of working memory (Ernst, Heishman, Spurgeon, & London, 2001). More recently, Durazzo, Meyerhoff, and Nixon (2012) have reported similar poorer performances by younger smokers compared with nonsmokers on multiple measures of auditory–verbal and visuospatial learning, visuospatial memory, cognitive efficiency, executive functioning, and processing. Recent reports suggest that khat use may be associated with cognitive deficits (Colzato, Ruiz, van den Wildenberg, Bajo, & Hommel, 2010; Colzato, Ruiz, van den Wildenberg, & Hommel, 2011, 2012; Hoffman & al’Absi, 2010; Hoffman & al’Absi, 2013). Taken together, these suggest the possibility that concurrent use of khat and tobacco may have deleterious effects on cognitive functions. However, this hypothesis has not been directly tested.
This study also examined the extent to which physiological activity during a cognitive task was linked with performance outcomes. It has been suggested that dysregulations in central motivational processes are associated with maladaptive physiological adjustments to environmental demands, which may lead to poor health behavior (Lovallo, 2011). Indeed, recent reports show a relationship between diminished cardiovascular levels and impaired cognitive ability (Ginty, Phillips, Der, Deary, & Carroll, 2011a, 2011b). This has not been examined in individuals who use khat and tobacco.
The purpose of this study was to examine the extent to which concurrent use of khat and tobacco was associated with diminished working memory. A mental arithmetic challenge task tapping cognitive processes related to attention and working memory was used to assess cognitive performance. It was hypothesized that concurrent users would have worse performance than khat-only users or nonusers. It was also predicted that lower levels of cardiovascular activity would be associated with worse performance.
METHODS
Participants
This study was part of a larger program that investigated neurocognitive, psychobiological, and social determinants associated with habitual khat use (al’Absi et al., 2013; Nakajima et al., 2013). Our previous study (Nakajima et al., 2013) reported data collected in the cities of Sana’a and Taiz in Yemen. However, due to minor differences in research protocol this study included data from Taiz only. Participant recruitment was conducted at Taiz University and in the surrounding communities. Interested participants were invited to an onsite screening to assess their eligibility. This screening included a brief interview regarding medical history and substance use including khat and tobacco. Participants were included if they were free from major medical or psychiatric diseases (e.g., stroke, hypertension, diabetes, major depression, anxiety disorder, substance use disorder). They must have completed at least a high school level education to ensure that they were able to comprehend tasks during the study. Khat-only users were defined as individuals who reported that they had been chewing khat for at least 2 years. In addition to the khat use, those who reported daily smoking cigarettes and/or water pipe use were classified as concurrent users. Those who had not used either substance were considered nonusers. Participants who passed the above-mentioned criteria read and signed a consent form approved by the research ethical committee at Taiz University. A total of 175 individuals (74 concurrent users, 49 khat-only users, and 52 nonusers) completed this study. The majority of them were college students.
Measures and Apparatus
A mental arithmetic test was conducted to assess processes related to attention and working memory. Participants were instructed to subtract the number 7 from a 4-digit number with their maximal effort and speed. They were asked to go back to the previous number and try again in case they made a mistake. The mental arithmetic challenge has been shown to effectively assess cognitive functions relying on short-term memory (al’Absi, Hugdahl, & Lovallo, 2002) and elicits sympathetic activation which results in increased cardiovascular activity (al’Absi et al., 1997). An automated blood pressure monitor (MicroLife Automatic BP monitor 3AC1; MicroLife) was used to measure systolic and diastolic blood pressure (SBP and DBP) and heart rate (HR) during the cognitive task. The Fagerström Test of Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991) was used to assess levels of nicotine dependence in concurrent users.
Procedure
The laboratory session was held between 9 and 10 a.m. The participants were tested individually. Upon arrival to the laboratory, the participant was greeted and seated in a comfortable chair in a testing room. A blood pressure cuff was then attached. The session consisted of a 30-min resting baseline during which the participant completed a battery of questionnaires such as demographic information and history of khat and tobacco use. BPs and HR were collected at the end of this period. The participant was then instructed to complete a mental arithmetic test (10min) and other tasks (not reported here). Cardiovascular measures were obtained four times (at 1-, 3-, 5-, and 7-min period) during the math task. After the task period, the participant rested for 15min.
Data Reduction and Analysis
Primary dependent variables related to mental math perfor mance were the following: (a) the number of correct responses, (b) the number of error responses, (c) total attempts (the sum of error and correct responses), and (d) accuracy rate (number of correct responses divided by total attempts). Each variable was analyzed by a 3 group (concurrent users of khat and tobacco, khat-only users, and nonusers) × 2 gender (men and women) analysis of variance (ANOVA). Due to significant skew in cardiovascular data, for each measure (e.g., SBP collected at the 1-min period), levels that exceeded ±2 SD were removed. As a result, completed data (i.e., none of measures collected during math was removed) for SBP were available from 145 individuals (57 concurrent, 43 khat-only, and 45 nonuser), DBP were available from 141 individuals (53 concurrent, 46 khat-only, and 42 nonuser), and HR were available from 145 individuals (59 concurrent, 43 khat-only, and 43 nonuser). These were analyzed by 3 group × 2 gender × 4 time period multivariate analysis of variances using Wilk’s criterion. Baseline cardiovascular measures (using the same correction method as previously mentioned) and demographic variables were analyzed by 3 group × 2 gender ANOVAs. Bonferroni corrections were made for follow-up comparisons. A detailed description of cardiovascular and self-report measures from a larger sample of tobacco and khat users (N = 308) will be reported elsewhere. Correlational and regression analyses were conducted to examine linkages between cardiovascular measures and math performance. Regression models were also conducted to test whether khat and tobacco use variables (e.g., frequency) were related to performance measures. Reported results varied in degrees of freedom due to occasional missing data.
RESULTS
Sample Characteristics
The average age of the current sample was 23.8 years (SD: 4.8; see Table 1). Concurrent users were older than nonusers (main effect of group: F[2,169] = 4.43, p = .01; follow-up tests: p = .01). Khat-only users did not differ from other groups in terms of age (ps > .33). Body mass index was comparable across three groups (p = .44). Men (M = 107.8, SEM = 1.4) exhibited greater baseline SBP than women (M = 102.8, SEM = 1.4; F[1, 157] = 6.33, p = .01). No other gender or group differences were observed in baseline cardiovascular measures (ps > .07). Because age was different among three groups, we conducted a preliminary analysis including age as a covariate in models examining math performance and cardiovascular measures. We found that age was not significant in any of these models. Therefore, age was not included as a covariate in reported results.
Table 1.
Demographic Variables, Baseline Cardiovascular Measures, and Khat and Tobacco Use
| Nonusers (n = 52) | Khat-only users (n = 49) | Concurrent users (n = 74) | |
|---|---|---|---|
| Women (%) | 51.9 | 44.9 | 48.6 |
| Age (years)a | 22.3 (0.6) | 23.8 (0.7) | 24.8 (0.5) |
| Body mass index (kg/m2) | 22.0 (0.5) | 21.2 (0.5) | 21.6 (0.4) |
| Baseline systolic BPb | 105.8 (1.8) | 105.2 (1.9) | 104.9 (1.5) |
| Baseline diastolic BP | 71.6 (1.6) | 68.2 (1.6) | 71.2 (1.4) |
| Baseline heart rate | 86.1 (1.6) | 88.9 (1.6) | 86.2 (1.4) |
| Khat use | |||
| Age started using khatb | n/a | 16.4 (0.5) | 16.7 (0.4) |
| Hours (per session)a | n/a | 4.2 (0.3) | 5.1 (0.3) |
| Times (per week)b | n/a | 4.9 (0.3) | 5.4 (0.2) |
| Duration (years)b | n/a | 5.2 (0.6) | 6.1 (0.5) |
| Tobacco use | |||
| Cigarettes (per day) | n/a | n/a | 6.1 (0.6) |
| Water pipe (per day) | n/a | n/a | 1.1 (0.1) |
| Duration (years) | n/a | n/a | 4.2 (0.4) |
| FTND | n/a | n/a | 1.7 (0.2) |
Note. BP = blood pressure; n/a = not applicable; FTND = Fagerström Test of Nicotine Dependence. Entries show M and SEM.
aGroup effect was significant.
bGender effect was significant.
Detailed results of group and gender differences in reported khat and tobacco use have been published elsewhere using a larger sample (Nakajima et al., 2013). In short, on average, khat users (khat-only and concurrent users combined) started chewing when they were 17-years-old (SD: 3.8). They chewed khat close to 5hr/day (SD: 2.4), 5 times a week (SD: 2.2) for almost 6 years (SD: 4.6; Table 1). Reported age at the start of chewing was earlier, and intensity and frequency of khat use were greater in men than in women (Fs[1,119] > 6.9, ps < .01). Concurrent users had smoked for 4 years (SD: 3.8). The mean FTND score was 1.7 (SD: 2.0), indicating very low dependence (Heatherton et al., 1991). Concurrent men reported smoking 12 cigarettes/day (SD: 6.9), whereas concurrent women reported smoking less than a cigarette per day (SD: 0.3). In contrast, men reported smoking <1 water pipe head/day (SD: 0.5), whereas women reported smoking two heads during the day (SD: 1.7). We note that these results were similar to those reported previously (Nakajima et al., 2013).
Mental Arithmetic Performance
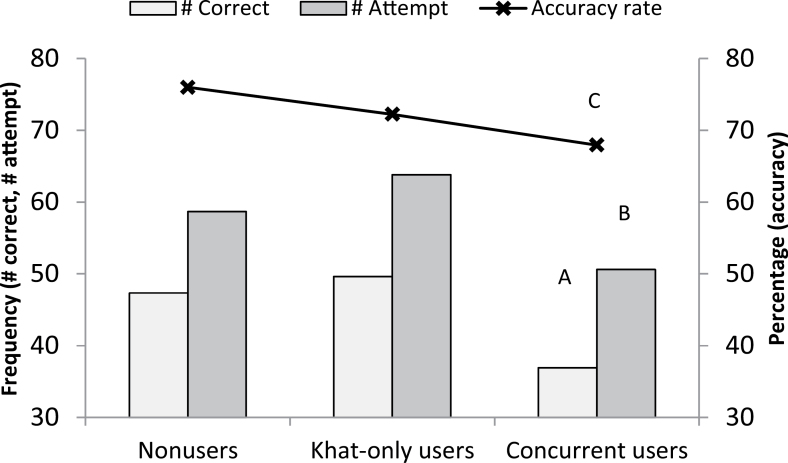
Concurrent users showed a lower number of correct responses than khat-only users and nonusers (group effect: F[2,169] = 5.74, p = .004; follow-up tests: ps ≤ .03) and fewer attempts than khat-only users (group effect: F[2,169] = 5.40, p = .005; follow-up tests: p = .005; see Figure 1). Concurrent users also had a lower accuracy rate than nonusers (group effect: F[2,169] = 3.63, p = .03; follow-up tests: p = .03). Khat-only users and nonusers were comparable in these measures (p > .76). Relative to women, men had a greater number of correct responses (men: M = 54.8, SEM = 2.4; women: M = 34.5, SEM = 2.5; F[1,169] = 34.4, p < .001), attempts (men: M = 66.8, SEM = 2.4; women: M = 48.6, SEM = 2.5; F[1,169] = 27.7, p < .001), and accuracy (men: M = 0.79 [i.e., 79%], SEM = 0.02; women: M = 0.65 [i.e., 65%], SEM = 0.02; F[1,169] = 30.1, p < .001). There were no group × gender differences in math performance variables (ps > .18; see Table 2).
Figure 1.
Group differences in mental arithmetic performance. A: Concurrent users showed a lower number of correct responses than khat-only users and nonusers. B: Concurrent users had fewer attempts than khat-only users. C: Concurrent users had a lower accuracy rate than nonusers.
Table 2.
Mental Math Performance and Cardiovascular Measures During Mental Arithmetic Challenge
| Nonusers | Khat-only users | Concurrent users | |
|---|---|---|---|
| Math performance | |||
| Number of correct responsea,b | 47.4 (3.1) | 49.6 (3.2) | 36.9 (2.6) |
| Number of error | 11.3 (1.0) | 14.2 (1.1) | 13.7 (0.9) |
| Number of attempta,b | 58.7 (3.1) | 63.8 (3.2) | 50.6 (2.6) |
| Accuracy rate (%)a,b | 76.0 (2.3) | 72.2 (2.4) | 67.9 (1.9) |
| Systolic BPa,b,c,d | |||
| 1-min period | 120.5 (2.0) | 107.7 (2.1) | 109.0 (1.8) |
| 3-min period | 113.9 (1.9) | 108.1 (2.0) | 109.5 (1.7) |
| 5-min period | 112.5 (1.9) | 105.4 (1.9) | 105.3 (1.7) |
| 7-min period | 109.2 (2.0) | 105.6 (2.1) | 103.6 (1.8) |
| Mean of periods | 114.0 (1.5) | 106.7 (1.5) | 106.9 (1.3) |
| Diastolic BPa,b,c,d | |||
| 1-min period | 82.9 (2.0) | 75.4 (1.9) | 73.9 (1.8) |
| 3-min period | 78.3 (2.0) | 73.4 (1.9) | 74.0 (1.7) |
| 5-min period | 77.5 (1.7) | 74.5 (1.7) | 73.4 (1.6) |
| 7-min period | 74.3 (1.9) | 72.0 (1.8) | 68.8 (1.7) |
| Mean of periods | 78.2 (1.4) | 73.9 (1.4) | 72.5 (1.3) |
| Heart ratee | |||
| 1-min period | 89.0 (1.6) | 90.6 (1.6) | 87.3 (1.4) |
| 3-min period | 91.1 (1.7) | 91.8 (1.7) | 87.5 (1.4) |
| 5-min period | 89.1 (1.6) | 91.3 (1.6) | 87.5 (1.4) |
| 7-min period | 88.1 (1.8) | 89.6 (1.8) | 88.0 (1.5) |
| Mean of periods | 89.3 (1.5) | 90.9 (1.5) | 87.6 (1.3) |
Note. BP = blood pressure. Otherwise indicated, entries show M and SEM.
aGroup effect was significant.
bGender effect was significant.
cGroup × gender interaction was significant.
dTime effect was significant.
eGroup × gender × time interaction was significant.
Cardiovascular Activity During the Mental Arithmetic Challenge
Significant main effects of group were found in SBP (F[2,139] = 8.48, p < .001) and DBP (F[2,135] = 4.69, p = .01) indicating lower SBP in concurrent and khat-only groups relative to nonusers (ps < .002), and lower DBP in concurrent users relative to nonusers (p = .01). These group differences were further qualified by significant group × gender interactions (ps < .02) indicating greater SBP and DBP in men than in women among nonusers and khat-only users; however, this gender difference was not observed among concurrent users. A significant group × gender × time interaction in HR (F[6,274] = 2.63, p = .02) with a change score analysis (differences in HR between 1- and 3-min period) found a group × gender interaction (F[2,150] = 3.94, p = .02), reflecting a tendency of gender differences in nonusers (p = .08) and in khat-only users (p = .08), but no such difference was found in concurrent users (p = .93). SBP (F[3,137] = 9.21, p < .001) and DBP (F[3,133] = 7.36, p < .001) decreased over time during the math task in all participants. In both measures, levels at 7-min period were lower than those at 1-min period; ps < .001; see Table 2).
Associations Between Math Performance and Cardiovascular Measures
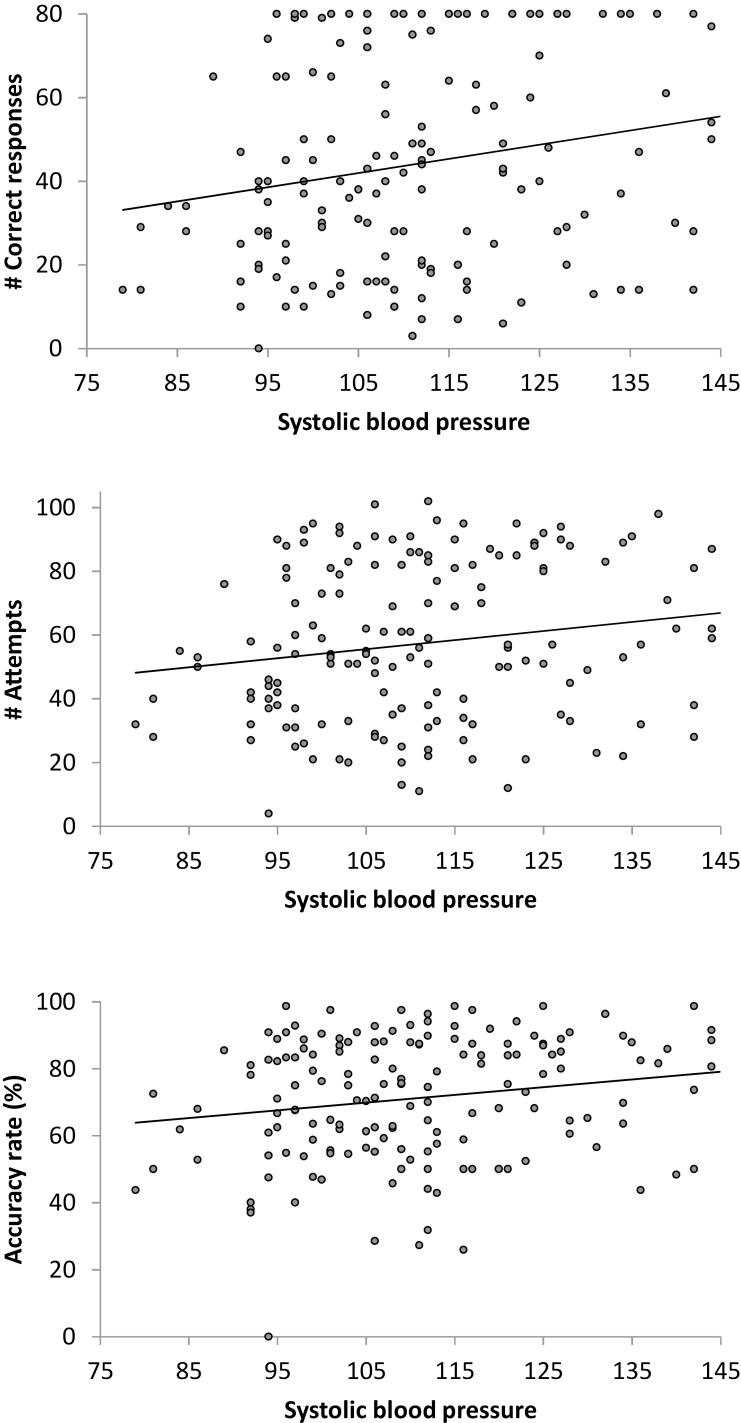
Correlational analysis found that greater SBP at 3-min period was associated with greater number of correct responses (r = .20, p =.01) and attempts (r = .17, p = .03), and higher accuracy (r = .18, p = .02; see Figure 2). Mean SBP across four periods during the math task were positively related with accuracy rate (r = .15, p = .049). DBP and HR were not related to mental arithmetic measures (ps > .09). Multiple regression models were conducted to examine whether group (nonusers as a reference) and SBP at 3-min period predicted performance measures. Gender was also included in the model because of its linkages with mental arithmetic performance. The results indicated that concurrent use predicted the number of correct responses (β = −.22, t = −2.61, p = .01), accuracy rate (β = −.27, t = −3.11, p = .002), and the number of attempts (β = −.17, t = −1.98, p = .049). SBP was not significant in these models (ps > .32).
Figure 2.
Positive associations between systolic blood pressure (at 3-min period) and math performance.
Multiple regression models were also conducted to test whether khat and tobacco use variables predicted working memory. Gender was adjusted in these models. Reported hours of khat chewing per session predicted the number of correct response (β = −.25, t = −2.93, p = .004), accuracy rate (β = −.23, t = −2.75, p = .01), and the number of attempts (β = −.28, t = −3.31, p = .001). Similarly, reported years of khat chewing was predictive of the number of correct response (β = −.22, t = −2.73, p = .01), accuracy rate (β = −.19, t = −2.37, p = .02), and the number of attempts (β = −.20, t = −2.35, p = .02). Reported water pipe use per day predicted accuracy rate (β = −.27, t = −2.17, p = .03). FTND predicted the number of correct response (β = −.26, t = −2.22, p = .03) and the total number of attempts (β = −.25, t = −1.99, p = .05). Collectively, these findings indicated that greater duration and frequency of khat and tobacco use as well as levels of nicotine dependence were associated with poorer math performance.
DISCUSSION
To the best of our knowledge, this study was among the first to demonstrate that a combination of khat and tobacco use is related to impairment in working memory and task-related physiological activity. Concurrent users had the lowest number of correct responses and fewer attempts than khat-only users, and lower accuracy rate than nonusers. Increase in reported hours per session and duration (in years) in khat use, as well as water pipe use per day and severity of nicotine dependence, were associated with decrease in mental arithmetic performance. Both SBP and DBP were lower in concurrent users than in nonusers, and lower SBP levels during mental stress were correlated with poorer math performance. These findings are in agreement with previous work reporting relationships between khat and cognitive performance (Colzato et al., 2010, 2011, 2012; Hoffman & al’Absi, 2010; Hoffman & al’Absi, 2013), tobacco and cognitive deficits (Durazzo et al., 2012; Ernst et al., 2001; Hill et al., 2003; Nooyens et al., 2008; Paul et al., 2006; Sabia et al., 2008; Starr et al., 2007), and diminished physiological stress reactivity and cognitive ability (Ginty et al., 2011a, 2011b).
Although mechanisms specific to the relationship between concurrent use of khat and tobacco and impaired cognitive function are not yet well understood, research has shown linkages between cognitive deficits and the use of tobacco. Compared with nonsmokers, smokers have been shown to have impaired performance on measures of auditory–verbal learning and/or verbal memory (Hill et al., 2003; Nooyens et al., 2008; Paul et al., 2006; Sabia et al., 2008; Starr et al., 2007) and working memory (Ernst et al., 2001). These findings have also been reported among young smokers (Durazzo et al., 2012). Studies have also shown linkages between cognitive dysfunctions and amphetamine use. Significant deficits in several different cognitive processes dependent upon brain fronto-striatal and limbic circuits have also been observed in studies of chronic methamphetamine users, including deficits in psychomotor functions, complex information processing speed, attention and working memory, episodic memory, and executive functions, including response inhibition and novel problem solving (Gonzalez et al., 2004; Ornstein et al., 2000; Paulus et al., 2002; Rogers et al., 1999a, 1999b; Salo et al., 2001, 2002; Sim et al., 2002; Simon et al., 2000, 2002; Simon, Dacey, Glynn, Rawson, & Ling, 2004; Volkow et al., 2001; Woods et al., 2005). Attentional deficits have been noted in the Stroop Color Word and Trailmaking tests (Kalechstein, Newton, & Green, 2003; Simon et al., 2000). Chronic methamphetamine users also exhibit deficits on measures of sustained attention and vigilance such as the Continuous Performance Test (CPT; Borgaro et al., 2003). A recent study completed by London and colleagues (2005) demonstrated that methamphetamine users made significantly more errors on an auditory version of the CPT than drug-free controls. These investigators noted that the signal detection index was significantly smaller in methamphetamine users than in controls, suggesting impairment in the ability to discriminate targets from nontargets. Deficits may also emerge on tasks with more complex processing demands that also involve working memory and decision making, such as an N-back task (London et al., 2005). Individuals with a history of methamphetamine use display working memory deficits in such tasks as the immediate recall component of the auditory verbal learning test (Volkow et al., 2001) and take 18%–30% longer to complete the working memory components of the California computerized assessment package (Chang et al., 2002). The current findings that greater frequency and intensity of khat use as well as higher levels of nicotine dependence were associated with poorer working memory indicate dose-dependent relationships, suggesting enhanced risk of concurrent use of tobacco and khat on cognitive ability.
This investigation found that lower SBP levels during the task were associated with poorer performance. Although the weak magnitude of correlations calls for caution in interpretation of the results, a recent seminal work (Lovallo, 2011) suggests that maladaptive (exaggerated or blunted) physiological stress response may be associated with alterations in central motivational processes that regulate homeostasis of the system. These processes involve layers of neurocognitive pathways in the brain that affect motivation, which may influence psychological, physiological, and behavioral outcomes (Lovallo, 2011; Phillips, 2011). It is, therefore, possible that poor math performance and low blood pressure levels observed among concurrent users of khat and tobacco may in part reflect malfunctions in the central motivational system. However, neurophysiological mechanisms of habitual substance use and central motivational processes have not been directly tested.
Math performance measures were comparable between khat-only users and nonusers, which appears to be inconsistent with findings on amphetamine users (Colzato et al., 2010, 2011, 2012; Hoffman & al’Absi, 2010; Hoffman & al’Absi, 2013). Reported history of khat chewing in the khat-only group was 5 years, which was a relatively short duration relative to possible khat effects on working memory. The extent to which khat chewing and amphetamine use have a different course of impact on central processes is not known. Effects of long-term khat use on working memory and other cognitive functions should be examined in future research.
The results of this study are limited by a number of factors. First, there was no smoking-only group. We encountered difficulty in recruiting individuals who smoke but do not chew khat. Cultural differences might have played a role. In Middle Eastern countries, khat is widely available and socially accepted (Cox & Rampes, 2003), whereas tobacco use especially by women is stigmatized in this region (Maziak, Asfar, & Mock, 2003). It has been proposed that khat use may be one determinant of tobacco use (Nakajima et al., 2013). More research is clearly needed to identify psychosocial characteristics of khat and tobacco users. Second, the current results cannot make any conclusions regarding causal directions between khat and tobacco use and working memory because of the cross-sectional nature of the research design. Thus, it is not clear whether concurrent use of khat and tobacco caused cognitive deficits or whether individuals with cognitive impairment are predisposed to use these substances. Future research should incorporate a longitudinal cohort to identify the directional relationship and examine whether this link is mediated by other psychosocial factors. Third, the observed findings may not best represent chronic effects of khat and tobacco on cognitive functions because the sample consisted of relatively young individuals with comparable backgrounds. For instance, the inclusion of concurrent users with low levels of nicotine dependence might affect results on math performance and cardiovascular measures. We note, however, this observation is consistent with recent work suggesting cultural influences on patterns of tobacco use. Tobacco is often used while khat chewing, and khat sessions are typically held in the afternoon. Thus, some questions in FTND such as tobacco use in the morning may not be sensitive in assessing nicotine dependence among concurrent users (Nakajima et al., 2012). This study should be replicated using a sample including wide ranges of age and regions, and individuals who are highly khat and tobacco use dependent. Finally, future research should assess levels of task engagement and perceived task difficulty to minimize potential confounds. Nevertheless, this study used a well-known stress task and collected working memory measures, and systematically assessed physiological measures to examine associations between khat and tobacco use, cognitive function, and physiological responses.
CONCLUSIONS
In conclusion, this study found that concurrent use of khat and tobacco was linked with decrements in working memory. Lower SBP levels during the cognitive task were related to worse performance. Frequency of khat use and levels of nicotine dependence predicted lower performance. These findings suggest linkages between concurrent use of khat and tobacco and cognitive deficits.
FUNDING
This work was supported by the Fogarty International Research Collaboration Award (FIRCA) grant from the Fogarty International Center and the National Institutes of Health (R03T W007219), R21 National Institute for Drug Abuse (DA024626), and Office of International Programs at the University of Minnesota.
DECLARATION OF INTERESTS
None declared.
ACKNOWLEDGMENTS
We would like to thank Drs A. Dokam, M. Alsoofi, N. S. Khalil, M. AlHabori, and A. N. Kasim for their help in coordinating the program.
The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- al’Absi M., Bongard S., Buchanan T., Pincomb G. A., Licinio J., Lovallo W. R. (1997). Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology, 34, 266–275. 10.1111/j.1469–8986.1997.tb02397.x [DOI] [PubMed] [Google Scholar]
- al’Absi M., Grabowski J. (2012). Concurrent use of tobacco and khat: Added burden on chronic disease epidemic. Addiction, 107, 451–452. 10.1111/j.1360-0443.2011.03684.x [DOI] [PubMed] [Google Scholar]
- al’Absi M., Hugdahl K., Lovallo W. R. (2002). Adrenocortical stress responses and altered working memory performance. Psychophysiology, 39, 95–99. 10.1111/1469–8986.3910095 [DOI] [PubMed] [Google Scholar]
- al’Absi M., Khalil N. S., Al Habori M., Hoffman R., Fujiwara K., Wittmers L. (2013). Effects of chronic khat use on cardiovascular, adrenocortical, and psychological responses to stress in men and women. The American Journal on Addictions, 22, 99–107. 10.1111/j.1521-0391.2013.00302.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali W. M., Al Habib K. F., Al-Motarreb A., Singh R., Hersi A., Al Faleh H. … Al Suwaidi J. (2011). Acute coronary syndrome and khat herbal amphetamine use: An observational report. Circulation, 124, 2681–2689. 10.1161/CIRCULATIONAHA.111.039768 [DOI] [PubMed] [Google Scholar]
- Ayana A. M., Mekonen Z. (2004). Khat (Catha edulis Forsk) chewing, sociodemographic description and its effect on academic performance, Jimma University students 2002. Ethiopian Medical Journal, 42, 125–136 [PubMed] [Google Scholar]
- Balint E. E., Falkay G., Balint G. A. (2009). Khat - a controversial plant. Wiener klinische Wochenschrift, 121, 604–614. 10.1007/s00508-009-1259-7 [DOI] [PubMed] [Google Scholar]
- Belew M., Kebede D., Kassaye M., Enquoselassie F. (2000). The magnitude of khat use and its association with health, nutrition and socio-economic status. Ethiopian Medical Journal, 38, 11–26 [PubMed] [Google Scholar]
- Borgaro S., Pogge D. L., DeLuca V. A., Bilginer L., Stokes J., Harvey P. D. (2003). Convergence of different versions of the continuous performance test: Clinical and scientific implications. Journal of Clinical and Experimental Neuropsychology, 25, 283–292. 10.1076/jcen.25.2.283.13646 [DOI] [PubMed] [Google Scholar]
- Chang L., Ernst T., Speck O., Patel H., DeSilva M., Leonido-Yee M., Miller E. N. (2002). Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry Research, 114, 65–79. 10.1016/S0925-4927(02)00004-5 [DOI] [PubMed] [Google Scholar]
- Colzato L. S., Ruiz M. J., van den Wildenberg W. P., Bajo M. T., Hommel B. (2010). Long-term effects of chronic khat use: Impaired inhibitory control. Frontiers in Psychology, 1, 219. 10.3389/fpsyg.2010.00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato L. S., Ruiz M. J., van den Wildenberg W. P., Hommel B. (2012). Khat use is associated with increased response conflict in humans. Human Psychopharmacology, 27, 315–321. 10.1002/hup.2229 [DOI] [PubMed] [Google Scholar]
- Colzato L. S., Ruiz M. J., van den Wildenberg W. P., Hommel B. (2011). Khat use is associated with impaired working memory and cognitive flexibility. PLoS ONE, 6, e20602. 10.1371/journal.pone.0020602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G., Rampes H. (2003). Adverse effects of khat: A review. Advances in Psychiatric Treatment, 9, 456–463. 10.1192/apt.9.6.456 [Google Scholar]
- Durazzo T. C., Meyerhoff D. J., Nixon S. J. (2012). A comprehensive assessment of neurocognition in middle-aged chronic cigarette smokers. Drug and Alcohol Dependence, 122, 105–111. 10.1016/j.drugalcdep.2011.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Heishman S. J., Spurgeon L., London E. D. (2001). Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology, 25, 313–319. 10.1016/S0893-133X(01)00257-3 [DOI] [PubMed] [Google Scholar]
- Feyissa A. M., Kelly J. P. (2008). A review of the neuropharmacological properties of khat. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 32, 1147–1166. 10.1016/j.pnpbp.2007.12.033 [DOI] [PubMed] [Google Scholar]
- Ginty A. T., Phillips A. C., Der G., Deary I. J., Carroll D. (2011a). Cognitive ability and simple reaction time predict cardiac reactivity in the West of Scotland Twenty-07 Study. Psychophysiology, 48, 1022–1027. 10.1111/ j.1469-8986.2010.01164.x [DOI] [PubMed] [Google Scholar]
- Ginty A. T., Phillips A. C., Der G., Deary I. J., Carroll D. (2011b). Heart rate reactivity is associated with future cognitive ability and cognitive change in a large community sample. International Journal of Psychophysiology, 82, 167–174. 10.1016/j.ijpsycho.2011.08.004 [DOI] [PubMed] [Google Scholar]
- Gonzalez R., Rippeth J. D., Carey C. L., Heaton R. K., Moore D. J., Schweinsburg B. C. … Grant I. (2004). Neurocognitive performance of methamphetamine users discordant for history of marijuana exposure. Drug and Alcohol Dependence, 76, 181–190. 10.1016/j.drugalcdep.2004.04.014 [DOI] [PubMed] [Google Scholar]
- Griffiths P. (1998). Qat use in London: A study of qat use among a sample of Somalis living in London. London: Home Office, Central Drugs Prevention Unit [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127. 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Hill R. D., Nilsson L. G., Nyberg L., Bäckman L. (2003). Cigarette smoking and cognitive performance in healthy Swedish adults. Age and Ageing, 32, 548–550. 10.1093/ageing/afg067 [DOI] [PubMed] [Google Scholar]
- Hoffman R., al’Absi M. (2010). Khat use and neurobehavioral functions: Suggestions for future studies. Journal of Ethnopharmacology, 132, 554–563. 10.1016/j.jep.2010.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R., al’Absi M. (2013). Working memory and speed of information processing in chronic khat users: Preliminary findings. European Addiction Research, 19, 1–6. 10.1159/000338285 [DOI] [PubMed] [Google Scholar]
- Kalechstein A. D., Newton T. F., Green M. (2003). Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. The Journal of Neuropsychiatry and Clinical Neurosciences, 15, 215–220. 10.1176/appi.neuropsych.15.2.215 [DOI] [PubMed] [Google Scholar]
- Kassim S., Islam S., Croucher R. E. (2011). Correlates of nicotine dependence in U.K. resident Yemeni khat chewers: A cross-sectional study. Nicotine & Tobacco Research, 13, 1240–1249. 10.1093/ntr/ntr180 [DOI] [PubMed] [Google Scholar]
- London E. D., Berman S. M., Voytek B., Simon S. L., Mandelkern M. A., Monterosso J. … Ling W. (2005). Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biological Psychiatry, 58, 770–778. 10.1016/j.biopsych. 2005.04.039 [DOI] [PubMed] [Google Scholar]
- Lovallo W. R. (2011). Do low levels of stress reactivity signal poor states of health? Biological Psychology, 86, 121–128. 10.1016/j.biopsycho.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziak W., Asfar T., Mock J. (2003). Why most women in Syria do not smoke: Can the passive barrier of traditions be replaced with an information-based one? Public Health, 117, 237–241. 10.1016/S0033-3506(03)00070-2 [DOI] [PubMed] [Google Scholar]
- Nakajima M., al’Absi M., Dokam A., Alsoofi M., Khalil N. S. (2012). An examination of the Fagerström Test for Nicotine Dependence among concurrent tobacco and khat users. Journal of Psychoactive Drugs, 44, 437–441. 10.1080/02791072.2012.737224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M., al’Absi M., Dokam A., Alsoofi M., Khalil N. S., Al Habori M. (2013). Gender differences in patterns and correlates of khat and tobacco use. Nicotine & Tobacco Research, 15, 1130–1135. 10.1093/ntr/nts257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooyens A. C., van Gelder B. M., Verschuren W. M. (2008). Smoking and cognitive decline among middle-aged men and women: The Doetinchem Cohort Study. American Journal of Public Health, 98, 2244–2250. 10.2105/AJPH.2007.130294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein T. J., Iddon J. L., Baldacchino A. M., Sahakian B. J., London M., Everitt B. J., Robbins T. W. (2000). Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology, 23, 113–126. 10.1016/S0893-133X(00)00097-X [DOI] [PubMed] [Google Scholar]
- Paul R. H., Brickman A. M., Cohen R. A., Williams L. M., Niaura R., Pogun S. … Gordon E. (2006). Cognitive status of young and older cigarette smokers: Data from the international brain database. Journal of Clinical Neuroscience, 13, 457–465.10.1016/j.jocn.2005.04.012 [DOI] [PubMed] [Google Scholar]
- Paulus M. P., Hozack N. E., Zauscher B. E., Frank L., Brown G. G., Braff D. L., Schuckit M. A. (2002). Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology, 26, 53–63 doi:10.1038/S0893-133X(01)00334-7 [DOI] [PubMed] [Google Scholar]
- Phillips A. C. (2011). Blunted cardiovascular reactivity relates to depression, obesity, and self-reported health. Biological Psychology, 86, 106–113. 10.1016/j.biopsycho.2010.03.016 [DOI] [PubMed] [Google Scholar]
- Rogers R. D., Everitt B. J., Baldacchino A., Blackshaw A. J., Swainson R., Wynne K. … Robbins T. W. (1999a). Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: Evidence for monoaminergic mechanisms. Neuropsychopharmacology,20, 322–339. 10.1016/S0893-133X(98)00091-8 [DOI] [PubMed] [Google Scholar]
- Rogers R. D., Owen A. M., Middleton H. C., Williams E. J., Pickard J. D., Sahakian B. J., Robbins T. W. (1999b). Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. The Journal of Neuroscience, 19, 9029–9038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabia S., Marmot M., Dufouil C., Singh-Manoux A. (2008). Smoking history and cognitive function in middle age from the Whitehall II study. Archives of Internal Medicine, 168, 1165–1173. 10.1001/archinte.168.11.1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R., Nordahl T., Possin K., Leamon M., Gibson D., Galloway G. … Sullivan E. (2001). Reduced cognitive inhibition in methamphetamine-dependent individuals. Abstract presented at Society of Neuroscience, San Diego, CA [DOI] [PubMed] [Google Scholar]
- Salo R., Nordahl T. E., Possin K., Leamon M., Gibson D. R., Galloway G. P. … Sullivan E. V. (2002). Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Research, 111, 65–74. 10.1016/S0165-1781(02)00111-7 [DOI] [PubMed] [Google Scholar]
- Sim T., Simon S. L., Domier C. P., Richardson K., Rawson R. A., Ling W. (2002). Cognitive deficits among methamphetamine users with attention deficit hyperactivity disorder symptomatology. Journal of Addictive Diseases, 21, 75–89. 10.1300/J069v21n01_07 [DOI] [PubMed] [Google Scholar]
- Simon S. L., Dacey J., Glynn S., Rawson R., Ling W. (2004). The effect of relapse on cognition in abstinent methamphetamine abusers. Journal of Substance Abuse Treatment, 27, 59–66. 10.1016/j.jsat.2004.03.011 [DOI] [PubMed] [Google Scholar]
- Simon S. L., Domier C., Carnell J., Brethen P., Rawson R., Ling W. (2000). Cognitive impairment in individuals currently using methamphetamine. The American Journal on Addictions, 9, 222–231 doi:10.1080/10550490050148053 [DOI] [PubMed] [Google Scholar]
- Simon S. L., Domier C. P., Sim T., Richardson K., Rawson R. A., Ling W. (2002). Cognitive performance of current methamphetamine and cocaine abusers. Journal of Addictive Diseases, 21, 61–74. 10.1300/J069v21n01_06 [DOI] [PubMed] [Google Scholar]
- Starr J. M., Deary I. J., Fox H. C., Whalley L. J. (2007). Smoking and cognitive change from age 11 to 66 years: A confirmatory investigation. Addictive Behaviors, 32, 63–68. 10.1016/j.addbeh.2006.03.020 [DOI] [PubMed] [Google Scholar]
- Volkow N. D., Chang L., Wang G. J., Fowler J. S., Leonido-Yee M., Franceschi D. … Miller E. N. (2001). Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. The American Journal of Psychiatry, 158, 377–382. 10.1176/appi.ajp.158.3.377 [DOI] [PubMed] [Google Scholar]
- Woods S. P., Rippeth J. D., Conover E., Gongvatana A., Gonzalez R., Carey C. L, … HIV Neurobehavioral Research Center Group (2005). Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology, 19, 35–43. 10.1037/0894-4105.19.1.35 [DOI] [PubMed] [Google Scholar]