Abstract
Introduction:
Schizophrenia is associated with a high prevalence of cigarette smoking. The aims of this study were to compare smokers with schizophrenia (SS) and control smokers without psychiatric illness (CS) on (a) cigarette craving and nicotine withdrawal symptom severity during a 72-hr smoking abstinence period; (b) nicotine reinforcement, before and after abstinence; and (c) latency to smoking lapse following abstinence. We also explored mediators of smoking lapse in SS and CS.
Methods:
SS (n = 28) and CS (n = 27) underwent a nicotine versus denicotinized cigarette puff choice task before and after a 72-hr period of smoking abstinence that was experimentally controlled by providing cash reinforcement contingent on biochemical verification of abstinence. Twenty-four hours after the second choice task, participants could receive a low-value reinforcer if they had continued to abstain since the previous day. Those who remained abstinent were recontacted a week later to determine time of their smoking lapse.
Results:
SS reported more severe cigarette craving and nicotine withdrawal symptoms throughout the 72-hr abstinence period, had greater nicotine preference after abstinence, and lapsed back to smoking significantly sooner than CS. The relationship between group and smoking lapse latency was mediated by baseline depression and nicotine withdrawal symptom severity but not by effects of abstinence on craving or nicotine reinforcement.
Conclusions:
Overall, these results indicate that negative affect is a key contributor to poor smoking cessation outcomes among those with schizophrenia.
INTRODUCTION
Schizophrenia is associated with a high prevalence of cigarette smoking and premature mortality from smoking-related disease (Brown, Kim, Mitchell, & Inskip, 2010; Hennekens, Hennekens, Hollar, & Casey, 2005). Experimental comparisons of smokers with and without schizophrenia have identified several factors that may contribute to the higher prevalence and severity of nicotine dependence in schizophrenia, including topography characteristics associated with higher nicotine intake (Tidey, Rohsenow, Swift, Kaplan, & Adolfo, 2008; Williams et al., 2005, 2011), low sensitivity to alternative reinforcers (AhnAllen et al., 2012, Spring, Pingitore, & McChargue, 2003), and deficits in working memory and task persistence (Sacco et al., 2005; Steinberg et al., 2012). However, few studies have examined the extent to which cigarette craving, nicotine withdrawal symptoms, and nicotine reinforcement contribute to smoking relapse in this population.
Among smokers without serious mental illness, cigarette craving and nicotine withdrawal symptoms are important predictors of ongoing smoking and relapse during quit attempts (Kenford et al., 2002; Killen and Fortmann, 1997; Piasecki, Jorenby, Smity, Fiore, & Baker, 2003). If smokers with schizophrenia (SS) experience more severe craving and withdrawal than control smokers (CS) without psychiatric illness, these states may contribute to the poorer cessation outcomes among SS. SS and CS have similar craving levels when they have been abstinent for 5 or 16hr (Fonder et al., 2005; Tidey et al., 2008; Tidey, Rohsenow, Kaplan, Swift, & AhnAllen, 2013; Weinberger et al., 2007), but longer abstinence durations have not been examined. Similarly, two studies reported that 5-hr abstinence increased withdrawal symptoms to a similar extent in SS and CS (Tidey et al., 2008, 2013), but another study found that 16-hr abstinence did not increase withdrawal symptoms in either group (Weinberger et al., 2007), and withdrawal symptoms during longer abstinence periods have not been compared in SS and CS.
Subjective effects of smoking reinstatement after abstinence also predict relapse in smokers without psychiatric illness (Shiffman, Ferguson, & Gwaltney, 2006; Strong et al., 2011). Furthermore, in experimental studies, those who are randomly assigned to smoke after abstinence relapse more quickly than those who are randomized to remain abstinent, suggesting that this relationship is due to the direct effects of smoking rather than factors such as motivation (Chornock, Stitzer, Gross, & Leischow, 1992; Juliano, Donny, Houtsmuller, & Stitzer, 2006). Because the neuropathology of schizophrenia may confer heightened vulnerability to the reinforcing effects of nicotine (Berg & Chambers, 2008; Chambers, Krystal, & Self, 2001), a plausible contributor to early relapse in SS is that they experience stronger nicotine reinforcement during a smoking lapse than CS. SS have been reported to make more hypothetical choices for smoking versus alternative reinforcers than CS (MacKillop and Tidey, 2011; Spring et al., 2003), but these studies did not investigate whether this was specifically due to differential sensitivity to the reinforcing effects of nicotine.
This study had four aims. The primary aim was to compare craving and withdrawal symptoms across a 72-hr abstinence period in SS and CS. We used high-value, abstinence-contingent incentives to gain experimental control over abstinence, as in previous studies (Alessi, Badger, & Higgins, 2004; Heil, Tidey, Holmes, Higgins, & Badger, 2003). This enabled us to examine the direct effects of abstinence, unconfounded by factors that affect one’s ability to remain abstinent. A 72-hr abstinence period was selected because lapses within the first few days of quit attempts are highly predictive of smoking cessation failure (Hughes, Keely, & Naud, 2004; Kenford et al., 1994). The second aim was to compare the reinforcing effects of nicotine, before and after abstinence, in SS and CS. The third aim was to compare latency to smoking lapse in SS and CS, and the fourth, exploratory aim was to examine predictors of lapse in SS and CS. We hypothesized that (a) SS would experience more severe craving and nicotine withdrawal than CS across the abstinence period, (b) SS would experience stronger reinforcing effects of nicotine than CS and this would increase to a greater extent in SS versus CS during abstinence, (c) smoking lapse latency would be shorter for SS than CS, and (d) negative affect during abstinence and relative reinforcing effects of nicotine following abstinence would mediate the group difference on lapse latency.
METHODS
Participants
Participants were men and women, aged ≥ 18 years, who met Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria for schizophrenia or schizoaffective disorder (SS) or did not have an Axis I disorder (CS), as confirmed by the Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 1994). All participants smoked 20–50 cigarettes/day, had Fagerström Test of Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991) scores of 6 or higher, and indicated that they wanted to quit smoking someday. Potential participants were excluded if they were currently receiving or seeking smoking treatment, had unstable medication or psychiatric symptoms, were using medications that could affect study measures, had positive urine drug or pregnancy tests at baseline, or had positive breath alcohol levels at any session. Procedures were approved by the Brown University Institutional Review Board. Participants who completed at least one session were included in analyses (28 SS and 27 CS).
Procedures
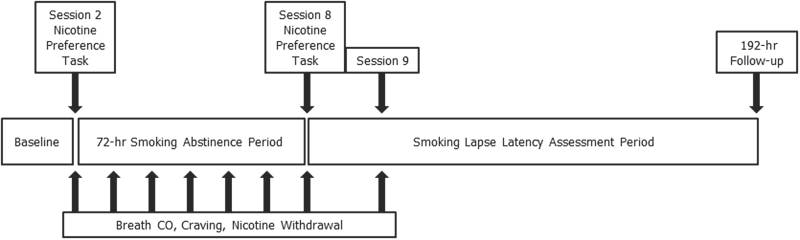
A schematic of the study design is shown in Figure 1. Participants underwent nine sessions. In Session 1, participants completed individual difference measures (described in Measures section) and practiced using the smoking topography equipment (CReSS; Borgwaldt KC) that was used in the nicotine preference task in subsequent sessions. In Session 2, participants arrived at the laboratory on a Monday morning, provided a breath carbon monoxide (CO) sample, completed measures of craving and nicotine withdrawal, and participated in the nicotine preference task (described in Measures section). Participants were asked to remain abstinent from smoking from that point until Thursday morning and return to the laboratory at 09:00 and 16:00 each day to provide breath CO samples to verify abstinence. CO samples meeting the abstinence criteria (≤50% of baseline CO on Monday afternoon, ≤4 ppm at all other timepoints) were reinforced with cash, starting with $25 on Monday afternoon and increasing by $5 at each subsequent sample. Those not meeting abstinence criteria were discontinued from further participation. After abstinence was verified, participants completed the craving and nicotine withdrawal measures. On Thursday morning (Session 8), the nicotine preference task was repeated. For ethical reasons, participants could opt out of participating in this task on Thursday and still receive full compensation for participation, as long as they attended and completed all other procedures for that session. At the end of this session, participants were reminded that they would receive $10 cash reinforcement if they continued to be abstinent, as verified by breath CO ≤ 4 ppm, for the next 24hr. Because we anticipated that many participants would lapse soon after the discontinuation of the high-value abstinence incentives, this $10 abstinence incentive was provided to prevent floor effects that would reduce our ability to detect differences in lapse latency among participants (Juliano et al., 2006). On Friday (Session 9), breath CO was measured from all participants, and time of first lapse was collected from those who had lapsed. One week later, those who had been abstinent at Session 9 were recontacted and time of first lapse was assessed using a timeline followback interview, a valid method for assessing smoking and other drug use in people with and without Axis I disorders (Brown et al., 1998; Carey, 1997; DeMarce, Burden, Lash, Stephens, & Grambow, 2007). Participants were compensated up to $275 (including abstinence incentives) for completing this study.
Figure 1.
Schematic of the study design.
Measures
Baseline Characteristics
Baseline individual difference measures included demographic characteristics, smoking history measures, and the Contemplation Ladder (Biener & Abrams, 1991), a 10-point measure of motivation to quit smoking. A trained interviewer assessed schizophrenia symptom severity in SS at Sessions 1 and 8 using the Brief Psychiatric Rating Scale (BPRS; Overall & Gorham, 1962), which consists of 18 items that are rated from 1 (not present) to 7 (extremely severe), yielding a total score and scores on 5 factors: thinking disturbance, anxiety–depression, hostility–suspiciousness, tension–excitement, and anergia (Burger, Yonker, Calsyn, Morse, & Klinkenberg, 2003). The 20-item Center for Epidemiological Studies Depression Scale (CES-D; Radloff, 1977) was used to assess past-week depression severity in all participants.
Sessions 2–8
Cigarette craving was assessed using the Questionnaire on Smoking Urges-brief form (QSU-brief). The 10 items were scored from 1 (strongly disagree) to 7 (strongly agree). This measure yields two factors: F1, which represents desire to smoke, and F2, which represents anticipation of relief of negative affect and urgent desire to smoke (Cox, Tiffany, & Christen, 2001). Nicotine withdrawal symptoms were measured using the Minnesota Nicotine Withdrawal Scale (MNWS), with the craving item omitted (Hughes & Hatsukami, 1986). Symptoms were rated from 0 (not present) to 4 (severe), and a total symptom score was calculated by averaging item scores.
During the nicotine preference task, mood was assessed using the Diener and Emmons Mood Scale (Diener & Emmons, 1984), which consists of four positive (happy, joyful, pleased, and enjoyment/fun) and five negative items (depressed/blue, unhappy, frustrated, worried/anxious, and angry/hostile), rated on 0–100-mm Visual Analog Scales. This measure was sensitive to nicotine administration in a previous study (Perkins et al., 2008). Cigarettes were rated using the Hedonic Rating Scale (Shiffman et al., 2006), which consists of two items, “Was smoking pleasant?” and “Was the cigarette satisfying?”, rated on 10-point scales and averaged to create a single score. Hedonic rating predicted latency from an initial lapse to smoking relapse in nonpsychiatric smokers (Shiffman et al., 2006).
Nicotine Preference Task
The nicotine preference task consisted of a phase in which participants sampled nicotine-containing and denicotinized cigarettes under double-blind conditions, followed by a phase in which they made a series of choices between the cigarettes (Perkins, Grobe, Weiss, Fonte, & Caggiula, 1996; Rukstalis et al., 2005). After providing a breath CO sample and rating their cigarette craving, withdrawal symptoms, and mood using the measures described previously, participants were asked to take four puffs from a cigarette labeled Cigarette A, using the CReSS topography measurement device. The cigarettes used in the task were Quest 1 cigarettes (Vector Tobacco), which contain 0.6mg of nicotine and 10mg of tar, and Quest 3 cigarettes, which contain <0.05mg of nicotine and 10mg of tar. Cigarette order was counterbalanced across participants, and cigarette labels were covered with opaque tape, which varied in color between sessions and among participants. After the fourth puff of Cigarette A, participants completed the Hedonic Rating Scale, and then rested for 20min to allow the effects of Cigarette A to dissipate. Participants then repeated the cigarette sampling procedure with the other cigarette (Cigarette B) and rated it using the Hedonic Rating Scale. After a 20-min break, participants underwent 4 choice trials, scheduled 20min apart. In each trial, participants lit both cigarettes without inhaling, placed them in the ashtray, and were invited to take four puffs from either cigarette. At the end of the task, participants rated their craving, withdrawal symptoms, and mood and provided a breath CO sample.
Data Analysis
Group comparisons on demographic and smoking history measures were conducted using independent-samples t-tests for continuous variables and chi-square tests for categorical variables. Analyses of effects of Group (SS and CS) and Time (Sessions 2–8) on QSU and MNWS scores were conducted using generalized estimating equation (GEE) models (Zeger, Liang, & Albert, 1988), which allow for varying numbers of observations per participant while controlling for autocorrelation (AR1 structure). GEE was also used to analyze the effects of Group and Time (Session 1 and Session 8) on BPRS scores in SS. Mixed 2×2 analysis of variance tests (ANOVAs) were used to examine effects of Group and Abstinence (Session 2 and Session 8) on percent nicotine choices (number of nicotine puff choices divided by total choices, multiplied by 100) and within-session changes (post- minus pre-session values) in CO, QSU, MNWS, and mood scores. Mixed 2×2 × 2 ANOVAs were conducted to examine the effects of Group, Abstinence, and Nicotine Content (nicotine cigarette and denicotinized cigarette) on average puff volumes and hedonic rating scores. Significant interactions were followed by simple effects tests. Six participants (1 SS and 5 CS) opted not to smoke in Session 8 and were not included in these analyses.
Smoking lapse latency was defined as hours between the end of Session 8 and time to first lapse. Lapse latencies were compared in SS and CS using a t test. Mediation of the relationship between group and lapse latency was examined using an SPSS macro written by Hayes (Preacher & Hayes, 2004). Hypothesized mediators included baseline CES-D and Contemplation Ladder scores, Session 8 QSU and MNWS scores, and Session 8 percent nicotine choice. Simple mediator models calculated the direct effect of group on lapse latency (c path) with no mediator in the equation, as well as the group → mediator path (a), the mediator → latency path (b), and the effect of group on lapse latency with the mediator in the equation (cʹ path). The indirect effect of group on latency via each mediator (c–cʹ) is equal to the product of the coefficients of the a and b paths (i.e., “ab”). Because the distribution of “ab” is not normally distributed, 10,000 bootstrap resamples were used to obtain lower and upper 95% CIs for each indirect effect (Preacher & Hayes, 2008). All variables were first standardized so that path coefficients could be directly compared across variables with different metrics. Finally, step-wise regression was used to examine whether Session 1 BPRS scores (entered in Step 1) and Session 8 BPRS scores (Step 2) predicted lapse latency in SS. GEE, mediation, and regression analyses were conducted using the SPSS mainframe statistical package (SPSS Inc.). All other analyses were conducted using SPSS Statistics Version 20 for Windows (IBM). Differences were considered significant when p ≤ .05.
RESULTS
Sample Characteristics
Participants’ demographic and smoking history characteristics are shown in Table 1. The groups differed significantly on employment and average CES-D score. SS had low-to-moderate psychiatric symptom levels, and most (75%) were taking second-generation (atypical) antipsychotics. Other psychiatric medication use was also common in SS, with 57% taking antidepressant, 39% taking anxiolytic, and 36% taking mood-stabilizing medications. There was no psychiatric medication use among CS.
Table 1.
Baseline Characteristics of Study Participants
| SS (n = 28) | CS (n = 27) | |
|---|---|---|
| Age [M (SD)] | 44.0 (10.6) | 43.9 (10.8) |
| Male | 57% | 63% |
| Race | ||
| White | 69% | 82% |
| African American | 19% | 11% |
| Hispanic ethnicity | 7% | 4% |
| Employed full- or part-time*** | 0% | 26% |
| Years of education | 11.4 (2.9) | 12.2 (2.2) |
| Cigarettes/day | 21.6 (8.0) | 23.0 (8.4) |
| Fagerström Test of Nicotine Dependence score | 6.3 (1.7) | 6.2 (1.7) |
| Contemplation Ladder score | 6.0 (1.5) | 6.1 (1.1) |
| Years of daily smoking | 24.9 (11.4) | 25.6 (11.4) |
| Baseline CO level (ppm) | 37.0 (18.8) | 30.6 (15.5) |
| CES-D score*** | 19.9 (13.6) | 8.0 (6.8) |
| BPRS score | 36.5 (8.8) | |
| Antipsychotic drug class | ||
| Second-generation only | 75% | |
| First-generation only | 11% | |
| Both | 14% | |
Notes. BPRS = Brief Psychiatric Rating Scale; CO = carbon monoxide; CES-D = Center for Epidemiological Studies Depression Scale; CS = control smokers; SS = smokers with schizophrenia.
***p < .001.
Effects of 72-Hour Abstinence
There were no differences between groups on percentages that met the CO abstinence criteria at any timepoint. These percentages were as follows: at 6hr, 96% of SS and 96% of CS; at 24hr, 86% of SS and 93% of CS; at 30hr, 86% of SS and 89% of CS; at 48hr, 86% of SS and 85% CS; at 54hr, 86% of SS and 85% of CS; and at 72hr, 75% of SS and 78% of CS.
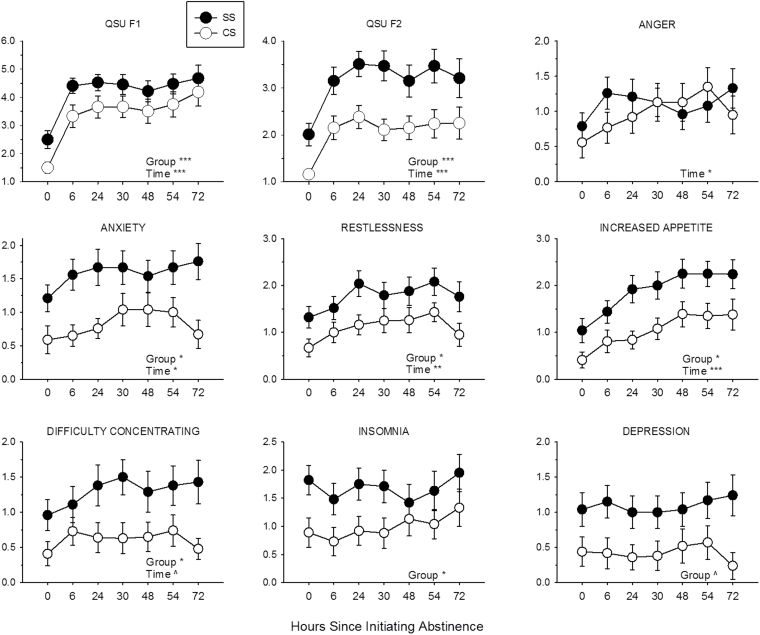
As shown in Figure 2, Time significantly predicted QSU scores such that these scores increased during abstinence (QSU F1: β = 0.42, SE = 0.06, 95% CI [0.31, 0.54], p < .0001 and QSU F2: β = 0.22, SE = 0.04, 95% CI [0.13, 0.30], p < .0001). Group also significantly predicted QSU scores such that these scores were higher among SS (QSU F1: β = 0.89, SE = 0.31, 95% CI [0.28, 1.50], p = .0001 and QSU F2: β = 0.77, SE = 0.17, 95% CI [0.43, 1.11], p < .0001). Time × Group interactions on QSU scores were not significant. Because the groups had significantly different CES-D scores at baseline and CES-D score can predict craving and nicotine withdrawal symptoms during abstinence (e.g., Leventhal et al., 2008), these data were reanalyzed with CES-D score included as a covariate. In these analyses, CES-D score did not significantly predict QSU F1 or F2 score (ps = .10 and .53, respectively). With CES-D score in the model, the relationship between Group and QSU F1 score became nonsignificant (β = 0.63, SE = 0.35, 95% CI [−0.06, 1.31], p = .07), but Group continued to significantly predict QSU F2 score (β = 0.66, SE = 0.24, 95% CI [0.19, 1.14], p < .01). Effects of Time and Time × Group interactions on QSU scores were unchanged.
Figure 2.
Effects of 72-hr abstinence on Questionnaire on Smoking Urges (QSU) factor and Minnesota Nicotine Withdrawal Scale (MNWS) item scores in SS (filled circles) and CS (open circles). Points represent M ± SEM. Significant and trend main effects of time and group are indicated (*p < .05; **p < .01; ***p < .001; ^p < .10).
Time significantly predicted total MNWS scores such that scores increased during abstinence (β = 0.07, SE = 0.02, 95% CI [0.03, 0.11], p = .0003; data not shown). As shown in Figure 2, Time significantly predicted scores on the MNWS items: anger (β = 0.07, SE = 0.04, 95% CI [0.01, 0.14], p < .05), anxiety (β = 0.06, SE = 0.03, 95% CI [0.003, 0.11], p < .05), restlessness (β = 0.07, SE = 0.03, 95% CI [0.02, 0.13], p < .01), and increased appetite (β = 0.19, SE = 0.04, 95% CI [0.12, 0.27], p < .0001). The relationship between Time and difficulty concentrating approached significance (β = 0.05, SE = 0.03, 95% CI [0.10, 1.78], p = .07) and Time did not significantly affect depression or insomnia (ps = .92 and .19, respectively). Group significantly predicted MNWS total scores such that these scores were higher among SS (β = 0.58, SE = 0.23, 95% CI [0.12, 1.04], p = .014; data not shown). SS had significantly higher scores on the MNWS items: anxiety (β = 0.65, SE = 0.26, 95% CI [0.15, 1.16], p < .05), restlessness (β = 0.65, SE = 0.27, 95% CI [0.12, 2.38], p < .05), increased appetite (β = 0.59, SE = 0.27, 95% CI [0.05, 1.13], p < .05), difficulty concentrating (β = 0.56, SE = 0.28, 95% CI [0.015, 1.10], p < .05), and insomnia (β = 0.80, SE = 0.34, 95% CI [0.14, 1.47], p < .05). The relationship between Group and depression approached significance (β = 0.59, SE = 0.32, 95% CI [−0.04, 1.21], p = .06) and Group did not significantly predict anger (p = .44). There were no significant Time × Group interactions on MNWS scores. These data were reanalyzed with CES-D score included as a covariate. In these analyses, CES-D score significantly predicted total MNWS score (β = 0.04, SE = 0.01, 95% CI [0.02, 0.06], p = .0002) and scores on all items except for increased appetite. With CES-D score in the model, Group no longer significantly predicted total MNWS or any MNWS item score. Relationships between Time and MNWS scores were unchanged.
In SS, BPRS–anergia factor scores were higher at Session 8 than at Session 1 (β = 1.27, SE = 0.55, 95% CI [0.19, 2.35], p < .05). No other BPRS factor score changed significantly during abstinence (Table 2). In SS, CES-D score was significantly related to BPRS total score (β = 0.35, SE = 0.07, 95% CI [0.22, 0.48], p < .0001) and scores on the BPRS thinking disorder (β = 0.06, SE = 0.02, 95% CI [0.03, 0.09], p < .001), anxiety–depression (β = 0.19, SE = 0.02, 95% CI [0.15, 0.24], p < .0001), and hostility–suspiciousness factors (β = 0.07, SE = 0.03, 95% CI [0.01, 0.13], p < .05), but not to scores on the activity or anergia factors. Including CES-D score as a covariate in these analytic models did not change the relationship between time and BPRS–anergia score.
Table 2.
Psychiatric Symptoms in Participants With Schizophrenia Before and After 72-Hr Abstinence (M [SD])
| BPRS score | Session 1 | Session 8 | |
|---|---|---|---|
| All enrolled (n = 30) | Study completers (n = 21) | ||
| Total | 36.5 (8.6) | 35.5 (7.2) | 35.9 (9.2) |
| Thinking disorder | 6.7 (2.2) | 6.9 (2.3) | 6.0 (2.4) |
| Anergia | 8.9 (3.2)a | 8.6 (2.7)a | 10.0 (3.1)b |
| Anxiety–depression | 10.3 (3.9) | 9.9 (3.1) | 9.5 (3.9) |
| Hostility–suspicion | 5.9 (2.4) | 5.5 (2.1) | 5.7 (2.2) |
| Activity | 4.8 (1.4) | 4.6 (1.3) | 4.7 (1.2) |
Note. BPRS = Brief Psychiatric Rating Scale.
Significant differences (p < .05) between means are indicated with different superscript letters (a and b).
Nicotine Reinforcement and Smoking Reinstatement
There was a significant Group × Abstinence interaction on percent nicotine choice (F [1, 34] = 4.46, p < .05). Post-hoc simple effects tests indicated that SS and CS significantly differed on percent nicotine choice at Session 8 (SS: 85.0±7.7 [M ± SD]; CS: 61.3±8.6; p < .05) but not at Session 2 (SS: 69.1±8.3; CS: 77.7±9.3). However, percent nicotine choice did not significantly increase across sessions among SS or decrease across sessions among CS (ps = .13 and .16, respectively). There were significant main effects of nicotine content on hedonic rating and average puff volume (F [1, 34] = 9.34, p < .01 and F [1, 34] = 17.32, p < .001, respectively). Averaging across groups and sessions, participants gave higher hedonic ratings for nicotine puffs than for denicotinized puffs (nicotine: 5.40±3.37 and denicotinized: 4.08±3.19) and took larger puffs when smoking denicotinized cigarettes than when smoking nicotine cigarettes (denicotinized: 64.0±15.1ml; nicotine: 57.7±15.7ml). A Group × Abstinence interaction on puff volume approached significance (F [1, 34] = 3.94, p = .055), with simple effects tests indicating that CS took larger puffs (averaged across cigarette type) than SS in Session 2 (CS: 66.0±11.7ml; SS: 55.5±16.4ml; p < .05) but not Session 8 (CS: 63.1±13.6ml; SS: 58.7±20.7ml).
Post- minus pre-session change scores for other measures collected during the nicotine preference task in Sessions 2 and 8 are shown in Table 3. There were significant effects of abstinence on changes in CO and QSU F1 and QSU F2 scores (F [1, 34] = 69.11, p < .001; F [1, 34] = 26.88, p < .001; F [1, 34] = 13.00, p = .001, respectively). Averaging across groups, participants had larger increases in CO and decreases in QSU F1 and F2 scores after smoking in Session 8 than in Session 2. There was a significant main effect of group on within-session change in positive mood (F [1, 34] = 4.87, p < .05). Averaged across sessions, smoking increased positive mood in SS but decreased positive mood in CS.
Table 3.
Post- Minus Pre-Session Change Scores (M [SD]) of Measures Collected During the Nicotine Preference Task in Sessions 2 and 8
| Session 2 (preabstinence) | Session 8 (postabstinence) | p values | |||||
|---|---|---|---|---|---|---|---|
| SS | CS | SS | CS | Group | Abstinence | Group × abstinence | |
| CO (ppm) | +0.6 (8.4) | +3.4 (7.5) | +11.4 (4.1) | +12.3 (4.2) | .32 | <.001 | .42 |
| QSU F1 | −0.2 (2.0) | +0.1 (0.5) | −1.5 (2.3) | −2.4 (2.0) | .60 | <.001 | .16 |
| QSU F2 | −0.0 (1.2) | −0.0 (0.1) | −1.1 (1.5) | −0.7 (1.0) | .53 | .001 | .52 |
| MNWS | −0.0 (0.4) | −0.0 (0.4) | −0.2 (1.0) | −0.2 (1.0) | .93 | .13 | .94 |
| Positive mood | +0.2 (0.8) | −0.3 (1.2) | +0.4 (1.3) | −0.4 (1.1) | .03 | .89 | .47 |
| Negative mood | −0.3 (1.0) | −0.3 (1.0) | +0.1 (1.1) | −0.5 (1.6) | .32 | .75 | .25 |
Note. CO = carbon monoxide; MNWS = Minnesota Nicotine Withdrawal Scale; QSU = Questionnaire on Smoking Urges.
Lapse Latency and Mediation
The groups differed significantly on lapse latency (t [40] = 2.52, p < .05). Average latencies were 12.3±14.0hr in SS and 53.7±74.1hr in CS. Seventy-six percent of SS and 52% of CS lapsed within 24hr of Session 8, as verified by breath CO at Session 9. Only one SS remained abstinent for >48hr; in contrast, 33% of CS remained abstinent for >48hr, including 4 CS who were still abstinent at the end of the follow-up period, all of whom had opted not to smoke in Session 8.
Mediation analyses indicated that baseline CES-D score mediated the relationship between group and lapse latency (ab = −.19; percentile CIs [CI p] = −.46 to −.06 and bias-corrected CIs [CI bc] = −.42 to −.05; R 2 = .17; p < .05). In a separate model, Session 8 MNWS score also mediated the relationship between group and lapse latency (ab = −.18; CI p = −.47 to −.05 and CI bc = −.39 to −.02; R 2 = .18; p < .05), but mediation was no longer supported when either Session 2 MNWS score or baseline CES-D score was entered in the model as a covariate. The relationship between group and lapse latency was not mediated by baseline Contemplation Ladder score, Session 8 QSU score, or Session 8 percent nicotine choice.
Regression analyses in SS indicated that baseline BPRS total and anxiety–depression factor scores were significantly associated with lapse latency (ps < .05), and the relationship between baseline anergia factor score and lapse latency approached significance (p = .07), when entered on Step 1. Session 1 scores were no longer significant when both variables were entered into the regression equation. No Session 8 BPRS scores were associated with lapse latency after controlling for Session 1 BPRS scores.
DISCUSSION
The results of this study provide novel information on mechanisms contributing to the low smoking cessation rate in people with schizophrenia, by demonstrating that, relative to CS, SS experience more severe cigarette craving and nicotine withdrawal symptoms throughout the first 72-hr of smoking abstinence, stronger reinforcing effects of nicotine after abstinence, and resume smoking significantly sooner after abstinence. Furthermore, the relationship between schizophrenia and latency to lapse is mediated by baseline depression and nicotine withdrawal symptom severity, but not by effects of abstinence on craving or nicotine reinforcement. Overall, these results indicate that negative affect is a key contributor to poor smoking cessation outcomes in schizophrenia.
To our knowledge, this is the first study to systematically compare the effects of >16-hr abstinence in SS and CS. Previous studies found that SS reported more severe withdrawal symptoms than CS when nonabstinent, but that 5-hr abstinence increased withdrawal symptoms to a similar extent in both groups (Tidey et al., 2008, 2013). Our current results are consistent with these findings and indicate that over a 72-hr abstinence period, nicotine withdrawal symptoms remain higher in SS than CS without any indication of an interaction between these factors. Furthermore, the fact that group differences on withdrawal symptoms were eliminated by covarying baseline CES-D score indicates that they are due to underlying group differences on depression severity. Previous studies have reported similar smoking urge levels in SS and CS after 5- or 16-hr abstinence (Fonder et al., 2005; Tidey et al., 2008, 2013). In contrast, we now report that SS experience higher urge levels over a 72-hr abstinence period, which is partially attributable to underlying group differences in depression severity. However, even after covarying baseline CES-D scores, SS continued to have higher QSU factor 2 scores during abstinence, suggesting that schizophrenia is uniquely associated with an aspect of craving that is not due to depression. Experimental studies comparing abstinence effects in SS and smokers with major depressive disorder would be useful for clarifying the extent to which mechanisms underlying smoking in these disorders overlap.
Another important finding was that the relative reinforcing effects of nicotine were higher in SS than CS after 72-hr abstinence. This is consistent with a study using an animal model of schizophrenia (Berg & Chambers, 2008) and with studies reporting that SS made more choices for smoking versus alternative reinforcers than CS (MacKillop & Tidey, 2011; Spring et al., 2003). However, our findings differed from our prediction in that we did not observe a larger pre- to post-abstinence increase in nicotine preference in SS than CS; rather, abstinence increased nicotine preference nonsignificantly in SS and decreased nicotine preference nonsignificantly in CS. Moreover, nicotine preference did not predict lapse latency in either group. Thus, our results do not appear to be consistent with the hypothesis that the poor smoking cessation outcomes in SS are due to stronger reinforcing effects of nicotine during smoking reinstatement.
Baseline depression severity, nicotine withdrawal symptoms, total BPRS score, and BPRS anxiety–depression factor scores were significantly associated with smoking lapse latency in SS. Although anergia factor scores (i.e., negative schizophrenia symptoms) increased in SS during abstinence, neither these nor positive symptom scores were related to lapse latency. Results of this study, therefore, provide only limited support for the hypothesis that the association between schizophrenia and smoking is due to self-medication of psychiatric symptoms; specifically, our results indicate that negative affect contributes to early smoking lapse in SS, but do not support the idea that self-medication of positive or negative schizophrenia symptoms contributes to their smoking. It must be acknowledged, however, that this study was underpowered to detect mediation effects.
Results of this study should be interpreted in light of its other limitations as well, one of which is that study participants were not intrinsically motivated to quit smoking; rather, incentives were provided to engender abstinence. It is possible that different relationships between abstinence effects and smoking lapse may exist among smokers who are intrinsically motivated to quit. A second limitation involves two of the measures used in this study. The CES-D, although frequently used in clinical studies, was originally designed for epidemiological research (Radloff, 1977), and the BPRS, while having sound psychometric properties, is a relatively short scale that may be less sensitive to symptom changes than more extensive scales (Mortimer, 2007). Given the overlap between depression symptoms and negative schizophrenia symptoms, and the fact that other smoking studies have not reported elevated depression symptoms in SS versus CS (e.g., George et al., 2002), future studies assessing mediators of smoking lapse in this population should use instruments such as the Calgary Depression Scale (Addington, Addington, & Maticka-Tyndale, 1993), which has minimal overlap with negative symptoms, and the Positive and Negative Syndrome Scale (Kay, Fiszbein, & Opler, 1987) or Scale for the Assessment of Negative Symptoms (Andreasen, 1984) that may better differentiate between the roles of depression and negative symptoms in mediating smoking behavior in SS. A third limitation is that we did not assess several factors that contribute to smoking cessation outcomes in SS, such as self-efficacy for cessation, task persistence, and other measures of executive functioning (Mann-Wrobel, Bennett, Weiner, Buchanan, & Ball, 2011; Moss et al., 2009; Steinberg et al., 2012). The relative contributions of these factors could be examined in future studies using the current model.
Notwithstanding these limitations, this study provides novel information that may help to improve smoking cessation approaches for SS. The significant role of negative affect in predicting lapse latency in this study suggests that smoking treatment approaches for SS should focus on reducing depression and nicotine withdrawal symptom severity, both before and during abstinence. This study design also provides an efficient model for testing whether medications that reduce these affective states improve smoking cessation outcomes in SS.
FUNDING
This work was supported by the National Institute on Drug Abuse (R21DA026829 and U54DA031659).
DECLARATION OF INTERESTS
None declared.
ACKNOWLEDGMENTS
We thank Laura Dionne and Jennifer Schmidlin for their careful assistance with data management and Suzanne Sales for her expert assistance with data analysis. Portions of these data were presented at annual meetings of the College on Problems of Drug Dependence and the Society for Research on Nicotine and Tobacco. Work on this manuscript was conducted at the Center for Alcohol and Addiction Studies, Brown University, Providence, RI, USA.
REFERENCES
- Addington D., Addington J., Maticka-Tyndale E. (1993). Assessing depression in schizophrenia: The Calgary Depression Scale. British Journal of Psychiatry Supplement, 163 (Suppl 22), 39–44 [PubMed] [Google Scholar]
- AhnAllen C. G., Liverant G. I., Gregor K. L., Kamholz B. W., Levitt J. J., Gulliver S. B, … Kaplan G. B. (2012). The relationship between reward-based learning and nicotine dependence in smokers with schizophrenia. Psychiatry Research, 196, 9–14. 10.1016/j.psychres.2011.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi S. M., Badger G. J., Higgins S. T. (2004). An experimental examination of the initial weeks of abstinence in cigarette smokers. Experimental and Clinical Psychopharmacology, 12, 276–287 doi:10.1037/1064-1297.12.4.276 [DOI] [PubMed] [Google Scholar]
- Andreasen N. C. (1984). Scale for the Assessment of Negative Symptoms (SANS). Iowa City, Iowa: University of Iowa [Google Scholar]
- Berg S. A., Chambers R. A. (2008). Accentuated behavioral sensitization to nicotine in the neonatal ventral hippocampal lesion model of schizophrenia. Neuropharmacology, 54, 1201–1207. 10.1016/j.neuropharm.2008.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L., Abrams D. B. (1991). The Contemplation Ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology, 10, 360–365. 10.1037//0278-6133.10.5.360 [DOI] [PubMed] [Google Scholar]
- Brown R. A., Burgess E. S., Sales S. D., Whiteley J. A., Evans D. M., Miller I. W. (1998). Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors, 12, 101–112 doi:10.1037/0893-164X.12.2.101 [Google Scholar]
- Brown S., Kim M., Mitchell C., Inskip H. (2010). Twenty-five year mortality of a community cohort with schizophrenia. The British Journal of Psychiatry, 196, 116–121. 10.1192/bjp.bp.109.067512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger G. K., Yonker R. D., Calsyn R. J., Morse G. A., Klinkenberg W. D. (2003). A confirmatory factor analysis of the Brief Psychiatric Rating Scale in a homeless sample. International Journal of Methods in Psychiatric Research, 12, 192–196 doi:10.1002/mpr.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey K. B. (1997). Reliability and validity of the Time-line Follow-Back Interview among psychiatric outpatients: A preliminary report. Psychology of Addictive Behaviors, 11, 26–33 doi:10.1037/0893-164X.11.1.26 [Google Scholar]
- Chambers R. A., Krystal J. H., Self D. W. (2001). A neurobiological basis for substance abuse comorbidity in schizophrenia. Biological Psychiatry, 50, 71–83 doi:10.1016/S0006-3223(01)01134-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chornock W. M., Stitzer M. L., Gross J., Leischow S. (1992). Experimental model of smoking re-exposure: Effects on relapse. Psychopharmacology, 108, 495–500 doi:10.1007/BF02247427 [DOI] [PubMed] [Google Scholar]
- Cox L. S., Tiffany S. T., Christen A. G. (2001). Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research, 3, 7–16. 10.1080/14622200020032051 [DOI] [PubMed] [Google Scholar]
- DeMarce J. M., Burden J. L., Lash S. J., Stephens R. S., Grambow S. C. (2007). Convergent validity of the Timeline Followback for persons with comorbid psychiatric disorders engaged in residential substance use treatment. Addictive Behaviors, 32, 1582–1592 doi:10.1016/j.addbeh.2006.11.015 [DOI] [PubMed] [Google Scholar]
- Diener E., Emmons R. A. (1984). The independence of positive and negative affect. Journal of Personality and Social Psychology, 47, 1105–1117 doi:10.1037/0022-3514.47.5.1105 [DOI] [PubMed] [Google Scholar]
- First M. B., Spitzer R. L., Gibbon M., Williams J. B. (1994). Structured clinical interview for DSM-IV axis-I disorders – non-patient edition. New York, NY: Biometric Research Department [Google Scholar]
- Fonder M. A., Sacco K. A., Termine A., Boland B. S., Seyal A. A., Dudas M. M, … George T. P. (2005). Smoking cue reactivity in schizophrenia: Effects of a nicotinic receptor antagonist. Biological Psychiatry, 57, 802–808. 10.1016/j.biopsych.2004.12.027 [DOI] [PubMed] [Google Scholar]
- George T. P., Vessicchio J. C., Termine A., Sahady D. M., Head C. A., Pepper W. T, … Wexler B. E. (2002). Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology, 26, 75–85 doi:10.1016/S0893-133X(01)00296-2 [DOI] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K.-O. (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127. 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Heil S. H., Tidey J. W., Holmes H. W., Badger G. J., Higgins S. T. (2003). A contingent payment model of smoking cessation: Effects on abstinence and withdrawal. Nicotine & Tobacco Research, 5, 205–213 doi:10.1080/1462220031000074864 [DOI] [PubMed] [Google Scholar]
- Hennekens C. H., Hennekens A. R., Hollar D., Casey D. E. (2005). Schizophrenia and increased risks of cardiovascular disease. American Heart Journal, 150, 1115–1121. 10.1016/j.ahj.2005.02.007 [DOI] [PubMed] [Google Scholar]
- Hughes J. R., Hatsukami D. (1986). Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry, 43, 289–294. 10.1001/archpsyc.1986.01800030107013 [DOI] [PubMed] [Google Scholar]
- Hughes J. R., Keely J., Naud S. (2004). Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction, 99, 29–38 doi:10.1111/j.1360-0443.2004.00540.x [DOI] [PubMed] [Google Scholar]
- Juliano L. M., Donny E. C., Houtsmuller E. J., Stitzer M. L. (2006). Experimental evidence for a causal relationship between smoking lapse and relapse. Journal of Abnormal Psychology, 115, 166–173 doi:10.1037/0021-843X.115.1.166 [DOI] [PubMed] [Google Scholar]
- Kay S. R., Fiszbein A., Opler L. A. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13, 261–276 doi:10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- Kenford S. L., Fiore M. C., Jorenby D. E., Smith S. S., Wetter D., Baker T. B. (1994). Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA, 271, 589–594 doi:10.1001/jama.1994.03510320029025 [DOI] [PubMed] [Google Scholar]
- Kenford S. L., Smith S. S., Wetter D. W., Jorenby D. E., Fiore M. C., Baker T. B. (2002). Predicting relapse back to smoking: Contrasting affective and physical models of dependence. Journal of Consulting and Clinical Psychology, 70, 216–227 doi:10.1037/0022-006X.70.1.216 [PubMed] [Google Scholar]
- Killen J. D., Fortmann S. P. (1997). Craving is associated with smoking relapse: Findings from three prospective studies. Experimental and Clinical Psychopharmacology, 5, 137–142 doi:10.1037/1064-1297.5.2.137 [DOI] [PubMed] [Google Scholar]
- Leventhal A. M., Ramsey S. E., Brown R. A., LaChance H. R., Kahler C. W. (2008). Dimensions of depressive symptoms and smoking cessation. Nicotine & Tobacco Research, 10, 507–517. 10.1080/14622200801901971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J., Tidey J. W. (2011). Cigarette demand and delayed reward discounting in nicotine-dependent individuals with schizophrenia and controls: An initial study. Psychopharmacology, 216, 91–99. 10.1007/s00213-011-2185-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann-Wrobel M. C., Bennett M. E., Weiner E. E., Buchanan R. W., Ball M. P. (2011). Smoking history and motivation to quit in smokers with schizophrenia in a smoking cessation program. Schizophrenia Research, 126, 277–283. 10.1016/j.schres.2010.10.030 [DOI] [PubMed] [Google Scholar]
- Mortimer A. M. (2007). Symptom rating scales and outcome in schizophrenia. The British Journal of Psychiatry Supplement, 50, s7–s14 doi:10.1192/bjp.191.50.57 [DOI] [PubMed] [Google Scholar]
- Moss T. G., Sacco K. A., Allen T. M., Weinberger A. H., Vessicchio J. C., George T. P. (2009). Prefrontal cognitive dysfunction is associated with tobacco dependence treatment failure in smokers with schizophrenia. Drug and Alcohol Dependence, 104, 94–99. 10.1016/j.drugalcdep.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall J. E., Gorham D. R. (1962). The Brief Psychiatric Rating Scale. Psychological Reports, 10, 799–812. 10.2466/pr0.1962.10.3.799 [Google Scholar]
- Perkins K. A., Grobe J. E., Weiss D., Fonte C., Caggiula A. (1996). Nicotine preference in smokers as a function of smoking abstinence. Pharmacology, Biochemistry, and Behavior, 55, 257–263 doi:10.1016/S0091-3057(96)00079-2 [DOI] [PubMed] [Google Scholar]
- Perkins K. A., Lerman C., Stitzer M., Fonte C. A., Briski J. L., Scott J. A., Chengappa K. N. (2008). Development of procedures for early screening of smoking cessation medications in humans. Clinical Pharmacology and Therapeutics, 84, 216–221. 10.1038/clpt.2008.30 [DOI] [PubMed] [Google Scholar]
- Piasecki T. M., Jorenby D. E., Smith S. S., Fiore M. C., Baker T. B. (2003). Smoking withdrawal dynamics: II. Improved tests of withdrawal-relapse relations. Journal of Abnormal Psychology, 112, 14–27 doi:10.1037/0021-843X.112.1.14 [PubMed] [Google Scholar]
- Preacher K. J., Hayes A. F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40, 879–891 doi:10.3758/BRM.40.3.879 [DOI] [PubMed] [Google Scholar]
- Preacher K. J., Hayes A. F. (2004). SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers, 36, 717–731 doi:10.3758/BF03206553 [DOI] [PubMed] [Google Scholar]
- Radloff L. S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401 doi:10.1177/ 014662167700100306 [Google Scholar]
- Rukstalis M., Jepson C., Strasser A., Lynch K. G., Perkins K., Patterson F., Lerman C. (2005). Naltrexone reduces the relative reinforcing value of nicotine in a cigarette smoking choice paradigm. Psychopharmacology, 180, 41–48 doi:10.1007/s00213-004-2136-8 [DOI] [PubMed] [Google Scholar]
- Sacco K. A., Termine A., Seyal A., Dudas M. M., Vessicchio J. C., Krishnan-Sarin S, … George T. P. (2005). Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: Involvement of nicotinic receptor mechanisms. Archives of General Psychiatry, 62, 649–659 doi:10.1001/archpsyc.62.6.649 [DOI] [PubMed] [Google Scholar]
- Shiffman S., Ferguson S. G., Gwaltney C. J. (2006). Immediate hedonic response to smoking lapses: Relationship to smoking relapse, and effects of nicotine replacement therapy. Psychopharmacology, 184, 608–618 doi:10.1007/s00213-005-0175-4 [DOI] [PubMed] [Google Scholar]
- Spring B., Pingitore R., McChargue D. E. (2003). Reward value of cigarette smoking for comparably heavy smoking schizophrenic, depressed, and nonpatient smokers. The American Journal of Psychiatry, 160, 316–322 doi:10.1176/appi.ajp.160.2.316 [DOI] [PubMed] [Google Scholar]
- Steinberg M. L., Williams J. M., Gandhi K. K., Foulds J., Epstein E. E., Brandon T. H. (2012). Task persistence predicts smoking cessation in smokers with and without schizophrenia. Psychology of Addictive Behaviors, 26, 850–858. 10.1037/a0028375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong D. R., Leventhal A. M., Evatt D. P., Haber S., Greenberg B. D., Abrams D., Niaura R. (2011). Positive reactions to tobacco predict relapse after cessation. Journal of Abnormal Psychology, 120, 999–1005. 10.1037/a0023666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey J. W., Rohsenow D. J., Swift R. M., Kaplan G. B., Adolfo A. B. (2008). Effects of smoking abstinence, smoking cues and nicotine replacement in smokers with schizophrenia and controls. Nicotine & Tobacco Research, 10, 1047–1056. 10.1080/14622200802097373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey J. W., Rohsenow D. J., Kaplan G. B., Swift R. M., AhnAllen C. G. (2013). Separate and combined effects of very low nicotine cigarettes and nicotine replacement in smokers with schizophrenia and controls. Nicotine & Tobacco Research, 15, 121–129. 10.1093/ntr/nts098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger A. H., Sacco K. A., Creeden C. L., Vessicchio J. C., Jatlow P. I., George T. P. (2007). Effects of acute abstinence, reinstatement, and mecamylamine on biochemical and behavioral measures of cigarette smoking in schizophrenia. Schizophrenia Research, 91, 217–225. 10.1016/j.schres.2006.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. M., Gandhi K. K., Lu S. E., Kumar S., Steinberg M. L., Cottler B., Benowitz N. L. (2011). Shorter interpuff interval is associated with higher nicotine intake in smokers with schizophrenia. Drug and Alcohol Dependence, 118, 313–319. 10.1016/j.drugalcdep.2011.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. M., Ziedonis D. M., Abanyie F., Steinberg M. L., Foulds J., Benowitz N. L. (2005). Increased nicotine and cotinine levels in smokers with schizophrenia and schizoaffective disorder is not a metabolic effect. Schizophrenia Research, 79, 323–335.10.1016/j.schres.2005.04.016 [DOI] [PubMed] [Google Scholar]
- Zeger S. L., Liang K. Y., Albert P. S. (1988). Models for longitudinal data: A generalized estimating equation approach. Biometrics, 44, 1049–1060 doi:10.2307/2531734 [PubMed] [Google Scholar]