Abstract
Introduction
Idiopathic acute eosinophilic pneumonia (AEP) is characterized by hypoxemia, pulmonary infiltrates and pulmonary eosinophilia. Data is limited and the purpose of this study is to better understand this disorder.
Methods
A search of the computerized patient records from January 1, 1997 to October 15, 2010 for patients with suspicion of “eosinophilic pneumonia” was conducted. Included patients were 18 years or older with an acute febrile illness, hypoxemia, diffuse pulmonary infiltrates on imaging, and pulmonary eosinophilia. Patients were excluded with other known causes of pulmonary eosinophilia.
Results
Of 195 patients with pulmonary eosinophilia, 8 patients had “definite” or “probable” and 4 patients had “possible” idiopathic AEP. Three patients were categorized as “probable” idiopathic AEP due to exceeding expected maximal 30-day symptom duration and/or a maximal recorded temperature less than 38 °C. Four patients were defined as “possible” idiopathic AEP given histories of polymyalgia rheumatica, eczema or allergic rhinitis. Of the 8 included patients, 63% were male with a median age of 53. Median duration of symptoms was 21 days. Median nadir oxygen saturation was 83%. Median eosinophil count on bronchoalveolar lavage was 36%. Two patients required intubation. Two patients were current smokers, one of whom had reported a change in smoking habits. All patients were treated with steroids (median of two months).
Conclusions
As diagnostic methods and pharmacologic knowledge improve, the number of patients meeting criteria for idiopathic AEP remains small. Much remains to be learned about this truly rare condition, and current criteria may exclude milder presentations of the disease.
Keywords: Idiopathic acute eosinophilic pneumonia, Smoking, Pulmonary eosinophilia
1. Introduction
Acute eosinophilic pneumonia (AEP) is a rare disorder marked by hypoxemia, pulmonary infiltrates and pulmonary eosinophilia [1,2]. AEP occurs secondary to medications or hypersensitivity reactions to an inhaled antigen (such as tobacco smoke) [3–8]. Hematopoietic stem transplant and autoimmune diseases are associated with eosinophilic lung disease and also need to be distinguished from idiopathic AEP [1,9].
Current data on idiopathic AEP is limited. Diagnostic criteria vary but typically include an acute febrile illness, hypoxemic respiratory failure, diffuse pulmonary infiltrates on imaging, and pulmonary eosinophilia with exclusion of known causes of pulmonary eosinophilia [2]. Reported case series demonstrate variations in duration of illness, definition of fever (or documentation of anti-pyretic use), definition of pulmonary eosinophilia, documentation of hypoxia and exclusion of atopy, allergic rhinitis and asthma [2,8,10–12]. The extent to which known causes of pulmonary eosinophilia were excluded also varies.
We performed a single-center retrospective case review and literature review to summarize available data. Our objective was to focus on idiopathic AEP (by carefully excluding known causes of pulmonary eosinophilia) and examining disease characteristics in our series and prior reports.
2. Methods
This protocol was approved by the Institutional Review Board (#10-006298). We searched the computerized medical records from January 1, 1997 to October 15, 2010 of patients consenting to research for the term “eosinophilic pneumonia”. Those patients were screened for presence of pulmonary eosinophilia (demonstrated eosinophils on lung biopsy or BAL with >25% eosinophils) [2,10]. Patients met criteria for inclusion if aged 18 years or older with an acute febrile illness (<45 days in duration), hypoxemia or desaturation (nadir oxygen saturation (SpO2) < 90% or PaO2 <60 mm Hg), diffuse pulmonary infiltrates on chest imaging (chest radiograph or chest computed tomography), and pulmonary eosinophilia (as defined above). Patients were excluded with asthma or other known causes of pulmonary eosinophilia. Patients meeting these criteria were categorized as “definite” idiopathic AEP. Patients with a maximal temperature of 37.2 ○C or above (but less than 38.0 ○C) without subjective fevers or illness duration greater than 30 days were characterized as “probable” idiopathic AEP. Charts were reviewed for demographics, laboratory and imaging findings, and clinical course.
3. Results
195 patients with suspected “eosinophilic pneumonia” demonstrated pulmonary eosinophilia (as defined above). Of these 195, 12 patients were included and 183 were excluded for various reasons (see Fig. 1). Thirteen patients were excluded due to relapse of symptoms on steroid taper and 2 patients were excluded due to lack of diffuse infiltrates on chest imaging. Five patients had all characteristics consistent with the diagnosis of idiopathic AEP. Three patients were categorized as “probable” idiopathic AEP due to exceeding expected 30-day symptom duration (37 and 38 days) and/or maximal temperature less than 38 ○C and are included in analysis. Four patients were defined as “possible” idiopathic AEP given history of polymyalgia rheumatica, eczema or allergic rhinitis and are described but not included in analysis. Clinical features of included patients are summarized in Table 1 and Table 2.
Fig. 1.
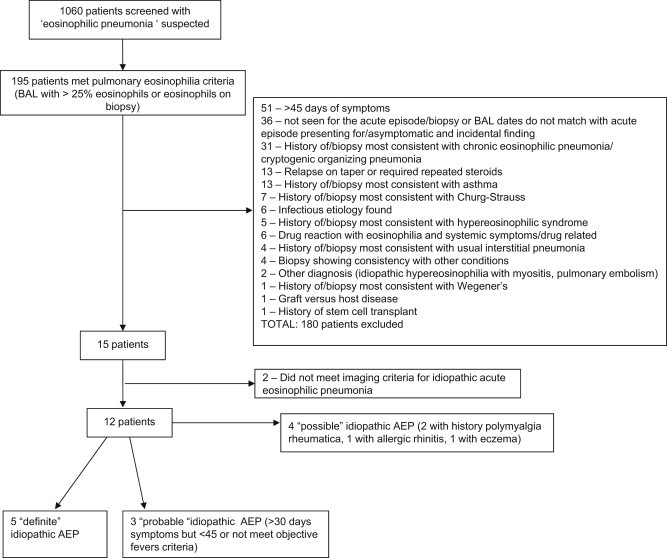
Trial flow including reasons for exclusion.
Table 1.
Description of cases at our institution.
| Patient | Age at diagnosis | Sex | Maximal temperature (celsius) | Fevers or chills | Nadir SpO2 (%) | Symptom duration (days) | Bilateral infiltrates modality | BAL eosinophils (%) | Eosinophils present on biopsy |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | M | 38.6 | Yes | 79 | 7 | CXR | 53 | Yes |
| 2 | 32 | F | 37.6 | Yes | 68 | 15 | CT | 34 | Not performed |
| 3 | 61 | M | 39.7 | Yes | 83 | 4 | CXR | 9 | Yes |
| 4 | 74 | M | 37.8 | Yes | 85 | 21 | CXR | 42 | Yes |
| 5 | 76 | M | 37.4 | Yes | 86 | 21 | CXR | 37 | No |
| 6 | 19 | F | 39.0 | Yes | 61 | 38 | CXR | 28 | Not performed |
| 7 | 79 | F | 37.5 | No | 82 | 37 | CXR | 20 | Yes |
| 8 | 22 | M | 37.2 | No | 83 | 30 | CXR | 87 | Yes |
| 9a | 73 | F | 38.0 | No | 81 | 7 | CT | 6 | Yes |
| 10b | 72 | M | 37.0 | Yes | 89 | 14 | CXR | 80 | Not performed |
| 11c | 57 | M | 37.3 | Yes | 83 | 35 | CT | Not performed | Yes |
| 12d | 22 | M | 38.7 | Yes | 88 | 2 | CXR | 60 | Not performed |
Patients who meet all criteria for idiopathic acute eosinophilic pneumonia (cases 1–5), probable idiopathic acute eosinophilic pneumonia (cases 6–8), or possible eosinophilic pneumonia despite history of atopic disease (cases 9–12). CXR: chest radiograph. CT: Computed tomography.
Patient with history of polymyalgia rheumatica.
Patient with history of polymyalgia rheumatica.
Patient with history of eczema, rheumatoid arthritis.
Patient with history of allergic rhinitis (required intubation).
Table 2.
Summary of clinical features of definite and probable idiopathic AEP cases at our institution.
| Age | 53 years old (19–79) |
| Male | 63% |
| Duration of symptoms | 21 days (4–38) |
| Maximum temperature | 37.7 °C (37.2–39.7) |
| Nadir SpO2 | 83% (61–86) |
| White blood cell count on admission (×109/L) | 13.2 (7.3–27.8) |
| Eosinophil count on admission (×109/L) | 0.34 (0.01–10.56) |
| BAL eosinophils | 36% (9–87) |
| Duration of hospitalization | 7 days (1–12) |
| Intubation | 25% (1–3 days) |
| Duration of steroids therapy | 60 days (35–285) |
All numbers above are median (range) unless otherwise noted.
4. Discussion
4.1. Presenting symptoms
While idiopathic AEP is by definition an acute illness, the symptom duration has been variably defined as less than 7 [11] or less than 30 days [2] without significant differences in the clinical manifestations. We examined all cases of possible idiopathic AEP of less than 45 days duration and describe two cases with symptoms slightly longer than 30 days as “probable” AEP. Although it can be argued that a longer duration of symptoms may lead to confusion with chronic eosinophilic pneumonia, the average duration of symptoms in chronic eosinophilic pneumonia is significantly longer (19.7 weeks) and the disease characteristics are distinct [13].
Likewise the definition of “febrile illness” varies in the reported literature including temperatures as low as 37.2 °C [6,12]. We used subjective fevers or a temperature of 38 °C. We have included one patient without fever who was using anti-pyretic agents. Little attention has been made to the presence or absence of anti-pyretic agents or cooling device use in prior reported cases. Furthermore, patients with sepsis may have an abnormally low temperature in lieu of fever. It is unknown if this is also true of AEP as such patients have by definition been excluded from AEP criteria. With this in mind clinicians should consider AEP in patients in the absence of fever if other features are compatible.
Hypoxemia has been typically described as a PaO2 less than 60 mm Hg or a pulse oximetry saturation of less than 90% on room air [2]. However, one recent case series did not use hypoxia/hypoxemia as an inclusion criteria for potential cases of idiopathic AEP [12]. In reviewing the literature, little attention has been paid to desaturation with activity. Some of our patients did not have hypoxia at rest but had hypoxia with minimal exertion. Desaturation with activity should be considered as a criterion for AEP. In patients without hypoxia but with other classic features AEP should be considered.
Pulmonary eosinophilia is perhaps the most straightforward criteria. Our case series included cases diagnosed by lung biopsy or by BAL consistent with prior reports [2,10]. Interestingly one prior report described a patient with a low eosinophil count on bronchial wash with a subsequent elevated BAL eosinophil count [14]. This emphasizes the need for a formal BAL in order to assure accurate diagnostics.
Finally, Philit et al. described “bilateral diffuse infiltrates on chest radiography” as a criterion for AEP [2]. Others have included “diffuse pulmonary infiltrates” without stipulating type of imaging or bilateral nature [12]. We have included diffuse pulmonary infiltrates by either chest CT or CXR in our inclusion criteria and believe clinicians should use similar criteria in considering this diagnosis.
4.2. Exclusion of “causes of pulmonary eosinophilia”
Perhaps the most controversial decision is whether to include or exclude patients with atopy or asthma from the case series. Some authors have proposed that patients with atopy have a predisposition to AEP [14] while others have excluded such patients as having an alternate explanation of pulmonary eosinophilia.4 We have highlighted in Table 3 the variability in the literature regarding atopy inclusion. We did not include asthmatic patients and have deemed those with allergic rhinitis or atopy as “possible” cases of AEP. Given the controversy, clinicians should be aware that atopy and asthma are not universally considered exclusive of AEP.
Table 3.
Summary of available IAEP case-series.
| Number of patients | Male | Female | Age | Current smokers | Number requiring intubation | BAL eosinophils % | Patients with atopic disease/asthma included | |
|---|---|---|---|---|---|---|---|---|
| Allen et al., 1989 [23] | 4 | 3 | 1 | 34 (Mean) | Unclear | 2/4 | 42 (Mean) | No asthma |
| Umeki et al., 1992 [24] | 5 | 4 | 1 | 37.7 (Mean) | 3/5 | Unclear | 20.3 (Mean for 3 patients) | Yes |
| Ogawa et al., 1993 [21] | 5 | 5 | 0 | 28.2 (Mean) | 3/5 | Unclear | 42.4 (Mean) | Yes |
| Hayakawa et al., 1994 [20] | 13 | 9 | 4 | 37.2 (Mean) | Unclear | Unclear | 40.3 (Mean) | Yes |
| Cheon et al., 1996 [25] | 6 | 4 | 2 | 29 (Mean) | 1/5 | Unclear | Unclear | No |
| Pope-Harman et al., 1996 [11] | 15 | 9 | 6 | 28.9 (Mean) | 6/15 | 8/15 | 36.9 (Mean) | Unclear |
| Tazelaar et al., 1997 [10] | 9 | 4 | 5 | 53 (mean) | 3/9 | Unclear | 1 (Only 1 patient underwent BAL) | Yes |
| Shiota et al., 2000 [22] | 2 | 2 | 0 | 23 (Mean) | 2/2 | 0/2 | 47 (Mean) | No |
| Philit et al., 2002 [2] | 22 | 13 | 9 | 29 (Mean) | 8/22 | 8/22 | 54.4 (mean) | Yes |
| Shorr et al., 2004 [8] | 18 | 16a | 2 | 22 (Median) | 18/18 | 12/18 | 40.5 (Median) | Unclear |
| Miyazaki et al., 2007 [26] | 17 | 11 | 6 | 19 (Median) | 16/17 | 0/17 | 65 (Median) | Yes |
| Daimon et al., 2008 [27] | 29 | 14 | 15 | 26 (Mean) | Unclear | Unclear | Unclear | No |
| Uchiyama et al., 2008 [6] | 33 | 23 | 10 | 19.3 (Mean) | 32/33 | Unclear | 52.8 (Mean) | Yes |
| Rhee et al., 2013 [12] | 137 | 137a | 0 | 20 (Median) | 135/137 | 3/137 | 40 (Median) | Yes |
| Our study | 8 | 5 | 3 | 53 (Median) | 2/8 | 2/8 | 36 (Median) | No |
| Total | 323 | 259 | 64 | n/a | 229/276 | 35/223 | Range 1–54.4% | At least 8 studies |
Military personnel study.
4.3. Disease severity
Our case series demonstrates variation in disease severity with only two patients requiring intubation for respiratory failure. Approximately 16% of all reported patients required intubation (Table 3). We do not report any deaths, shock, or extra-corporeal membrane oxygenation use but such reports exist in the literature [8,14–19]. Conversely many case series include patients with spontaneous resolution without steroid use [2,6,20,21]. All of our patients received some duration of corticosteroid therapy.
4.4. Treatment
Treatment in our case series ranged from 35 to 285 days of corticosteroid taper with a median duration of 60 days. The literature also contains a spectrum of treatment regimens from 125 mg methylprednisolone every six hours followed by prolonged steroid taper to no treatment at all. Although not demonstrated in our current case series, a recent retrospective report by Rhee et al. seems to support a two-week duration of corticosteroid treatment [12].
4.5. Relapses
It is believed that relapse is not consistent with a diagnosis of AEP [1,20] and therefore we excluded 13 patients from our case series with relapse of symptoms. It should be noted however that relapses have been reported [12,22] as has positive cigarette smoke provocation test in AEP patients [6,7]. Clinicians should be aware of this potential when counseling their patients.
4.6. Sex and age trends
Approximately 80% of reported patients are males (Table 3). It is unclear whether this reflects smoking or occupational differences by gender (for example, two case series are of military personnel [8,12]) or an actual tendency. Likewise young age has been previously reported, however, the oldest patient in our case series is 79-years old. This suggests the diagnosis should be considered regardless of age in patients with pulmonary infiltrates of non-infectious etiology.
4.7. Smoking history
Interestingly the patients in our series did not have a new or increased smoking history contrary to prior published associations [2,6–8]. Eighty-three percent of all reported patients are current smokers (Table 3). It is difficult to differentiate between new-onset smokers or individuals with alterations in smoking habits due to reporting styles and differing cutoffs in the articles presented. Only one of our eight patients had recently altered smoking habits (patient 6). So while smoking is a known association, individuals who lack this history should not be discounted.
5. Conclusions
Idiopathic AEP is a truly rare condition comprising approximately 4% of cases of pulmonary eosinophilia at our institution. Inclusion criteria vary but consideration should be made for patients with pulmonary eosinophilia by BAL or biopsy and diffuse pulmonary infiltrates. Other criteria include approximately one month of symptoms, desaturation at rest or with exercise, and subjective or objective fevers. Not all reported cases appear to meet all criteria and disease severity can range from self-resolving to fatal. Idiopathic AEP should remain in the differential regardless of age, sex, or smoking association although these remain diagnostic clues.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
Funding: Mayo Foundation.
Footnotes
Notation of prior abstract publication/presentation: Presented at the CHEST 2011 in Honolulu, Hawaii on October 26, 2011. Ajani S. Kennedy CC. Idiopathic Acute Eosinophilic Pneumonia is a truly rare disorder. Chest 140 (4): 755A.
References
- 1.Allen J.N., Davis W.B. Eosinophilic lung diseases. Am J Respir Crit Care Med. 1994;150:1423–1438. doi: 10.1164/ajrccm.150.5.7952571. [DOI] [PubMed] [Google Scholar]
- 2.Philit F., Etienne-Mastroianni B., Parrot A., Guerin C., Robert D., Cordier J.F. Idiopathic acute eosinophilic pneumonia: a study of 22 patients. Am J Respir Crit Care Med. 2002;166:1235–1239. doi: 10.1164/rccm.2112056. [DOI] [PubMed] [Google Scholar]
- 3.Seebach J., Speich R., Fehr J., Tuchschmid P., Russi E. Gm-csf-induced acute eosinophilic pneumonia. Br J Haematol. 1995;90:963–965. doi: 10.1111/j.1365-2141.1995.tb05227.x. [DOI] [PubMed] [Google Scholar]
- 4.Badesch D.B., King T.E., Jr., Schwarz M.I. Acute eosinophilic pneumonia: a hypersensitivity phenomenon? Am Rev Respir Dis. 1989;139:249–252. doi: 10.1164/ajrccm/139.1.249. [DOI] [PubMed] [Google Scholar]
- 5.Rom W.N., Weiden M., Garcia R., Yie T.A., Vathesatogkit P., Tse D.B. Acute eosinophilic pneumonia in a new york city firefighter exposed to world trade center dust. Am J Respir Crit Care Med. 2002;166:797–800. doi: 10.1164/rccm.200206-576OC. [DOI] [PubMed] [Google Scholar]
- 6.Uchiyama H., Suda T., Nakamura Y., Shirai M., Gemma H., Shirai T. Alterations in smoking habits are associated with acute eosinophilic pneumonia. Chest. 2008;133:1174–1180. doi: 10.1378/chest.07-2669. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe K., Fujimura M., Kasahara K., Yasui M., Myou S., Kita T. Acute eosinophilic pneumonia following cigarette smoking: a case report including cigarette-smoking challenge test. Intern Med. 2002;41:1016–1020. doi: 10.2169/internalmedicine.41.1016. [DOI] [PubMed] [Google Scholar]
- 8.Shorr A.F., Scoville S.L., Cersovsky S.B., Shanks G.D., Ockenhouse C.F., Smoak B.L. Acute eosinophilic pneumonia among us military personnel deployed in or near Iraq. JAMA. 2004;292:2997–3005. doi: 10.1001/jama.292.24.2997. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimi M., Nannya Y., Watanabe T., Asai T., Ichikawa M., Yamamoto G. Acute eosinophilic pneumonia is a non-infectious lung complication after allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2009;89:244–248. doi: 10.1007/s12185-008-0240-y. [DOI] [PubMed] [Google Scholar]
- 10.Tazelaar H.D., Linz L.J., Colby T.V., Myers J.L., Limper A.H. Acute eosinophilic pneumonia: histopathologic findings in nine patients. Am J Respir Crit Care Med. 1997;155:296–302. doi: 10.1164/ajrccm.155.1.9001328. [DOI] [PubMed] [Google Scholar]
- 11.Pope-Harman A.L., Davis W.B., Allen E.D., Christoforidis A.J., Allen J.N. Acute eosinophilic pneumonia. A summary of 15 cases and review of the literature. Medicine. 1996;75:334–342. doi: 10.1097/00005792-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Rhee C.K., Min K.H., Yim N.Y., Lee J.E., Lee N.R., Chung M.P. Clinical characteristics and corticosteroid treatment of acute eosinophilic pneumonia. Eur Respir J. 2013;41:402–409. doi: 10.1183/09031936.00221811. [DOI] [PubMed] [Google Scholar]
- 13.Marchand E., Reynaud-Gaubert M., Lauque D., Durieu J., Tonnel A.B., Cordier J.F. Idiopathic chronic eosinophilic pneumonia. A clinical and follow-up study of 62 cases. The groupe d'etudes et de recherche sur les maladies “orphelines” pulmonaires (germ"o"p) Medicine. 1998;77:299–312. doi: 10.1097/00005792-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Janz D.R., O'Neal H.R., Jr., Ely E.W. Acute eosinophilic pneumonia: a case report and review of the literature. Crit Care Med. 2009;37:1470–1474. doi: 10.1097/CCM.0b013e31819cc502. [DOI] [PubMed] [Google Scholar]
- 15.Buddharaju V.L., Saraceno J.L., Rosen J.M., Spivack S.D., Smith T.C., Ilves R. Acute eosinophilic pneumonia associated with shock. Crit Care Med. 1999;27:2014–2016. doi: 10.1097/00003246-199909000-00048. [DOI] [PubMed] [Google Scholar]
- 16.Kawayama T., Fujiki R., Morimitsu Y., Rikimaru T., Aizawa H. Fatal idiopathic acute eosinophilic pneumonia with acute lung injury. Respirology. 2002;7:373–375. doi: 10.1046/j.1440-1843.2002.00413.x. [DOI] [PubMed] [Google Scholar]
- 17.Noirot A., Floriot C., Boudon B., Delacour J.L., Wagschal G., Daoudal P. Acute eosinophilic pneumonia with fatal outcome. Presse Med. 1990;19:920. [PubMed] [Google Scholar]
- 18.Sauvaget E., Dellamonica J., Arlaud K., Sanfiorenzo C., Bernardin G., Padovani B. Idiopathic acute eosinophilic pneumonia requiring ecmo in a teenager smoking tobacco and cannabis. Pediatr Pulmonol. 2010;45:1246–1249. doi: 10.1002/ppul.21314. [DOI] [PubMed] [Google Scholar]
- 19.Lim S.Y., Suh G.Y., Jeon K. Acute eosinophilic pneumonia presenting as life-threatening hypoxaemia necessitating extracorporeal membrane oxygenation. Int J Tuberc Lung Dis. 2012;16:1711–1712. doi: 10.5588/ijtld.12.0555. [DOI] [PubMed] [Google Scholar]
- 20.Hayakawa H., Sato A., Toyoshima M., Imokawa S., Taniguchi M. A clinical study of idiopathic eosinophilic pneumonia. CHEST J. 1994;105:1462–1466. doi: 10.1378/chest.105.5.1462. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa H., Fujimura M., Matsuda T., Nakamura H., Kumabashiri I., Kitagawa S. Transient wheeze. Eosinophilic bronchobronchiolitis in acute eosinophilic pneumonia. CHEST J. 1993;104:493–496. doi: 10.1378/chest.104.2.493. [DOI] [PubMed] [Google Scholar]
- 22.Shiota Y., Kawai T., Matsumoto H., Hiyama J., Tokuda Y., Marukawa M. Acute eosinophilic pneumonia following cigarette smoking. Intern Med. 2000;39:830–833. doi: 10.2169/internalmedicine.39.830. [DOI] [PubMed] [Google Scholar]
- 23.Allen J.N., Pacht E.R., Gadek J.E., Davis W.B. Acute eosinophilic pneumonia as a reversible cause of noninfectious respiratory failure. N Engl J Med. 1989;321:569–574. doi: 10.1056/NEJM198908313210903. [DOI] [PubMed] [Google Scholar]
- 24.Umeki S. Reevaluation of eosinophilic pneumonia and its diagnostic criteria. Arch Intern Med. 1992;152:1913–1919. [PubMed] [Google Scholar]
- 25.Cheon J.E., Lee K.S., Jung G.S., Chung M.H., Cho Y.D. Acute eosinophilic pneumonia: Radiographic and ct findings in six patients. AJR Am J Roentgenol. 1996;167:1195–1199. doi: 10.2214/ajr.167.5.8911179. [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki E., Nureki S., Ono E., Ando M., Matsuno O., Fukami T. Circulating thymus- and activation-regulated chemokine/ccl17 is a useful biomarker for discriminating acute eosinophilic pneumonia from other causes of acute lung injury. Chest. 2007;131:1726–1734. doi: 10.1378/chest.06-2596. [DOI] [PubMed] [Google Scholar]
- 27.Daimon T., Johkoh T., Sumikawa H., Honda O., Fujimoto K., Koga T. Acute eosinophilic pneumonia: Thin-section ct findings in 29 patients. Eur J Radiol. 2008;65:462–467. doi: 10.1016/j.ejrad.2007.04.012. [DOI] [PubMed] [Google Scholar]


