Abstract
Pulmonary Epithelioid Hemangioendothelioma is a rare and low grade tumor of endothelial origin found in the lungs. At onset patients are usually asymptomatic or present with non-specific symptoms. Chest imaging shows the presence of multiple, bilateral small nodules and diagnosis usually requires a lung biopsy. At this time there is no standardized treatment regimen and the prognosis is variable.
Keywords: PEH – Pulmonary Epithelioid Hemangioendothelioma, CT – Cat Scan, PET – Positron Emission Tomography, EHE – Epithelioid Hemangioendothelioma, IVBAT – Intravascular Bronchiolo-Alveolar Tumor
Our patient is a 65-year-old male that presented to the emergency room with constant left sided anterior chest pain for the previous five days. Prior to his presentation he was asymptomatic and in the process of recovering from surgery on his trigger finger. He has degenerative disk disease of the lumbar spine, well controlled diabetes, hypercholesterolemia, and hypertension. The patient has a 20-pack-year smoking history and quit 32 years ago. On physical examination, there was localized tenderness in the area where the patient reported chest pain and the lung examination was normal. A CT angiogram ruled out a pulmonary embolism but did reveal multiple, well defined, and irregularly shaped, bilateral nodules. The largest of the nodules in the right lower lobe measured 2 cm in size. The CT scan also revealed a possible lytic lesion in the left second rib, and a sclerotic lesion located on the posterior portion of the 10th rib. A fine needle aspirate of the right lower lobe lesion as well as that of the left second rib were non-diagnostic, but did reveal atypical cells. A CT scan of the abdomen and pelvis showed no abnormalities and a FDG-PET scan showed faint tracer uptake in one of the right lower lobe nodules. This nodule had an SUV of 3.5 and the background demonstrated an SUV of 2.7. The left second rib demonstrated an increased hyper metabolic activity of SUV of 14. The patient underwent a VATS lung biopsy with wedge resection of the nodule in the right lower lobe. The wedge resection contained multiple firm white nodules. One nodule measured 1 × 0.6 × 0.5 cm, a second nodule measured 0.6 × 0.3 × 0.2 cm and additional smaller nodules measured up to 0.2 cm in diameter. Microscopic examination revealed multiple nodular areas of eosinophilic material containing cells with small uniform nuclei. Some of these cells had prominent intra-cytoplasmic vacuoles. These cells stained positive with immunohistochemical markers for endothelial cells, CD31 and CD34, supporting the diagnosis of Epithelioid Hemangioendothelioma (EHE) (Figs. 1–4).
Fig. 1.
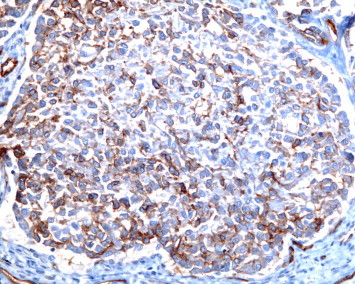
CD-31 stain at 200× magnification.
Fig. 2.
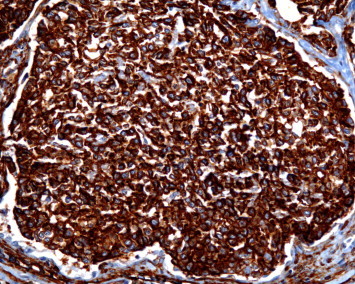
CD-34 stain at 200× magnification.
Fig. 3.
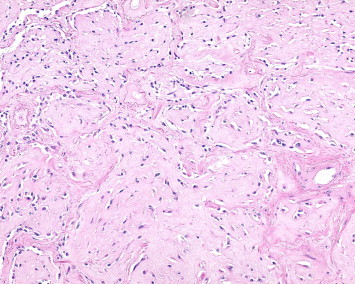
Hematoxylin and eosin at 200× magnification.
Fig. 4.
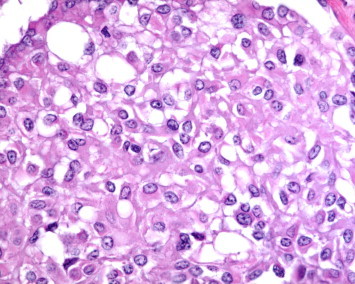
Hematoxylin and eosin at 400× magnification.
EHE is a rare vascular tumor of intermediate behavior that was first described by Weiss in 1982 as an Intravascular Broncho-Alveolar Tumor (IVBAT).17 Pulmonary Epithelioid Hemangioendothelioma (PEH) is a rare, low grade tumor of endothelial origin17,6 and can be found in many organ systems including the lungs, liver, bone, soft tissue and can be found sequentially, or simultaneously.5 When this occurs it can be hard to determine of the tumor is multicentric or a primary tumor with metastases to other tissues.5 PEH is initially recognized on a chest radiograph or CT scan with the presence of unilateral or bilateral nodules which range in size up to 2 cm3,10 and are usually found near medium sized vessels. The problem with this radiological presentation is that it is non-specific. Many physicians would consider a PET scan an important tool used to rule out malignant lung diseases; However, in our patient, only one nodule showed FDG uptake and a negative PET scan in PEH patients is not unheard of.4 There are two possible explanations that attempt to relate the malignancy of a nodule to the FDG uptake. The first is that a larger nodule may show a higher FDG uptake, but the PET scan can fail to detect nodules smaller than 6 mm in diameter. Secondly, a greater FDG uptake can represent a more clinically malignant potential and the lack of tracer uptake can be evidence of a more benign character.
The diagnosis of PEH is made on the basis of histopathological features which are confirmed using immunohistochemical staining. CD34, CD31 and Factor VIII are the well-recognized endothelial cell markers although a new marker, Nuclear Fli-1, a protein that is expressed in endothelial cells as well as in T-cells and megakaryocytes, is being considered for use as a marker for PEH. Gill et al. found that Nuclear Fli-1 was detected in 100 percent of their EHE cases and found that FLI-1, CD34 and CD31 were the markers most sensitive for EHE. It was also found that FLI-1 immuno-staining demonstrated better sensitivity than CD34 and better specificity than CD31.7 PEH is closely associated with arterioles, venules, and lymphatic vessels and often display an intra-alveolar growth pattern.17,18 EHE and subsequently PEH demonstrates reactivity for factor VIII-related antigen (99%), CD31 (86%), and CD34 (94%).18 Calcification is commonly seen on the histology, but radiological calcification is quite rare8 and PEH does not show necrosis, cytological atypia or a high mitotic index.13 Cellular pleomorphism, mitotic activity, necrosis and extensive cellular spindling are cytologic features that predict aggressive behavior. Unfortunately there is no clear definition of malignant EHE or malignant PEH. PEH is known to have biological behavior intermediate between a hemangioma and a conventional angiosarcoma.
Pulmonary involvement is relatively rare with pulmonary EHE being approximately 19% of all EHE cases.10 Other commonly involved organ systems are the liver and bone with multi-organ involvement quite common in EHE. Multi-organ involvement of both the bone and lung is even rarer at 10% of all multi-organ involvement with the most common being liver and bone involvement.10 In a study of 93 patients distant metastasis was confirmed in 47 patients.2 The most common was hepatic metastasis (22.6%) followed by pleural metastasis (20.4%) and lymph node metastasis (10.8%).2
The diagnosis of EHE is often incidental (Table 1) as a majority of patients are usually asymptomatic or have minor symptoms. Our patient was relatively asymptomatic except for the reported anterior rib pain. Given the rarity of this tumor, the demographic information is quite variable. Previous reports have suggested that EHE is often found in women in ratio's ranging from 3:1 female to male ratio to a 2:1 female to male involvement. Patients age in range from 19 to 70 years, with an average onset in the 4th decade. Patients have been reported to live for up to 20 years5,8 with no changes in tumor size or aggressiveness. There is even a report of a patient surviving 10 years without any treatment.14
Table 1.
Review of the clinical, pathological and radiological features of published cases and reviews of PEH.
| Name of study/Authors | Female/Male | Age | Symptoms | Radiography | PET | Metastasis | Immuno features |
|---|---|---|---|---|---|---|---|
| Our case | Male | 66 | Chest pain & left anterior rib pain | Multiple bilateral | Pos 1 nodule | None | CD31, CD34 |
| Amin case report | Female | 70 | Non-related | Unilateral single nodule | Not performed | Lymph node and pleural | VIII, CD34 |
| Amin lit review (n = 93) | Female/Male (73%/27%) | 40.1 | Symptomatic/Asymptomatic (50.5%/49.5%) | Unilateral/Bilateral(12.2%/78.9%) | Not measured | Distant/Hepatic/Pleural/Lymph (50.5%/22.6%/20.4%/10.8%) | Factor VIII, CD31, CD34, vimentin |
| Bagan (n = 80) | Female/Male (63.8%/36.2%) | 39.4 | Symptomatic/Asymptomatic (42.%/48.7) | Unilateral/Bilateral (23.7%/76.2%) | No | 38 70% | VIII, CD 31, CD34 |
| Cazzuffi case | Male | 67 | Dyspnea and cough | Multiple bilateral | Negative | Spleen and liver | Factor VIII, CD31, Cd34 |
| Cazzuffi data | 3:1 Ratio | 40.1 | Weight loss, fatigue, and respiratory symptom | Multiple bilateral | Positive%?? | Lymph, liver, bone | Factor VIII, CD31, Cd34 |
| Cronin | Female | 35 | Multiple bilateral | None | No | Factor VIII, CD31, CD34 | |
| Jinghong | Female | 20 | No complaints | Multiple Bilateral | Not performed | No | FactornVIII, CD 31, CD34 |
| Joo Lee | Female | 31 | Upper back, and bilateral shoulder pain | Unilateral and vertebral bodies | Bone scan | Multiple bone metastasis | a-sma, Vimentin, weak for CD34 |
| Mhoyan | Female | 25 | Chest pain, generalized weakness | Multiple bilateral | Not performed | No | Factor VIII, CD 31, CD34 |
| Okamura | Female | 19 | None | Multiple bilateral | Pos 1 nodule | None | Factor VIII, CD34 |
| Shraim | Male | 51 | Dry cough, SOBexertion, | Multiple pleural | Not performed | Skin, anterior ab wall | Factorn VIII, CD 31, CD34 |
| Tochigi | Woman | 5th Decade | None | Unilateral multiple | Not performed | No | Vimentin, CD31, FLI-1, CD34 |
Pulmonary involvement of EHE can be quite variable in its prognosis and the suggested risk factors have been quite variable. When PEH was first reported as IVBAT by Dial et al. in 1983, they observed that the poor prognostic factors were the presence of respiratory symptoms at presentation, lymphatic spread, pleural invasion, extensive intravascular spread, and hepatic metastases. Further research by Kitaichi et al., found that pleural effusion and spindle tumor cells were unfavorable prognostic factors. A medline literature review by Bagan et al. found that pleural effusion, significant loss of weight, and anemia were factors of a poor prognosis. When analyzing the long term survival of PEH patients, Bagan et al. discovered that patients could be placed into two categories: asymptomatic patients with nodules (median survival of 15 years) and patients with symptoms of vascular endothelial cell proliferation. The 5-year survival of patients with pleural effusion was 2% whereas survival of the population without effusion was 73%.3 The 5-year survival rate was at one point reported to be between 47 and 71% but an examination of an internet based EHE registry by Lau et al. found the 1 year survival rate to be 90% and 73% at 5 years. In Amin's review of 93 patients, they were able to reveal that male, symptomatic patients, pleural effusion, metastases and lymph node metastases were significant risk factors. The study of the online registry by Lau et al. found that the only significant risk factors were male sex, diagnosis in middle age, uncontained spread, and involvement of three of more bones all correlated with poor survival.
Various therapeutic modalities have been used in attempts to either stop or slow the progression of PEH. Because of the rarity of this condition there is no standard of agreed upon treatment. In patients with a unilateral nodule a surgical resection is possible.9 In situations of surgical resection as a form of treatment, use of a PET scan allows one to resect aggressive PEH nodules.16 Patients with lymph node metastases have undergone a surgical resection but due to the low number of patients, the prognostic value remains unclear.15 The use of various chemotherapies has been reported for metastatic or unresectable PEH, but with variable effectiveness.12 The use of interferon-2a found that pulmonary lesions regressed slightly and some beneficial results have been obtained with the use of bevacizumab. Other proposed treatments include azathioprine,1,4,11 thalidomide and multiple wedge resection. It is interesting to note that corticosteroids have been suggested as a possible form of treatment3,4 because the neoplastic cells express glucocorticoid receptors. For patients with diffuse PEH it is has been suggested that hormonal therapy could be used if the neoplastic cells express estrogen or progesterone receptors. Although no cases have been reported to be positive for ER-a or PR, there is a single report of a patient testing positive for ER-B.2 Lau et al.' study of the online registry found that 26% of patients chose medical treatments and 22% chose to simply wait and observe the disease. However, all of these treatment options represent single case reports or an analysis of a cohort of single case reports and lack convincing evidence of benefit.
Author contribution
Sidharth R. Mehta – Graduate Assistant and First Author.
Dr. Arvind Das – Managing Pulmonologist and Second Author.
Dr. Barnard – Anatomic Pathologist and Reviewer/Author.
Dr. Marcus – Pathology Resident and Reviewer/Author.
Conflict of interest statement
All authors confirm that there have been no financial incentives provided to report on this case, nor do they have any financial bias.
References
- 1.Al-Shraim M., Mahboub B., Neligan P.C., Chamberlain D., Ghazarian D. Primary pleural epithelioid haemangioendothelioma with metastases to the skin. A case report and literature review. J Clin Pathol. 2005;58:107–109. doi: 10.1136/jcp.2004.018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amin R., Hiroshima K., Kokubo T. Risk factors and independent predictors of survival in patients with pulmonary epithelioid haemangioendothelioma. Review of the literature and case report. Respirology. 2006;11:818–825. doi: 10.1111/j.1440-1843.2006.00923.x. [DOI] [PubMed] [Google Scholar]
- 3.Bagan P., Hassan M., Barthes F. Prognostic factors and surgical indications of pulmonary epithelioid hemangioendothelioma: a review of the literature. Ann Thorac Surg. 2006;82:2010–2013. doi: 10.1016/j.athoracsur.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 4.Cazzuffi R., Calia N., Ravenna F. Primary pulmonary epithelioid hemangioendothelioma: a rare cause of PET-negative pulmonary nodules. Case Rep Med. 2011 doi: 10.1155/2011/262674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cronin P., Arenberg D. Pulmonary epithelioid hemangioendothelioma: an unusual case and a review of the literature. Chest. 2004;125:789–792. doi: 10.1378/chest.125.2.789. [DOI] [PubMed] [Google Scholar]
- 6.Dalil D.H., Liebow A.A., Gmelich J.T. Intravascular, bronchiolar, and alveolar tumor of the lung(IVBAT): an analysis of twenty cases of a peculiar sclerosing endothelial tumor. Cancer. 1983;51:452–464. doi: 10.1002/1097-0142(19830201)51:3<452::aid-cncr2820510317>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Gill R., O'Donnell R., Horvai A. Utility of immunohistochemsitry for endothelial markers in distinguishing epithelioid hemangioendothelioma from carcinoma metastatic to bone. Arch Pathol Lab Med. 2009;133:967–972. doi: 10.5858/133.6.967. [DOI] [PubMed] [Google Scholar]
- 8.Jinghon X., Lirong C. Pulmonary epithelioid hemangioendothelioma accompanied by bilateral multiple calcified nodules in lung. Diagn Pathol. 2011;6:21. doi: 10.1186/1746-1596-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitaichi M., Nagai S., Nishimura K. Pulmonary epithelioid haemangioendothelioma in 21 patients, including three with partial spontaneous regression. Eur Respir J. 1998;12:89–96. doi: 10.1183/09031936.98.12010089. [DOI] [PubMed] [Google Scholar]
- 10.Lau K., Massad M., Pollack C. Clinical patterns and outcome in epithelioid hemangioendothelioma with or without pulmonary involvement: insights from an internet registry in the study of a rare cancer. Chest. 2011;140:1312–1318. doi: 10.1378/chest.11-0039. [DOI] [PubMed] [Google Scholar]
- 11.Ledson M.J., Convery R., Carty A., Evans C.C. Epithelioid hemangioendothelioma. Thorax. 1999;54:560–561. doi: 10.1136/thx.54.6.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y.J., Chung M.J., Jeong K.C. Pleural epithelioid hemangioendothelioma. Yonsei Med J. 2008;6:1036–1040. doi: 10.3349/ymj.2008.49.6.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mhoyan A., Weidner N., Shabaik A. Epithelioid hemangioendothelioma of the lung diagnosed by transesophageal endoscopic ultrasound-guided fine needle aspiration. Acta Cytol. 2004;4:555–559. doi: 10.1159/000326417. [DOI] [PubMed] [Google Scholar]
- 14.Roudier-Pujol C., Enjolras O., Lacronique J. Multifocal epithelioid hemangioendothelioma with partial remission after interferon alfa-2a treatment. Ann Dermatol Venereol. 1994;121:898–904. [PubMed] [Google Scholar]
- 15.Tochigi N., Tsuta K., Maeshima A.M. Malignant pulmonary epithelioid hemangioendothelioma with hilar lymph node metastasis. Ann Diagn Pathol. 2011;15:207–212. doi: 10.1016/j.anndiagpath.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe S., Yano F., Kita T. F-FDG-PET/CT as an indicator for resection of pulmonary epithelioid hemangioendothelioma. Ann Nucl Med. 2008;22:521–524. doi: 10.1007/s12149-007-0159-z. [DOI] [PubMed] [Google Scholar]
- 17.Weiss S.W., Enzinger F.M. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer. 1982;50:970–981. doi: 10.1002/1097-0142(19820901)50:5<970::aid-cncr2820500527>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.Weissferdt A., Moran C. Primary vascular tumors of the lungs: a review. Ann Diagn Pathol. 2010;14:296–308. doi: 10.1016/j.anndiagpath.2010.03.001. [DOI] [PubMed] [Google Scholar]


