Abstract
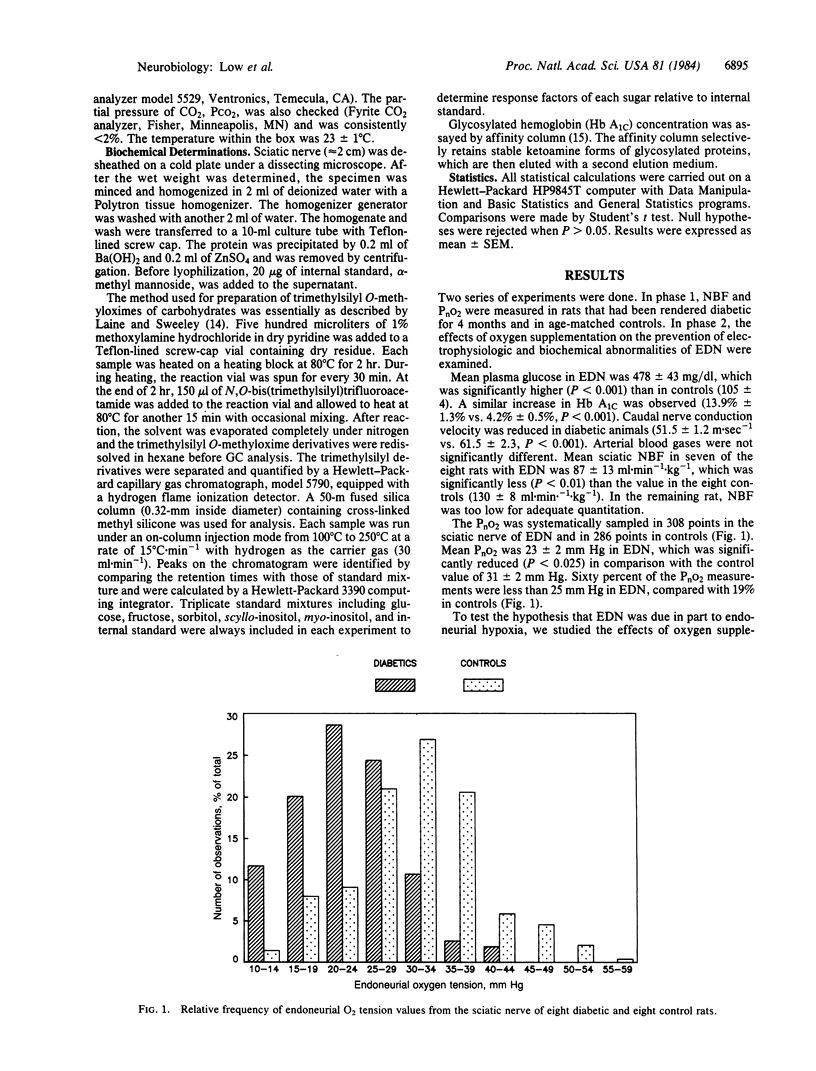
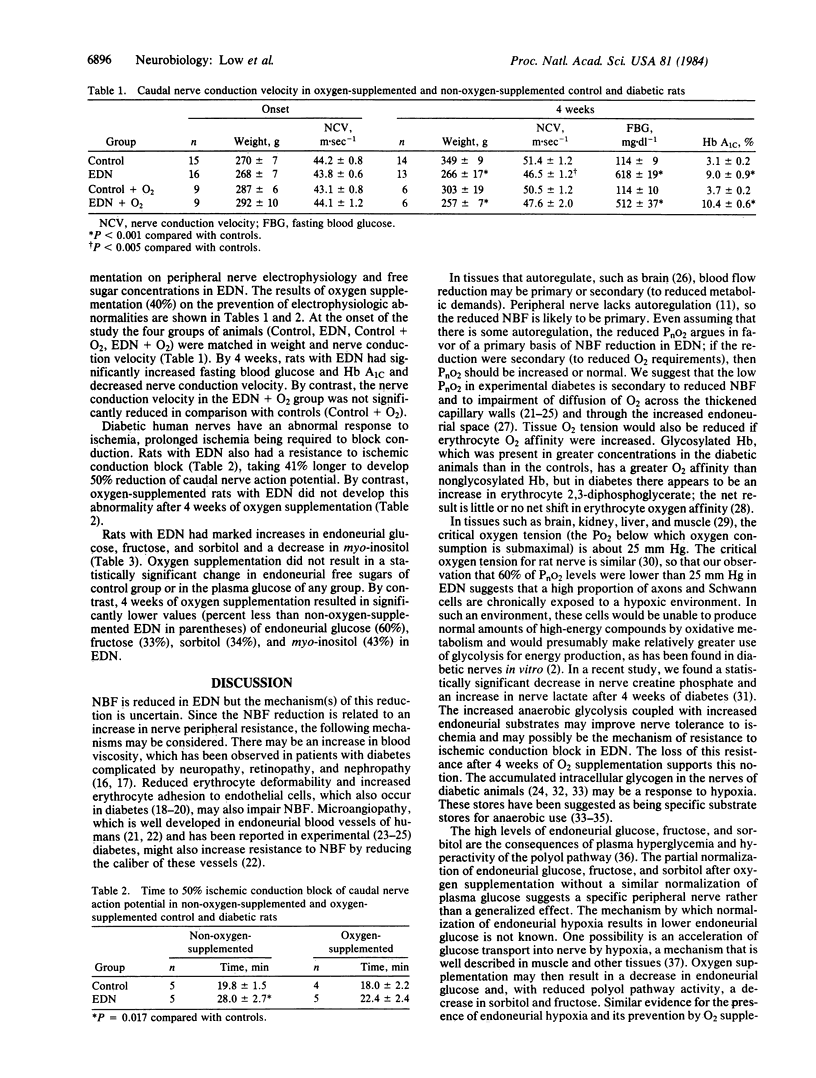
Endoneurial hypoxia has been suggested as a mechanism of human and experimental diabetic neuropathy (EDN). We found that rats rendered diabetic for 4 months had reduced nerve blood flow (NBF) and nerve oxygen tension (PnO2). The NBF was reduced by at least 33% in EDN and 60% of the oxygen tensions in the endoneurial O2 histogram were less than 25 mm Hg (3.3 kPa) in EDN compared with only 19% in the controls. To test the hypothesis that EDN may in part be due to hypoxia, we studied the effectiveness of oxygen supplementation in preventing some electrophysiologic and biochemical abnormalities. Rats with EDN had reduced caudal nerve conduction velocity and had a resistance to ischemic conduction block. When a matched groups of rats with EDN were O2 supplemented for 4 weeks, the time to 50% block of nerve conduction and nerve conduction velocity was no longer statistically different from controls. Endoneurial free sugars (glucose, fructose, sorbitol) were markedly increased in EDN. Oxygen supplementation resulted in no change in plasma glucose; by contrast, these increased endoneurial free sugars were significantly reduced (towards normal) by 60%, 33%, and 34%, respectively. myo-Inositol, however, was further decreased by oxygen supplementation. These findings of a partial prevention of electrophysiologic and biochemical abnormalities support a role of hypoxia in the pathogenesis of EDN.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUKLAND K., BOWER B. F., BERLINER R. W. MEASUREMENT OF LOCAL BLOOD FLOW WITH HYDROGEN GAS. Circ Res. 1964 Feb;14:164–187. doi: 10.1161/01.res.14.2.164. [DOI] [PubMed] [Google Scholar]
- Barnes A. J., Locke P., Scudder P. R., Dormandy T. L., Dormandy J. A., Slack J. Is hyperviscosity a treatable component of diabetic microcirculatory disease? Lancet. 1977 Oct 15;2(8042):789–791. doi: 10.1016/s0140-6736(77)90724-3. [DOI] [PubMed] [Google Scholar]
- Blümcke S., Themann H., Niedorf H. R. Deposition of glycogen during the degeneration and regeneration of the sciatic nerves of rabbits. (Light and electron microscopic studies). Acta Neuropathol. 1965 Oct 4;5(1):69–81. doi: 10.1007/BF00689164. [DOI] [PubMed] [Google Scholar]
- Brismar T. Potential clamp analysis of the effect of anoxia on the nodal function of rat peripheral nerve fibres. Acta Physiol Scand. 1981 Aug;112(4):495–496. doi: 10.1111/j.1748-1716.1981.tb06851.x. [DOI] [PubMed] [Google Scholar]
- Brismar T., Sima A. A. Changes in nodal function in nerve fibres of the spontaneously diabetic BB-Wistar rat: potential clamp analysis. Acta Physiol Scand. 1981 Dec;113(4):499–506. doi: 10.1111/j.1748-1716.1981.tb06928.x. [DOI] [PubMed] [Google Scholar]
- Das P. K., Bray G. M., Aguayo A. J., Rasminsky M. Diminished ouabain-sensitive, sodium-potassium ATPase activity in sciatic nerves of rats with streptozotocin-induced diabetes. Exp Neurol. 1976 Oct;53(1):285–288. doi: 10.1016/0014-4886(76)90299-5. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Lambert E. H., Windebank A. J., Lais A. A., Sparks M. F., Karnes J., Sherman W. R., Hallcher L. M., Low P. A., Service F. J. Acute hyperosmolar hyperglycemia causes axonal shrinkage and reduced nerve conduction velocity. Exp Neurol. 1981 Mar;71(3):507–514. doi: 10.1016/0014-4886(81)90028-5. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H., Merola L. O., Field R. A. Sorbitol pathway: presence in nerve and cord with substrate accumulation in diabetes. Science. 1966 Jan 14;151(3707):209–210. doi: 10.1126/science.151.3707.209. [DOI] [PubMed] [Google Scholar]
- Greene D. A., De Jesus P. V., Jr, Winegrad A. I. Effects of insulin and dietary myoinositol on impaired peripheral motor nerve conduction velocity in acute streptozotocin diabetes. J Clin Invest. 1975 Jun;55(6):1326–1336. doi: 10.1172/JCI108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Sodium- and energy-dependent uptake of myo-inositol by rabbit peripheral nerve. Competitive inhibition by glucose and lack of an insulin effect. J Clin Invest. 1982 Nov;70(5):1009–1018. doi: 10.1172/JCI110688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Winegrad A. I. Effects of acute experimental diabetes on composite energy metabolism in peripheral nerve axons and Schwann cells. Diabetes. 1981 Nov;30(11):967–974. doi: 10.2337/diab.30.11.967. [DOI] [PubMed] [Google Scholar]
- Grover-Johnson N., Spencer P. S. Peripheral nerve abnormalities in aging rats. J Neuropathol Exp Neurol. 1981 Mar;40(2):155–165. doi: 10.1097/00005072-198103000-00007. [DOI] [PubMed] [Google Scholar]
- Jakobsen J. Axonal dwindling in early experimental diabetes. I. A study of cross sectioned nerves. Diabetologia. 1976 Dec;12(6):539–546. doi: 10.1007/BF01220629. [DOI] [PubMed] [Google Scholar]
- Jakobsen J. Peripheral nerves in early experimental diabetes: expansion of the endoneurial space as a cause of increased water content. Diabetologia. 1978 Feb;14(2):113–119. doi: 10.1007/BF01263449. [DOI] [PubMed] [Google Scholar]
- Jefferys J. G., Palmano K. P., Sharma A. K., Thomas P. K. Influence of dietary myoinositol on nerve conduction and inositol phospholipids in normal and diabetic rats. J Neurol Neurosurg Psychiatry. 1978 Apr;41(4):333–339. doi: 10.1136/jnnp.41.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine R. A., Sweeley C. C. Analysis of trimethylsilyl O-methyloximes of carbohydrates by combined gas-liquid chromatography-mass spectrometry. Anal Biochem. 1971 Oct;43(2):533–538. doi: 10.1016/0003-2697(71)90284-3. [DOI] [PubMed] [Google Scholar]
- Low P. A., Dyck P. J., Schmelzer J. D. Chronic elevation of endoneurial fluid pressure is associated with low-grade fiber pathology. Muscle Nerve. 1982 Feb;5(2):162–165. doi: 10.1002/mus.880050214. [DOI] [PubMed] [Google Scholar]
- Low P. A., Schmelzer J. D. Peripheral nerve conduction studies in galactose-poisoned rats. Demonstration of increased resistance to ischemic conduction associated with endoneurial edema due to sugar alcohol accumulation. J Neurol Sci. 1983 Jun;59(3):415–421. doi: 10.1016/0022-510x(83)90026-6. [DOI] [PubMed] [Google Scholar]
- Low P. A., Tuck R. R. Effects of changes of blood pressure, respiratory acidosis and hypoxia on blood flow in the sciatic nerve of the rat. J Physiol. 1984 Feb;347:513–524. doi: 10.1113/jphysiol.1984.sp015079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G. D., Lowe J. M., Drummond M. M., Reith S., Belch J. J., Kesson C. M., Wylie A., Foulds W. S., Forbes C. D., MacCuish A. C. Blood viscosity in young male diabetics with and without retinopathy. Diabetologia. 1980 May;18(5):359–363. doi: 10.1007/BF00276814. [DOI] [PubMed] [Google Scholar]
- McMillan D. E., Utterback N. G., La Puma J. Reduced erythrocyte deformability in diabetes. Diabetes. 1978 Sep;27(9):895–901. doi: 10.2337/diab.27.9.895. [DOI] [PubMed] [Google Scholar]
- Moore S. A., Peterson R. G., Felten D. L., O'Connor B. L. Glycogen accumulation in tibial nerves of experimentally diabetic and aging control rats. J Neurol Sci. 1981 Nov-Dec;52(2-3):289–303. doi: 10.1016/0022-510x(81)90012-5. [DOI] [PubMed] [Google Scholar]
- Powell H., Knox D., Lee S., Charters A. C., Orloff M., Garrett R., Lampert P. Alloxan diabetic neuropathy: electron microscopic studies. Neurology. 1977 Jan;27(1):60–66. doi: 10.1212/wnl.27.1.60. [DOI] [PubMed] [Google Scholar]
- Samaja M., Melotti D., Carenini A., Pozza G. Glycosylated haemoglobins and the oxygen affinity of whole blood. Diabetologia. 1982 Nov;23(5):399–402. doi: 10.1007/BF00260950. [DOI] [PubMed] [Google Scholar]
- Schlaepfer W. W., Gerritsen G. C., Dulin W. E. Segmental demyelination in the distal peripheral nerves of chronically diabetic Chinese hamsters. Diabetologia. 1974 Nov;10 (Suppl):541–548. doi: 10.1007/BF01221984. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein H., Volger E. Red-cell aggregation and red-cell deformability in diabetes. Diabetes. 1976;25(2 Suppl):897–902. [PubMed] [Google Scholar]
- Sidenius P., Jakobsen J. Retrograde axonal transport. A possible role in the development of neuropathy. Diabetologia. 1981 Feb;20(2):110–112. doi: 10.1007/BF00262011. [DOI] [PubMed] [Google Scholar]
- Sidenius P., Jakobsen J. Reversibility and preventability of the decrease in slow axonal transport velocity in experimental diabetes. Diabetes. 1982 Aug;31(8 Pt 1):689–693. doi: 10.2337/diab.31.8.689. [DOI] [PubMed] [Google Scholar]
- Silver I. A. Polarography and its biological applications. Phys Med Biol. 1967 Jul;12(3):285–299. doi: 10.1088/0031-9155/12/3/201. [DOI] [PubMed] [Google Scholar]
- Sima A. A., Lorusso A. C., Thibert P. Distal symmetric polyneuropathy in the spontaneously diabetic BB-wistar rat. An ultrastructural and teased fiber study. Acta Neuropathol. 1982;58(1):39–47. doi: 10.1007/BF00692696. [DOI] [PubMed] [Google Scholar]
- Sima A. A. Peripheral neuropathy in the spontaneously diabetic BB-Wistar-rat. An ultrastructural study. Acta Neuropathol. 1980;51(3):223–227. doi: 10.1007/BF00687389. [DOI] [PubMed] [Google Scholar]
- Spritz N., Singh H., Marinan B. Metabolism of peripheral nerve myelin in experimental diabetes. J Clin Invest. 1975 May;55(5):1049–1056. doi: 10.1172/JCI108005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M. A., Sherman W. R., Kurien M. M., Moonsammy G. I., Wisgerhof M. Polyol accumulations in nervous tissue of rats with experimental diabetes and galactosaemia. J Neurochem. 1967 Nov;14(11):1057–1066. doi: 10.1111/j.1471-4159.1967.tb09516.x. [DOI] [PubMed] [Google Scholar]
- Tuck R. R., Schmelzer J. D., Low P. A. Endoneurial blood flow and oxygen tension in the sciatic nerves of rats with experimental diabetic neuropathy. Brain. 1984 Sep;107(Pt 3):935–950. doi: 10.1093/brain/107.3.935. [DOI] [PubMed] [Google Scholar]
- Vital C., Vallat J. M., Le Blanc M., Martin F., Coquet M. Les neuropathies periphériques du diabète sucré. Etude ultrastructurale de 12-cas biopsies. J Neurol Sci. 1973 Apr;18(4):381–398. doi: 10.1016/0022-510x(73)90133-0. [DOI] [PubMed] [Google Scholar]
- Wautier J. L., Paton R. C., Wautier M. P., Pintigny D., Abadie E., Passa P., Caen J. P. Increased adhesion of erythrocytes to endothelial cells in diabetes mellitus and its relation to vascular complications. N Engl J Med. 1981 Jul 30;305(5):237–242. doi: 10.1056/NEJM198107303050501. [DOI] [PubMed] [Google Scholar]
- Yue D. K., McLennan S., Church D. B., Turtle J. R. The measurement of glycosylated hemoglobin in man and animals by aminophenylboronic acid affinity chromatography. Diabetes. 1982 Aug;31(8 Pt 1):701–705. doi: 10.2337/diab.31.8.701. [DOI] [PubMed] [Google Scholar]


