Abstract
Lung transplantation is an accepted therapy for patients with end-stage lung disease and offers a major survival benefit in selected patients. The most important indications are chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis besides cystic fibrosis and pulmonary arterial hypertension. The incidence of lung cancer in patients after Ltx is 20–25 times higher than in the general population. Diagnosis is often difficult in IPF patients because of the diffuse lung abnormalities due to the underlying fibrosis. Moreover, the lung cancer may mimic a pulmonary infection. Symptoms are often aspecific, diagnosis is difficult, and prognosis is extremely poor. We describe three patients who were transplanted for idiopathic pulmonary fibrosis and who developed a primary lung cancer.
Keywords: Idiopathic pulmonary fibrosis, Lung cancer, Lung transplantation
1. Introduction
Lung transplantation (Ltx) is an accepted therapy for patients with end-stage lung disease and offers a major survival benefit in selected patients. The most important indications are chronic obstructive pulmonary disease (COPD) (29%) and idiopathic pulmonary fibrosis (IPF) (24%) besides cystic fibrosis and pulmonary arterial hypertension.1 The incidence of lung cancer is 4.1% in patients after Ltx, this is 20–25 times higher than in the general population.2 Diagnosis is often difficult in IPF patients because of the diffuse lung abnormalities due to the underlying fibrosis. Moreover, the lung cancer may mimic a pulmonary infection. We describe three patients who were transplanted for idiopathic pulmonary fibrosis and who developed a primary lung cancer.
2. Cases
Patient A, a 48-year old male with IPF presented 7 years after successful single Ltx with dyspnoea, weight loss and cough. At that time he was renovating his house. A high resolution computed tomography of the chest showed an increasing opacity in the native lung replacing the fibrotic lesions and noduli and ground glass opacities in the transplant lung (Fig. 1). The differential diagnosis consisted of fungus infection (exposure during renovating), rejection and malignancy. No abnormalities were seen on bronchoscopy but biopsies of the transplant lung showed a large cell carcinoma of the lung with lymphangitis carcinomatosa. No extrathoracic metastases were found on 18fluorodeoxyglucose positron emission tomography (18FDG-PET). Due to his poor performance (WHO 4) no oncological treatment was started and he died shortly after. Patient B, a 58-year old male with IPF, underwent a bilateral Ltx shortly after a single left Ltx failed due to rejection. In the explanted right lung a squamous cell carcinoma was found with mediastinal lymph metastases. No extrathoracic metastases were found on 18FDG-PET. The lung cancer was staged as pT2N2M0 and chemo-radiotherapy was started. 14 months later local progression appeared, shortly after initiation of second line chemotherapy he died. Patient C, a 53-year old female with IPF complained of left pretibial pain before transplantation. A bone scintigraphy showed uptake in the left tibia, 18FDG-PET showed uptake in both lungs and the left tibia. Uptake in the tibia was suggestive for hypertrophic osteo-arthropathy and was interpreted as compatible with her IPF as was the pulmonal uptake. At the time of transplantation, however, she was diagnosed with an adenocarcinoma in both explanted lungs. New bone scintigraphy showed multiple lesions suggestive for skeletal metastases. She died shortly after. A summary is presented in Table 1.
Fig. 1.
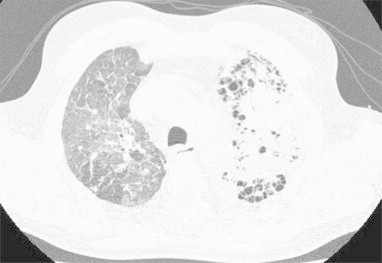
Chest HRCT of patient A: increasing opacity in the left native lung replacing the fibrotic lesions and noduli and ground glass opacities in the transplant lung.
Table 1.
Summary patients A–C.
| Patient A | Patient B | Patient C | |
|---|---|---|---|
| Age at diagnosis IPFa/Ltxb | 46/48 years | 53/57 years | 47/53 years |
| Histologic confirmation IPF | yes, UIPc | yes, UIP | yes, UIP |
| Smoking status | ex, 30 packyears | ex, 26 packyears | never |
| Treatment IPF (all acetylcysteine/prednisone) | cyclophosfamide | azathioprine | azathioprine |
| Singe or bilateral Ltx | single (right) | After rejection single (left) Ltx bilateral Ltx | bilateral |
| Histology explanted lung | UIP, no malignancy | left: UIP right: squamous cell carcinoma |
massive bilateral adenocarcinoma with lymphangitis carcinomatosa |
| Time Ltx to diagnosis of lung cancer | 8 years, three months | in explanted lung | in explanted lung |
| Stage lung cancer | TxNxM1a (stage IV) | T2N2M0 (stage IIIA) | T4N2M1b (stage IV) |
| Treatment lung cancer | none, performance too poor | radical chemo-radiotherapy, progression: chemotherapy | palliative radiotherapy symptomatic skeletal metastases, performance too poor for further therapy |
| Time diagnosis lung cancer to death | 3 months | 1 year, 10 months | 3 months |
idiopathic pulmonary fibrosis.
lung transplantation
usual interstitial pneumonia.
3. Discussion
After Ltx the incidence of lung cancer is increased in contrast to other solid organ transplant recipients.3,4 Lung cancer arises in the majority of cases in the native lung but sometimes is found unexpectedly in the explanted lung. Risk factors are IPF itself, smoking, older age, male gender, prolonged immunosuppression and single Ltx.1
3.1. IPF
Causal mechanisms and frequency of lung cancer in IPF are difficult to determine. This is partly due to a yearlong lack of uniform diagnostic criteria for IPF, making interpretation of the literature difficult. Uniform diagnostic criteria are now established by the ATS/ERS in 2002 and better diagnosis is now expected.5 A recent study found a rate ratio of 4.96 for developing lung cancer in IPF patients compared to the general population; this was independent of smoking status.6 Due to inflammation and repeat injury induced by IPF, genetic errors may develop. Eventually this can result in lung cancer.5
3.2. Smoking, age and gender
83–100% of transplanted patients who developed lung cancer had a smoking history of at least 30 packyears.3,7 Patient A and B had a smoking history of 30 and 26 packyears respectively, but patient C was a life time non-smoker. Increasing age and male predominance are also recognized risk factors.8,9
3.3. Immunosuppressive drug therapy
Long-term immunosuppression is thought to be carcinogenic due to inhibition of immune-mediated tumour surveillance and direct carcinogenic effects.8,9 In contrast to other solid organ transplants, immunosuppression post Ltx is much more intensified due to the common development of acute and chronic rejection. This may be the explanation why other solid organ transplant recipients do not have an increased risk of developing lung cancer.3 Aggressive tumour behaviour can also be attributed to the same mechanism.3
3.4. Single versus bilateral Ltx
In a study of Dickson et al, 9/131 (6.9%) single lung transplanted patients developed lung cancer in the native lung. 8 were transplanted for COPD and 1 for IPF, lung cancer developed after a mean of 52 months following transplantation.8 Patient A also developed a large cell carcinoma in the native lung but only after 99 months. When a bilateral Ltx is performed, lung cancer is rarely accounted although when it does, it mostly arises from the native lung epithelium. The hazard ratio for developing lung cancer is 4.31 for single Ltx versus bilateral Ltx after adjusting for age, native disease and smoking.8 In 2% of patients lung cancer is unexpectedly found in the explanted lung.1 This occurred in patient B and C although in patient C, retrospectively, there was a suspicion of malignancy on bone scintigraphy and 18FDG-PET. Reports of lung cancer arising from the donor long are rare. This may be due to a younger donor age and frequently a non-smoking status of the donor, although this concept is rapidly changing as more and more extended criteria donor lungs are now being used.1 Leuven and many other centers worldwide have shifted to bilateral Ltx in more than 95% of IPF and COPD/emphysema patients, the statistically lower incidence of primary lung cancer after bilateral Ltx being one of the reasons.
3.5. Symptoms and diagnosis
Only a minority of patients is asymptomatic, but symptoms are usually aspecific7 or mimic an infection or rejection,3 as in patient A. Chest CT scanning is more sensitive than chest x-ray to diagnose lung cancer.4 18FDG-PET-scan may be false positive due to the underlying fibrosis or infection, as was wrongly suggested in patient C. Mean time from Ltx to diagnosis of lung cancer is 40–52 months, in patient A this was much longer.3,7,8 Mean age at diagnosis is 59 years (range 52–64 years).3 Adenocarcinoma and squamous cell carcinoma represent the most frequent pathological types, followed by small cell carcinoma.7,9
3.6. Treatment and prognosis
Although disease is often diagnosed in an early stage, prognosis remains extremely poor. Clinical course is frequently recurrent, aggressive and fatal, as we encountered in all three patients. Due to the underlying disease and immunosuppressive drugs, therapeutic options are limited.7,8,10 Post-transplant survival rates of patients with lung cancer at 1 and 2 years were 50% and 33% respectively.3 This in contrast with a 1 and 2 year survival of 90% and 85% respectively in lung transplanted patients without lung cancer in Leuven, Belgium.
4. Conclusions
Transplanted IPF patients are at increased risk for developing primary lung cancer. Risk factors are IPF itself, smoking, older age, male gender, immunosuppressive drug therapy and single Ltx. Symptoms are often aspecific, diagnosis is difficult, and prognosis is extremely poor. These cases stress the importance of actively searching for lung cancer before as well as after Ltx in patients with IPF.
Conflict of interest
The authors declare that they have no competing interests.
Funding
No funding source.
Author contributions
L. Hendriks and M. Drent have written the case report, the others have given significant comments on the case histories.
Footnotes
The authors declare that the manuscript has not been previously published in a journal, nor is it before another journal for consideration. The abstract was presented as a poster presentation at the WASOG & BAL meeting 2011 Maastricht.
Contributor Information
L.E.L. Hendriks, Email: lizzahendriks@yahoo.com.
M. Drent, Email: http://www.ildcare.eu, m.drent@hetnet.nl.
E.H.J. van Haren, Email: e.v.haren@atriummc.nl.
J.A. Verschakelen, Email: johny.verschakelen@uz.kuleuven.ac.be.
G.M. Verleden, Email: geert.verleden@uzleuven.be.
References
- 1.Mathew J., Kratzke R.A. Lung cancer and lung transplantation: a review. J Thorac Oncol. 2009;4:753–760. doi: 10.1097/JTO.0b013e31819afdd9. [DOI] [PubMed] [Google Scholar]
- 2.Bellil Y., Edelman M.J. Bronchogenic carcinoma in solid organ transplant recipients. Curr Treat Options Oncol. 2006;6:77–81. doi: 10.1007/s11864-006-0034-5. [DOI] [PubMed] [Google Scholar]
- 3.Arcasoy S.M., Hersh C., Christie J.D. Bronchogenic carcinoma complicating lung transplantation. J Heart Lung Transplant. 2001;20:1044–1053. doi: 10.1016/s1053-2498(01)00301-1. [DOI] [PubMed] [Google Scholar]
- 4.Collins J., Kazerooni E.A., Lacomis J. Bronchogenic carcinoma after lung transplantation: frequency, clinical characteristics and imaging findings. Radiology. 2002;224:131–138. doi: 10.1148/radiol.2241011189. [DOI] [PubMed] [Google Scholar]
- 5.Daniels C.E., Jett J.R. Does interstitial lung disease predispose to lung cancer? Curr Opin Pulm Med. 2005;11:431–437. doi: 10.1097/01.mcp.0000170521.71497.ba. [DOI] [PubMed] [Google Scholar]
- 6.Le Jeune I., Gribbin J., West J., Smith C., Cullinan P., Hubbard R. The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Resp Med. 2007;101:2534–2540. doi: 10.1016/j.rmed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Minai O., Shah S., Mazzone P. Bronchogenic carcinoma after lung transplantation: characteristics and outcomes. J Thorac Oncol. 2008;3:1404–1409. doi: 10.1097/JTO.0b013e31818e1259. [DOI] [PubMed] [Google Scholar]
- 8.Dickson R.P., Dave R.D., Rea J.B., Palmer S.M. High frequency of bronchogenic carcinoma after single-lung transplantation. J Heart Lung Transplant. 2006;25:1297–1301. doi: 10.1016/j.healun.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Y., Seneviratne C.K., Koss M. Idiopathic pulmonary fibrosis and malignancy. Curr Opin Pulm Med. 2001;7:278–282. doi: 10.1097/00063198-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Choi Y.H., Leung A.N., Miro S., Poirier C., Hunt S., Theodore J. Primary bronchogenic carcinoma after heart or lung transplantation: radiologic and clinical findings. J Thorac Imag. 2000;15(1):36–40. doi: 10.1097/00005382-200001000-00008. [DOI] [PubMed] [Google Scholar]


