Abstract
Sarcoidosis is a multisystem granulomatous inflammatory disease of unknown etiology. There is evidence that Tumor Necrosis Factor alpha (TNF-α) antagonists are useful in the treatment of advanced or refractory disease. However, sarcoidosis-like reaction has been reported with TNF-α blockade in other inflammatory conditions. Here we report a case of sarcoid-like reaction in a patient with psoriatic arthritis shortly after initiation of adalimumab therapy. Stopping adalimumab and systemic anti-inflammatory therapy with corticosteroids resulted in resolution of pulmonary symptoms and chest radiographic findings. Though TNF-α plays a critical role in pathogenesis of sarcoidosis, the development of sarcoid reaction with TNF-α blockade is paradoxical and the mechanism of this response remains unknown. TNF-α induced sarcoid-reaction could involve multiple organs. Its development with one agent does not preclude therapy with other TNF-α blockers.
Keywords: TNF blockers, Adalimumab, Sarcoid reaction
1. Introduction
Tumor Necrosis Factor (TNF) is a pro-inflammatory cytokine produced by activated macrophages, CD 4+ lymphocytes, NK cells and other cells. Hence, agents blocking TNF-α are widely used for the treatment of various immune mediated inflammatory conditions such as rheumatoid arthritis [1], psoriatic arthritis [2] and ankylosing spondylitis. TNF- α blockers have also proven effective in treatment of granulomatous inflammatory diseases such as sarcoidosis [3–5], Crohn's disease and granulomatosis with polyangiitis (Wegener's) [6], conditions where TNF-α is critical in pathogenesis. Most commonly used TNF-α antagonists include etanercept which is a fusion protein that binds TNF-α by mimicking the soluble TNF receptor, infliximab and adalimumab, which are monoclonal antibodies against TNF-α. These agents have demonstrated variable therapeutic efficacy in sarcoidosis and other inflammatory conditions, presumably owing to different binding characteristics to TNF-α. In a recent study, adalimumab compared to etanercept or infliximab was more effective in the treatment of psoriasis [7] thus making it an important option in therapy. However, like other TNF antagonists, adverse effects have been observed with adalimumab.
Although TNF-α antagonists are effective in treatment of sarcoidosis, a paradoxical sarcoid-like reaction [8] has been seen in approximately 1/2800 patients treated for inflammatory arthropathies [9]. A survey of the literature revealed 52 cases [10–21] where the use of TNF-α antagonists has led to the development of a sarcoid-like reaction. Among those cases 33 were treated with etanercept, 12 with Infliximab and 7 with adalimumab. A majority of the adalimumab cases had a diagnosis of rheumatoid arthritis with only one case of sarcoid-like reaction in a patient with psoriasis. Here, we describe another case of psoriatic arthritis being treated with adalimumab who developed a sarcoid-like reaction that showed complete resolution with discontinuation of adalimumab in combination with anti-inflammatory therapy.
2. Case report
31-year-old white female presented to the pulmonary clinic for evaluation of fevers. She had been diagnosed with psoriatic arthritis 18 months prior. Her initial treatment was with infliximab monotherapy. Subsequently, cyclosporine was added due to limited benefit from infliximab. Due to lack of response in joint symptoms, infliximab was switched to adalimumab. Two months later, she started having fevers for which the adalimumab was held and fever workup performed. However, due to recurrence of joint pain, one additional dose of adalimumab was given 6–8 weeks prior to evaluation in the pulmonary clinic. For the persistent fevers and shortness of breath a chest radiograph was performed which showed infiltrates prompting further evaluation with chest CT Scan which is shown in Fig. 1 (panel A and B). As there was a mediastinal mass (concerning for lymphoma), a PET scan was also done which demonstrated increased uptake in the lymph nodes, but the mass behind the sternum did not demonstrate high metabolic activity, suggesting it was thymic hyperplasia (FDG PET images not shown). Transbronchial biopsies were performed which showed non-necrotizing granulomas (Fig. 2). Histopathological stains were negative for malignancy and no acid-fast bacilli or fungal elements were identified. Antigens for Histoplasma and Legionella (serogroup 1) in urine were also negative. Fungal antibodies were also negative. No bacterial organisms were identified (including Mycobacterium tuberculosis) on culture of the bronchoalveolar lavage fluid. Adalimumab was held and systemic anti-inflammatory treatment with prednisone was initiated which resulted in improvement with the imaging abnormalities, fevers and the pulmonary symptoms. Chest CT scan obtained at seven months had minimal residual infiltrates, which completely resolved on the CT scan done at 23 months (Fig. 1 panel C and D).
Fig. 1.
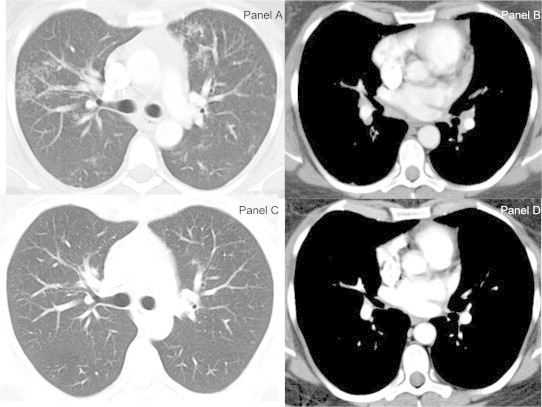
Chest CT scan images lung windows (panel A and C) and mediastinal windows (panel B and D). At presentation the lung windows demonstrated bilateral asymmetric perilymphatic distributed micronodular opacities (Galaxy Sign- Panel A) and bilateral hilar and subcarinal lymphadenopathy (panel B). Subsequent chest CT scan images at 23 months showed complete resolution of the parenchymal micronodules with significant improvement of the lymphadenopathy except mildly enlarged right hilar lymph node.
Fig. 2.
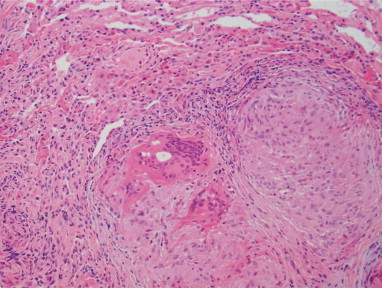
Transbronchial biopsies performed at presentation reveal non-caseating, well-formed granulomas involving lung tissue. No fungal or acid-fast organisms were identified (with appropriate controls).
3. Discussion
TNF-α antagonists have shown potential for remedying symptoms of immune-mediated inflammatory arthropathies including psoriatic arthritis. However, a number of recent reports have shown an association of sarcoid-like reaction in psoriatic arthritis patients getting anti TNF-α therapy, resulting in the formation of non-necrotizing granulomas in multiple organs. Here we describe a patient with a diagnosis of psoriatic arthritis who developed sarcoid-like reaction in the lung while being treated with adalimumab. The patient presented with symptoms after initiating treatment with adalimumab, diagnostic evaluation for the presenting symptoms showed bilateral hilar and subcarinal lymphadenopathy with parenchymal infiltrates and biopsy of the lesions revealed non-necrotizing granulomas similar to those seen in sarcoidosis. Adalimumab was withdrawn and prednisone was initiated which gradually led to the resolution of symptoms and improvement in imaging abnormalities. Long term follow up revealed no imaging abnormality at approximately 2 years after the diagnosis of sarcoid reaction from treatment with adalimumab.
The molecular mechanism underlying the paradoxical sarcoid-like reaction upon treatment with TNF- α antagonists is presently unknown despite improved understanding of the early pathogenesis of Sarcoidosis [22]. In response to an unidentified trigger, immune response is initiated by mobilization and activation of CD4+ T helper cells resulting in secretion of cytokines such as IFN-γ and Interleukin-2. These cytokines act as chemo-attractants and recruit fresh macrophages, neutrophils and other lymphocytes. Subsequent activation of macrophages release TNF-α that mediates formation of granulomas by activating multiple signaling pathways resulting in increased collagen synthesis, angiogenesis and activation of NFκB signaling pathway [23]. Thus, inhibition of TNF-α response by its antagonists should result in less granuloma formation. However, it is postulated that the cytokine imbalance caused by neutralization of peripheral TNF-α activate specific auto-reactive T-cells which result in sarcoid-like reaction in patients treated with TNF-α antagonists [24].
In addition to sarcoidosis, this paradoxical inflammatory reaction with TNF blockade has been shown in other autoimmune disease as well. There have been case reports of drug-induced lupus [25,26], psoriasis [27] and vasculitis [28,29] caused by anti-TNF therapy. Interstitial lung disease other than sarcoidosis has also been reported with TNF blockadeutz. When presenting with sarcoid-like reactions, all three agents discussed have been reported to cause both pulmonary and extra pulmonary disease. However, the three commonly used TNF blockers are not equivalent. It is interesting to note that a large majority of the case reports of anti-TNF-α therapy induced sarcoid-like reaction involve etanercept, which is not effective in the treatment of sarcoidosis and may even exacerbate the disease [30]. These variations could be attributed to different modes of action of TNF-α antagonists [6,31]. While infliximab and adalimumab bind both soluble monomeric and trimeric TNF-α and trans-membrane TNF-α, etanercept binds only to soluble trimeric TNF-α with reduced affinity to the trans-membrane portion of TNF. Also, the complex interaction between these agents and other occupational/environmental exposures [32], genetic factors [33] and other concomitant use of immunosuppressive medications are potentially important though poorly understood. Paradoxical inflammatory response with one TNF-α does not preclude use of other TNF blocking agents. However new therapeutic options including ustekinumab, abatacept, IL-17 inhibitors, apremilast, JAK inhibitors, and possibly IL-6 inhibitors [34] might be useful for patients who develop sarcoid reaction to TNF-α blockers.
References
- 1.Haraoui B. The anti-tumor necrosis factor agents are a major advance in the treatment of rheumatoid arthritis. J Rheumatol Suppl. 2005;72:46–47. [PubMed] [Google Scholar]
- 2.Haraoui B. Differentiating the efficacy of the tumor necrosis factor inhibitors. Semin Arthritis Rheum. 2005;34:7–11. doi: 10.1016/j.semarthrit.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Judson M.A., Baughman R.P., Costabel U., Flavin S., Lo K.H., Kavuru M.S. Efficacy of infliximab in extrapulmonary sarcoidosis: results from a randomized trial. Eur Respir J. 2008;31:1189–1196. doi: 10.1183/09031936.00051907. [DOI] [PubMed] [Google Scholar]
- 4.Rossman M.D., Newman L.S., Baughman R.P., Teirstein A., Weinberger S.E., Miller W., Jr. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2006 Oct;23(3):201–208. [PubMed] [Google Scholar]
- 5.Baughman R.P., Drent M., Kavuru M., Judson M.A., Costabel U., du Bois R. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174:795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 6.Wallis R.S., Ehlers S. Tumor necrosis factor and granuloma biology: explaining the differential infection risk of etanercept and infliximab. Semin Arthritis Rheum. 2005;34:34–38. doi: 10.1016/j.semarthrit.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Kirson N.Y., Rao S., Birnbaum H.G., Kantor E., Wei R.S., Cifaldi M. Matching-adjusted indirect comparison of adalimumab vs etanercept and infliximab for the treatment of psoriatic arthritis. J Med Econ. 2013;16:479–489. doi: 10.3111/13696998.2013.768530. [DOI] [PubMed] [Google Scholar]
- 8.Clementine R.R., Lyman J., Zakem J., Mallepalli J., Lindsey S., Quinet R. Tumor necrosis factor-alpha antagonist-induced sarcoidosis. J Clin Rheumatol. 2010;16:274–279. doi: 10.1097/RHU.0b013e3181efa190. [DOI] [PubMed] [Google Scholar]
- 9.Daïen C.I., Monnier A., Claudepierre P., Constantin A., Eschard J.P., Houvenagel E. Sarcoid-like granulomatosis in patients treated with tumor necrosis factor blockers: 10 cases. Rheumatology. 2009;48:883–886. doi: 10.1093/rheumatology/kep046. [DOI] [PubMed] [Google Scholar]
- 10.Ognenovski V.M., Ojo T.C., Fox D.A. Etanercept-associated pulmonary granulomatous inflammation in patients with rheumatoid arthritis. J Rheumatol. 2008;35:2279–2282. doi: 10.3899/jrheum.080383. [DOI] [PubMed] [Google Scholar]
- 11.Louie G.H., Chitkara P., Ward M.M. Relapse of sarcoidosis upon treatment with etanercept. Ann Rheum Dis. 2008;67:896–898. doi: 10.1136/ard.2007.078840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Stoep D., Braunstahl G.J., van Zeben J., Wouters J. Sarcoidosis during anti-tumor necrosis factor-alpha therapy: no relapse after rechallenge. J Rheumatol. 2009;36:2847–2848. doi: 10.3899/jrheum.090307. [DOI] [PubMed] [Google Scholar]
- 13.Gifre L., Ruiz-Esquide V., Xaubet A., Gomez-Puerta J.A., Hernandez M.V., Sanmarti R. Lung sarcoidosis induced by TNF antagonists in rheumatoid arthritis: a case presentation and a literature review. Arch Bronconeumol. 2011;47:208–212. doi: 10.1016/j.arbres.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Bachmeyer C., Blum L., Petitjean B., Kemiche F., Pertuiset E. Granulomatous tattoo reaction in a patient treated with etanercept. J Eur Acad Dermatol Venereol. 2007;21:550–552. doi: 10.1111/j.1468-3083.2006.01949.x. [DOI] [PubMed] [Google Scholar]
- 15.Dhaille F., Viseux V., Caudron A., Dadban A., Tribout C., Boumier P. Cutaneous sarcoidosis occurring during anti-TNF-alpha treatment: report of two cases. Dermatology. 2010;220:234–237. doi: 10.1159/000275676. [DOI] [PubMed] [Google Scholar]
- 16.Popper H.H. Epithelioid cell granulomatosis of the lung: new insights and concepts. Sarcoidosis, Vasculitis, Diffuse Lung Dis Off J WASOG/World Assoc Sarcoidosis Other Granulomatous Disord. 1999;16:32–46. [PubMed] [Google Scholar]
- 17.Cathcart S., Sami N., Elewski B. Sarcoidosis as an adverse effect of tumor necrosis factor inhibitors. J Drugs Dermatol. 2012;11:609–612. [PubMed] [Google Scholar]
- 18.Takahashi H., Kaneta K., Honma M., Ishida-Yamamoto A., Ashida T., Kohgo Y. Sarcoidosis during infliximab therapy for Crohn's disease. J Dermatol. 2010;37:471–474. doi: 10.1111/j.1346-8138.2010.00861.x. [DOI] [PubMed] [Google Scholar]
- 19.Baughman R.P., Iannuzzi M. Tumour necrosis factor in sarcoidosis and its potential for targeted therapy. BioDrugs. 2003;17:425–431. doi: 10.2165/00063030-200317060-00005. [DOI] [PubMed] [Google Scholar]
- 20.Tong D., Manolios N., Howe G., Spencer D. New onset sarcoid-like granulomatosis developing during anti-TNF therapy: an under-recognised complication. Intern Med J. 2012;42:89–94. doi: 10.1111/j.1445-5994.2011.02612.x. [DOI] [PubMed] [Google Scholar]
- 21.Hubscher O., Re R., Iotti R. Pulmonary rheumatoid nodules in an etanercept-treated patient. Arthritis Rheum. 2003;48:2077–2078. doi: 10.1002/art.11154. [DOI] [PubMed] [Google Scholar]
- 22.Iannuzzi M.C., Rybicki B.A., Teirstein A.S. Sarcoidosis. N Engl J Med. 2007;357:2153–2165. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 23.Ziegenhagen M.W., Muller-Quernheim J. The cytokine network in sarcoidosis and its clinical relevance. J Intern Med. 2003;253:18–30. doi: 10.1046/j.1365-2796.2003.01074.x. [DOI] [PubMed] [Google Scholar]
- 24.Marcella S., Welsh B., Foley P. Development of sarcoidosis during adalimumab therapy for chronic plaque psoriasis. Australas J Dermatol. 2011;52:e8–11. doi: 10.1111/j.1440-0960.2010.00660.x. [DOI] [PubMed] [Google Scholar]
- 25.Debandt M., Vittecoq O., Descamps V., Le Loet X., Meyer O. Anti-TNF-alpha-induced systemic lupus syndrome. Clin Rheumatol. 2003;22:56–61. doi: 10.1007/s10067-002-0654-5. [DOI] [PubMed] [Google Scholar]
- 26.Williams V.L., Cohen P.R. TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF alpha antagonists. Int J Dermatol. 2011;50:619–625. doi: 10.1111/j.1365-4632.2011.04871.x. [DOI] [PubMed] [Google Scholar]
- 27.Wollina U., Hansel G., Koch A., Schonlebe J., Kostler E., Haroske G. Tumor necrosis factor-alpha inhibitor-induced psoriasis or psoriasiform exanthemata: first 120 cases from the literature including a series of six new patients. Am J Clin Dermatol. 2008;9:1–14. doi: 10.2165/00128071-200809010-00001. [DOI] [PubMed] [Google Scholar]
- 28.Ramos-Casals M., Brito-Zeron P., Cuadrado M.J., Khamashta M.A. Vasculitis induced by tumor necrosis factor-targeted therapies. Curr Rheumatol Rep. 2008;10:442–448. doi: 10.1007/s11926-008-0072-z. [DOI] [PubMed] [Google Scholar]
- 29.Mohan N., Edwards E.T., Cupps T.R., Slifman N., Lee J.H., Siegel J.N. Leukocytoclastic vasculitis associated with tumor necrosis factor-alpha blocking agents. J Rheumatol. 2004;31:1955–1958. [PubMed] [Google Scholar]
- 30.Utz J.P., Limper A.H., Kalra S., Specks U., Scott J.P., Vuk-Pavlovic Z. Etanercept for the treatment of stage II and III progressive pulmonary sarcoidosis. Chest. 2003;124:177–185. doi: 10.1378/chest.124.1.177. [DOI] [PubMed] [Google Scholar]
- 31.Ehlers S. Tumor necrosis factor and its blockade in granulomatous infections: differential modes of action of infliximab and etanercept? Clin Infect Dis. 2005;41(Suppl. 3):S199–S203. doi: 10.1086/429998. [DOI] [PubMed] [Google Scholar]
- 32.Newman L.S., Rose C.S., Bresnitz E.A., Rossman M.D., Barnard J., Frederick M. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324–1330. doi: 10.1164/rccm.200402-249OC. [DOI] [PubMed] [Google Scholar]
- 33.Luisetti M., Beretta A., Casali L. Genetic aspects in sarcoidosis. Eur Respir J. 2000;16:768–780. doi: 10.1034/j.1399-3003.2000.16d31.x. [DOI] [PubMed] [Google Scholar]
- 34.Mease P. Update on treatment of psoriatic arthritis. Bull NYU Hosp Jt Dis. 2012;70:167–171. [PubMed] [Google Scholar]


