Abstract
Pulmonary alveolar proteinosis (PAP) is a rare and diffuse lung process, characterized by the presence of alveolar spaces filled with amorphous eosinophilic material. Impaired macrophage function and impaired host defence due to abnormalities of surfactant proteins may favor the growth of microorganisms. The association of alveolar proteinosis with mycobacterial infections is rarely reported. The PAP and superinfection with pulmonary tuberculosis is defined by radiologic and histopathologic in a 46 year-old patient. The patients with PAP should be monitored for superinfection. It may cause the disease progression and radiological, clinical symptoms may improve with treatment of superinfection.
Keywords: Pulmonary alveolar proteinosis, Tuberculosis, Radiology
1. Introduction
Pulmonary alveolar proteinosis (PAP) was first described in 1958 by Rosen et al. and is a rare lung disease characterized by the abnormal accumulation of PAS-positive (periodic acid-Schiff) phospholipoprotein material in the alveoli.1 Two forms are described: primary or idiopathic, which occurs in the absence of another illness or a known environmental exposure; and secondary, when associated with another morbid condition, especially infectious or neoplastic, in various states of immunosuppression, as well as in those resulting from the inhalation of chemical agents and mineral particles (silica, aluminum, titanium, and some insecticides). Several etiological agents have been identified in this population: Aspergillus sp., Nocardia sp., Mycobacterium sp., Cryptococcus neoformans, Histoplasma capsulatum, Pneumocystis carinii, and virus.2,3 The association of alveolar proteinosis and pulmonary tuberculosis has been rarely reported. Educational aims are the discussion of the radiologic, histopathologic and clinical association of PAP and pulmonary tuberculosis in a case.
2. Case report
A 46-year-old, life long non-smoker male was admitted to the hospital with the complaints of dyspnea, cough and fever. He had fatigue, non-productive cough and progressive dyspnea during two months and fever for a week. He is working as a welder. His medical history was normal. Physical examination revealed the bilateral fine crackles. The purulent sputum was present. Chest roentgenogram demonstrated the bilateral alveolar and interstitial opacities with paracardiac non-homogenous opacity on right hemithorax (Fig. 1A). The hemoglobin value was 10.1, peripheral blood leukocyte count was 12,000 cell/cu mm and erythrocytes sedimentation rate was 60 mm/h. Arterial blood gas values during room-air breathing revealed that the pH: 7.52, pO2: 60 mmHg, pCO2: 24 mmHg and SaO2: 94%. Other laboratory values were normal. Thorax CT revealed the bilateral ground-glass opacities associated with thickened interlobular septa, called to as “crazy paving” pattern (Fig. 2). Also, alveolar consolidation was observed on right middle lobe. Fiberoptic bronchoscopy showed the hyperemic bronchial mucosa. AFB-staining and cytological examination of bronchial lavage fluids were negative and benign, respectively. Transbronchial biopsy was performed. Histopathologic examination of transbronchial biopsy showed the alveolar spaces filled with granular eosinophilic materials which were Periodic acid-Schiff (PAS) positive (Fig. 3). The patient wanted to get discharged on 2nd day of hospitalization and he did not want to receive any treatment. Ten days later, he was admitted to the hospital with fever. Chest radiograph on second admission was similar to the first admission. The direct smear of the sputum showed acid-fast bacilli with AFB-staining. The culture of previously taken bronchial lavage fluid grew Mycobacterium tuberculosis. Antituberculosis treatment combined with regimen of isoniazid, morfozinamid, rifampicin and ethambutol was started. The symptomatic and radiologic improvements were observed after the treatment (Figs. 1A and 4).
Fig. 1.
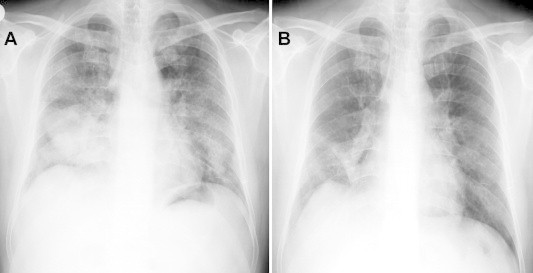
Chest roentgenogram is showing the bilateral alveolar, interstitial opacities with paracardiac non-homogenous opacity on right hemithorax (1A) and regression is seen after six months later with antituberculous therapy (1B).
Fig. 2.
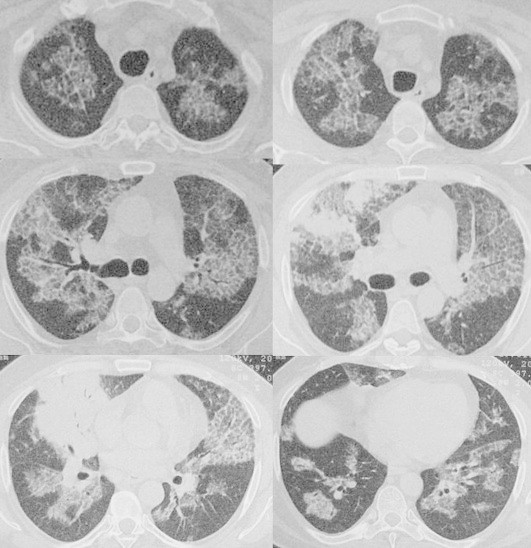
Thorax CT scans are showing the bilateral ground-glass opacities associated with thickened interlobular septa, called to as “crazy paving” pattern.
Fig. 3.
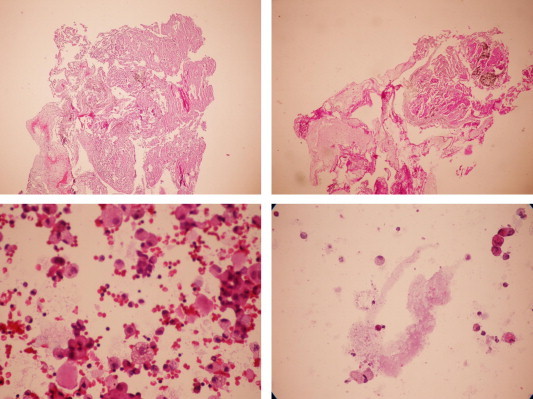
Histopathologic examination of transbronchial biopsy is showing the alveolar spaces filled with granular eosinophilic materials which were Periodic acid-Schiff (PAS) positive.
Fig. 4.
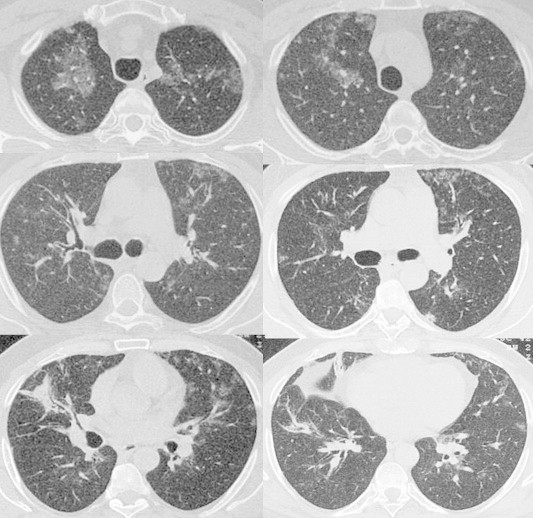
The radiologic improvements are showing on thorax CT scans after the six months with antituberculous treatment.
3. Discussion
Pulmonary alveolar proteinosis (PAP) is a rare and diffuse lung process, etiology undetermined, characterized by the presence of alveolar spaces filled with amorphous eosinophilic material.1,4 The accumulation in alveolar spaces is probably caused by defective clearance of lipoproteinaceous material by alveolar macrophages. Recent animal experiments have suggested that GM-CSF (granulocyte-macrophage colony stimulating factor) deficiency might play a role in the pathogenesis.5,6
Secondary PAP could be associated with 3 main clinical settings: Infection of the lung, most commonly with Norcardia astroides, TB, Mycobacterium avium-intracellulare, or P. carinii. Hematologic malignancies and other conditions that alter the patient’s immune status, e.g., lymphoma, leukemia, or AIDS. And exposure to inhaled chemicals and minerals, e.g., fumes, dusts, silica, aluminum, insecticides, or titanium leading to type II pneumocyte destruction.7,8 The material filling alveolar spaces in secondary PAP is mainly cell debris and fibrin.9 Bilateral air space consolidation is a typical but non-specific feature appearing on chest radiography. High resolution computed tomography scanning (HRCT) reveals ground-glass opacification usually associated with thickened interlobular septa, distinctly visible within the affected lung, referred to as “crazy paving” pattern and under the light microscopy the alveoli and terminal bronchioles are filled with a granular lipoproteinaceous material which stains a deep pink with PAS stain, as seen in our patients. A major and typical complication of PAP is infection with Nocardia species, Mycobacterium species, C. neoformans, H. capsulatum, P. carinii and viruses. This susceptibility to unusual organism is multifactorial. Impaired macrophage function and impaired host defence due to abnormalities of surfactant proteins may favor the growth of microorganisms. The association of alveolar proteinosis with mycobacterial infections is rarely reported. This association may not be fortutious and they usually described with M. tuberculosis infection was superimposed on the pulmonary alveolar proteinosis, which acted a predisposing factor. Very few cases were defined with pulmonary tuberculosis accompanied to PAP. Two of them as superinfection of the proteinosis in the adult patients with acquired immune deficiency syndrome, one case in a HIV positive infected child and one with the association proteinosis and diabetes mellitus.2–4,9,10
We think that the M. tuberculosis was evolved as a superinfection on PAP. Because of the crazy pattern seen bilaterally in our patient. If the PAP were secondary to tuberculosis, the crazy pattern would be expected as localized.
In conclusion; superinfection of M. tuberculosis may raise risk for patients with PAP. The patients with PAP should be monitored for superinfection. It may cause the disease progression and radiological, clinical symptoms may improve with treatment of superinfection.
References
- 1.Rosen S.H., Castleman B., Liebow A.A. Pulmonary alveolar proteinosis. N Engl J Med. 1958;258:1123–1142. doi: 10.1056/NEJM195806052582301. [DOI] [PubMed] [Google Scholar]
- 2.Ruben F.L., Talamo T.S. Secondary pulmonary alveolar proteinosis occurring in two patients with acquired immune deficiency syndrome. Am J Med. 1986;80:1187–1190. doi: 10.1016/0002-9343(86)90683-2. [DOI] [PubMed] [Google Scholar]
- 3.Pereira-Silva J.L., Marinho M.M.M.A., Veloso T.V.B., Coelho Filho J.C. Pulmonary alveolar proteinosis and tuberculosis in a diabetic patient: a rare or a seldom diagnosed association? Braz J Infect Dis. 2002;6(4):188–195. doi: 10.1590/s1413-86702002000400006. [DOI] [PubMed] [Google Scholar]
- 4.Lin C.Y., Sheu C.Y., Chen P.J. Pulmonary alveolar proteinosis: report of four cases. Chin J Radiol. 1999;24(6):243–247. [Google Scholar]
- 5.Parker L.A., Debra B.N. Recurrent alveolar proteinosis following double lung transplantation. Chest. 1997;111:1457–1458. doi: 10.1378/chest.111.5.1457. [DOI] [PubMed] [Google Scholar]
- 6.Godwin J.D., Nestor L.M., Julie E.T. Pulmonary alveolar proteinosis: CT findings. Radiology. 1998;169:609–613. doi: 10.1148/radiology.169.3.3186983. [DOI] [PubMed] [Google Scholar]
- 7.Bennet M.W., Eric J.S., Rodney A.S., David J.P. Diagnosing pulmonary alveolar proteinosis: a review and update. Chest. 1997;111:460–466. doi: 10.1378/chest.111.2.460. [DOI] [PubMed] [Google Scholar]
- 8.Gacouin A., Le Tulzo Y., Suprin E. Acute respiratory failure caused by secondary alveolar proteinosis in a patient with acute myeloid leukemia: a case report. Intensive Care Med. 1998;24:265–267. doi: 10.1007/s001340050563. [DOI] [PubMed] [Google Scholar]
- 9.Wendy E.Z., Felix S.C. Pulmonary alveolar proteinosis. Radiologic-pathologic conferences of the Massachusetts General Hospital. AJR. 1993;61:26. [Google Scholar]
- 10.Dragomir A., Cıontu M., Martius M. Superinfection with Mycobacterium tuberculosis in a patient with pulmonary alveolar proteinosis. Medica-a J of Clin Med. 2008;1(3):59–63. [Google Scholar]


