Abstract
A 70-year-old woman, who has had a diagnosis of sarcoidosis since she was 38 years old, showed newly appearing diffuse ground-glass opacities in the bilateral lung field, and bilateral enlargement of the hilar and mediastinal lymph nodes. Based on findings from bronchoalveolar lavage fluid (BALF) and pathology analysis, eosinophilic pneumonia accompanied by sarcoidosis was suspected. Both disease conditions (sarcoidosis and BALF eosinophilia) worsened and improved simultaneously, and she showed two similar episodes during the follow-up. This case prompted us to conduct a retrospective investigation of eosinophil percentage in peripheral blood and BALF in 178 patients (excluding our patient) who had received a diagnosis of sarcoidosis between 2000 and 2009 in our department. Among the 178 patients, the highest eosinophil percentage in BALF was 2.6%; in contrast, peripheral blood eosinophilia was very common. Thus we concluded that, for subjects with sarcoidosis, marked eosinophilia in BALF, as observed in the case of this 70-year-old woman, was exceptional.
Keywords: Sarcoidosis, Eosinophilia
1. Introduction
According to previous reports, BALF eosinophilia in patients with sarcoidosis is rare,1–3 and only three cases of sarcoidosis with eosinophilia in BALF have been reported.4–6 Here we report an interesting case of sarcoidosis with increased eosinophil percentage in peripheral blood and BALF. Of interest, both sarcoidosis and eosinophilia worsened and improved simultaneously, and no previous reports have shown a simultaneous change in the severity of these two disease conditions. Furthermore, we present the results of a retrospective analysis of 178 patients (excluding our patient) who had received a diagnosis of sarcoidosis in our department between 2000 and 2009. Finally, we discuss the percentage of these 178 subjects that presented with an increase of eosinophils in peripheral blood and BALF.
2. Case report
A 70-year-old woman, who has had a diagnosis of sarcoidosis since she was 38 years old, was admitted to our hospital in October 2008. She had been followed without treatment after the initial diagnosis, but she developed cough and dyspnea in September 2008. She had never smoked and had no apparent history of exposure to allergens, had not been exposed to environmental changes, and had not used new medicines or dietary supplements for at least 6 months before admission. She had no history of any allergic diseases, such as bronchial asthma, allergic rhinitis, or atopic dermatitis. She lived in an urban area and had never traveled to areas with a high prevalence of parasites. Laboratory data revealed increases in peripheral eosinophil counts (10.0%, 869/μL), total serum IgE levels [509.3 IU/L (normal: 0–295)], serum angiotensin converting enzyme (ACE) activity [33.5 IU/L (normal: 8.3–21.5)], and soluble interleukin-2 receptor (sIL2R) [3932 U/mL (normal: 0–459)] relative to data from three months before (eosinophil: 5.0%, 330/μL, ACE: 23.2 IU/L). The results of IgE multiple antigen simultaneous tests revealed positive for Dermatophagoides farinae and negative for Aspergillus. She was also negative for antineutrophil cytoplasmic antibodies to both myeloperoxidase and proteinase 3. No evidence of parasites were found in stool samples. High-resolution computed tomography (CT) revealed diffuse ground-glass opacity in the bilateral middle and lower lobe, and enlargement of bilateral hilar and mediastinal lymph nodes. Analysis of BALF obtained from the right S4+5 segment revealed elevated eosinophil (15.2%) and lymphocyte (51.2%) counts and a high CD4/8 ratio (26.2), but no evidence of microbial culture. Lung biopsy revealed infiltration of eosinophils in alveolar walls and alveolar spaces (Fig 1a) with granulomatous tissue containing multinucleated giant cells (Fig 1b), but no evidence of angiitis. Transbronchial needle biopsy from the #7 lymph node also revealed non-caseating epithelioid cell granuloma (Fig. 1c) with no apparent pathogens, such as fungus or mycobacterium. Eosinophilic pneumonia accompanied by sarcoidosis was suspected. Her respiratory symptoms, the ground-glass opacities by chest X-ray and peripheral eosinophilia, elevated serum ACE activity and sIL2R remitted spontaneously without treatment within several months. When her clinical course was evaluated retrospectively, it became evident that she had had a similar episode in 2004; at that time, she had developed a mild cough, ground-glass opacities in the lower lobe of the left lung, peripheral eosinophilia, and elevated serum ACE activity (Fig. 2). All these symptoms remitted spontaneously within several months without treatment.
Fig. 1.
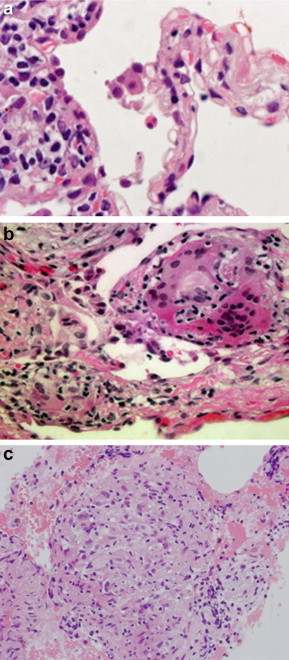
Pathological findings of a transbronchial lung biopsy specimen (a and b) and transbronchial needle biopsy specimen from the #7 lymph node (c) (hematoxylin and eosin stain, a 1000x, b 200x, c 40x). (a) Infiltration of eosinophils in alveolar walls and alveolar spaces were observed. (b) Granulomatous tissue with multinucleated giant cells was evident in lung parenchyma. (c) Non-caseating epithelioid cell granuloma was evident.
Fig. 2.
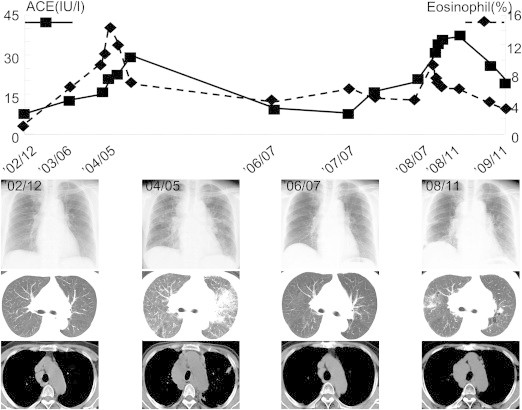
Clinical course from December 2002 to October 2010. Chest Xp and CT showed consolidation in left lung field in 2004; additionally, serum ACE activity and percentage of peripheral eosinophil were elevated. In 2008, ground-glass opacities were evident in the right lung filed, and serum ACE activity and the percentage of peripheral eosinophil were again elevated, but spontaneously remitted.
3. Retrospective investigation of eosinophil percentage in peripheral blood and BALF in patients with sarcoidosis in our department
The case described above prompted us to conduct a retrospective study on eosinophil percentage in peripheral blood and BALF in patients with sarcoidosis; 178 patients (excluding our patient) who had received a diagnosis of sarcoidosis—as per the ATS/ERS/WASOG Statement on sarcoidosis7—between January 2000 and December 2009 at First Department of Medicine, Hokkaido University Hospital were enrolled in this study. Among these 178 patients, the highest eosinophil percentage in BALF was 2.6% (Table 1); in contrast, the eosinophil percentage in peripheral blood was highly variable and reached a maximum of 22.0% in one case. We categorized each subject into one of three groups (<4%, 4%–10%, and ≥10%) according to the peripheral blood eosinophil percentage as described by Renston et al.8; we then evaluated the presence of several allergic diseases on the basis of medical records (Table 1). Although some patients with peripheral blood eosinophilia had comorbidities, such as asthma or allergic rhinitis, others did not have additional clinically apparent diseases. Percentage of peripheral blood eosinophils of <4% or ≥4% were not associated with the stage of sarcoidosis according to the results of the chi-square test.
Table 1.
Characteristics of 178 patients with sarcoidosis categorized according to percentage of peripheral (p) eosinophil.
| All | p eosinophil < 4% | 4% ≤ p eosinophil < 10% | 10% ≤ p eosinophil | |
|---|---|---|---|---|
| Number | 178 | 115 | 58 | 5 |
| Male/female | 62/116 | 37/78 | 24/34 | 1/4 |
| Agea | 56 (17–79) | 60 (17–79) | 50 (20–75) | 61 (24–76) |
| Smoking (No/Ex/Current) | 80/40/58 | 53/25/37 | 24/14/20 | 3/1/1 |
| Stage (0/I/II/III) | 31/88/45/14 | 23/54/29/9 | 8/30/16/4 | 0/4/0/1 |
| ACE (IU/l)a | 23.6 (0.9–48.3) | 23.6 (4.1–48.3) | 24.2 (0.9–42.2) | 29.8 (22.1–36.4) |
| BAL | ||||
| Total cells (×104/ml BALF)a | 17.0 (2.2–92.2) | 17.9 (2.2–89.8) | 17.4 (4.3–92.2) | 37.6 (17–51.5) |
| Macrophage (%)a | 64.6 (8.2–96.8) | 65.3 (8.2–96.4) | 64.6 (22.8–96.8) | 70.3 (51.9–91.8) |
| Lymphocytes (%)a | 34.3 (2.8–92.4) | 33.6 (3.6–92.4) | 34.8 (2.8–76.3) | 38.3 (5.6–47.1) |
| Neutrophil (%)a | 0.2 (0–7.0) | 0.2 (0–7.0) | 0.2 (0–2.0) | 0 (0–1.0) |
| Eosinophil (%)a | 0 (0–2.6) | 0 (0–2.4) | 0 (0–2.0) | 1.3 (0–2.6) |
| CD4/CD8a | 4.59 (0.72–30.2) | 4.20 (0.72–15.5) | 5.17(0.79–30.2) | 5.18 (4.01–16.1) |
| Peripheral eosinophil (/ml)a | 148.8(0–1034) | 92.8(0–277)b | 250 (146–795) | 589 (332–1034) |
| Peripheral eosinophil (%)a | 2.8 (0–22.0) | 2.0 (0–3.9)b | 5.0 (4.0–9.7) | 12.0 (10.7–22.0) |
| Asthma (%) | 6 (3.4) | 2 (1.7) | 1 (0.2) | 3 (60.0) |
| Allergic rhinitis (%) | 7 (3.9) | 5 (4.3) | 2 (3.4) | 0 (0) |
| Atopic dermatitis (%) | 1 (0.5) | 1 (0.8) | 0 (0) | 0 (0) |
| Drug allergy (%) | 14 (7.9) | 10 (8.7) | 2 (3.4) | 2 (40.0) |
| Food allergy (%) | 4 (2.2) | 2 (1.7) | 2 (3.4) | 0 (0) |
Median (range).
p < 0.05 (compared with p eosinophil ≥4%).
4. Discussion
Several studies have paid special attention to eosinophil percentage in BALF in patients with sarcoidosis, and eosinophils represented over 1% of all the inflammatory cells only in a few cases.1–3 Similarly, among the 178 patients examined in this retrospective study, the highest eosinophil percentage in BALF was 2.6%. To the best of our knowledge, only three sarcoidosis cases with elevated eosinophil percentage in BALF have been reported,4–6 and in these cases, the authors speculated the co-occurrence of chronic eosinophilic pneumonia.
In contrast, Renston et al. reported that 41% of patients with sarcoidosis showed an increase of peripheral blood eosinophil percentage more than 4%.8 In the population used in this project, peripheral blood eosinophilia, which was defined as >4%, was observed in 35.4% patients (63/178). Some patients had comorbidities (i.e. asthma, allergic rhinitis) that would potentially cause eosinophilia. However, others did not show any evidence of comorbidities on the basis of medical records. Considering both the report by Renston et al. and the present findings, sarcoidosis might be directly associated with peripheral eosinophilia in some patients. That is, peripheral eosinophilia in patients with sarcoidosis might not always indicate coexisting allergic diseases.
The mechanism underlying the co-occurrence of sarcoidosis and BALF eosinophilia is unclear. Tani et al. demonstrated that concentrations of IL-4, IL-5, and IFN-γ were increased in BALF4 in their case. Shijubo et al. discussed the possibility that factors related to migration of both CD4-positive lymphocytes and eosinophils, such as lymphocyte-chemoattractant factor (LCF) and IL-2,5 were involved. In these two previous reports, there was no description of the clinical course of concomitant sarcoidosis or BALF eosinophilia. Our case of the 70-year-old woman is unique and interesting because both sarcoidosis and BALF eosinophilia worsened and improved simultaneously.
In summary, we report a case of sarcoidosis with a concomitant increase in eosinophil percentage in peripheral blood and BALF; both disease conditions worsened and improved simultaneously. In addition, as a result of a retrospective investigation of eosinophil percentage in 178 patients with sarcoidosis in our department, we concluded that BALF eosinophilia in patients with sarcoidosis is extremely rare, whereas the coexistence of sarcoidosis and peripheral eosinophilia is very common.
Disclosure of conflict of interest
The authors state that they have no conflict of interest.
References
- 1.Winterbauer R.H., Lammert J., Selland M., Wu R., Corley D., Springmeyer S.C. Bronchoalveolar lavage cell populations in the diagnosis of sarcoidosis. Chest. 1993;104:352–361. doi: 10.1378/chest.104.2.352. [DOI] [PubMed] [Google Scholar]
- 2.Ziegenhagen M.W., Rothe M.E., Schlaak M., Muller-Quernheim J. Bronchoalveolar and serological parameters reflecting the severity of sarcoidosis. Eur Respir J. 2003;21:407–413. doi: 10.1183/09031936.03.00010403. [DOI] [PubMed] [Google Scholar]
- 3.Danila E., Jurgauskine L., Malickaite R. BAL fluid cells and pulmonary function in different radiographic stages of newly diagnosed sarcoidosis. Adv Med Sci. 2008;53:228–233. doi: 10.2478/v10039-008-0014-z. [DOI] [PubMed] [Google Scholar]
- 4.Tani K., Kashio M., Sano N., Nakamura Y., Ogushi F., Sone S. A case of sarcoidosis associated with chronic eosinophilic pneumonia. J Med Invest. 1998;45:131–136. [PubMed] [Google Scholar]
- 5.Shijubo N., Fujishima T., Morita S., Nakata H., Satoh M., Uno E. Idiopathic chronic eosinophilic pneumonia associated with noncaseating epithelioid granulomas. Eur Respir J. 1995;8:327–330. doi: 10.1183/09031936.95.08020327. [DOI] [PubMed] [Google Scholar]
- 6.Nakano Y., Kurihara N., Miyamoto O., Takamatsu K., Adachi N., Fujiwara H. A case of sarcoidosis beginning with extensive ground-glass pattern on chest X-ray, accompanied with high fever and eosinophilia. Nihon Kyobu Shikkan Gakkai Zasshi. 1989;27:98–106. [PubMed] [Google Scholar]
- 7.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 8.Renston J.P., Goldman E.S., Hsu R.M., Tomashefski J.F., Jr. Peripheral blood eosinophilia in association with sarcoidosis. Mayo Clin Proc. 2000;75:586–590. doi: 10.4065/75.6.586. [DOI] [PubMed] [Google Scholar]


