Abstract
Non-tuberculosis mycobacteria (NTM) have emerged as an important pathogen in lung infection. NTM infection is rarely accompanied by pleural involvement, and empyema necessitatis caused by NTM is very uncommon. We report a very rare case of Mycobacterium abscessus pulmonary disease with empyema accompanied by empyema necessitatis. The patient was successfully treated by percutaneous tube drainage of the empyema and empyema necessitatis with aggressive antibiotics treatment and surgical resection.
Keywords: Empyema, Empyema necessitatis, Mycobacterium abscessus
1. Introduction
The prevalence of non-tuberculosis mycobacteria (NTM) infection is increasingly reported worldwide1, 2, 3 and has become an emerging threat to public health. In South Korea, Mycobacterium abscessus is the second most common pathogen responsible for lung disease caused by NTM, following the Mycobacterium avium-intracellulare complex (MAC).4 Although the most common clinical manifestation of NTM infection is lung disease, the reported cases of empyema due to NTM are very rare5, 6, 7 and no cases of empyema necessitatis caused by NTM have been reported so far. Here, we report a very unusual case of severe M. abscessus infection causing empyema necessitatis in an immunocompetent patient.
2. Case report
A 57 year old man was referred to our hospital for further evaluation and management of empyema necessitatis. The patient was an alcoholic and had an 80 pack-year smoking history. Seven years earlier, he had been treated for pulmonary tuberculosis (TB) for 3 months at another hospital, but stopped taking pills and failed to follow up.
Since two years ago, the patient had experienced chronic coughing. Three months prior to admission, his coughing became aggravated and he experienced right anterior chest wall swelling with crepitus. He visited a nearby clinic one month prior to admission. A chest computed tomography (CT) scan taken at that clinic showed focal consolidation and cavitation in the right upper lobe with a finding suggestive of bronchopleural fistula (Fig. 1). Because his sputum was positively stained for acid-fast bacilli (AFB), he was started on anti-TB medication. Although he had kept taking pills regularly, his right anterior chest wall swelling had become aggravated in size and turned into abscess. Then he was referred to our hospital for further management.
Fig. 1.
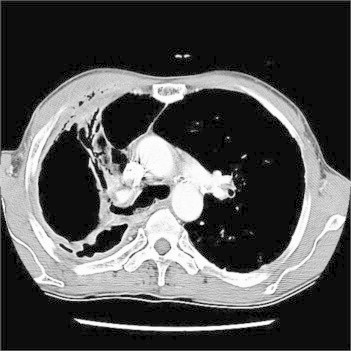
Initial chest CT scan showed that cavitation with focal consolidation, pleural effusion and pleural wall thickness in right upper lobe. There were diffuse infiltration and air collection at the soft tissue of right anterior chest wall, which suggest the presence of bronchopleural fistula.
On physical examination, the patient was alert and in no distress. His height was 160 cm and body weight was 44 kg. His body temperature was 36.9 °C, blood pressure was 100/60 mmHg, pulse was 70 beats per minute with a regular rhythm, and respiratory rate was 20 breaths per minute. Inspiratory crackles and decreased breathing sound were heard in the right upper anterior chest field. Complete blood count revealed WBC of 17,000/mm3 (neutrophils 85%), hemoglobin 12.3 g/dL and platelets 412 k/mm3. C-reactive protein concentration was 8.7 mg/dL. Routine chemical laboratory data were all within normal range. The patient was negative for antibody to human immunodeficiency virus.
Sputum AFB smear and culture were repeated and anti-TB medication was continued. Compared with previous chest CT scan taken one month earlier, his chest CT scan showed increased amount of pleural effusion with newly developed empyema necessitatis in right anterior chest wall (Fig. 2A). To drain his pleural fluid, one chest tube was inserted into the pleural space and another tube was inserted into the empyema necessitatis. The drained pleural fluid was grossly pus and the fluid contained 550,000 WBC/mm3 (with a differential of 99% neutrophil) with a pH 7.0. The pleural fluid had a protein concentration of 5.2 g/dL, a glucose concentration of 5 mg/dL, a lactic acid dehydrogenase concentration of 1233 U/L and it was stained positively for AFB.
Fig. 2.
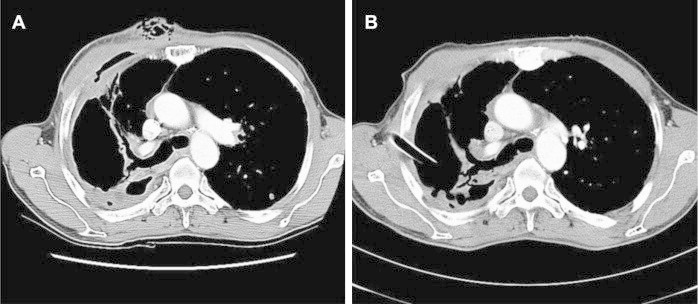
A. Chest CT scan one month later showed that progression of lung inflammation and bronchopleural fistula with increased amount of pleural effusion. Note the newly developed large bulging contour with irregular air density at right anterior chest wallindicates the empyema necessitatis formation. B. Chest CT scan three month after treatment initiation showed that decreased size of empyema, disappearance of bronchopleural fistula in right upper lobe and improved empyema necessitatis at right anterior chest wall. Chest tube was inserted into the right pleural space.
Nucleic acid amplification tests for Mycobacterium tuberculosis in the sputum and pleural fluid samples were negative using a commercial DNA probe. The PCR-restriction fragment length polymorphism analysis targeting rpoB gene identified M. abscessus from the patient's sputum and pleural fluid specimens.8 The AFB culture of sputum and pleural fluid eventually yielded M. abscessus growth.
Based on these clinical findings and laboratory data, the patient was diagnosed as having pulmonary and pleural infection with empyema necessitatis caused by M. abscessus. Anti-TB medication was discontinued and antibiotic treatment for M. abscessus was initiated (oral clarithromycin 1000 mg/day, intravenous cefoxitin 10 g/day and intravenous amikacin 750 mg/day). Even six weeks after initiation of antibiotics, however, pleural fluid was still positive for AFB. Ciprofloxacin 800 mg/day was added and then the AFB stain became negative.
A chest radiography and CT scan taken about three months after treatment initiation against M. abscessus showed improvement of empyema necessitatis, pleural and lung parenchymal infection (Fig. 2B). After removal of the drainage tube, round-shaped skin defect with soft tissue exposure occurred (Fig. 3A), and he underwent skin graft implantation (Fig. 3B). Six months after initiation of the treatment, he received right upper lobectomy of lung, which showed chronic granulomatous inflammation with multifocal microabscesses microscopically (Fig. 4). Treatment was completed after a total of one and half years of antibiotic treatment.
Fig. 3.
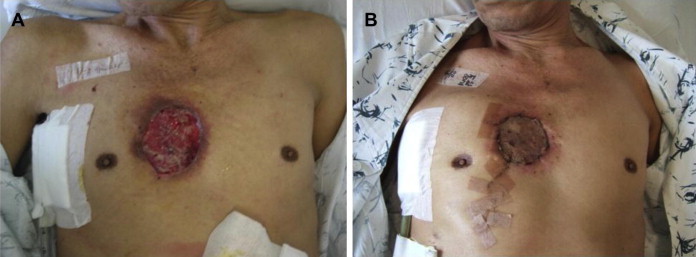
A. The anterior chest wall abscess with a skin defect after removal of the drainage catheter. B. After skin graft implantation.
Fig. 4.
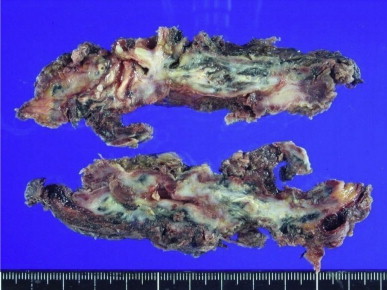
Pathologic specimen of right upper lobe showed that the external surface of the lung was brownish red, fibrous and irregular. There were chronic granulomatous inflammation with bronchiectasis, multifocal microabscesses and intrapulmonary/pleural fibrosis microscopically.
3. Discussion
We have described here a very rare case of empyema necessitatis caused by M. abscessus, in which the lung parenchyma as well as the pleura and overlying soft tissue were all involved. Although the clinical and radiological characteristics of NTM infection resemble those of TB, NTM infection is rarely accompanied by pleural involvement.7 There have been a few case reports of pleural effusion caused by NTM such as MAC,9, 10, 11, 12 Mycobacterium kansasii,13, 14 Mycobacterium scrofulaceum.15 Only a few cases of chronic empyema due to MAC were also reported.5, 6, 7 In the case of M. abscessus, only one case of M. abscessus empyema in a lung transplant recipient was reported.16 None of these cases, however, reported the development of empyema necessitatis in the patients with pulmonary and pleural infection caused by NTM. To our knowledge, this is the first case report of empyema necessitatis caused by NTM, specifically M. abscessus.
American Thoracic Society guidelines described M. abscessus disease as a chronic incurable infection for most patients,17 which reflect the difficulty of treatment against this organism. It was also stated that, however, curative therapy is more likely to be obtained with limited disease and a combination of surgical resection of involved lung and chemotherapy.17 Because pulmonary-pleural infection was limited in our patients, he had been treated with multidrug chemotherapy including oral and intravenous antibiotics combined with tubal drainage and surgical resection, which lead to successful treatment. Since only one case of empyema due to M. abscessus in a lung transplant recipient has been reported,16 the treatment outcome of M. abscessus empyema is unclear, although this recipient died because of multiorgan failure. Our case suggests that, however, even more severe form of M. abscessus lung disease extending chest wall, can be cured with aggressive medical therapy and operation if the infection is confined to resectable area.
Several studies have reported the treatment outcome of M. abscessus pulmonary disease. In South Korea, Lyu et al. reported 80.5% treatment success rate in 41 patients,18 and Jeon et al. reported 58% treatment success rate in 65 patients,19 of which treatment success rates tend to be higher than other countries.20, 21 Recently, it was revealed that M. abscessus comprises three closely related species: M. abscessus, Mycobacterium massiliense and Mycobacterium bolletii.4 In South Korea, M. abscessus and M. massiliense are isolated in almost equal numbers among M. abscessus complex infections, whereas M. bolletii is rare.4 According to the report, the microbiologic treatment response rate was higher in patients with M. massiliense lung disease than in those with M. abscessus lung disease.4 However, in our study, the clinical isolate of M. abscessus could not be further identified to this subspecies level.
The pathway of NTM infection to empyema is uncertain, but there are two theories about the process.5 The first theory is the development of the empyema from the lung infection. The second theory entails the development of the empyema after a minor trauma. A Chest CT scan in our case showed that the possible presence of bronchopleural fistula, which suggests that empyema in the present case resulted from pulmonary parenchymal infection through a bronchopleural fistula. Empyema necessitatis is generally thought to be complication of empyema in which the pleural infection spreads outside of the pleural space to involve the soft tissue of the chest wall. Bronchopleural fistula associated with tuberculosis usually follows a surgical procedure but can also occur spontaneously.22, 23 Park et al. suggested that development of spontaneous bronchopleural fistula due to pulmonary MAC infection could be possible.7 Our case also support this suggestion that spontaneous bronchopleural fistula can happen in pulmonary NTM infection, including M. abscessus.
In summary, our case suggests that NTM such as M. abscessus is capable of causing empyema and empyema necessitatis as well as lung parenchymal infection. Physicians should be aware of the possibility of pulmonary infection and empyema associated with empyema necessitatis caused by M. abscessus even in immunocompetent patients.
Conflict of interest statement
No author has a conflict of interest to disclose.
References
- 1.Marras T.K., Chedore P., Ying A.M., Jamieson F. Isolation prevalence of pulmonary non tuberculous mycobacteria in Ontario, 1997–2003. Thorax. 2007;62:661–666. doi: 10.1136/thx.2006.070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai C.C., Tan C.K., Chou C.H. Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000–2008. Emerg Infect Dis. 2010;16:294–296. doi: 10.3201/eid1602.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrejak C., Thomsen V.Ø., Johansen I.S. Nontuberculous pulmonary mycobacteriosis in Denmark: incidence and prognostic factors. Am J Respir Crit Care Med. 2010;181:514–521. doi: 10.1164/rccm.200905-0778OC. [DOI] [PubMed] [Google Scholar]
- 4.Koh W.J., Jeon K., Lee N.Y. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med. 2011;183:405–410. doi: 10.1164/rccm.201003-0395OC. [DOI] [PubMed] [Google Scholar]
- 5.Kotani K., Hirose Y., Endo S., Yamamoto H., Makihara S. Surgical treatment of atypical Mycobacterium intracellulare infection with chronic empyema: a case report. J Thorac Cardiovasc Surg. 2005;130:907–908. doi: 10.1016/j.jtcvs.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 6.Asai K., Urabe N. Acute empyema with intractable pneumothorax associated with ruptured lung abscess caused by Mycobacterium avium. Gen Thorac Cardiovasc Surg. 2011;59:443–446. doi: 10.1007/s11748-010-0687-7. [DOI] [PubMed] [Google Scholar]
- 7.Park S.U., Koh W.J., Kwon O.J. Acute pneumonia and empyema caused by Mycobacterium intracellulare. Intern Med. 2006;45:1007–1010. doi: 10.2169/internalmedicine.45.1665. [DOI] [PubMed] [Google Scholar]
- 8.Lee H., Park H.J., Cho S.N., Bai G.H., Kim S.J. Species identification of mycobacteria by PCR restriction fragment length polymorphism of the rpoB gene. J Clin Microbiol. 2000;38:2966–2971. doi: 10.1128/jcm.38.8.2966-2971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okada Y., Ichinose Y., Yamaguchi K., Kanazawa M., Yamasawa F., Kawashiro T. Mycobacterium avium-intracellulare pleuritis with massive pleural effusion. Eur Respir J. 1995;8:1428–1429. doi: 10.1183/09031936.95.08081428. [DOI] [PubMed] [Google Scholar]
- 10.Kawamoto H., Yamagata M., Nakashima H. Development of a case of Mycobacterium avium complex disease from right pleural effusion. Nihon Kokyuki Gakkai Zasshi. 2000;38:706–709. [PubMed] [Google Scholar]
- 11.Kobayashi K., Yano S., Kato K., Saito S., Tokushima T. A case of Mycobacterium avium pulmonary disease accompanied with pleural effusion. Kekkaku. 2002;77:725–728. [PubMed] [Google Scholar]
- 12.Yanagihara K., Tomono K., Sawai T. Mycobacterium avium complex pleuritis. Respiration. 2002;69:547–549. doi: 10.1159/000066463. [DOI] [PubMed] [Google Scholar]
- 13.Kamiya H., Toyota E., Kobayashi N., Kudo K. A case of pulmonary Mycobacterium kansasii infection complicated with pleural effusion. Kekkaku. 2004;79:397–400. [PubMed] [Google Scholar]
- 14.Olafsson E.J., Naum C.C., Sarosi G.A., Mastronarde J.G. Bilateral pleural effusions and right pneumothorax in a 25-year-old man. Chest. 2004;126:986–992. doi: 10.1378/chest.126.3.986. [DOI] [PubMed] [Google Scholar]
- 15.Fusegawa H., Ookubo Y., Nishiumi M., Fujino T. A case of pulmonary Mycobacterium scrofulaceum infection presented as pleurisy. Kekkaku. 2005;80:469–473. [PubMed] [Google Scholar]
- 16.Fairhurst R.M., Kubak B.M., Shpiner R.B., Levine M.S., Pegues D.A., Ardehali A. Mycobacterium abscessus empyema in a lung transplant recipient. J Heart Lung Transplant. 2002;21:391–394. doi: 10.1016/s1053-2498(01)00339-4. [DOI] [PubMed] [Google Scholar]
- 17.Griffith D.E., Aksamit T., Brown-Elliott B.A. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 18.Lyu J., Jang H.J., Song J.W. Outcomes in patients with Mycobacterium abscessus pulmonary disease treated with long-term injectable drugs. Respir Med. 2011;105:781–787. doi: 10.1016/j.rmed.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Jeon K., Kwon O.J., Lee N.Y. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med. 2009;180:896–902. doi: 10.1164/rccm.200905-0704OC. [DOI] [PubMed] [Google Scholar]
- 20.Jarand J., Levin A., Zhang L., Huitt G., Mitchell J.D., Daley C.L. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis. 2011;52:565–571. doi: 10.1093/cid/ciq237. [DOI] [PubMed] [Google Scholar]
- 21.Griffith D.E., Girard W.M., Wallace R.J., Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis. 1993;147:1271–1278. doi: 10.1164/ajrccm/147.5.1271. [DOI] [PubMed] [Google Scholar]
- 22.Kim H.Y., Song K.S., Goo J.M., Lee J.S., Lee K.S., Lim T.H. Thoracic sequelae and complications of tuberculosis. Radiographics. 2001;21:839–858. doi: 10.1148/radiographics.21.4.g01jl06839. [DOI] [PubMed] [Google Scholar]
- 23.Lois M., Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest. 2005;128:3955–3965. doi: 10.1378/chest.128.6.3955. [DOI] [PubMed] [Google Scholar]


