Abstract
The development of HIV vaccines has been hampered by the lack of an animal model that can accurately predict vaccine efficacy. Chimpanzees can be infected with HIV-1 but are not practical for research. However, several species of macaques are susceptible to the Simian Immunodeficiency Viruses (SIV) that causes a disease in macaques that closely mimics HIV in humans. Thus, macaque-SIV models of HIV infection have become a critical foundation for AIDS vaccine development. Here, we examine the multiple variables and considerations that must be taken into account to use this NHP model effectively. These include the species and subspecies of macaques, virus strain, dose and route of administration and macaque genetics including Major Histocompatibility Complex molecules that affect immune responses and other virus restriction factors. We illustrate how these NHP models can be used to carry out studies of immune responses in mucosal and other tissues than could not easily be performed on human volunteers. Futhermore macaques are an ideal model system to optimize adjuvants, test vaccine platforms, and identify correlates of protection that can advance the HIV vaccine field. We also illustrate techniques used to identify different macaque lymphocyte populations and review some poxvirus vaccine candidates that are in various stages of clinical trials. Understanding how to effectively use this valuable model will greatly increase the likelihood of finding a successful vaccine for HIV.
Introduction
Once HIV was shown to be the etiologic agent of AIDS(Barre-Sinoussi et al., 1983; Gallo et al., 1984), the hunt began for an animal model that could advance vaccine and pathogenesis studies. Small mammals including mice and rats are not susceptible to HIV infection. While some non-human primates can be infected with HIV, infection rarely causes an AIDS like disease. Other lentiviruses can cause immunodeficiencies, feline immunodeficiency virus infection of cats shares some features of HIV infection, but the best available model is simian immunodeficiency virus (SIV) infection of macaques that closely mimics HIV disease progression in humans(Gardner and Luciw, 1989).
HIV was introduced in the human population as a result of cross-species transmission of SIVs from African non-human primates. Two viruses, SIVcpz found in chimpanzees Pan troglodytes troglodytes (Heeney et al., 2006; Keele et al., 2006; Sharp et al., 2005) and SIVsm found in sooty mangabeys Cercocebus atys (Santiago et al., 2005), gave rise to HIV 1 and HIV 2 respectively. African hosts of SIV remain disease free, but transmission to new hosts such as HIV to humans or SIV to Asian primates results in immunopathologic sequalae and progression to AIDS. HIV infection of humans and SIV infection of Asian macaques share many similarities including: mucosal transmission, tropism for CD4 T cells and macrophages, alterations in immune activation, and, in the advanced stages of disease, lymphomas and infections with normally benign or opportunistic pathogens. These similarities have led to the use of non-human primates as models for HIV, and lentiviral infection of macaques is the most widely studied non-human primate model.
One vital use of animal models is in the testing of HIV vaccine candidates. Macaques are the current ‘gold standard’ animal species for testing HIV vaccines. Vaccine studies in macaques can be modeled based on the patient cohort to be used in clinical trials as most modes of HIV transmission including mother to child, hetero or homosexual transmission and intravenous drug users can be mimicked by varying the route (oral, vaginal, penile, rectal or intravenous) and amount of virus inoculum (single high dose versus repeated low dose) used to challenge vaccinated animals. The ability of the vaccine to prevent or delay virus acquisition, control virus replication and disease progression can then be determined. These virologic outcomes are used to compare the relative efficacy of different vaccine strategies, and should guide the choice of HIV vaccines to be advanced to clinical trials.
We review the different macaque models and virologic considerations that should be made when designing a vaccine study. These include the choice of macaque species, MHC alleles and polymorphism of genetic restricting factors, challenge virus, virus dose, route of administration as well as some vaccine candidates.
Species of Macaques
Macaques have greatly contributed to our understanding of HIV pathogenesis. Initial descriptions of an AIDS like disease similar to humans, was made in macaques (Henrickson et al., 1983; Letvin et al., 1983; Stromberg et al., 1984) and early transmission studies showed that the passage of body-fluids or cells from an infirmed animal could induce immunosuppression in healthy animals (Letvin et al., 1985; London et al., 1983; Murphey-Corb et al., 1986). There are many species of macaques, but only three have gained prominence in HIV vaccine and pathogenesis studies and they all belong to the Cercopithecoidea superfamily. They are cynomolgus macaques (Macaca nemestrina), pig-tail macaques (Macaca fascicularis) and rhesus macaques (Macaca mulatta)(Baroncelli et al., 2008). Of the three-macaque species, rhesus macaques are the most frequently used and thus the viral and cellular dynamics following SIV or Simian Human Immunodeficiency Virus (SHIV) challenge have been well characterized in blood, lymphoid and mucosal compartments of these species. A plethora of information exists to facilitate research with rhesus macaques including the sequence of the entire rhesus genome (Gibbs et al., 2007) and the cross reactivity between human and macaques of several commercially available reagents. Furthermore ex-vivo assays designed to measure innate and adaptive responses in humans are often easily adaptable to rhesus macaques. Macaques of Indian origin are the best studied subspecies and multiple MHC alleles particularly those associated with the control of SIV replication such as Mamu A01, B08 and B17 (Loffredo et al., 2007b; Mothe et al., 2003; Pal et al., 2002; Yant et al., 2006) have been described and will be discussed in detail later. This has been extremely important for vaccine studies where animals with protective alleles can either be avoided or distributed evenly between vaccine and placebo groups to eliminate biased results. Immunodominant epitopes induced by vaccination can also be carefully monitored with rhesus MHC tetramers that are readily available from the NIH tetramer core facility or other commercial sources.
A major limitation to performing research with Indian rhesus macaques is the availability of animals, especially specific pathogen-free animals (Cohen, 2000). Export of these animals from India was banned in 1978, leaving breeding colonies the main source for research animals (Cohen, 2000). Vaccine studies generally require large numbers of animals to determine statistically significant differences between vaccinated animals and controls. For this reason the demand for Indian rhesus macaques has outpaced the supply, and researchers have sought various alternatives. One alternative that is gaining popularity is the use of rhesus macaques of Chinese origin that are imported from China. The subspecies of Chinese rhesus macaques infected with the commonly used SIV variants: SIVmac251 or 239 was initially considered a less relevant model due to lower plasma virus loads(Joag et al., 1994; Ling et al., 2002; Marthas et al., 2001) differences in T cell responses (Marcondes et al., 2006) and the slower disease course (Trichel et al., 2002) compared to Indian rhesus macaques. However most of these studies used SIV strains that had been expanded and adapted to grow in Indian rhesus macaques. Indeed when SIVmac251 was passaged in vivo in Chinese rhesus macaques, the resulting virus stock yielded virus loads similar to those in Indian rhesus macaques (Burdo et al., 2005). This experience emphasizes that the choice of virus, how it is prepared or passaged, and the macaque species and even sub-species can have a profound impact on the outcome of the infection and should therefore be carefully considered when testing vaccines.
SIV/SHIV infection of cynomolgus macaques is an additional model of HIV infection. Currently their use in research is much more limited than that of rhesus macaques, but several factors are increasing their use in HIV vaccine studies. These include the recent completion of their genome sequence (Higashino et al., 2012), advances in the ability to ascertain their MHC type and their availability relative to other non-human primates.. Similar to Chinese rhesus macaques, cynomolgus macaques infected with SIV/SHIV also demonstrate reduced virus loads, CD4 T cell loss and a slower disease course when compared to Indian rhesus macaques (Reimann et al., 2005). However, high virus loads and disease progression can be obtained when virus is adapted to cynomolgus monkeys (Borsetti et al., 2008). A population of cynomolgus macaques found on the Island of Mauritius may be of particularly use in HIV vaccine research. This population descended from a small group of monkeys and thus have low MHC diversity: greater than half of the of animals have the MHC 1 alleles Mafa-B*430101, Mafa-B*440101 and Mafa-B*460101 (Krebs et al., 2005). This MHC homogeneity reduces variability between animals after vaccination and allows better comparisons of vaccine regimens. Furthermore, since variation is reduced within the group, fewer animals are needed to obtain statistically significant results. It should be noted, however, that cynomolgus macaques of Chinese or Vietnamese origin, unlike those of Mauritian origin, have heterogenous MHC alleles, so the origin of cynomolgus macaques should be determined when planning HIV vaccine studies.
Interest in pig-tail macaques (Macaca nemestrina) was initially sparked by the fact that they can be infected with HIV-1(Agy et al., 1992). However, most animals with this type of HIV experience only minor changes in CD4 T cell count and transient viremia (Frumkin et al., 1993); in addition, in this case in vivo passage does not increase pathogenicity (Agy et al., 1997). In contrast, pigtail macques do support robust replication of pathogenic strains of HIV-2 and possibly other HIV isolates (McClure et al., 2000). This is partially explained by the presence of a deletion in the TRIM 5 gene (Brennan et al., 2007), a host restriction factor present in most Old World primates to be discussed later. Despite the fact that HIV-2 induces pathogenic changes in pigtail macaques, SIV or SHIV viruses are typically used to challenge vaccinated animals. Vaccine research in pigtail macaques has advanced with the characterization of their MHC alleles and the identification of Mane-A10, an immunodominant Gag epitope associated with lower SIV viral loads (Smith et al., 2005a); nevertheless these macaques are the least commonly used macaques in HIV vaccine studies..
Challenge Virus
The two main outcomes used to evaluate vaccine efficacy are prevention of HIV transmission and control of virus replication. Animal models also use these two virologic outcomes as measurements of efficacy. Selecting the ‘appropriate virus’ to use in a vaccine study is extremely important, as a virus that replicates poorly may be easily controlled by a vaccine-induced immune response causing an over-estimation of vaccine efficacy, whereas a virus that is overtly pathogenic may overwhelm any immune system and cause an underestimation of vaccine efficacy.
Since the ultimate goal is an HIV vaccine, using HIV as the challenge virus is a natural first choice. This would potentially allow the same immunogens that are to be used in humans to be tested in non-human primates. Early studies tested vaccine candidates by infecting chimpanzees with HIV-1(Boyer et al., 1997; Fultz et al., 1992; Girard et al., 1991; Girard et al., 1997) or macaques with HIV-2 (Andersson et al., 1996; Franchini et al., 1995; Looney et al., 1998; Myagkikh et al., 1996) but the lack of persistent virus replication, CD4 loss and disease progression makes HIV infection of primates a poor vaccine model.
The creation of SIV/HIV chimeras called SHIVs was greeted in the HIV vaccine field with much enthusiasm. SHIVs typically have the env, tat, rev and vpu of HIV while the remaining genes are from SIV. The SIV genetic backbone enables persistent replication and pathogenic SHIVs to be developed by passaging, with SHIV89.6p being the most extensively used (Reimann et al., 1996). As the envelope sequence is from HIV, SHIV’s have been used to evaluate the ability of vaccine induced or passively transferred neutralizing antibodies to interfere with envelope binding/entry and thus prevent SHIV infection(Hessell et al., 2009; Lakhashe et al., 2011). The pathogenic SHIVs created for vaccine usage often used the SIVmac239 backbone and HIV env genes that express the chemokine receptor CXCR4 or both CXCR4 and CCR5 receptors to enter cells. These viruses cause profound depletion of naïve T cells during the acute phase and rapid disease progression unlike what is typically seen in SIV or HIV infections(Harouse et al., 1999; Joag et al., 1996). In order to better model HIV infection, pathogenic SHIVs that express the CCR5 receptor for entry were created(Nishimura et al., 2010), as most HIV infections are established with CCR5-using viruses. This second generation of SHIVs target memory CD4 cells that express CCR5 and are abundant in the gastrointestinal tract similar to HIV and SIV. To date SHIVs containing HIV envelopes from Clades A, B, C and E isolates have been created(Sina et al., 2011), allowing for the selection of a SHIV challenge or preclinical model that best fits the circulating HIV clade of the putative patient population desired for clinical trials.
Despite the many advantages of SHIVs, the intriguing disadvantage is their apparent susceptibility to vaccine induced immune control(Feinberg and Moore, 2002). This is probably best demonstrated by Adenovirus vaccine vectors expressing SIV Gag, which when tested in SHIV models demonstrated good control of virus replication(Shiver et al., 2002). However, when similar vaccines were tested in SIVmac239 models, no protection from infection or sustained control of virus replication was observed(Casimiro et al., 2005; McDermott et al., 2005). These findings were highlighted in 2008 when the results of the Merck Step trial were released(Buchbinder et al., 2008). This trial vaccinated individuals with Ad5 expressing HIV gag/pol/nef and failed to prevent HIV transmission or reduce virus replication in vaccinees. The results of the Step trial were vastly different from the preclinical SHIV models using Ad5 gag, but have been recapitulated recently by studies vaccinating animals with Ad5 gag/pol/nef and challenging with SIVmac251 or SIVsmE660 delivered by a mucosal route (Qureshi et al., 2012; Reynolds et al., 2012).
The discovery of SIV infection of macaques and its ability to cause an AIDS-like disease(Daniel et al., 1985; Letvin et al., 1985), led many investigators to select SIV infection of rhesus macaques as their challenge model for testing HIV vaccines. SIVmac251 is probably the most stringent challenge to date. It was isolated from a rhesus macaque and replicates robustly, with peak virus loads 2–3 logs higher than observed during HIV infection of humans(Haase, 2011). In addition, SIVmac251 causes severe depletion of mucosal CD4 T cells and is very difficult to neutralize, similar to some primary HIV strains. While several vaccines tested in this model system can reduce peak viremia, few cause persistent long lasting control of virus replication. There are several stocks of SIVmac251, each a heterogeneous swarm of viruses that can transmit multiple variants across mucosal tissues(Keele et al., 2009). The infectivity and also virologic properties differ between virus stocks, making comparisons across vaccine regimens or between studies difficult. SIVmac239 is a molecular clone related to but not derived from SIVmac251 and has similar pathogenic properties to SIVmac251. The clonality of SIVmac239 makes it the choice of many vaccine researchers as it may reduce the variability of the challenge outcome allowing for a better comparison of vaccination regimens. However, the main disadvantage to SIVmac239 is also its main advantage, as one can question the wisdom of testing a vaccine with a clonal virus when vaccinated individuals need to be protected against the swarm of HIV viruses in circulation.
SIVs isolated from sooty mangabeys (SIVsm) and in particular SIVsmE660 are being increasingly used in HIV vaccine studies. SIVsmE660 is an uncloned pathogenic virus isolate and stocks of this virus contain multiple heterogeneous variants similar to SIVmac251. The env diversity of SIVmac251 and SIVsmE660 has been likened to that observed in an individual infected with HIV for 1–2 years(Keele et al., 2009). Most vaccine studies have been performed with vaccine immunogens that are either identical or very closely matched (homologous) to the challenge virus. However, to mimic HIV infection, researchers are opting for heterologous challenges. SIVsmE660 shares approximately 82% identity to SIVmac251(Shedlock et al., 2009), so selecting one as the vaccine immunogen, and the other as the virus challenge, creates a model that approximates the sequence variability between some HIV clades. SIVsmE660 is also more sensitive to neutralization than SIVmac251, allowing for its utility in vaccine studies aimed at inducing neutralizing antibodies. However its ease of neutralization and sensitivity to rhesus TRIM5 alpha needs to be accounted for when evaluating vaccine studies. This was demonstrated in one study where a significant protection from infection was observed in vaccinated macaques challenged with SIVsmE660, while similarly vaccinated animals challenged with SIVmac251 were all infected(Letvin et al., 2011).
Route, Mode and Dose of Virus infection
Most HIV infections are established across mucosal surfaces, making the cervico-vaginal, penile, oral or rectal epithelia the site of virus entry and their underlying tissues the likely site of virus expansion and dissemination. In order to similarly model transmission, non- human primate challenge models using vaginal, rectal, oral and penile applied cell-free virus have been developed(Keele and Estes, 2011). Intra-rectal challenge is the most used mucosal challenge model as well-titered stocks show reproducible rates of virus acquisition, and males are more readily available than sexually mature females, that are also used for breeding. Modeling vaginal transmission of HIV has proven much more problematic. There is often considerable variability in the rate of virus transmission and despite multiple challenges some naïve animals often do not become infected. This variability may be linked to hormone induced changes in the thickness of the vaginal epithelia, frequency of CD4 and CCR5 expressing target cells, microbial populations causing overt inflammation, changes in the pH, or varying amounts of mucus in the vaginal tract. Some investigators have chosen to use hormone treatment with depot medroxyprogesterone to synchronize animals, but progesterone treatment severely thins the vaginal epithelia in macaques(Marx et al., 1996) and has many immunomodulatory effects. Conversely, carefully monitoring the menstrual cycle of animals, or using repeated weekly challenges over the course of 6–12 weeks so that animals are challenged multiple times at each phase of the cycle could explain or overcome some of the variation. In addition, antibiotic treatment to resolve bacterial vaginosis and reduce inflammation can be beneficial.
A penile model for SIV transmission was recently developed by immersing the penis into cell free virus, but this mode of transmission is much less efficient than vaginal exposure and requires higher doses of virus to establish the infection (Ma et al., 2011). This finding is consistent with epidemiological data in discordant monogamous couples where the rate of male to female HIV transmission was greater than female to male (Gray et al., 2001; Padian et al., 1997). The penile shaft and glans in macaques have been found to contain a full complement of immune cells and SIV-specific immune responses can be detected in the penile tissues (Rothaeusler et al., 2012). The macaque penile challenge model has been used in vaccine studies(Qureshi et al., 2012) but the recent observation that TRIM5 polymorphisms affect SIVsmE660 infectivity via the penile route(Yeh et al., 2011) indicate that that careful genotyping of animals, and evaluating the capacity of the virus stock to infect via the penile route is needed before vaccine studies are initiated.
It has now been well documented that during heterosexual HIV infection only one or a few viral variants are usually transmitted from donor to recipient(Derdeyn et al., 2004; Keele et al., 2008; Salazar-Gonzalez et al., 2008). Single variant infections can also be obtained using macaque models with SIV delivered via the penile, vaginal or rectal routes (Keele et al., 2009; Ma et al., 2011; Stone et al., 2010). In general, HIV sexual transmission is an inefficient process with a low per contact transmission rate(Royce et al., 1997), so mucosal challenges in macaques with a high virus dose and a 100% infectivity rate per exposure do not model HIV infection. Furthermore, in the intra-rectal SIV challenge model the dose of the virus inoculum significantly affects the number of transmitted variants, with higher doses associated with multiple SIV variants(Liu et al., 2010). Thus, the field has shifted to using repeated low doses of viruses to assess vaccine efficacy. In this model, virus is atraumatically applied to the mucosa every 7–14 days with a goal of achieving an infectivity rate of 20–40% per exposure in unvaccinated controls. The ability of the vaccine to alter the rate of virus acquisition can then be determined. Furthermore, in an attempt to unravel protective vaccine-induced immune responses, the rate of acquisition or number of challenges to attain SIV infection, can be correlated with immunologic parameters. Several vaccines have demonstrated partial efficacy using this model(Barouch et al., 2012; Hansen et al., 2011; Lai et al., 2011; Xiao et al., 2012). Interestingly antibodies or the combination of antibodies and T cells appear to correlate with either protection from or a delay in virus acquisition in several vaccine strategies(Barouch et al., 2012; Lai et al., 2011; Xiao et al., 2012). These findings are consistent with the RV144 Thai Trial where antibodies to gp120 were also found to be a correlate of a reduced risk of HIV transmission(Haynes et al., 2012). In contrast, cell mediated immune responses in repeated low dose studies, appear to be related to virus suppression once the infection is established(Barouch et al., 2012; Hansen et al., 2011). The challenge dose also has profound effects on the viral kinetics, innate immune responses and the elapse of time(Liu et al., 2010). We have also observed significantly different vaccine outcomes when vaccinated animals were challenged with either a single high dose or repeated intermediate doses of virus. Protection from SIV infection or high virus loads was only observed in animals challenged with intermediate virus doses (Vaccari et. al. manuscript in submission). These findings have implications for HIV vaccines where a vaccine may be more effective in a low risk population, for example in populations exposed via heterosexual transmission, and less effective in a high risk population, for example in populations exposed to homosexual transmission or drug usage.. High virus doses that transmit multiple variants likely overwhelm the immune system as virus expansion, aberrant immune activation and apoptosis may outpace the expansion of vaccine-induced immune responses and compromise their functionality.
The mucosal repeated low dose challenge macaque model described above has an infectivity rate many times greater than observed during HIV transmission(Royce et al., 1997) and the quantity of virus used is higher than reported in vaginal secretions or semen(Baeten et al., 2011; Pilcher et al., 2007). Thus, an argument could be made for further decreasing the challenge dose; however this could allow for an overestimation of vaccine efficacy in the animal model that then does not translate to protection in humans. In addition, due to the variability inherent in repeated low dose mucosal challenges, vaccinologists require larger group sizes to attain statistical power and reducing the infectivity will proportionally increase the length of the study and also the cost.
Most vaccine and pathogenesis studies tested in macaques are performed with cell-free virus; however, cell-associated virus transmission from infected leukocytes in seminal fluid or vaginal secretions could be a significant mode of HIV transmission. Cell-to-cell transfer of HIV in culture is extremely efficient (Bomsel, 1997; Tan et al., 1993) and in-vivo infected cells may adhere to epithelial surfaces or migrate through the epithelial barrier and transfer virus to target cells(Anderson, 2010). In addition, it is likely that infected cells protect virions from the low pH, mucus trapping and antiviral defenses in the mucosa. The first non-human primate models of cell-associated HIV/SIV infection were reported in 1998(Girard et al., 1998; Sodora et al., 1998). These studies showed that while intravenous challenge with cell-associated virus was extremely efficient and required very few cells, intra-vaginal challenge with such virus was much less efficient(Sodora et al., 1998). Recently repeated challenge models with SIV-infected splenocytes or peripheral blood cells applied to the vaginal tract of macaques have been developed. Genital ulcers(Kaizu et al., 2006; Weiler et al., 2008) or progesterone treatment to thin the epithelium(Salle et al., 2010) can facilitate infection; however, a standardized repeated low-dose challenge model that does not require artificial epithelial disruption or thinning would better recapitulate HIV infection and may be important to the vaccine field. Vaccine-induced protection from cell-associated virus may differ from what is needed to protect from cell-free virus, reinforcing the need for relevant models of cell-associated HIV transmission.
MHC haplotype/Trim5α/cyp-mediated resistance to viral challenge
Numerous observations have shown that host genetic factors play important roles in disease progression in human immunodeficiency virus type 1 (HIV-1) infection (O’Brien and Moore, 2000; Rowland-Jones et al., 2001). The major histocompatibility complex (MHC) is one of the critical factors associated with susceptibility or resistance to HIV-1 infection (de Sorrentino et al., 2000). Moreover, innate restriction factors such as Trim5α/cyp, APOBEC3G, or Tetherin, can inhibit HIV replication at different stages of infection (Mogensen et al., 2010). These intrinsic genetic/cellular hindrances of viral replication represent a natural barrier against HIV infection. When using non-human primates (NHP) as a model to develop HIV vaccines, the allelic diversity of these restriction factors may have an impact on the susceptibility of the macaques to various viruses and thus influence the outcomes of the studies. Here, we briefly review these factors to provide guidelines for NHP experimental design.
1. MHC, mamu B*08, B*17, A*01
MHC class I glycoproteins (MHC-I) are expressed on all nucleated cells, and their function in cells involved in immunologic responses is to present foreign peptides of intracellular origin to cytotoxic T cells, which are then activated to cause the destruction of infected cells (Gallimore et al., 1995). The MHC-I alleles have consistently been reported to impact HIV viral setpoint and the rate of disease progression. Whole-genome association studies demonstrated the central role of HLA class I in controlling HIV-1 infection (Fellay et al., 2007; Pereyra et al., 2010). For example, HIV-infected HLA-B*27 or B*57 –positive individuals can mount strong CTL responses and show better viral control (Feeney et al., 2004; Leslie et al., 2004).
The Rhesus macaque MHC genes, in contrast to the highly polymorphic genes of the major MHC class I genes in humans (HLA-A, HLA-B, and HLA-C), uses an alternative strategy: express multiple dominant Mamu-A and Mamu-B transcripts per chromosome with high expression levels. Rhesus macaques may possess one to three A and two to four B locus genes (Boyson et al., 1996; Urvater et al., 2000). In another words, rhesus macaques are characterized by not only alternative gene combinations, but also diversity in gene number to ensure that different individuals mount distinct responses against the same pathogen (Otting et al., 2005). Thus, compared to the human lymphocyte antigen (HLA) complex, the macaque MHC region encodes many more class I genes.
In common with human MHC alleles, certain monkey MHC alleles are associated with successful control of SIV infections:
Mamu-A*01
Mamu-A*01 was significantly associated with lower set-point viral load and longer survival time after infection with SIVmac251 (Muhl et al., 2002). Pal et al. showed that Mamu-A*01 may positively influence the results of vaccine studies against SIVmac251 (Pal et al., 2002). Both the presence of the Mamu-A*01 genotype and vaccination of rhesus macaques with ALVAC-SIV-gag-pol-env contributed to the restriction of SIVmac251 replication during primary infection, preservation of CD4+ T cells, and delayed disease progression following intra-rectal (but not intravenous) challenge of the animals to SIVmac251(Pal et al., 2002). In addition, a significant delay in CD4+ T cell loss was observed in Mamu-A*01-positive macaques (Pal et al., 2002). IN contrast, Mamu-A*01-positive macaques did not significantly restrict primary viremia of intravenous or intra-rectal challenge with SHIV89.6P or SHIVKU2 (Pal et al., 2002). These findings contrast to to those of Zhang et al. who observed that pathogenic SHIV 89.6P- infected Mamu-A*01-positive rhesus monkeys, exhibited significantly delayed disease progression (Zhang et al., 2002). The delay corresponded not only to the above mentioned Mamu-A*01-restricted dominant CTL response but also to a lower viral load in lymph nodes and, importantly, to minimal destruction of LN structure during early infection (Zhang et al., 2002). To determine the effect of different MHC class I alleles on viral replication, Mothé et al screened eight common MHC class I alleles in 53 SIVmac239-infected animals: Mamu-A*01, Mamu-A*02, Mamu-A*08, Mamu-A*11, Mamu-B*01, Mamu-B*03, Mamu-B*04, and Mamu-B*17 (Mothe et al., 2003; O’Connor et al., 2003). Their studies extended the finding of association of Mamu-A*01 with enhanced control of viral replication from SIVmac251 to SIVmac239 replication. Moreover, they demonstrated a dramatic association between Mamu-A*01 and -B*17 expression and slowed disease progression. In addition, they showed that two epitopes restricted by Mamu-A*01 and one epitope restricted by Mamu-B*17 were the dominant acute-phase CTL responses in animals expressing these alleles (O’Connor et al., 2003).
Mamu B*08
Mamu B*08-positive macaques were found to be over-represented among elite controllers (EC) (i.e., individuals exhibiting greatly increase ability to control challenge with SIV): 38% of ECs were Mamu-B*08-positive compared to 3% of progressors (Loffredo et al., 2007b). Subsequent studies on ECs found evidence for selective pressure mediated by Mamu-B*08-restricted CD8+ T cells in all of the newly identified epitopes in a cohort of SIVmac239-chronically infected macaques (Loffredo et al., 2007a). The same group found that Mamu-B*08-restricted CD8+ T-cell responses dominated the acute phase and accounted for 23.3% to 59.6% of the total SIV-specific immune responses. Additionally, the ECs mounted strong and broad CD8+ T-cell responses against several epitopes in Vif and Nef (Loffredo et al., 2008). Overall, 50% of Mamu-B*08-positive Indian rhesus macaques control SIVmac239 replication and became an EC. A study aimed at defining a detailed peptide-binding motif revealed that despite substantial sequence differences between Mamu-B*08 and human protective allele HLA-B*2705, the peptide-binding repertoires of these two MHC class I molecules share remarkable similarity (Loffredo et al., 2009). All Mamu-B*08-restricted epitopes contain an R at the position 2 primary anchor and 10/? also possess either R or K at the N terminus (Loffredo et al., 2009). The association of Mamu-B*08 with a protective human MHC class I allele implicates CD8+ T cells and/or natural killer cells in the ability of this MHC molecule to associate with control of viral replication. This possibility is inf in fact supported by a study of escape point mutations of eight Mamu-B*08-restricted CD8+ T cell epitopes which confirmed that these epitope-specific CD8+ T cell responses may play a role in establishing the control of viral replication in Mamu-B*08+ macaques (Valentine et al., 2009).
Mamu B*17
In rhesus macaques, Mamu-B*17 is another MHC allele that is associated with reduced HIV replication and is over-represented in ECs. An analysis of 181 SIVmac239-infected rhesus macaques revealed that Mamu-B*17 was associated with a 26-fold reduction in plasma virus concentrations (Yant et al., 2006). The Mamu-B*17-restricted CD8+ T cell repertoire is focused primarily on a limited number of epitopes, akin to the protective effects described for HLA-B*57 in HIV-infected individuals (Wu et al., 2011). Besides the dominant epitopes in Mamu-B*17(+) SIVmac239-infected rhesus macaques, which spontaneously controlled viral replication, strong CD8+ T lymphocyte responses against a cryptic epitope, RHLAFKCLW, were identified (Maness et al., 2007). cRW9-specific CD8+ CTL selected for viral variation in vivo and effectively suppressed SIV replication in vitro, suggesting that they might play a key role in the SIV-specific response (Maness et al., 2007).
Besides the above-mentioned protective alleles in rhesus macaques, in pigtail macaques, animals with Mane-A*10(+) have been shown to have lower set point SIV levels than Mane-A*10(−) animals (Fernandez et al., 2005; Smith et al., 2005b).
2. Trim5α/cyp, APOBEC3G and other innate factors
It is known that HIV-1 infects humans and chimpanzees but not Old World monkeys such as the rhesus macaques (Rh) and cynomolgus macaques (CM). The observation that resistance against HIV-1 infection was dominant in heterokaryons between human and old world monkey cells suggested the presence of inhibitory factors against HIV-1 infection in old world monkey cells (Munk et al., 2002). In 2004, Stremlau et al. first identified Trim5α as the host antiviral factor in Old World monkeys (Stremlau et al., 2004). TRIM5 α is a tripartite motif (TRIM) protein composed of RING, B-box 2, coiled-coil, and B30.2(SPRY) domains (Stremlau et al., 2005). The major determinant of anti-HIV-1 potency is the B30.2(SPRY) domain (Stremlau et al., 2005). Both Rh and CM TRIM5α restrict HIV-1 infection but fail to restrict SIV isolated from a macaque monkey. Further studies confirmed that human and simian Trim5α was responsible for the poor species-specific retroviral infectivity in Old World monkeys (Hatziioannou et al., 2004; Keckesova et al., 2004; Perron et al., 2004; Yap et al., 2004).
Trim5α demonstrated different levels of resistance to different retroviral infections between human and macaques and among different species of macaques (species-specific restriction, shown in table 1). For example, rhesus monkey TRIM5 more potently blocks HIV-1 infection than human TRIM5α. Within and between primate species, SIV isolated from sooty mangabeys (SIVsm) and SIV isolated from African green monkeys (SIVagm) replicate in their natural hosts (VandeWoude and Apetrei, 2006) and CD4+ human cells. SIVmac evolved from SIVsm in captive macaques, and replicates efficiently in Rh (Himathongkham and Luciw, 1996; Shibata et al., 1995) and CM (Akari et al., 1996) as well as in human CD4+ cells but not in African green monkey cells. Sequence comparison between Trim5α alleles suggested that a variable region in the SPRY domain might be responsible for the variability of susceptibility of SIV (Stremlau et al., 2005). This possibility is consistent with evidence that the SPRY domain interacts directly with the incoming viral capsid and thus interrupts the uncoating events required for the continuation of reverse transcription (Kootstra et al., 2003).
Table 1.
Species-specific restriction by Trim5α (Nakayama and Shioda, 2012)
| Trim5α | HIV-1 | SIVmac | SIVagm |
|---|---|---|---|
| Human | No | No | No |
| Rhesus | Yes | No | Yes |
| Cynomolgus | Yes | No | N.D |
As Trim5α may influence vaccines and pathogenesis studies, it is important to be aware the existence of intra-species variations in the Rh Trim5α, which could influence the susceptibility to viral infection, and in those animals where infection has been established, the set-point of viral loads (Newman et al., 2006; Sawyer et al., 2005; Song et al., 2005). The PRYSPRY sequence of the B30.2 domain of the Trim5α is highly variable (Kaiser et al., 2007). Newman et al. identified six alleles of Rh-Trim5α with different restriction profiles (Wilson et al., 2008a); for example, Mamu 1 and 3 alleles restrict HIV-1, 2, but not SIVmac239; while Mamu 4 and 5 alleles restrict HIV-1, but not HIV-2 and SIV mac239. Lim et al. independently reported 11 Trim5α alleles based on 339TFP341-to-Q polymorphisms (Lim et al., 2010a) and found that natural variation in the TRIM5α B30.2 (SPRY) domain influenced the efficiency of SIVmac capsid binding and the in vitro susceptibility of cells from the monkeys to SIVmac infection (Lim et al., 2010b). Rh with a Q allele were associated with higher levels of plasma viral load, and more rapid progression to AIDS (Lim et al., 2010a). Therefore, it is necessary to perform Trim5α genotyping when using SIVsm. However, Fenizia et al, recently reported that Trim5α did not affect the susceptibility to SIVmac251 and that the Gag sequences of SIVmac251 and SIVmac239 stocks have mutations that interfere with their binding to TRIM5α (Fenizia et al., 2011).
Soon after the discovery of Trim5α in 2004, a fusion protein composed of the N-terminal half of Trim5α, RING, B-box2, coiled-coil, and cyclophilin A (CypA), which was encoded by a retrotransposed cDNA to replace PRYSPRY, was identified in owl monkeys (Nisole et al., 2004; Sayah et al., 2004). Initially, the expression of TRIMcyp was thought to be restricted only to owl monkeys, but later studies found that the cyp insert also exists at least in three macaques: Rh, CM, and Pigtail (Pt). The common ancestor of these monkeys must have had the authentic cypA, which has been shown to restrict HIV-1, but only weakly HIV-2 (Price et al., 2009a; Virgen et al., 2008). Mutations in the monkeys either enhance or decrease the antiviral activity of TRIMcyp against HIV-1 or 2 (Price et al., 2009a). The rhesus TRIMCyp is encoded by a single, but common, allele (Mamu7) of the rhesus TRIM5 gene. The antiviral specificity of the rhesus TRIMCyp is distinct, restricting infection of HIV-2 and feline immunodeficiency virus but not HIV-1 (Wilson et al., 2008b). Neither M. nemestrina nor M. fascicularis TRIMCyp could restrict HIV-1 or SIVmac in an in vitro infectivity assay (Brennan et al., 2008). On the other hand, TRIMcyp frequency in CM was higher than in Rh, and TRIMcyp frequency is higher in eastern Asia than in Western Aisa (Dietrich et al., 2011; Saito et al., 2012). As to SIV infection, Rh TRIMcyp failed to restrict SIVmac239 (Brennan et al., 2008; Wilson et al., 2008b), but not SIVsm (Kirmaier et al., 2010). Therefore, to establish a monkey model for the study of HIV-1/AIDS, depending on the challenge virus and the species used in the study, Trim5α and/or TRIMcyp are factors that need to be considered.
Besides Trim5α, other restriction factors capable of suppressing HIV/SIV replications include APOBEC3G, and Tetherin (Mogensen et al., 2010). These factors can inhibit HIV replication at different stages of the viral replication cycle. However, HIV has evolved strategies to overcome these factors, i.e. Vif for APOBEC3G, Vpu/nef/env for tetherin. APOBEC3G polymorphisms have been associated with rate of disease progression (Sobieszczyk et al., 2011). HIV-1 vif can potently suppress human APOBEC3G, but not Rh APOBEC3G. This might partially explain the restriction of HIV-1 replication in monkey cells. Macaque APOBEC3G–associated viral control has been observed in several SIV vaccine studies (Sui et al., 2011; Sui et al., 2010; Wang et al., 2009; Wang and Lehner, 2011). Tetherin, also called BST2 or CD317, is an interferon-inducible membrane protein, which interferes with the detachment of viral particles (Neil et al., 2008; Van Damme et al., 2008). Another recently identified host factor is SAMHD, whose activity was confined to differentiated uncycling cells like macrophages and mDC (Hrecka et al., 2011; Laguette et al., 2011). Vpx can counteract SAMHD1. As HIV-1 lacks Vpx, it is not clear whether monkey SAMHD1 restricts HIV-1 replication.
Protective immunity to reduce viral acquisition and viral load
The ultimate goal of an HIV vaccine is to achieve sterilizing immunity, which will completely prevent infection. In reality however, what has been reported, and will likely be achieved in the foreseeable future, is a partial protection from infection and vaccine-induced control of virus replication. While less desirable, a substantial reduction in viral replication will decrease the risk of progression to AIDS and virus transmission. To test HIV-1 vaccine efficacy, we need an animal model that accurately recapitulates human infection, where the immune correlates of protection can be determined and this information used to improve HIV vaccines. In this regard, NHP models have proven to be invaluable for the following reasons:.
1. Use of NHP as a model to measure mucosal immunity
In as much as over 85% percent of HIV transmission is mucosal, primarily via either the genital or the rectal route, it is critical to develop strategies to prevent HIV mucosal transmission. A growing body of evidence suggests that mucosal immunity plays an intimate and fundamental role in HIV-1 transmission and disease development. Macaque models have been widely used to demonstrate the early events during SIV infection in the genital mucosa, and work from Reynolds et al. clearly showed that natural mucosal SIV-specific CTL are “too little and too late” (Reynolds et al., 2005). Therefore, it is generally accepted that HIV-1 vaccines may need to elicit potent mucosal immune responses, which might include mucosal mucosal IgA, IgG, SIV-specific CTL and the activation of NK and NKT cells.
Effective induction of mucosal immunity often occurs in the mucosa-associated lymphoid tissues, so mucosal sampling and measurements of mucosal immune responses are essential and can be optimized in macaques. However, intensive sampling of mucosal tissues in humans is less realistic. Longitudinal sampling of blood, lymphoid, gastrointestinal and bronchial tissue is now routinely done in vaccine and pathogenesis studies in macaques. Mononuclear cells obtained from lung lavage, and vaginal or rectal pinch biopsies have been characterized using multi-parameter flow cytometry to compare the frequency and function of mucosal immune cells. Examples of some of the markers used to evaluate the phenotype of T, B, NK and NK T cells in blood and the gastrointestinal tract in macaques are presented in Figures 1 and 2. Similarly, mucosal secretions can be obtained using Weck cel or cotton swabs and then used to determine the class, titer and avidity of antibodies at the site of virus infection. The careful evaluation of vaccine-induced vaginal and rectal responses in macaque models may elucidate the quality and quantity of mucosal immune responses needed to prevent SIV/HIV mucosal transmission.
Figure 1.
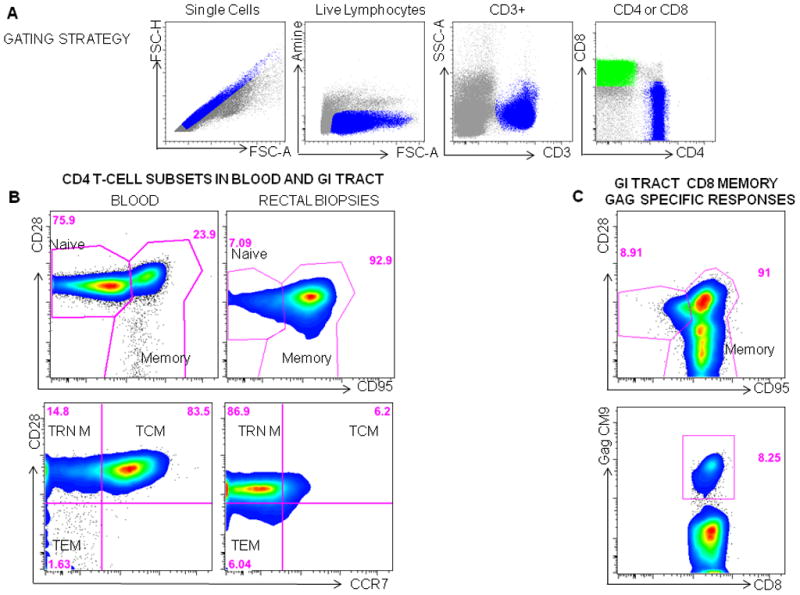
Measuring T-cell Phenotype in macaques. A Gating strategy: Mononuclear cells are first gated on single cells, then live lymphocytes or cells that are negative for the Amine dye are gated, followed by gating CD3+ T cells and then CD8+(green) and CD4+ (blue) subsets. B. Four CD4 T cell populations can be identified in the blood or rectum in macaques using CD28, CD95 and CCR7. First naïve (CD28+CD95−) cells and memory (CD95+CD28+/−) cells are gated. The memory population can then be differentiated by CD28 and CCR7 into central memory cells (TCM:CD28+CCR7+), transitional memory (TRN M: CD28+CCR7−) and effector/effector memory cells (TEM: CD28−CCR7−)cells. C Mononuclear cells isolated from rectal biopsies obtained from a vaccinated SIV infected animal, 10 days post SIV infection are gated using the strategy shown in A, CD8 memory T cells are then gated (CD95+CD28+/−) and the frequency of SIV Gag specific memory cells to the CM9 epitope are determined using an MHC Pentamer.
Figure 2.
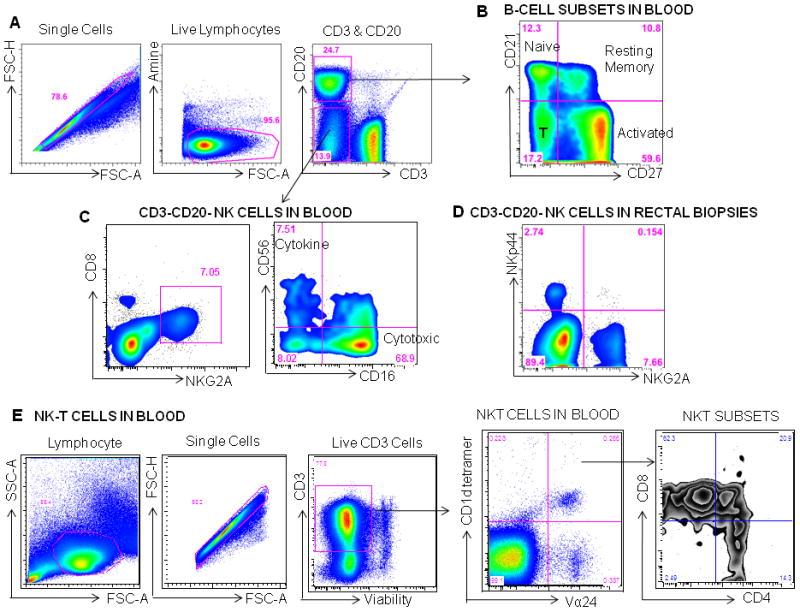
Measuring B cell and NK and NK-T cell subsets macaques. A Gating Strategy: Mononuclear cells are first gated on single cells, then live lymphocytes, then CD20+ B cells and CD20−CD3− cells are gated. B CD20+ B cells can be phenotyped based on CD27 and CD21 staining. Naïve B cells are (CD21+CD27−), resting memory B cells are CD21+CD27+, while activated B cells are CD21−CD27+ and tissue bound (T) or differentiated B cells are CD21−CD27−. C Cells that are not T cells or B cells (CD3−CD20−) in blood are gated for dual expression of CD8 and NKG2A and then phenotyped by CD56 and CD16. Using these markers NK cells that have cytotoxic function CD16+ and those that are cytokine producing CD56+ and have more of a regulatory function can be identified. In the rectal mucosa classic NK cells can be defined as NKG2A+ and can be either cytokine producing or cytotoxic. A second subset has been recently described in the intestinal tract, which expresses NKp44 and produces IL17 and TNFα. These cells are thought to play a role in mucosal regulation and maintaining the integrity of the epithelial barrier. E Gating Strategy for NKT cells in blood: lymphocytes are first gated on single cells, then live CD3+ cells, and then Vα24+ CD1d tetramer+ NKT cells are gated. Within Vα24+ CD1d tetramer+ NKT cells, CD4+, CD8+ and CD4+CD8+ subsets are identified.
2. Use of NHP as a model to test which component of the various HIV proteins should be included in a vaccine to induce protective immunity
One important consideration in the development of an HIV-1 vaccine is to define the vaccine composition and the contributions of each component to vaccine efficacy. The generation of virus-specific T cell responses has been the goal of HIV vaccine development, as sterilizing immunity through the induction of neutralizing antibodies is not currently feasible. Several studies were performed at the population level to define the selection of T cell antigens for an HIV vaccine. Kiepiela et al. showed in a cohort of 578 HIV-infected individuals from South Africa that only Gag-specific CD8+ T cell responses were associated with lowering viremia, while Env-specific and Accessory/Regulatory protein–specific CD8+ T cell responses were associated with higher viremia (Kiepiela et al., 2007). Similarly, Ranasinghe et al. conducted a comprehensive analysis of HIV-specific CD4+ T cells in 93 subjects at different stages of HIV infection and found that CD4+ T cell responses targeting Gag were robustly associated with lower levels of viremia and Gag/Env ratios were a potent marker of viral control, with a high frequency and magnitude of Gag responses and low proportion of Env responses associated with effective immune control (Ranasinghe et al., 2012). However, as these authors pointed out, we should also be aware that these data, which were obtained from cohorts of HIV-infected individuals, might have different implications for their role in controlling natural chronic infection versus their potential role as effective immunogens in an HIV vaccine (Kiepiela et al., 2007; Ranasinghe et al., 2012). Thus, these data suggest the possibility that such responses may mediate successful control of viremia when vaccinated HIV-negative individuals subsequently become HIV infected. However, the NHP model is invaluable to determine prospectively which antigen should be included in an HIV vaccine.
So far, the NHP model has not provided a consistent answer. Some NHP models suggested the potential for Env vaccination to enhance SIV replication and disease (Staprans et al., 2004), whereas in a SHIV89.6 challenge model, Amara et al. showed that inclusion of Gag-Pol, in addition to Env, were important for persistent virus control and protection against CD4+ cell loss (Amara et al., 2002). Other studies using NHP also suggested that non-gag proteins, in addition to Gag, function as CD8+ T cell targets and therefore contributed to the control of SIV in vaccinated animals (Casimiro et al., 2005; Hel et al., 2006; Wilson et al., 2006). Therefore correctly extrapolating data from NHP vaccine studies to humans is important. As discussed above, the genetic background of the macaques, homologous vs heterologous viral challenges, etc., all play important roles in determining the outcomes.
3. Use of NHP as a model to evaluate/optimize molecular adjuvants to induce better innate and adaptive immune responses in NHP for subsequent human trials
The efficacy of an HIV vaccine can be improved by using adjuvants that stimulate antigen-presenting cells (APCs), especially dendritic cells (DCs), which play a crucial role in immune responses against infections. DCs serve as a bridge between innate and adaptive immunity and adjuvants act in part by signaling through Toll-like receptors (TLRs) on DCs to trigger adaptive immune responses in both CD4+ and CD8+ T cells that recognize antigenic peptides presented by MHC molecules on those DCs. Studies using TLR agonists as potential adjuvants for HIV vaccine development are under way. Animal models are important tools to test novel adjuvant formulations. However, in mice, a commonly used animal model, there are important differences in the TLR expression patterns on APCs as compared to humans. For example, in humans, B cells and pDCs are the only immune cells that are known to express TLR9 and thus capable of activation by CpG oligodeoxynucleotides (ODNs) (Krug et al., 2001), whereas in contrast, TLR9 is broadly expressed on all major DC subtypes (pDCs and mDCs) as well as on B cells, macrophages and monocytes in mice (Edwards et al., 2003; Hemmi et al., 2000). Moreover, in humans, functional TLR7 and 8 are found on B cells, pDCs and mDCs and on monocytes (Hornung et al., 2002; Jarrossay et al., 2001; Kadowaki et al., 2001; Krug et al., 2001), while in mice, TLR8 does not appear to be functional (Edwards et al., 2003). Therefore, direct extrapolation from mouse data to humans in this case could be misleading, and these differences make mouse models less suitable for testing these adjuvants for human translation. In this regard, based on their close relationship to humans, NHPs such as rhesus macaques are the best animal model. Indeed, work from Ketloy et al. clearly proved that NHPs share the same expression and functionality of TLRs with humans (Ketloy et al., 2008). Their detailed analysis showed that blood DC subsets of rhesus macaques expressed the same sets of TLRs (TLR3, 4, 7, 8 and 9) as those of humans but were substantially different from the corresponding mouse DC subsets (Ketloy et al., 2008). Additionally, TLR expression patterns in macaque mo-DCs, monocytes and B cells were also similar to those in humans. Overall, therefore, rhesus macaques are better animal models than mice for the evaluation of TLR ligands as adjuvants for the design of human vaccines. Several studies have already used TLR ligands as adjuvants in rhesus macaques (Sui et al., 2010; Verthelyi et al., 2002; Wille-Reece et al., 2005), and translation of these studies to the human setting should have a good foundation.
4. Understanding the difference of antibody repertories between NHP and human
The humoral immune response is one of the important arms of adaptive immunity for HIV vaccine development. Macaques have been intensively used as models for studying vaccine development and the induction and function of humoral responses induced by variety of vaccine platforms. Therefore, to know the extent of similarity between antibody repertoires of macaques and humans is important. On average, rhesus Ig VH, DH, JH, and CH gene segments share both a similar organization and greater than 90% sequence identity with their human counterparts (Andris et al., 1997; Bible et al., 2003; Helmuth et al., 2000; Link et al., 2005; Meek et al., 1991; Scinicariello et al., 2004). In rhesus and chimpanzee, the pattern of DH and JH gene segment utilization in the expressed repertoire was similar, although not identical, to humans (Link et al., 2005). However, the distribution of CDR-H3 lengths proved quite different in the adults of these three species: the rhesus repertoire of muCDR-H3 transcripts did not contain the longer hypervariable intervals that humans express; whereas the chimpanzee repertoire included more long CDR-H3 structures than the human repertoire. These differences in CDR length arise from differences in the somatic mechanisms of N addition and terminal nucleotide loss (Link et al., 2005). However, more sensitive sequencing techniques using naïve B cells is needed to advance our understanding of antibody repertoires in different species and how these differences affect the humoral response to specific antigens.
5. Use of NHP as a model to search for the correlates of protection
Identifying protective correlates is one major goal of HIV vaccine development. However, despite tremendous efforts, clear immune correlates of protection against viral acquisition and/or disease progression remain unknown. The paucity of successful HIV vaccine clinical trials makes it difficult to identify correlates of protection in humans. However, a validated macaque model can be used to determine if a potential immune correlate or surrogate found in humans is causally related to protection. A number of vaccine platforms have been tested in NHP models and some of them showed protection against viral acquisition and replication. Among these studies, correlates of protection have been identified. In this review, we summarize immune responses that were reported to be associated with protection from virus acquisition or high levels of virus replication and provide illustrative examples for each type of immune correlate of protection that has been identified (Table 2). Cellular CD4+ and CD8+ T cell responses and humoral responses have all been implicated in control of SIV/SHIV replication in various experimental systems. However, validation of the animal models necessarily comes from human clinical trials, and to date while limited reduction of acquisition of infection has been found, no trial has reported significant vaccine induced control of HIV replication after infection. Thus, there remains a need to improve the animal model such that correlates in the animal model translate to humans.
Table 2.
Selected examples of correlates of protection in NHP models
| Correlates of protection from: --disease or high viral load (d) -- or acquisition (a) |
Challenge virus/route/dose | Vaccine platforms | Reference | ||
|---|---|---|---|---|---|
|
| |||||
| Cellular immune responses | CD8 | general | SIVmac251 | DNA/NYVAC | (Hel et al., 2002a) |
|
| |||||
| TCM | SIVmac251d | DNA/Poxvirus | (Vaccari et al., 2005) | ||
|
| |||||
| TEM | SIVmac259 intrarectala | Recombinant CMV | (Hansen et al., 2011) | ||
|
| |||||
| Polyfunctional T cell | SIVmac239d | Live attenuated SHIV89.6 | (Genesca et al., 2012) | ||
| SIVmac251d | Peptide/Poxvirus With adjuvant | (Sui et al., 2010) | |||
| SHIV89.6Pd | DNA/Ad5/Poxvirus | (Sun et al., 2008) | |||
|
| |||||
| Avidity of T cell | SIVmac251d | DNA/MVA | (O’Connor et al., 2002) | ||
|
| |||||
| Mucosal high- avidity T cell (Rectal) | SHIVKUIId | Peptide/Poxvirus | (Belyakov et al., 2006) | ||
|
| |||||
| Public clonotype | SIVmac239d | DNA/Ad5 | (Price et al., 2009b) | ||
|
| |||||
| CD4 | general | SIVmac251 | DNA/NYVAC | (Hel et al., 2002a) | |
|
| |||||
| TCM | SIVmac251 | DNA/MVA | (Vaccari et al., 2008) | ||
|
| |||||
| TEM | N/A | N/A | N/A | ||
|
| |||||
| Humoral immune responses | Neutralization antibodies | HIV (chimpanzees) SHIVSF162P3a | passive immunization with antibody | (Emini et al., 1992) (Hessell et al., 2009) | |
|
| |||||
| Binding antibodies | SHIV89.6P | Replicating Ad5/gp120 | (Demberg et al., 2007) | ||
|
| |||||
| ADCC | SIVmac251 | Replicating Ad5/gp120 boost | (Gomez- Roman et al., 2005) | ||
|
| |||||
| Avidity of the antibodies | SHIV89.6P | Replicating Ad5/gp120 | (Xiao et al., 2010) | ||
| SIVsmE660 | DNA/MVA | (Lai et al., 2012) | |||
| SHIV162.P3 | DNA/MVA | (Zhao et al., 2009) | |||
|
| |||||
| Mucosal antibodies IgA | SIVmac251a | Replicating recombinant Ad5/gp120 boost | (Xiao et al., 2012) | ||
|
| |||||
| Innate immunity | APOBEC3G | SIVmac251d | Peptide/MVA with adjuvant | (Sui et al., 2010) | |
N/A: Not Available
NB: This table was not meant to be comprehensive, but only to provide examples
Vaccine Candidates
Over the past 30 years several vaccine candidates have been evaluated for HIV. While live attenuated retroviruses are immunogenic, safety concerns including reversion and recombination preclude their use. Three of the most promising vaccine platforms are DNA, recombinant peptides or proteins and viral vectors. Proteins induce mainly humoral responses while peptides, DNA and viral vectors can induce both cell mediated as well as humoral responses. The immunogenicity and efficacy of these vaccine regimens alone or in combination with each other are being evaluated in various stages of pre-clinical animal studies as well as clinical trials in humans. Four phase III HIV vaccine clinical trials have been completed(Buchbinder et al., 2008; Flynn et al., 2005; Pitisuttithum et al., 2006; Rerks-Ngarm et al., 2009), but only one, the RV144 Thai trial showed significant efficacy (31.2%) (Rerks-Ngarm et al., 2009). The Thai trial vaccinated individuals with the canarypox vector ALVAC expressing HIV genes, in combination with the HIV-gp120 protein. The results of the RV144 Thai trial have invigorated HIV vaccine research and there is renewed interest in finding ways of improving the immunogenicity of ALVAC and other pox viral vectors that are known to be safe in humans. To illustrate the use of NHP models to mimic human clinical studies we will review pox viral vectors including ALVAC. Many examples exist of other vaccine platforms including adenovirus, DNA, peptides and proteins and some of these have been described in earlier sections, but we are limiting our detailed description to three pox viral vectors for the sake of space and brevity.
Pox viral vectors have many advantages including large genomes allowing for the insertion of multiple foreign genes, thermostability, and diminishing pre-existing vector immunity as global vaccinia vaccination was terminated in the 1970s following smallpox eradication. Attenuated pox viruses currently used in HIV vaccination strategies were derived by multiple passages in chick embryo fibroblasts or through deletion of virulence, host adaptive or immune evasion genes(Franchini et al., 2004; Pantaleo et al., 2010). In addition to ALVAC, the attenuated vaccinia strains Modified Vaccina Ankara (MVA) and New York Vaccinia virus (NYVAC) have been shown to be safe in immunocompromised macaques(Edghill-Smith et al., 2003; Stittelaar et al., 2001) and HIV infected humans(Cosma et al., 2003) and are being tested in clinical trials(Esteban, 2009).
ALVAC is the most extensively studied HIV vaccine pox vector. It has an abortive infection in primate cells(Taylor et al., 1995) but it does enter antigen presenting cells, induce maturation of dendritic cells (Yu et al., 2006), and stimulate a type 1 IFN responses(Harenberg et al., 2008). Several preclinical macaque studies with ALVAC expressing either HIV or SIV genes demonstrated the ability of ALVAC to stimulate cellular responses, and protect vaccinated animals from either virus infection or high virus loads(Andersson et al., 1996; Hel et al., 2002b; Myagkikh et al., 1996; Nacsa et al., 2004; Pal et al., 2002; Pal et al., 2006; Van Rompay et al., 2005). The humoral response induced by ALVAC is significantly improved or boosted by administering adjuvanted soluble envelope proteins(Pal et al., 2006). Macaque preclinical studies demonstrating utility of this ALVAC-prime, protein boost approach provided the groundwork for clinical trials with ALVAC and gp120(Andersson et al., 1996; Pal et al., 2002). ALVAC induces lower T cell responses compared to many other viral vectors, so there was skepticism regarding moving this platform forward to phase III clinical trials. However, in-spite of low T cell responses, protective efficacy with ALVAC, similar to that of the RV144 Thai Trial, was also demonstrated in ALVAC vaccinated infant macaques using a repeated low dose oral challenge model(Van Rompay et al., 2005). This finding highlights the need to better characterize the immune response induced by ALVAC/gp120 vaccines. Specifically, little is known about the innate immune response triggered by ALVAC and how this innate response facilitates the development of CD4 helper T cells and antibodies that do not neutralize most primary SIV isolates. In addition, characterization of mucosal responses in terms of phenotype, function and duration following ALVAC vaccination is required. Measuring immune responses immediately following vaccination and at mucosal sites is difficult in humans, but these studies are underway in macaques and will greatly enhance our understanding of the immunogenicity of ALVAC.
As the RV144 Thai trial significantly protected some vaccinees from HIV infection, an in-depth analysis of correlates of reduced risk of HIV acquisition was performed. Envelope specific antibodies directed to the V1V2 region of gp120 were found to be an inverse correlate of risk(Haynes et al., 2012). The V1V2 region of gp120 is the binding site of some broadly neutralizing antibodies(Gorny et al., 2005; McLellan et al., 2011; Walker et al., 2009) and also binds to the α4β7 integrin on CD4 T cells that can facilitate the infection of mucosal T cells(Nawaz et al., 2011). Interestingly, antibodies to the V1V2 region of gp120 with increased avidity were also observed in animals protected from a low dose mucosal SIVmac251 challenge (Pegu et. al J Virol in submission). Using macaque models one can directly test the protective efficacy of passively infused V1V2 antibodies and optimize vaccine strategies that induce them. Follow up analysis of the RV144 Thai Trial has recently demonstrated no long term clinical benefit of vaccination in HIV-infected vaccinees(Rerks-Ngarm et al., 2012). No virologic benefit or preservation of CD4 T cells was also observed in macaques challenged with repeated low doses of SIV (Pegu et. al J Virol in submission). Thus, the animal model closely mirrors the outcome of the RV144 Thai trial, and can be used to test multiple methods aimed at not only improving protective efficacy but also preventing disease progression should breakthrough infections occur.
NYVAC an alternate pox-virus vector, was created by deleting 18 genes from the Copenhagen vaccinia vaccine strain(Tartaglia et al., 1992). These included host range genes, and as a result replication is blocked at an early stage in human cells. The immunogenicity of NYVAC is similar to ALVAC in primates and both induce cell-mediated responses(Abimiku et al., 1995; Casimiro et al., 2004; Hel et al., 2002b) and have been shown to partially protect from infection or high virus load in HIV/SIV macaque models(Benson et al., 1998; Franchini et al., 1995; Myagkikh et al., 1996). However, unlike ALVAC, repeated vaccination with NYVAC induces vector specific immunity that can dampen the immune response to encoded genes. Enhanced B and T cell responses can be achieved by heterologous priming with DNA followed by boosting with NYVAC(Hel et al., 2001). This DNA/NYVAC regimen induces robust CD4+ T cell responses with increased proliferative capacity in macaques(Hel et al., 2002a) and in humans as measured in phase 1 clinical trials(Harari et al., 2008; McCormack et al., 2008). Importantly, systemically administered NYVAC vaccination induces T cell responses in the vaginal and rectal lamina propria in macaque (Stevceva et al., 2002) and in the ileum and rectum in humans(Perreau et al., 2011).
Increased immunogenicity is likely to improve vaccine efficacy, so modified NYVAC vectors aimed at inducing better innate and adaptive responses have been created(Kibler et al., 2011; Quakkelaar et al., 2011). Replication competent NYVAC was made by the re-introduction of host range genes to permit replication in human cells. In addition the removal of genes that block IFN pathways have demonstrated increased CD8 T cell responses in mice(Gomez et al., 2012). However, the protective efficacy of NYVAC and its ability to alter the clinical course of HIV infection remains unknown. Furthermore as antibodies were the correlate of protection from HIV in the RV144 Thai trial, a detailed characterization of the functional capacity of antibodies induced by NYVAC and how they compare to those induced by ALVAC is needed along with a comparison of efficacy in a repeated low dose mucosal challenge model in macaques.
The other vaccinia vaccine derivative that is being widely evaluated as an HIV vaccine candidate is MVA. MVA was attenuated by 570 passages on chicken embryo fibroblasts and its replication in human cells is blocked at the virion assembly stage, so there is expression of both early and late genes. MVA can infect human macrophages and dendritic cells, engaging toll like receptors (TLRs) and activating multiple signaling pathways and transcription factors including MyD88, IRFs, STAT1, NFκB, ultimately leading to the induction of chemokines, cytokines and increased antigen processing and presentation(Delaloye et al., 2009; Guerra et al., 2007).
As in the case of NYVAC, repeated immunization with MVA induces vector specific immunity that can reduce the recall response to the expressed antigen, so DNA priming followed by MVA boosting is often used. This dual or other combinatorial strategy with MVA have been shown to protect from infection or high virus load in many preclinical macaque models using SIV or SHIV challenges(Amara et al., 2001; Cox et al., 2012; Hanke et al., 1999; Manrique et al., 2009; Sui et al., 2010; Vaccari et al., 2008). Recently, using a repeated low dose mucosal challenge, a DNA prime-MVA boost regimen has also been shown to significantly delay SIV acquisition(Barouch et al., 2012; Lai et al., 2012). Intriguingly, delayed SIV infection was correlated with antibody responses while control of virus replication correlated with T cell responses. These results indicate that protective B and T cell responses can be induced by this vaccination strategy and methods aimed at increasing the immunogenicity in macaques may translate to a clinical benefit in humans.
Several phase I safety and immunogenicity clinical trials with DNA/MVA are in progress or have been completed and while they demonstrate good immunogenicity(Goepfert et al., 2011; Goonetilleke et al., 2006; Sandstrom et al., 2008) the relative efficacy of this strategy remains unknown. The addition of DNA encoding cytokines (IFNγ, IL-12, IL-2) or the deletion of genes such as C6L and the IL-18 binding protein in MVA show enhanced immunogenicity in murine(Falivene et al., 2012; Garcia-Arriaza et al., 2011) or primate models(Bertley et al., 2004; Manrique et al., 2008). Granulocyte-macrophage colony stimulating factor (GMCSF) has been tested in macaque models and been shown to increase B and T cell responses and protective efficacy or reduce SIV/SHIV viremia (Lai et al., 2011; Lai et al., 2007). Whether the protective immunity induced by ALVAC in humans is also induced by MVA remains to be determined. MVA induces a higher frequency of CD8 T cell response as opposed to the primarily CD4-inducing ALVAC. While a head-to-head comparison in a repeated low dose model in adult macaques has not been performed, in infant macaques, MVA induced higher env binding titers and T cell responses compared to ALVAC but failed to significantly reduce SIV acquisition(Van Rompay et al., 2005).
Macaque models are being used to directly compare these three promising HIV pox viral vectors as well as methods to improve the protective efficacy of each. Hopefully the results from these studies will help advance the ‘best’ vaccine candidates to HIV vaccine clinical trials.
Acknowledgments
We would like to acknowledge Namal P.M. Liyanage and Luca Schifanella for help with the figure and Teresa Habina and Lisa Smith for editorial support.
References
- Abimiku AG, Franchini G, Tartaglia J, Aldrich K, Myagkikh M, Markham PD, Chong P, Klein M, Kieny MP, Paoletti E, et al. HIV-1 recombinant poxvirus vaccine induces cross-protection against HIV-2 challenge in rhesus macaques. Nat Med. 1995;1:321–329. doi: 10.1038/nm0495-321. [DOI] [PubMed] [Google Scholar]
- Agy MB, Frumkin LR, Corey L, Coombs RW, Wolinsky SM, Koehler J, Morton WR, Katze MG. Infection of Macaca nemestrina by human immunodeficiency virus type-1. Science. 1992;257:103–106. doi: 10.1126/science.1621083. [DOI] [PubMed] [Google Scholar]
- Agy MB, Schmidt A, Florey MJ, Kennedy BJ, Schaefer G, Katze MG, Corey L, Morton WR, Bosch ML. Serial in vivo passage of HIV-1 infection in Macaca nemestrina. Virology. 1997;238:336–343. doi: 10.1006/viro.1997.8832. [DOI] [PubMed] [Google Scholar]
- Akari H, Mori K, Terao K, Otani I, Fukasawa M, Mukai R, Yoshikawa Y. In vitro immortalization of Old World monkey T lymphocytes with Herpesvirus saimiri: its susceptibility to infection with simian immunodeficiency viruses. Virology. 1996;218:382–388. doi: 10.1006/viro.1996.0207. [DOI] [PubMed] [Google Scholar]
- Amara RR, Smith JM, Staprans SI, Montefiori DC, Villinger F, Altman JD, O’Neil SP, Kozyr NL, Xu Y, Wyatt LS, Earl PL, Herndon JG, McNicholl JM, McClure HM, Moss B, Robinson HL. Critical Role for Env as well as Gag-Pol in Control of a Simian-Human Immunodeficiency Virus 89.6P Challenge by a DNA Prime/Recombinant Modified Vaccinia Virus Ankara Vaccine. J Virol. 2002;76:6138–6146. doi: 10.1128/JVI.76.12.6138-6146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara RR, Villinger F, Altman JD, Lydy SL, O’Neil SP, Staprans SI, Montefiori DC, Xu Y, Herndon JG, Wyatt LS, Candido MA, Kozyr NL, Earl PL, Smith JM, Ma HL, Grimm BD, Hulsey ML, Miller J, McClure HM, McNicholl JM, Moss B, Robinson HL. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292:69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- Anderson DJ. Finally, a macaque model for cell-associated SIV/HIV vaginal transmission. J Infect Dis. 2010;202:333–336. doi: 10.1086/653620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson S, Makitalo B, Thorstensson R, Franchini G, Tartaglia J, Limbach K, Paoletti E, Putkonen P, Biberfeld G. Immunogenicity and protective efficacy of a human immunodeficiency virus type 2 recombinant canarypox (ALVAC) vaccine candidate in cynomolgus monkeys. J Infect Dis. 1996;174:977–985. doi: 10.1093/infdis/174.5.977. [DOI] [PubMed] [Google Scholar]
- Andris JS, Miller AB, Abraham SR, Cunningham S, Roubinet F, Blancher A, Capra JD. Variable region gene segment utilization in rhesus monkey hybridomas producing human red blood cell-specific antibodies: predominance of the VH4 family but not VH4–21 (V4–34) Mol Immunol. 1997;34:237–253. doi: 10.1016/s0161-5890(97)00021-7. [DOI] [PubMed] [Google Scholar]
- Baeten JM, Kahle E, Lingappa JR, Coombs RW, Delany-Moretlwe S, Nakku-Joloba E, Mugo NR, Wald A, Corey L, Donnell D, Campbell MS, Mullins JI, Celum C. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3:77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroncelli S, Negri DR, Michelini Z, Cara A. Macaca mulatta, fascicularis and nemestrina in AIDS vaccine development. Expert Rev Vaccines. 2008;7:1419–1434. doi: 10.1586/14760584.7.9.1419. [DOI] [PubMed] [Google Scholar]
- Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, Sanmiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EM, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Belyakov IM, Kuznetsov VA, Kelsall B, Klinman D, Moniuszko M, Lemon M, Markham PD, Pal P, Clements JD, Lewis MG, Strober S, Franchini G, Berzofsky JA. Impact of Vaccine-induced Mucosal High Avidity CD8+ CTLs in Delay of AIDS-viral Dissemination from Mucosa. Blood. 2006;107:3258–3264. doi: 10.1182/blood-2005-11-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson J, Chougnet C, Robert-Guroff M, Montefiori D, Markham P, Shearer G, Gallo RC, Cranage M, Paoletti E, Limbach K, Venzon D, Tartaglia J, Franchini G. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIV dependence on route of challenge exposure. J Virol. 1998;72:4170–4182. doi: 10.1128/jvi.72.5.4170-4182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertley FM, Kozlowski PA, Wang SW, Chappelle J, Patel J, Sonuyi O, Mazzara G, Montefiori D, Carville A, Mansfield KG, Aldovini A. Control of simian/human immunodeficiency virus viremia and disease progression after IL-2-augmented DNA-modified vaccinia virus Ankara nasal vaccination in nonhuman primates. J Immunol. 2004;172:3745–3757. doi: 10.4049/jimmunol.172.6.3745. [DOI] [PubMed] [Google Scholar]
- Bible JM, Howard W, Robbins H, Dunn-Walters DK. IGHV1, IGHV5 and IGHV7 subgroup genes in the rhesus macaque. Immunogenetics. 2003;54:867–873. doi: 10.1007/s00251-003-0536-2. [DOI] [PubMed] [Google Scholar]
- Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nature Medicine. 1997;3:42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- Borsetti A, Baroncelli S, Maggiorella MT, Bellino S, Moretti S, Sernicola L, Belli R, Ridolfi B, Farcomeni S, Negri DR, Cafaro A, Ensoli B, Titti F. Viral outcome of simian-human immunodeficiency virus SHIV-89.6P adapted to cynomolgus monkeys. Arch Virol. 2008;153:463–472. doi: 10.1007/s00705-007-0009-2. [DOI] [PubMed] [Google Scholar]
- Boyer JD, Ugen KE, Wang B, Agadjanyan M, Gilbert L, Bagarazzi ML, Chattergoon M, Frost P, Javadian A, Williams WV, Refaeli Y, Ciccarelli RB, McCallus D, Coney L, Weiner DB. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat Med. 1997;3:526–532. doi: 10.1038/nm0597-526. [DOI] [PubMed] [Google Scholar]
- Boyson JE, Shufflebotham C, Cadavid LF, Urvater JA, Knapp LA, Hughes AL, Watkins DI. The MHC class I genes of the rhesus monkey. Different evolutionary histories of MHC class I and II genes in primates. J Immunol. 1996;156:4656–4665. [PubMed] [Google Scholar]
- Brennan G, Kozyrev Y, Hu SL. TRIMCyp expression in Old World primates Macaca nemestrina and Macaca fascicularis. Proc Natl Acad Sci U S A. 2008;105:3569–3574. doi: 10.1073/pnas.0709511105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan G, Kozyrev Y, Kodama T, Hu SL. Novel TRIM5 isoforms expressed by Macaca nemestrina. J Virol. 2007;81:12210–12217. doi: 10.1128/JVI.02499-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Marcondes MC, Lanigan CM, Penedo MC, Fox HS. Susceptibility of Chinese rhesus monkeys to SIV infection. AIDS. 2005;19:1704–1706. doi: 10.1097/01.aids.0000186823.76230.33. [DOI] [PubMed] [Google Scholar]
- Casimiro DR, Bett AJ, Fu TM, Davies ME, Tang A, Wilson KA, Chen M, Long R, McKelvey T, Chastain M, Gurunathan S, Tartaglia J, Emini EA, Shiver J. Heterologous human immunodeficiency virus type 1 priming-boosting immunization strategies involving replication-defective adenovirus and poxvirus vaccine vectors. J Virol. 2004;78:11434–11438. doi: 10.1128/JVI.78.20.11434-11438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro DR, Wang F, Schleif WA, Liang X, Zhang ZQ, Tobery TW, Davies ME, McDermott AB, O’Connor DH, Fridman A, Bagchi A, Tussey LG, Bett AJ, Finnefrock AC, Fu TM, Tang A, Wilson KA, Chen M, Perry HC, Heidecker GJ, Freed DC, Carella A, Punt KS, Sykes KJ, Huang L, Ausensi VI, Bachinsky M, Sadasivan-Nair U, Watkins DI, Emini EA, Shiver JW. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with dna and recombinant adenoviral vaccine vectors expressing Gag. J Virol. 2005;79:15547–15555. doi: 10.1128/JVI.79.24.15547-15555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. AIDS research. Vaccine studies stymied by shortage of animals. Science. 2000;287:959–960. doi: 10.1126/science.287.5455.959. [DOI] [PubMed] [Google Scholar]
- Cosma A, Nagaraj R, Buhler S, Hinkula J, Busch DH, Sutter G, Goebel FD, Erfle V. Therapeutic vaccination with MVA-HIV-1 nef elicits Nef-specific T-helper cell responses in chronically HIV-1 infected individuals. Vaccine. 2003;22:21–29. doi: 10.1016/s0264-410x(03)00538-3. [DOI] [PubMed] [Google Scholar]
- Cox JH, Ferrari MG, Earl P, Lane JR, Jagodzinski LL, Polonis VR, Kuta EG, Boyer JD, Ratto-Kim S, Eller LA, Pham DT, Hart L, Montefiori D, Ferrari G, Parrish S, Weiner DB, Moss B, Kim JH, Birx D, VanCott TC. Inclusion of a CRF01_AE HIV envelope protein boost with a DNA/MVA prime-boost vaccine: Impact on humoral and cellular immunogenicity and viral load reduction after SHIV-E challenge. Vaccine. 2012;30:1830–1840. doi: 10.1016/j.vaccine.2011.12.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel MD, Letvin NL, King NW, Kannagi M, Sehgal PK, Hunt RD, Kanki PJ, Essex M, Desrosiers RC. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985;228:1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- de Sorrentino AH, Marinic K, Motta P, Sorrentino A, Lopez R, Illiovich E. HLA class I alleles associated with susceptibility or resistance to human immunodeficiency virus type 1 infection among a population in Chaco Province, Argentina. J Infect Dis. 2000;182:1523–1526. doi: 10.1086/315854. [DOI] [PubMed] [Google Scholar]
- Delaloye J, Roger T, Steiner-Tardivel QG, Le Roy D, Knaup Reymond M, Akira S, Petrilli V, Gomez CE, Perdiguero B, Tschopp J, Pantaleo G, Esteban M, Calandra T. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 2009;5:e1000480. doi: 10.1371/journal.ppat.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Demberg T, Florese RH, Heath MJ, Larsen K, Kalisz I, Kalyanaraman VS, Lee EM, Pal R, Venzon D, Grant R, Patterson LJ, Korioth-Schmitz B, Buzby A, Dombagoda D, Montefiori DC, Letvin NL, Cafaro A, Ensoli B, Robert-Guroff M. A replication-competent adenovirus-human immunodeficiency virus (Ad-HIV) tat and Ad-HIV env priming/Tat and envelope protein boosting regimen elicits enhanced protective efficacy against simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol. 2007;81:3414–3427. doi: 10.1128/JVI.02453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdeyn CA, Decker JM, Bibollet-Ruche F, Mokili JL, Muldoon M, Denham SA, Heil ML, Kasolo F, Musonda R, Hahn BH, Shaw GM, Korber BT, Allen S, Hunter E. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science. 2004;303:2019–2022. doi: 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- Dietrich EA, Brennan G, Ferguson B, Wiseman RW, O’Connor D, Hu SL. Variable prevalence and functional diversity of the antiretroviral restriction factor TRIMCyp in Macaca fascicularis. J Virol. 2011;85:9956–9963. doi: 10.1128/JVI.00097-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edghill-Smith Y, Venzon D, Karpova T, McNally J, Nacsa J, Tsai WP, Tryniszewska E, Moniuszko M, Snodgrass SJ, Parrish J, Lewis MG, Berzofsky JA, Belyakov IM, Moss B, Tartaglia J, Bray M, Hirsh V, Golding H, Franchini G. Modeling a safer smallpox-vaccination regimen, for human immunodeficiency virus type 1-infected patients, in immunocompromised macaques. Journal of Infectious Diseases. 2003;188:1181–1191. doi: 10.1086/378518. [DOI] [PubMed] [Google Scholar]
- Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, Akira S, Reis e Sousa C. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 2003;33:827–833. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- Emini EA, Schleif WA, Nunberg JH, Conley AJ, Eda Y, Tokiyoshi S, Putney SD, Matsushita S, Cobb KE, Jett CM, Eichberg JW, Murthy KK. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992;355:728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- Esteban M. Attenuated poxvirus vectors MVA and NYVAC as promising vaccine candidates against HIV/AIDS. Hum Vaccin. 2009;5:867–871. doi: 10.4161/hv.9693. [DOI] [PubMed] [Google Scholar]
- Falivene J, Del Medico Zajac MP, Pascutti MF, Rodriguez AM, Maeto C, Perdiguero B, Gomez CE, Esteban M, Calamante G, Gherardi MM. Improving the MVA vaccine potential by deleting the viral gene coding for the IL-18 binding protein. PLoS One. 2012;7:e32220. doi: 10.1371/journal.pone.0032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney ME, Tang Y, Roosevelt KA, Leslie AJ, McIntosh K, Karthas N, Walker BD, Goulder PJ. Immune escape precedes breakthrough human immunodeficiency virus type 1 viremia and broadening of the cytotoxic T-lymphocyte response in an HLA-B27-positive long-term-nonprogressing child. J Virol. 2004;78:8927–8930. doi: 10.1128/JVI.78.16.8927-8930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg MB, Moore JP. AIDS vaccine models: challenging challenge viruses. Nat Med. 2002;8:207–210. doi: 10.1038/nm0302-207. [DOI] [PubMed] [Google Scholar]
- Fellay J, Shianna KV, Ge D, Colombo S, Ledergerber B, Weale M, Zhang K, Gumbs C, Castagna A, Cossarizza A, Cozzi-Lepri A, De Luca A, Easterbrook P, Francioli P, Mallal S, Martinez-Picado J, Miro JM, Obel N, Smith JP, Wyniger J, Descombes P, Antonarakis SE, Letvin NL, McMichael AJ, Haynes BF, Telenti A, Goldstein DB. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317:944–947. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenizia C, Keele BF, Nichols D, Cornara S, Binello N, Vaccari M, Pegu P, Robert-Guroff M, Ma ZM, Miller CJ, Venzon D, Hirsch V, Franchini G. TRIM5alpha does not affect simian immunodeficiency virus SIV(mac251) replication in vaccinated or unvaccinated Indian rhesus macaques following intrarectal challenge exposure. J Virol. 2011;85:12399–12409. doi: 10.1128/JVI.05707-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez CS, Stratov I, De Rose R, Walsh K, Dale CJ, Smith MZ, Agy MB, Hu SL, Krebs K, Watkins DI, O’Connor DH, Davenport MP, Kent SJ. Rapid viral escape at an immunodominant simian-human immunodeficiency virus cytotoxic T-lymphocyte epitope exacts a dramatic fitness cost. J Virol. 2005;79:5721–5731. doi: 10.1128/JVI.79.9.5721-5731.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- Franchini G, Gurunathan S, Baglyos L, Plotkin S, Tartaglia J. Poxvirus-based vaccine candidates for HIV: two decades of experience with special emphasis on canarypox vectors. Expert Rev Vaccines. 2004;3:S75–88. doi: 10.1586/14760584.3.4.s75. [DOI] [PubMed] [Google Scholar]
- Franchini G, Robert-Guroff M, Tartaglia J, Aggarwal A, Abimiku A, Benson J, Markham P, Limbach K, Hurteau G, Fullen J, et al. Highly attenuated HIV type 2 recombinant poxviruses, but not HIV-2 recombinant Salmonella vaccines, induce long-lasting protection in rhesus macaques. AIDS Res Hum Retroviruses. 1995;11:909–920. doi: 10.1089/aid.1995.11.909. [DOI] [PubMed] [Google Scholar]
- Frumkin LR, Agy MB, Coombs RW, Panther L, Morton WR, Koehler J, Florey MJ, Dragavon J, Schmidt A, Katze MG, et al. Acute infection of Macaca nemestrina by human immunodeficiency virus type 1. Virology. 1993;195:422–431. doi: 10.1006/viro.1993.1392. [DOI] [PubMed] [Google Scholar]
- Fultz PN, Nara P, Barre-Sinoussi F, Chaput A, Greenberg ML, Muchmore E, Kieny MP, Girard M. Vaccine protection of chimpanzees against challenge with HIV-1-infected peripheral blood mononuclear cells. Science. 1992;256:1687–1689. doi: 10.1126/science.256.5064.1687. [DOI] [PubMed] [Google Scholar]
- Gallimore A, Cranage M, Cook N, Almond N, Bootman J, Rud E, Silvera P, Dennis M, Corcoran T, Stott J, et al. Early suppression of SIV replication by CD8+ nef-specific cytotoxic T cells in vaccinated macaques. Nat Med. 1995;1:1167–1173. doi: 10.1038/nm1195-1167. [DOI] [PubMed] [Google Scholar]
- Gallo RC, Salahuddin SZ, Popovic M, Shearer GM, Kaplan M, Haynes BF, Palker TJ, Redfield R, Oleske J, Safai B, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Garcia-Arriaza J, Najera JL, Gomez CE, Tewabe N, Sorzano CO, Calandra T, Roger T, Esteban M. A candidate HIV/AIDS vaccine (MVA-B) lacking vaccinia virus gene C6L enhances memory HIV-1-specific T-cell responses. PLoS One. 2011;6:e24244. doi: 10.1371/journal.pone.0024244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MB, Luciw PA. Animal models of AIDS. FASEB J. 1989;3:2593–2606. doi: 10.1096/fasebj.3.14.2556312. [DOI] [PubMed] [Google Scholar]
- Genesca M, Ma ZM, Wang Y, Assaf B, Qureshi H, Fritts L, Huang Y, McChesney MB, Miller CJ. Live-Attenuated Lentivirus Immunization Modulates Innate Immunity and Inflammation while Protecting Rhesus Macaques from Vaginal Simian Immunodeficiency Virus Challenge. J Virol. 2012;86:9188–9200. doi: 10.1128/JVI.00532-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC, Wilson RK, Batzer MA, Bustamante CD, Eichler EE, Hahn MW, Hardison RC, Makova KD, Miller W, Milosavljevic A, Palermo RE, Siepel A, Sikela JM, Attaway T, Bell S, Bernard KE, Buhay CJ, Chandrabose MN, Dao M, Davis C, Delehaunty KD, Ding Y, Dinh HH, Dugan-Rocha S, Fulton LA, Gabisi RA, Garner TT, Godfrey J, Hawes AC, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Kirkness EF, Cree A, Fowler RG, Lee S, Lewis LR, Li Z, Liu YS, Moore SM, Muzny D, Nazareth LV, Ngo DN, Okwuonu GO, Pai G, Parker D, Paul HA, Pfannkoch C, Pohl CS, Rogers YH, Ruiz SJ, Sabo A, Santibanez J, Schneider BW, Smith SM, Sodergren E, Svatek AF, Utterback TR, Vattathil S, Warren W, White CS, Chinwalla AT, Feng Y, Halpern AL, Hillier LW, Huang X, Minx P, Nelson JO, Pepin KH, Qin X, Sutton GG, Venter E, Walenz BP, Wallis JW, Worley KC, Yang SP, Jones SM, Marra MA, Rocchi M, Schein JE, Baertsch R, Clarke L, Csuros M, Glasscock J, Harris RA, Havlak P, Jackson AR, Jiang H, Liu Y, Messina DN, Shen Y, Song HX, Wylie T, Zhang L, Birney E, Han K, Konkel MK, Lee J, Smit AF, Ullmer B, Wang H, Xing J, Burhans R, Cheng Z, Karro JE, Ma J, Raney B, She X, Cox MJ, Demuth JP, Dumas LJ, Han SG, Hopkins J, Karimpour-Fard A, Kim YH, Pollack JR, Vinar T, Addo-Quaye C, Degenhardt J, Denby A, Hubisz MJ, Indap A, Kosiol C, Lahn BT, Lawson HA, Marklein A, Nielsen R, Vallender EJ, Clark AG, Ferguson B, Hernandez RD, Hirani K, Kehrer-Sawatzki H, Kolb J, Patil S, Pu LL, Ren Y, Smith DG, Wheeler DA, Schenck I, Ball EV, Chen R, Cooper DN, Giardine B, Hsu F, Kent WJ, Lesk A, Nelson DL, O’Brien WE, Prufer K, Stenson PD, Wallace JC, Ke H, Liu XM, Wang P, Xiang AP, Yang F, Barber GP, Haussler D, Karolchik D, Kern AD, Kuhn RM, Smith KE, Zwieg AS. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- Girard M, Kieny M-P, Pinter A, Barre-Sinoussi F, Nara P, Kolbe H, Kusumi K, Chaput A, Reinhart T, Muchmore E, Ronco J, Kaczorek M, Gomard E, Gluckman J-C, Fultz PN. Immunization of chimpanzees confers protection against challenge with human immunodeficiency virus. Proc Natl Acad Sci USA. 1991;88:542–546. doi: 10.1073/pnas.88.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M, Mahoney J, Wei Q, van der Ryst E, Muchmore E, Barre-Sinoussi F, Fultz PN. Genital infection of female chimpanzees with human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1998;14:1357–1367. doi: 10.1089/aid.1998.14.1357. [DOI] [PubMed] [Google Scholar]
- Girard M, van der Ryst E, Barre-Sinoussi F, Nara P, Tartaglia J, Paoletti E, Blondeau C, Jennings M, Verrier F, Meignier B, Fultz PN. Challenge of chimpanzees immunized with a recombinant canarypox-HIV-1 virus. Virology. 1997;232:98–104. doi: 10.1006/viro.1997.8560. [DOI] [PubMed] [Google Scholar]
- Goepfert PA, Elizaga ML, Sato A, Qin L, Cardinali M, Hay CM, Hural J, DeRosa SC, DeFawe OD, Tomaras GD, Montefiori DC, Xu Y, Lai L, Kalams SA, Baden LR, Frey SE, Blattner WA, Wyatt LS, Moss B, Robinson HL. Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis. 2011;203:610–619. doi: 10.1093/infdis/jiq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez CE, Perdiguero B, Najera JL, Sorzano CO, Jimenez V, Gonzalez-Sanz R, Esteban M. Removal of vaccinia virus genes that block interferon type I and II pathways improves adaptive and memory responses of the HIV/AIDS vaccine candidate NYVAC-C in mice. J Virol. 2012;86:5026–5038. doi: 10.1128/JVI.06684-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roman VR, Patterson LJ, Venzon D, Liewehr D, Aldrich K, Florese R, Robert-Guroff M. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol. 2005;174:2185–2189. doi: 10.4049/jimmunol.174.4.2185. [DOI] [PubMed] [Google Scholar]
- Goonetilleke N, Moore S, Dally L, Winstone N, Cebere I, Mahmoud A, Pinheiro S, Gillespie G, Brown D, Loach V, Roberts J, Guimaraes-Walker A, Hayes P, Loughran K, Smith C, De Bont J, Verlinde C, Vooijs D, Schmidt C, Boaz M, Gilmour J, Fast P, Dorrell L, Hanke T, McMichael AJ. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J Virol. 2006;80:4717–4728. doi: 10.1128/JVI.80.10.4717-4728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny MK, Stamatatos L, Volsky B, Revesz K, Williams C, Wang XH, Cohen S, Staudinger R, Zolla-Pazner S. Identification of a new quaternary neutralizing epitope on human immunodeficiency virus type 1 virus particles. J Virol. 2005;79:5232–5237. doi: 10.1128/JVI.79.8.5232-5237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire-Mangen F, Lutalo T, Li X, vanCott T, Quinn TC. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- Guerra S, Najera JL, Gonzalez JM, Lopez-Fernandez LA, Climent N, Gatell JM, Gallart T, Esteban M. Distinct gene expression profiling after infection of immature human monocyte-derived dendritic cells by the attenuated poxvirus vectors MVA and NYVAC. J Virol. 2007;81:8707–8721. doi: 10.1128/JVI.00444-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- Hanke T, Neumann VC, Blanchard TJ, Sweeney P, Hill AV, Smith GL, McMichael A. Effective induction of HIV-specific CTL by multi-epitope using gene gun in a combined vaccination regime. Vaccine. 1999;17:589–596. doi: 10.1016/s0264-410x(98)00238-2. [DOI] [PubMed] [Google Scholar]
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Jr, Lifson JD, Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari A, Bart PA, Stohr W, Tapia G, Garcia M, Medjitna-Rais E, Burnet S, Cellerai C, Erlwein O, Barber T, Moog C, Liljestrom P, Wagner R, Wolf H, Kraehenbuhl JP, Esteban M, Heeney J, Frachette MJ, Tartaglia J, McCormack S, Babiker A, Weber J, Pantaleo G. An HIV-1 clade CDNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med. 2008;205:63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenberg A, Guillaume F, Ryan EJ, Burdin N, Spada F. Gene profiling analysis of ALVAC infected human monocyte derived dendritic cells. Vaccine. 2008;26:5004–5013. doi: 10.1016/j.vaccine.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harouse JM, Gettie A, Tan RC, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science. 1999;284:816–819. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- Hatziioannou T, Perez-Caballero D, Yang A, Cowan S, Bieniasz PD. Retrovirus resistance factors Ref1 and Lv1 are species-specific variants of TRIM5alpha. Proc Natl Acad Sci U S A. 2004;101:10774–10779. doi: 10.1073/pnas.0402361101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeney JL, Dalgleish AG, Weiss RA. Origins of HIV and the evolution of resistance to AIDS. Science. 2006;313:462–466. doi: 10.1126/science.1123016. [DOI] [PubMed] [Google Scholar]
- Hel Z, Nacsa J, Tryniszewska E, Tsai WP, Parks RW, Montefiori DC, Felber BK, Tartaglia J, Pavlakis GN, Franchini G. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J Immunol. 2002a;169:4778–4787. doi: 10.4049/jimmunol.169.9.4778. [DOI] [PubMed] [Google Scholar]
- Hel Z, Nacsa J, Tsai WP, Thornton A, Giuliani L, Tartaglia J, Franchini G. Equivalent immunogenicity of the highly attenuated poxvirus-based ALVAC-SIV and NYVAC-SIV vaccine candidates in SIVmac251-infected macaques. Virology. 2002b;304:125–134. doi: 10.1006/viro.2002.1722. [DOI] [PubMed] [Google Scholar]
- Hel Z, Tsai WP, Thornton A, Nacsa J, Giuliani L, Tryniszewska E, Poudyal M, Venzon D, Wang X, Altman J, Watkins DI, Lu W, von Gegerfelt A, Felber BK, Tartaglia J, Pavlakis GN, Franchini G. Potentiation of simian immunodeficiency virus (SIV)-specific CD4(+) and CD8(+) T cell responses by a DNA-SIV and NYVAC-SIV prime/boost regimen. J Immunol. 2001;167:7180–7191. doi: 10.4049/jimmunol.167.12.7180. [DOI] [PubMed] [Google Scholar]
- Hel Z, Tsai WP, Tryniszewska E, Nacsa J, Markham PD, Lewis MG, Pavlakis GN, Felber BK, Tartaglia J, Franchini G. Improved vaccine protection from simian AIDS by the addition of nonstructural simian immunodeficiency virus genes. J Immunol. 2006;176:85–96. doi: 10.4049/jimmunol.176.1.85. [DOI] [PubMed] [Google Scholar]
- Helmuth EF, Letvin NL, Margolin DH. Germline repertoire of the immunoglobulin V(H)3 family in rhesus monkeys. Immunogenetics. 2000;51:519–527. doi: 10.1007/s002510000170. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Henrickson RV, Maul DH, Osborn KG, Sever JL, Madden DL, Ellingsworth LR, Anderson JH, Lowenstine LJ, Gardner MB. Epidemic of acquired immunodeficiency in rhesus monkeys. Lancet. 1983;1:388–390. doi: 10.1016/s0140-6736(83)91503-9. [DOI] [PubMed] [Google Scholar]
- Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009;5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashino A, Sakate R, Kameoka Y, Takahashi I, Hirata M, Tanuma R, Masui T, Yasutomi Y, Osada N. Whole-genome sequencing and analysis of the Malaysian cynomolgus macaque (Macaca fascicularis) genome. Genome Biol. 2012;13:R58. doi: 10.1186/gb-2012-13-7-r58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himathongkham S, Luciw PA. Restriction of HIV-1 (subtype B) replication at the entry step in rhesus macaque cells. Virology. 1996;219:485–488. doi: 10.1006/viro.1996.0276. [DOI] [PubMed] [Google Scholar]
- Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474:658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Joag SV, Li Z, Foresman L, Stephens EB, Zhao LJ, Adany I, Pinson DM, McClure HM, Narayan O. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J Virol. 1996;70:3189–3197. doi: 10.1128/jvi.70.5.3189-3197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joag SV, Stephens EB, Adams RJ, Foresman L, Narayan O. Pathogenesis of SIVmac infection in Chinese and Indian rhesus macaques: effects of splenectomy on virus burden. Virology. 1994;200:436–446. doi: 10.1006/viro.1994.1207. [DOI] [PubMed] [Google Scholar]
- Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser SM, Malik HS, Emerman M. Restriction of an extinct retrovirus by the human TRIM5alpha antiviral protein. Science. 2007;316:1756–1758. doi: 10.1126/science.1140579. [DOI] [PubMed] [Google Scholar]
- Kaizu M, Weiler AM, Weisgrau KL, Vielhuber KA, May G, Piaskowski SM, Furlott J, Maness NJ, Friedrich TC, Loffredo JT, Usborne A, Rakasz EG. Repeated intravaginal inoculation with cell-associated simian immunodeficiency virus results in persistent infection of nonhuman primates. J Infect Dis. 2006;194:912–916. doi: 10.1086/507308. [DOI] [PubMed] [Google Scholar]
- Keckesova Z, Ylinen LM, Towers GJ. The human and African green monkey TRIM5alpha genes encode Ref1 and Lv1 retroviral restriction factor activities. Proc Natl Acad Sci U S A. 2004;101:10780–10785. doi: 10.1073/pnas.0402474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Estes JD. Barriers to mucosal transmission of immunodeficiency viruses. Blood. 2011;118:839–846. doi: 10.1182/blood-2010-12-325860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Li H, Learn GH, Hraber P, Giorgi EE, Grayson T, Sun C, Chen Y, Yeh WW, Letvin NL, Mascola JR, Nabel GJ, Haynes BF, Bhattacharya T, Perelson AS, Korber BT, Hahn BH, Shaw GM. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med. 2009;206:1117–1134. doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Van Heuverswyn F, Li Y, Bailes E, Takehisa J, Santiago ML, Bibollet-Ruche F, Chen Y, Wain LV, Liegeois F, Loul S, Ngole EM, Bienvenue Y, Delaporte E, Brookfield JF, Sharp PM, Shaw GM, Peeters M, Hahn BH. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006;313:523–526. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketloy C, Engering A, Srichairatanakul U, Limsalakpetch A, Yongvanitchit K, Pichyangkul S, Ruxrungtham K. Expression and function of Toll-like receptors on dendritic cells and other antigen presenting cells from non-human primates. Vet Immunol Immunopathol. 2008;125:18–30. doi: 10.1016/j.vetimm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Kibler KV, Gomez CE, Perdiguero B, Wong S, Huynh T, Holechek S, Arndt W, Jimenez V, Gonzalez-Sanz R, Denzler K, Haddad EK, Wagner R, Sekaly RP, Tartaglia J, Pantaleo G, Jacobs BL, Esteban M. Improved NYVAC-based vaccine vectors. PLoS One. 2011;6:e25674. doi: 10.1371/journal.pone.0025674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, Bishop K, van der Stok M, Nair K, Khan N, Crawford H, Payne R, Leslie A, Prado J, Prendergast A, Frater J, McCarthy N, Brander C, Learn GH, Nickle D, Rousseau C, Coovadia H, Mullins JI, Heckerman D, Walker BD, Goulder P. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- Kirmaier A, Wu F, Newman RM, Hall LR, Morgan JS, O’Connor S, Marx PA, Meythaler M, Goldstein S, Buckler-White A, Kaur A, Hirsch VM, Johnson WE. TRIM5 suppresses cross-species transmission of a primate immunodeficiency virus and selects for emergence of resistant variants in the new species. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kootstra NA, Munk C, Tonnu N, Landau NR, Verma IM. Abrogation of postentry restriction of HIV-1-based lentiviral vector transduction in simian cells. Proc Natl Acad Sci U S A. 2003;100:1298–1303. doi: 10.1073/pnas.0337541100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs KC, Jin Z, Rudersdorf R, Hughes AL, O’Connor DH. Unusually high frequency MHC class I alleles in Mauritian origin cynomolgus macaques. J Immunol. 2005;175:5230–5239. doi: 10.4049/jimmunol.175.8.5230. [DOI] [PubMed] [Google Scholar]
- Krug A, Towarowski A, Britsch S, Rothenfusser S, Hornung V, Bals R, Giese T, Engelmann H, Endres S, Krieg AM, Hartmann G. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31:3026–3037. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai L, Kwa S, Kozlowski PA, Montefiori DC, Ferrari G, Johnson WE, Hirsch V, Villinger F, Chennareddi L, Earl PL, Moss B, Amara RR, Robinson HL. Prevention of infection by a granulocyte-macrophage colony-stimulating factor co-expressing DNA/modified vaccinia Ankara simian immunodeficiency virus vaccine. J Infect Dis. 2011;204:164–173. doi: 10.1093/infdis/jir199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai L, Kwa SF, Kozlowski PA, Montefiori DC, Nolen TL, Hudgens MG, Johnson WE, Ferrari G, Hirsch VM, Felber BK, Pavlakis GN, Earl PL, Moss B, Amara RR, Robinson HL. SIVmac239 MVA vaccine with and without a DNA prime, similar prevention of infection by a repeated dose SIVsmE660 challenge despite different immune responses. Vaccine. 2012;30:1737–1745. doi: 10.1016/j.vaccine.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai L, Vodros D, Kozlowski PA, Montefiori DC, Wilson RL, Akerstrom VL, Chennareddi L, Yu T, Kannanganat S, Ofielu L, Villinger F, Wyatt LS, Moss B, Amara RR, Robinson HL. GM-CSF DNA: an adjuvant for higher avidity IgG, rectal IgA, and increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology. 2007;369:153–167. doi: 10.1016/j.virol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhashe SK, Velu V, Sciaranghella G, Siddappa NB, Dipasquale JM, Hemashettar G, Yoon JK, Rasmussen RA, Yang F, Lee SJ, Montefiori DC, Novembre FJ, Villinger F, Amara RR, Kahn M, Hu SL, Li S, Li Z, Frankel FR, Robert-Guroff M, Johnson WE, Lieberman J, Ruprecht RM. Prime-boost vaccination with heterologous live vectors encoding SIV gag and multimeric HIV-1 gp160 protein: efficacy against repeated mucosal R5 clade C SHIV challenges. Vaccine. 2011;29:5611–5622. doi: 10.1016/j.vaccine.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie AJ, Pfafferott KJ, Chetty P, Draenert R, Addo MM, Feeney M, Tang Y, Holmes EC, Allen T, Prado JG, Altfeld M, Brander C, Dixon C, Ramduth D, Jeena P, Thomas SA, St John A, Roach TA, Kupfer B, Luzzi G, Edwards A, Taylor G, Lyall H, Tudor-Williams G, Novelli V, Martinez-Picado J, Kiepiela P, Walker BD, Goulder PJ. HIV evolution: CTL escape mutation and reversion after transmission. Nat Med. 2004;10:282–289. doi: 10.1038/nm992. [DOI] [PubMed] [Google Scholar]
- Letvin NL, Daniel MD, Sehgal PK, Desrosiers RC, Hunt RD, Waldron LM, MacKey JJ, Schmidt DK, Chalifoux LV, King NW. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985;230:71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- Letvin NL, Eaton KA, Aldrich WR, Sehgal PK, Blake BJ, Schlossman SF, King NW, Hunt RD. Acquired immunodeficiency syndrome in a colony of macaque monkeys. Proc Natl Acad Sci U S A. 1983;80:2718–2722. doi: 10.1073/pnas.80.9.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin NL, Rao SS, Montefiori DC, Seaman MS, Sun Y, Lim SY, Yeh WW, Asmal M, Gelman RS, Shen L, Whitney JB, Seoighe C, Lacerda M, Keating S, Norris PJ, Hudgens MG, Gilbert PB, Buzby AP, Mach LV, Zhang JR, Balachandran H, Shaw GM, Schmidt SD, Todd JP, Dodson A, Mascola JR, Nabel GJ. Immune and Genetic Correlates of Vaccine Protection Against Mucosal Infection by SIV in Monkeys. Science Translational Medicine. 2011;3:81ra36. doi: 10.1126/scitranslmed.3002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SY, Chan T, Gelman RS, Whitney JB, O’Brien KL, Barouch DH, Goldstein DB, Haynes BF, Letvin NL. Contributions of Mamu-A*01 status and TRIM5 allele expression, but not CCL3L copy number variation, to the control of SIVmac251 replication in Indian-origin rhesus monkeys. PLoS Genet. 2010a;6:e1000997. doi: 10.1371/journal.pgen.1000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SY, Rogers T, Chan T, Whitney JB, Kim J, Sodroski J, Letvin NL. TRIM5alpha Modulates Immunodeficiency Virus Control in Rhesus Monkeys. PLoS Pathog. 2010b;6:e1000738. doi: 10.1371/journal.ppat.1000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling B, Veazey RS, Luckay A, Penedo C, Xu K, Lifson JD, Marx PA. SIV(mac) pathogenesis in rhesus macaques of Chinese and Indian origin compared with primary HIV infections in humans. AIDS. 2002;16:1489–1496. doi: 10.1097/00002030-200207260-00005. [DOI] [PubMed] [Google Scholar]
- Link JM, Larson JE, Schroeder HW. Despite extensive similarity in germline DH and JH sequence, the adult Rhesus macaque CDR-H3 repertoire differs from human. Mol Immunol. 2005;42:943–955. doi: 10.1016/j.molimm.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Liu J, Keele BF, Li H, Keating S, Norris PJ, Carville A, Mansfield KG, Tomaras GD, Haynes BF, Kolodkin-Gal D, Letvin NL, Hahn BH, Shaw GM, Barouch DH. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J Virol. 2010;84:10406–10412. doi: 10.1128/JVI.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo JT, Bean AT, Beal DR, Leon EJ, May GE, Piaskowski SM, Furlott JR, Reed J, Musani SK, Rakasz EG, Friedrich TC, Wilson NA, Allison DB, Watkins DI. Patterns of CD8+ immunodominance may influence the ability of Mamu-B*08-positive macaques to naturally control simian immunodeficiency virus SIVmac239 replication. J Virol. 2008;82:1723–1738. doi: 10.1128/JVI.02084-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo JT, Friedrich TC, Leon EJ, Stephany JJ, Rodrigues DS, Spencer SP, Bean AT, Beal DR, Burwitz BJ, Rudersdorf RA, Wallace LT, Piaskowski SM, May GE, Sidney J, Gostick E, Wilson NA, Price DA, Kallas EG, Piontkivska H, Hughes AL, Sette A, Watkins DI. CD8+ T cells from SIV elite controller macaques recognize Mamu-B*08-bound epitopes and select for widespread viral variation. PLoS One. 2007a;2:e1152. doi: 10.1371/journal.pone.0001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo JT, Maxwell J, Qi Y, Glidden CE, Borchardt GJ, Soma T, Bean AT, Beal DR, Wilson NA, Rehrauer WM, Lifson JD, Carrington M, Watkins DI. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J Virol. 2007b;81:8827–8832. doi: 10.1128/JVI.00895-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo JT, Sidney J, Bean AT, Beal DR, Bardet W, Wahl A, Hawkins OE, Piaskowski S, Wilson NA, Hildebrand WH, Watkins DI, Sette A. Two MHC class I molecules associated with elite control of immunodeficiency virus replication, Mamu-B*08 and HLA-B*2705, bind peptides with sequence similarity. J Immunol. 2009;182:7763–7775. doi: 10.4049/jimmunol.0900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London WT, Sever JL, Madden DL, Henrickson RV, Gravell M, Maul DH, Dalakas MC, Osborn KG, Houff SA, Gardner MB. Experimental transmission of simian acquired immunodeficiency syndrome (SAIDS) and Kaposi-like skin lesions. Lancet. 1983;2:869–873. doi: 10.1016/s0140-6736(83)90867-x. [DOI] [PubMed] [Google Scholar]
- Looney DJ, McClure J, Kent SJ, Radaelli A, Kraus G, Schmidt A, Steffy K, Greenberg P, Hu SL, Morton WR, Wong-Staal F. A minimally replicative HIV-2 live-virus vaccine protects M. nemestrina from disease after HIV-2(287) challenge. Virology. 1998;242:150–160. doi: 10.1006/viro.1997.8992. [DOI] [PubMed] [Google Scholar]
- Ma ZM, Keele BF, Qureshi H, Stone M, Desilva V, Fritts L, Lifson JD, Miller CJ. SIVmac251 is inefficiently transmitted to rhesus macaques by penile inoculation with a single SIVenv variant found in ramp-up phase plasma. AIDS Res Hum Retroviruses. 2011;27:1259–1269. doi: 10.1089/aid.2011.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness NJ, Valentine LE, May GE, Reed J, Piaskowski SM, Soma T, Furlott J, Rakasz EG, Friedrich TC, Price DA, Gostick E, Hughes AL, Sidney J, Sette A, Wilson NA, Watkins DI. AIDS virus specific CD8+ T lymphocytes against an immunodominant cryptic epitope select for viral escape. J Exp Med. 2007;204:2505–2512. doi: 10.1084/jem.20071261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique M, Kozlowski PA, Wang SW, Wilson RL, Micewicz E, Montefiori DC, Mansfield KG, Carville A, Aldovini A. Nasal DNA-MVA SIV vaccination provides more significant protection from progression to AIDS than a similar intramuscular vaccination. Mucosal Immunol. 2009;2:536–550. doi: 10.1038/mi.2009.103. [DOI] [PubMed] [Google Scholar]
- Manrique M, Micewicz E, Kozlowski PA, Wang SW, Aurora D, Wilson RL, Ghebremichael M, Mazzara G, Montefiori D, Carville A, Mansfield KG, Aldovini A. DNA-MVA vaccine protection after X4 SHIV challenge in macaques correlates with day-of-challenge antiviral CD4+ cell-mediated immunity levels and postchallenge preservation of CD4+ T cell memory. AIDS Res Hum Retroviruses. 2008;24:505–519. doi: 10.1089/aid.2007.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes MC, Penedo MC, Lanigan C, Hall D, Watry DD, Zandonatti M, Fox HS. Simian immunodeficiency virus-induced CD4+ T cell deficits in cytokine secretion profile are dependent on monkey origin. Viral Immunol. 2006;19:679–689. doi: 10.1089/vim.2006.19.679. [DOI] [PubMed] [Google Scholar]
- Marthas ML, Lu D, Penedo MC, Hendrickx AG, Miller CJ. Titration of an SIVmac251 stock by vaginal inoculation of Indian and Chinese origin rhesus macaques: transmission efficiency, viral loads, and antibody responses. AIDS Res Hum Retroviruses. 2001;17:1455–1466. doi: 10.1089/088922201753197123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, Mahoney CJ, Miller CJ, Claypool LE, Ho DD, Alexander NJ. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- McClure J, Schmidt AM, Rey-Cuille MA, Bannink J, Misher L, Tsai CC, Anderson DM, Morton WR, Hu SL. Derivation and characterization of a highly pathogenic isolate of human immunodeficiency virus type 2 that causes rapid CD4+ cell depletion in Macaca nemestrina. J Med Primatol. 2000;29:114–126. doi: 10.1034/j.1600-0684.2000.290304.x. [DOI] [PubMed] [Google Scholar]
- McCormack S, Stohr W, Barber T, Bart PA, Harari A, Moog C, Ciuffreda D, Cellerai C, Cowen M, Gamboni R, Burnet S, Legg K, Brodnicki E, Wolf H, Wagner R, Heeney J, Frachette MJ, Tartaglia J, Babiker A, Pantaleo G, Weber J. EV02: a Phase I trial to compare the safety and immunogenicity of HIV DNA-C prime-NYVAC-C boost to NYVAC-C alone. Vaccine. 2008;26:3162–3174. doi: 10.1016/j.vaccine.2008.02.072. [DOI] [PubMed] [Google Scholar]
- McDermott AB, O’Connor DH, Fuenger S, Piaskowski S, Martin S, Loffredo J, Reynolds M, Reed J, Furlott J, Jacoby T, Riek C, Dodds E, Krebs K, Davies ME, Schleif WA, Casimiro DR, Shiver JW, Watkins DI. Cytotoxic T-lymphocyte escape does not always explain the transient control of simian immunodeficiency virus SIVmac239 viremia in adenovirus-boosted and DNA-primed Mamu-A*01-positive rhesus macaques. J Virol. 2005;79:15556–15566. doi: 10.1128/JVI.79.24.15556-15566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O’Dell S, Patel N, Shahzad-ul-Hussan S, Yang Y, Zhang B, Zhou T, Zhu J, Boyington JC, Chuang GY, Diwanji D, Georgiev I, Kwon YD, Lee D, Louder MK, Moquin S, Schmidt SD, Yang ZY, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Burton DR, Koff WC, Walker LM, Phogat S, Wyatt R, Orwenyo J, Wang LX, Arthos J, Bewley CA, Mascola JR, Nabel GJ, Schief WR, Ward AB, Wilson IA, Kwong PD. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek K, Eversole T, Capra JD. Conservation of the most JH proximal Ig VH gene segment (VHVI) throughout primate evolution. J Immunol. 1991;146:2434–2438. [PubMed] [Google Scholar]
- Mogensen TH, Melchjorsen J, Larsen CS, Paludan SR. Innate immune recognition and activation during HIV infection. Retrovirology. 2010;7:54. doi: 10.1186/1742-4690-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothe BR, Weinfurter J, Wang C, Rehrauer W, Wilson N, Allen TM, Allison DB, Watkins DI. Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J Virol. 2003;77:2736–2740. doi: 10.1128/JVI.77.4.2736-2740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhl T, Krawczak M, Ten Haaft P, Hunsmann G, Sauermann U. MHC class I alleles influence set-point viral load and survival time in simian immunodeficiency virus-infected rhesus monkeys. J Immunol. 2002;169:3438–3446. doi: 10.4049/jimmunol.169.6.3438. [DOI] [PubMed] [Google Scholar]
- Munk C, Brandt SM, Lucero G, Landau NR. A dominant block to HIV-1 replication at reverse transcription in simian cells. Proc Natl Acad Sci U S A. 2002;99:13843–13848. doi: 10.1073/pnas.212400099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey-Corb M, Martin LN, Rangan SR, Baskin GB, Gormus BJ, Wolf RH, Andes WA, West M, Montelaro RC. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986;321:435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- Myagkikh M, Alipanah S, Markham PD, Tartaglia J, Paoletti E, Gallo RC, Franchini G, Robert-Guroff M. Multiple immunizations with attenuated poxvirus HIV type 2 recombinants and subunit boosts required for protection of rhesus macaques. AIDS Res Hum Retroviruses. 1996;12:985–992. doi: 10.1089/aid.1996.12.985. [DOI] [PubMed] [Google Scholar]
- Nacsa J, Radaelli A, Edghill-Smith Y, Venzon D, Tsai WP, Morghen Cde G, Panicali D, Tartaglia J, Franchini G. Avipox-based simian immunodeficiency virus (SIV) vaccines elicit a high frequency of SIV-specific CD4+ and CD8+ T-cell responses in vaccinia-experienced SIVmac251-infected macaques. Vaccine. 2004;22:597–606. doi: 10.1016/j.vaccine.2003.08.028. [DOI] [PubMed] [Google Scholar]
- Nakayama EE, Shioda T. TRIM5alpha and Species Tropism of HIV/SIV. Front Microbiol. 2012;3:13. doi: 10.3389/fmicb.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz F, Cicala C, Van Ryk D, Block KE, Jelicic K, McNally JP, Ogundare O, Pascuccio M, Patel N, Wei DL, Fauci AS, Arthos J. The Genotype of Early-Transmitting HIV gp120s Promotes alpha(4)beta(7) -Reactivity, Revealing alpha(4)beta(+)(7)/CD4(+) T cells As Key Targets in Mucosal Transmission. Plos Pathogens. 2011;7:e1001301. doi: 10.1371/journal.ppat.1001301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- Newman RM, Hall L, Connole M, Chen GL, Sato S, Yuste E, Diehl W, Hunter E, Kaur A, Miller GM, Johnson WE. Balancing selection and the evolution of functional polymorphism in Old World monkey TRIM5alpha. Proc Natl Acad Sci U S A. 2006;103:19134–19139. doi: 10.1073/pnas.0605838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Shingai M, Willey R, Sadjadpour R, Lee WR, Brown CR, Brenchley JM, Buckler-White A, Petros R, Eckhaus M, Hoffman V, Igarashi T, Martin MA. Generation of the pathogenic R5-tropic simian/human immunodeficiency virus SHIVAD8 by serial passaging in rhesus macaques. J Virol. 2010;84:4769–4781. doi: 10.1128/JVI.02279-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisole S, Lynch C, Stoye JP, Yap MW. A Trim5-cyclophilin A fusion protein found in owl monkey kidney cells can restrict HIV-1. Proc Natl Acad Sci U S A. 2004;101:13324–13328. doi: 10.1073/pnas.0404640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien SJ, Moore JP. The effect of genetic variation in chemokines and their receptors on HIV transmission and progression to AIDS. Immunol Rev. 2000;177:99–111. doi: 10.1034/j.1600-065x.2000.17710.x. [DOI] [PubMed] [Google Scholar]
- O’Connor DH, Allen TM, Vogel TU, Jing P, DeSouza IP, Dodds E, Dunphy EJ, Melsaether C, Mothé B, Yamamoto H, Horton H, Wilson N, Hughes AL, Watkins DI. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat Med. 2002;8:493–499. doi: 10.1038/nm0502-493. [DOI] [PubMed] [Google Scholar]
- O’Connor DH, Mothe BR, Weinfurter JT, Fuenger S, Rehrauer WM, Jing P, Rudersdorf RR, Liebl ME, Krebs K, Vasquez J, Dodds E, Loffredo J, Martin S, McDermott AB, Allen TM, Wang C, Doxiadis GG, Montefiori DC, Hughes A, Burton DR, Allison DB, Wolinsky SM, Bontrop R, Picker LJ, Watkins DI. Major histocompatibility complex class I alleles associated with slow simian immunodeficiency virus disease progression bind epitopes recognized by dominant acute-phase cytotoxic-T-lymphocyte responses. J Virol. 2003;77:9029–9040. doi: 10.1128/JVI.77.16.9029-9040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otting N, Heijmans CM, Noort RC, de Groot NG, Doxiadis GG, van Rood JJ, Watkins DI, Bontrop RE. Unparalleled complexity of the MHC class I region in rhesus macaques. Proc Natl Acad Sci U S A. 2005;102:1626–1631. doi: 10.1073/pnas.0409084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padian NS, Shiboski SC, Glass SO, Vittinghoff E. Heterosexual transmission of human immunodeficiency virus (HIV) in northern California: results from a ten-year study. Am J Epidemiol. 1997;146:350–357. doi: 10.1093/oxfordjournals.aje.a009276. [DOI] [PubMed] [Google Scholar]
- Pal R, Venzon D, Letvin NL, Santra S, Montefiori DC, Miller NR, Tryniszewska E, Lewis MG, VanCott TC, Hirsch V, Woodward R, Gibson A, Grace M, Dobratz E, Markham PD, Hel Z, Nacsa J, Klein M, Tartaglia J, Franchini G. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J Virol. 2002;76:292–302. doi: 10.1128/JVI.76.1.292-302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R, Venzon D, Santra S, Kalyanaraman VS, Montefiori DC, Hocker L, Hudacik L, Rose N, Nacsa JY, ES, Belyakov IM, Berzofsky JA, Washington Parks R, Markham P, Letvin NL, Tartaglia J, Franchini G. Systemic Immunization with an ALVAC-HIV-1/Protein Boost Vaccine Strategy Protects Rhesus Macaques from CD4+ T-Cell Loss and Reduces Both Systemic and Mucosal SHIVKU2 RNA Levels. Journal of Virology. 2006;80:3732–3742. doi: 10.1128/JVI.80.8.3732-3742.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G, Esteban M, Jacobs B, Tartaglia J. Poxvirus vector-based HIV vaccines. Curr Opin HIV AIDS. 2010;5:391–396. doi: 10.1097/COH.0b013e32833d1e87. [DOI] [PubMed] [Google Scholar]
- Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PI, Walker BD, Study TIHC. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330:1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreau M, Welles HC, Harari A, Hall O, Martin R, Maillard M, Dorta G, Bart PA, Kremer EJ, Tartaglia J, Wagner R, Esteban M, Levy Y, Pantaleo G. DNA/NYVAC vaccine regimen induces HIV-specific CD4 and CD8 T-cell responses in intestinal mucosa. J Virol. 2011;85:9854–9862. doi: 10.1128/JVI.00788-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron MJ, Stremlau M, Song B, Ulm W, Mulligan RC, Sodroski J. TRIM5alpha mediates the postentry block to N-tropic murine leukemia viruses in human cells. Proc Natl Acad Sci U S A. 2004;101:11827–11832. doi: 10.1073/pnas.0403364101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilcher CD, Joaki G, Hoffman IF, Martinson FE, Mapanje C, Stewart PW, Powers KA, Galvin S, Chilongozi D, Gama S, Price MA, Fiscus SA, Cohen MS. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS. 2007;21:1723–1730. doi: 10.1097/QAD.0b013e3281532c82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, Hu D, Tappero JW, Choopanya K. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- Price AJ, Marzetta F, Lammers M, Ylinen LM, Schaller T, Wilson SJ, Towers GJ, James LC. Active site remodeling switches HIV specificity of antiretroviral TRIMCyp. Nat Struct Mol Biol. 2009a;16:1036–1042. doi: 10.1038/nsmb.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DA, Asher TE, Wilson NA, Nason MC, Brenchley JM, Metzler IS, Venturi V, Gostick E, Chattopadhyay PK, Roederer M, Davenport MP, Watkins DI, Douek DC. Public clonotype usage identifies protective Gag-specific CD8+ T cell responses in SIV infection. J Exp Med. 2009b;206:923–936. doi: 10.1084/jem.20081127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quakkelaar ED, Redeker A, Haddad EK, Harari A, McCaughey SM, Duhen T, Filali-Mouhim A, Goulet JP, Loof NM, Ossendorp F, Perdiguero B, Heinen P, Gomez CE, Kibler KV, Koelle DM, Sekaly RP, Sallusto F, Lanzavecchia A, Pantaleo G, Esteban M, Tartaglia J, Jacobs BL, Melief CJ. Improved innate and adaptive immunostimulation by genetically modified HIV-1 protein expressing NYVAC vectors. PLoS One. 2011;6:e16819. doi: 10.1371/journal.pone.0016819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi H, Ma ZM, Huang Y, Hodge G, Thomas MA, DiPasquale J, DeSilva V, Fritts L, Bett AJ, Casimiro DR, Shiver JW, Robert-Guroff M, Robertson MN, McChesney MB, Gilbert PB, Miller CJ. Low-dose penile SIVmac251 exposure of rhesus macaques infected with adenovirus type 5 (Ad5) and then immunized with a replication-defective Ad5-based SIV gag/pol/nef vaccine recapitulates the results of the phase IIb step trial of a similar HIV-1 vaccine. J Virol. 2012;86:2239–2250. doi: 10.1128/JVI.06175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe S, Flanders M, Cutler S, Soghoian DZ, Ghebremichael M, Davis I, Lindqvist M, Pereyra F, Walker BD, Heckerman D, Streeck H. HIV-specific CD4 T cell responses to different viral proteins have discordant associations with viral load and clinical outcome. J Virol. 2012;86:277–283. doi: 10.1128/JVI.05577-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann KA, Li JT, Veazey R, Halloran M, Park IW, Karlsson GB, Sodroski J, Letvin NL. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann KA, Parker RA, Seaman MS, Beaudry K, Beddall M, Peterson L, Williams KC, Veazey RS, Montefiori DC, Mascola JR, Nabel GJ, Letvin NL. Pathogenicity of simian-human immunodeficiency virus SHIV-89.6P and SIVmac is attenuated in cynomolgus macaques and associated with early T-lymphocyte responses. J Virol. 2005;79:8878–8885. doi: 10.1128/JVI.79.14.8878-8885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Paris RM, Chunsutthiwat S, Premsri N, Namwat C, Bowonwatanuwong C, Li SS, Kaewkungkal J, Trichavaroj R, Churikanont N, de Souza MS, Andrews C, Francis D, Adams E, Flores J, Gurunathan S, Tartaglia J, O’Connell RJ, Eamsila C, Nitayaphan S, Ngauy V, Thongcharoen P, Kunasol P, Michael NL, Robb ML, Gilbert PB, Kim JH. Extended evaluation of the virologic, immunologic, and clinical course of volunteers who acquiredHIV-1 infection in a phase III vaccine trial of ALVAC-HIV and AIDSVAX(R) B/E. J Infect Dis. 2012 doi: 10.1093/infdis/jis478. [DOI] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Reynolds MR, Rakasz E, Skinner PJ, White C, Abel K, Ma ZM, Compton L, Napoe G, Wilson N, Miller CJ, Haase A, Watkins DI. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J Virol. 2005;79:9228–9235. doi: 10.1128/JVI.79.14.9228-9235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MR, Weiler AM, Piaskowski SM, Piatak M, Jr, Robertson HT, Allison DB, Bett AJ, Casimiro DR, Shiver JW, Wilson NA, Lifson JD, Koff WC, Watkins DI. A trivalent recombinant Ad5 gag/pol/nef vaccine fails to protect rhesus macaques from infection or control virus replication after a limiting-dose heterologous SIV challenge. Vaccine. 2012;30:4465–4475. doi: 10.1016/j.vaccine.2012.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothaeusler K, Ma ZM, Qureshi H, Carroll TD, Rourke T, McChesney MB, Miller CJ. Antiviral antibodies and T cells are present in the foreskin of simian immunodeficiency virus-infected rhesus macaques. J Virol. 2012;86:7098–7106. doi: 10.1128/JVI.00410-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland-Jones S, Pinheiro S, Kaul R. New insights into host factors in HIV-1 pathogenesis. Cell. 2001;104:473–476. doi: 10.1016/s0092-8674(01)00235-5. [DOI] [PubMed] [Google Scholar]
- Royce RA, Sena A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- Saito A, Kono K, Nomaguchi M, Yasutomi Y, Adachi A, Shioda T, Akari H, Nakayama EE. Geographical, genetic and functional diversity of antiretroviral host factor TRIMCyp in cynomolgus macaque (Macaca fascicularis) J Gen Virol. 2012;93:594–602. doi: 10.1099/vir.0.038075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Gonzalez JF, Bailes E, Pham KT, Salazar MG, Guffey MB, Keele BF, Derdeyn CA, Farmer P, Hunter E, Allen S, Manigart O, Mulenga J, Anderson JA, Swanstrom R, Haynes BF, Athreya GS, Korber BT, Sharp PM, Shaw GM, Hahn BH. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J Virol. 2008;82:3952–3970. doi: 10.1128/JVI.02660-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salle B, Brochard P, Bourry O, Mannioui A, Andrieu T, Prevot S, Dejucq-Rainsford N, Dereuddre-Bosquet N, Le Grand R. Infection of macaques after vaginal exposure to cell-associated simian immunodeficiency virus. J Infect Dis. 2010;202:337–344. doi: 10.1086/653619. [DOI] [PubMed] [Google Scholar]
- Sandstrom E, Nilsson C, Hejdeman B, Brave A, Bratt G, Robb M, Cox J, Vancott T, Marovich M, Stout R, Aboud S, Bakari M, Pallangyo K, Ljungberg K, Moss B, Earl P, Michael N, Birx D, Mhalu F, Wahren B, Biberfeld G. Broad immunogenicity of a multigene, multiclade HIV-1 DNA vaccine boosted with heterologous HIV-1 recombinant modified vaccinia virus Ankara. J Infect Dis. 2008;198:1482–1490. doi: 10.1086/592507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago ML, Range F, Keele BF, Li Y, Bailes E, Bibollet-Ruche F, Fruteau C, Noe R, Peeters M, Brookfield JF, Shaw GM, Sharp PM, Hahn BH. Simian immunodeficiency virus infection in free-ranging sooty mangabeys (Cercocebus atys atys) from the Tai Forest, Cote d’Ivoire: implications for the origin of epidemic human immunodeficiency virus type 2. J Virol. 2005;79:12515–12527. doi: 10.1128/JVI.79.19.12515-12527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer SL, Wu LI, Emerman M, Malik HS. Positive selection of primate TRIM5alpha identifies a critical species-specific retroviral restriction domain. Proc Natl Acad Sci U S A. 2005;102:2832–2837. doi: 10.1073/pnas.0409853102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayah DM, Sokolskaja E, Berthoux L, Luban J. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature. 2004;430:569–573. doi: 10.1038/nature02777. [DOI] [PubMed] [Google Scholar]
- Scinicariello F, Engleman CN, Jayashankar L, McClure HM, Attanasio R. Rhesus macaque antibody molecules: sequences and heterogeneity of alpha and gamma constant regions. Immunology. 2004;111:66–74. doi: 10.1111/j.1365-2567.2003.01767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PM, Shaw GM, Hahn BH. Simian immunodeficiency virus infection of chimpanzees. J Virol. 2005;79:3891–3902. doi: 10.1128/JVI.79.7.3891-3902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedlock DJ, Silvestri G, Weiner DB. Monkeying around with HIV vaccines: using rhesus macaques to define ‘gatekeepers’ for clinical trials. Nat Rev Immunol. 2009;9:717–728. doi: 10.1038/nri2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Sakai H, Kawamura M, Tokunaga K, Adachi A. Early replication block of human immunodeficiency virus type 1 in monkey cells. J Gen Virol. 1995;76 (Pt 11):2723–2730. doi: 10.1099/0022-1317-76-11-2723. [DOI] [PubMed] [Google Scholar]
- Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, Huang L, Harris VA, Long RS, Liang X, Handt L, Schleif WA, Zhu L, Freed DC, Persaud NV, Guan L, Punt KS, Tang A, Chen M, Wilson KA, Collins KB, Heidecker GJ, Fernandez VR, Perry HC, Joyce JG, Grimm KM, Cook JC, Keller PM, Kresock DS, Mach H, Troutman RD, Isopi LA, Williams DM, Xu Z, Bohannon KE, Volkin DB, Montefiori DC, Miura A, Krivulka GR, Lifton MA, Kuroda MJ, Schmitz JE, Letvin NL, Caulfield MJ, Bett AJ, Youil R, Kaslow DC, Emini EA. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- Sina ST, Ren W, Cheng-Mayer C. Coreceptor use in nonhuman primate models of HIV infection. J Transl Med. 2011;9(Suppl 1):S7. doi: 10.1186/1479-5876-9-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MZ, Dale CJ, De Rose R, Stratov I, Fernandez CS, Brooks AG, Weinfurter J, Krebs K, Riek C, Watkins DI, O’Connor DH, Kent SJ. Analysis of pigtail macaque major histocompatibility complex class I molecules presenting immunodominant simian immunodeficiency virus epitopes. J Virol. 2005a;79:684–695. doi: 10.1128/JVI.79.2.684-695.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MZ, Fernandez CS, Chung A, Dale CJ, De Rose R, Lin J, Brooks AG, Krebs KC, Watkins DI, O’Connor DH, Davenport MP, Kent SJ. The pigtail macaque MHC class I allele Mane-A*10 presents an immundominant SIV Gag epitope: identification, tetramer development and implications of immune escape and reversion. J Med Primatol. 2005b;34:282–293. doi: 10.1111/j.1600-0684.2005.00126.x. [DOI] [PubMed] [Google Scholar]
- Sobieszczyk ME, Lingappa JR, McElrath MJ. Host genetic polymorphisms associated with innate immune factors and HIV-1. Curr Opin HIV AIDS. 2011;6:427–434. doi: 10.1097/COH.0b013e3283497155. [DOI] [PubMed] [Google Scholar]
- Sodora DL, Gettie A, Miller CJ, Marx PA. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S119–123. [PubMed] [Google Scholar]
- Song B, Gold B, O’Huigin C, Javanbakht H, Li X, Stremlau M, Winkler C, Dean M, Sodroski J. The B30.2(SPRY) domain of the retroviral restriction factor TRIM5alpha exhibits lineage-specific length and sequence variation in primates. J Virol. 2005;79:6111–6121. doi: 10.1128/JVI.79.10.6111-6121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staprans SI, Barry AP, Silvestri G, Safrit JT, Kozyr N, Sumpter B, Nguyen H, McClure H, Montefiori D, Cohen JI, Feinberg MB. Enhanced SIV replication and accelerated progression to AIDS in macaques primed to mount a CD4 T cell response to the SIV envelope protein. Proc Natl Acad Sci U S A. 2004;101:13026–13031. doi: 10.1073/pnas.0404739101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevceva L, Alvarez X, Lackner AA, Tryniszewska E, Kelsall B, Nacsa J, Tartaglia J, Strober W, Franchini G. Both mucosal and systemic routes of immunization with the live, attenuated NYVAC/simian immunodeficiency virus SIV(gpe) recombinant vaccine result in gag-specific CD8(+) T-cell responses in mucosal tissues of macaques. J Virol. 2002;76:11659–11676. doi: 10.1128/JVI.76.22.11659-11676.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stittelaar KJ, Kuiken T, de Swart RL, van Amerongen G, Vos HW, Niesters HG, van Schalkwijk P, van der Kwast T, Wyatt LS, Moss B, Osterhaus AD. Safety of modified vaccinia virus Ankara (MVA) in immune-suppressed macaques. Vaccine. 2001;19:3700–3709. doi: 10.1016/s0264-410x(01)00075-5. [DOI] [PubMed] [Google Scholar]
- Stone M, Keele BF, Ma ZM, Bailes E, Dutra J, Hahn BH, Shaw GM, Miller CJ. A limited number of simian immunodeficiency virus (SIV) env variants are transmitted to rhesus macaques vaginally inoculated with SIVmac251. J Virol. 2010;84:7083–7095. doi: 10.1128/JVI.00481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- Stremlau M, Perron M, Welikala S, Sodroski J. Species-specific variation in the B30.2(SPRY) domain of TRIM5alpha determines the potency of human immunodeficiency virus restriction. J Virol. 2005;79:3139–3145. doi: 10.1128/JVI.79.5.3139-3145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg K, Benveniste RE, Arthur LO, Rabin H, Giddens WE, Jr, Ochs HD, Morton WR, Tsai CC. Characterization of exogenous type D retrovirus from a fibroma of a macaque with simian AIDS and fibromatosis. Science. 1984;224:289–282. doi: 10.1126/science.6200929. [DOI] [PubMed] [Google Scholar]
- Sui Y, Gagnon S, Dzutsev A, Zhu Q, Yu H, Hogg A, Wang Y, Xia Z, Belyakov IM, Venzon D, Klinman D, Strober W, Kelsall B, Franchini G, Berzofsky JA. TLR agonists and/or IL-15 adjuvanted mucosal SIV vaccine reduced gut CD4(+) memory T cell loss in SIVmac251-challenged rhesus macaques. Vaccine. 2011;30:59–68. doi: 10.1016/j.vaccine.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Zhu Q, Gagnon S, Dzutsev A, Terabe M, Vaccari M, Venzon D, Klinman D, Strober W, Kelsall B, Franchini G, Belyakov IM, Berzofsky JA. Innate and adaptive immune correlates of vaccine and adjuvant-induced control of mucosal transmission of SIV in macaques. Proc Natl Acad Sci U S A. 2010;107:9843–9848. doi: 10.1073/pnas.0911932107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Santra S, Schmitz JE, Roederer M, Letvin NL. Magnitude and quality of vaccine-elicited T-cell responses in the control of immunodeficiency virus replication in rhesus monkeys. J Virol. 2008;82:8812–8819. doi: 10.1128/JVI.00204-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Pearce-Pratt R, Phillips DM. Productive infection of a cervical epithelial cell line with human immunodeficiency virus: implications for sexual transmission. J Virol. 1993;67:6447–6452. doi: 10.1128/jvi.67.11.6447-6452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia J, Perkus ME, Taylor J, Norton EK, Audonnet JC, Cox WI, Davis SW, van der Hoeven J, Meignier B, MR, et al. NYVAC: a highly attenuated strain of vaccinia virus. Virology. 1992;188:217–232. doi: 10.1016/0042-6822(92)90752-b. [DOI] [PubMed] [Google Scholar]
- Taylor J, Meignier B, Tartaglia J, Languet B, VanderHoeven J, Franchini G, Trimarchi C, Paoletti E. Biological and immunogenic properties of a canarypox-rabies recombinant, ALVAC-RG (vCP65) in non-avian species. Vaccine. 1995;13:539–549. doi: 10.1016/0264-410x(94)00028-l. [DOI] [PubMed] [Google Scholar]
- Trichel AM, Rajakumar PA, Murphey-Corb M. Species-specific variation in SIV disease progression between Chinese and Indian subspecies of rhesus macaque. J Med Primatol. 2002;31:171–178. doi: 10.1034/j.1600-0684.2002.02003.x. [DOI] [PubMed] [Google Scholar]
- Urvater JA, McAdam SN, Loehrke JH, Allen TM, Moran JL, Rowell TJ, Rojo S, Lopez de Castro JA, Taurog JD, Watkins DI. A high incidence of Shigella-induced arthritis in a primate species: major histocompatibility complex class I molecules associated with resistance and susceptibility, and their relationship to HLA-B27. Immunogenetics. 2000;51:314–325. doi: 10.1007/s002510050625. [DOI] [PubMed] [Google Scholar]
- Vaccari M, Mattapallil J, Song K, Tsai WP, Hryniewicz A, Venzon D, Zanetti M, Reimann KA, Roederer M, Franchini G. Reduced protection from simian immunodeficiency virus SIVmac251 infection afforded by memory CD8+ T cells induced by vaccination during CD4+ T-cell deficiency. J Virol. 2008;82:9629–9638. doi: 10.1128/JVI.00893-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari M, Trindade CJ, Venzon D, -Zanetti M, Franchini G. Vaccine-induced CD8+ central memory T cells in protection from simian AIDS. J Immunol. 2005;175:3502–3507. doi: 10.4049/jimmunol.175.6.3502. [DOI] [PubMed] [Google Scholar]
- Valentine LE, Loffredo JT, Bean AT, Leon EJ, MacNair CE, Beal DR, Piaskowski SM, Klimentidis YC, Lank SM, Wiseman RW, Weinfurter JT, May GE, Rakasz EG, Wilson NA, Friedrich TC, O’Connor DH, Allison DB, Watkins DI. Infection with “escaped” virus variants impairs control of simian immunodeficiency virus SIVmac239 replication in Mamu-B*08-positive macaques. J Virol. 2009;83:11514–11527. doi: 10.1128/JVI.01298-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rompay KK, Abel K, Lawson JR, Singh RP, Schmidt KA, Evans T, Earl P, Harvey D, Franchini G, Tartaglia J, Montefiori D, Hattangadi S, Moss B, Marthas ML. Attenuated poxvirus-based simian immunodeficiency virus (SIV) vaccines given in infancy partially protect infant and juvenile macaques against repeated oral challenge with virulent SIV. J Acquir Immune Defic Syndr. 2005;38:124–134. doi: 10.1097/00126334-200502010-00002. [DOI] [PubMed] [Google Scholar]
- VandeWoude S, Apetrei C. Going wild: lessons from naturally occurring T-lymphotropic lentiviruses. Clin Microbiol Rev. 2006;19:728–762. doi: 10.1128/CMR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verthelyi D, Kenney RT, Seder RA, Gam AA, Friedag B, Klinman DM. CpG oligodeoxynucleotides as vaccine adjuvants in primates. J Immunol. 2002;168:1659–1663. doi: 10.4049/jimmunol.168.4.1659. [DOI] [PubMed] [Google Scholar]
- Virgen CA, Kratovac Z, Bieniasz PD, Hatziioannou T. Independent genesis of chimeric TRIM5-cyclophilin proteins in two primate species. Proc Natl Acad Sci U S A. 2008;105:3563–3568. doi: 10.1073/pnas.0709258105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Bergmeier LA, Stebbings R, Seidl T, Whittall T, Singh M, Berry N, Almond N, Lehner T. Mucosal immunization in macaques upregulates the innate APOBEC 3G anti-viral factor in CD4(+) memory T cells. Vaccine. 2009;27:870–881. doi: 10.1016/j.vaccine.2008.11.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Lehner T. Induction of innate immunity in control of mucosal transmission of HIV. Current Opinion in Hiv and Aids. 2011;6:398–404. doi: 10.1097/COH.0b013e3283499df7. [DOI] [PubMed] [Google Scholar]
- Weiler AM, Li Q, Duan L, Kaizu M, Weisgrau KL, Friedrich TC, Reynolds MR, Haase AT, Rakasz EG. Genital ulcers facilitate rapid viral entry and dissemination following intravaginal inoculation with cell-associated simian immunodeficiency virus SIVmac239. J Virol. 2008;82:4154–4158. doi: 10.1128/JVI.01947-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille-Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci U S A. 2005;102:15190–15194. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NA, Reed J, Napoe GS, Piaskowski S, Szymanski A, Furlott J, Gonzalez EJ, Yant LJ, Maness NJ, May GE, Soma T, Reynolds MR, Rakasz E, Rudersdorf R, McDermott AB, O’Connor DH, Friedrich TC, Allison DB, Patki A, Picker LJ, Burton DR, Lin J, Huang L, Patel D, Heindecker G, Fan J, Citron M, Horton M, Wang F, Liang X, Shiver JW, Casimiro DR, Watkins DI. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J Virol. 2006;80:5875–5885. doi: 10.1128/JVI.00171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Webb BL, Maplanka C, Newman RM, Verschoor EJ, Heeney JL, Towers GJ. Rhesus macaque TRIM5 alleles have divergent antiretroviral specificities. J Virol. 2008a;82:7243–7247. doi: 10.1128/JVI.00307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Webb BL, Ylinen LM, Verschoor E, Heeney JL, Towers GJ. Independent evolution of an antiviral TRIMCyp in rhesus macaques. Proc Natl Acad Sci U S A. 2008b;105:3557–3562. doi: 10.1073/pnas.0709003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Gao F, Liu J, Qi J, Gostick E, Price DA, Gao GF. Structural basis of diverse peptide accommodation by the rhesus macaque MHC class I molecule Mamu-B*17: insights into immune protection from simian immunodeficiency virus. J Immunol. 2011;187:6382–6392. doi: 10.4049/jimmunol.1101726. [DOI] [PubMed] [Google Scholar]
- Xiao P, Patterson LJ, Kuate S, Brocca-Cofano E, Thomas MA, Venzon D, Zhao J, DiPasquale J, Fenizia C, Lee EM, Kalisz I, Kalyanaraman VS, Pal R, Montefiori D, Keele BF, Robert-Guroff M. Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIV(mac251) challenge. J Virol. 2012;86:4644–4657. doi: 10.1128/JVI.06812-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao P, Zhao J, Patterson LJ, Brocca-Cofano E, Venzon D, Kozlowski PA, Hidajat R, Demberg T, Robert-Guroff M. Multiple vaccine-elicited non-neutralizing anti-envelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following SHIV89.6P challenge in rhesus macaques. J Virol. 2010;14:7161–7173. doi: 10.1128/JVI.00410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yant LJ, Friedrich TC, Johnson RC, May GE, Maness NJ, Enz AM, Lifson JD, O’Connor DH, Carrington M, Watkins DI. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J Virol. 2006;80:5074–5077. doi: 10.1128/JVI.80.10.5074-5077.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap MW, Nisole S, Lynch C, Stoye JP. Trim5alpha protein restricts both HIV-1 and murine leukemia virus. Proc Natl Acad Sci U S A. 2004;101:10786–10791. doi: 10.1073/pnas.0402876101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh WW, Rao SS, Lim SY, Zhang J, Hraber PT, Brassard LM, Luedemann C, Todd JP, Dodson A, Shen L, Buzby AP, Whitney JB, Korber BT, Nabel GJ, Mascola JR, Letvin NL. The TRIM5 gene modulates penile mucosal acquisition of simian immunodeficiency virus in rhesus monkeys. J Virol. 2011;85:10389–10398. doi: 10.1128/JVI.00854-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Jones B, Hu N, Chang H, Ahmad S, Liu J, Parrington M, Ostrowski M. Comparative analysis of tropism between canarypox (ALVAC) and vaccinia viruses reveals a more restricted and preferential tropism of ALVAC for human cells of the monocytic lineage. Vaccine. 2006;24:6376–6391. doi: 10.1016/j.vaccine.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Zhang ZQ, Fu TM, Casimiro DR, Davies ME, Liang X, Schleif WA, Handt L, Tussey L, Chen M, Tang A, Wilson KA, Trigona WL, Freed DC, Tan CY, Horton M, Emini EA, Shiver JW. Mamu-A*01 allele-mediated attenuation of disease progression in simian-human immunodeficiency virus infection. J Virol. 2002;76:12845–12854. doi: 10.1128/JVI.76.24.12845-12854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Lai L, Amara RR, Montefiori DC, Villinger F, Chennareddi L, Wyatt LS, Moss B, Robinson HL. Preclinical studies of human immunodeficiency virus/AIDS vaccines: inverse correlation between avidity of anti-Env antibodies and peak postchallenge viremia. J Virol. 2009;83:4102–4111. doi: 10.1128/JVI.02173-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


