Abstract
Background
Hepatitis C is a global public health problem. As many as 12 million people may be chronically infected in India and most are unaware of it.
Aims
To determine the incidence of hepatitis C in the Ratia block of the Fatehabad district, Haryana, India.
Method
This cross-sectional study was carried out by house-tohouse visits over 2 weeks. After obtaining written consent, a blood sample was drawn from suspected cases by a laboratory technician maintaining all necessary safety precautions and sterilization.
Results
Of the samples, 1,630 (22.3 per cent) were found to be positive for hepatitis C by ELISA, 253 (15.5 per cent) patients were previously hepatitis C positive, and adults (21–60 years) were affected maximally (70.0 per cent).
Conclusion
The study emphasises the need for public awareness campaigns at various levels and prevention of HCV infection. It also suggests the need to develop and strengthen evaluation methodology for the Integrated Disease Surveillance Project (IDSP).
Keywords: Hepatitis C, HCV, community
What this study adds:
-
What is known about this subject?
As many as 12 million people in India may be infected with hepatitis C, but most are unaware of it.
-
What new information is offered in this study?
This was an extensive study undertaken to find the prevalence of hepatitis C in a rural block, which covered more than 200,000 people. Lack of awareness coupled with the unscrupulous practices of healthcare providers have led to an alarming 22.6 per cent of the population sample being infected with the hepatitis C virus (HCV).
-
What are the implications for research, policy or practice?
This study highlights the urgent need to implement preventive measures to control the further spread of HCV infection among the rural community of Haryana, India. Public awareness campaigns are needed.
Background
Globally, 3–4 million people are infected with the hepatitis C virus every year. About 150 million people are chronically infected with hepatitis C virus and are at risk of developing liver cirrhosis and/or liver cancer. More than 350,000 people die every year from hepatitis C-related liver diseases.1 The WHO South-East Asia Region has about 30 million hepatitis C carriers, which is more than 1.6 per cent of the total population. More than 120,000 people in the region are estimated to die each year due to cirrhosis and liver cancer associated with hepatitis C.2 The countries with the high rates of prevalence include Egypt (22 per cent), Pakistan (4.8 per cent), and China (3.2 per cent). The main mode of transmission in these countries is attributed to the use of unsafe syringes and needles.1
In India, several studies on voluntary or mixed donors have noted prevalence below 2 per cent.3-8 About 12 million people may be chronically infected in India and most are unaware.9 The common modalities of the spread of hepatitis C infection are blood transfusions, injection drug use, unsafe therapeutic injections, and healthcare-related procedures. In developed countries, the predominant cause of hepatitis C infection is intravenous drug use, whereas in India blood transfusions and unsafe therapeutic injections are the predominant ways of transmitting hepatitis C. Hepatitis C infection does not occur in animals. No vaccine is available against hepatitis C.
Most descriptions of global HCV epidemiology rely heavily upon HCV sero-prevalence studies. These studies are typically cross-sectional in design and are done in a selected population, for instance, blood donors or patients with chronic liver disease, which are not a true representation of the community or region in which they reside. Populationbased studies representative of an entire community are far more useful and accurate in capturing the real scenario of the disease. Different HCV genomes have been isolated from different geographical regions and the HCV has been classified into six major genotypes (genotype 1–6). Although the genotype of the virus does not influence disease presentation or severity, it is a major predictor of the response to antiviral therapy. Most of the Indian studies revealed that genotype 3 predominates in the north, east and west India, whereas genotype 1 is common in south India. The epidemiology of hepatitis C in India has not been studied systematically. Most of the studies of the prevalence of hepatitis C have been based in blood banks, with the assumption that blood donors are surrogates for the population at large. However, this assumption may be incorrect.
A number of hepatitis C patients were referred to Pt B D Sharma PGIMS, Rohtak from the Community Health Centre (CHC), Ratia block, district Fatehabad, Haryana, India, in 2011. This study was planned to assess the incidence of hepatitis C in that block.
Method
The study was an epidemiology-based, cross-sectional type conducted in the Ratia block of district Fatehabad, Haryana, India, by house-to-house visits over two weeks (13 February to 25 February 2012). This block with a population of 218,410 has 17 wards, 3 colonies, 1 community health centre, 2 primary health centers, 27 subcenters, 78 villages, and 194 Anganwadi centers (AWCs). The team involved consisted of faculty members from the Department of Community Medicine, General Medicine and Microbiology, 3 senior residents, 27 junior residents and interns from the Department of Community Medicine Pt. B D Sharma PGIMS, Rohtak. The Director General of Health Services, Haryana, provided 24 trained laboratory technicians, multipurpose health workers (MPHW), Anganwadi workers, accredited social health activists (ASHA), voluntary workers, and logistics support.
The working definition of a suspected hepatitis C case for the survey was: any person having nausea, vomiting, anorexia, malaise, extreme fatigue, right upper quadrant tenderness, flu-like symptoms, and dark urine. A pre-tested semi-structured questionnaire was developed for interviewing the suspected hepatitis C cases. Twenty-four teams, each consisting of one doctor, one laboratory technician, one MPHW male or female, one Anganwadi worker, and one voluntary worker were made.
The doctors were trained to interview the suspected cases and the laboratory technicians were trained to take a blood sample in a vacuum container. After explaining the nature of investigation, all the suspected and previous positive cases were interviewed by the doctor. Written consent was taken and a blood sample was drawn from each by the laboratory technician, maintaining all necessary safety precautions and sterilization.
All the blood samples collected were transported to CHC, Ratia on the same day. Maintenance of the cold chain was ensured. They were subjected to a one-step rapid card test for qualitative detection of antibodies to hepatitis C virus in the serum. This one-step rapid card test device has a sensitivity of 95 per cent. The positive samples were transported to Pt B D Sharma PGIMS, Rohtak, Haryana, for an ELISA test for further confirmation. Ethical clearance was obtained before the start of this survey.
Results
In the present survey, a total of 7,533 samples were collected from suspected and previously positive cases of hepatitis C. The sample positivity rate of HCV was found to be 25.3 per cent (1,912/7,533) by the HCV one-step device/card device, while 22.6 per cent (1,630/7,533) of the samples were positive in the ELISA test.
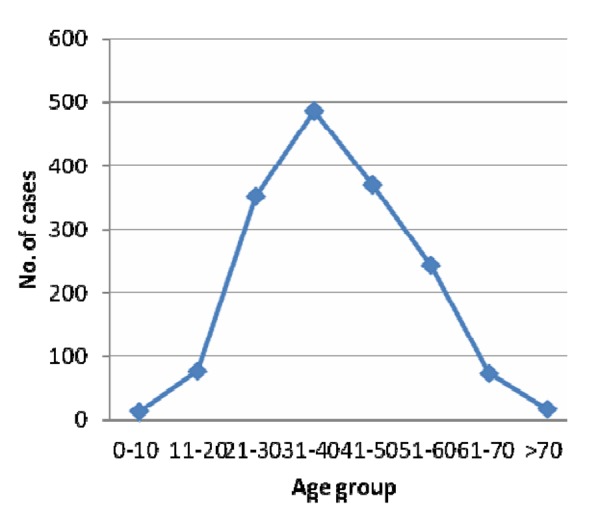
The present study showed that the maximum number of hepatitis C cases, 486 (29.8 per cent), were in the age group 31–40 years and only 0.8 per cent of cases were in the 0–10 year age group. A total of 1,202/1,630 (74.0 per cent) were infected with HCV in the age group 20–60 years (Table 1 and Figure 1). More than half of the cases were male, that is, 938 (57.5 per cent).
Table 1. Age-wise distribution of ELISA positive hepatitis C cases.
| Age group (years) | Number (per cent) |
|---|---|
| 0–10 | 13 (0.8) |
| 11–20 | 76 (4.7) |
| 21–30 | 351 (21.5) |
| 31–40 | 486 (29.8) |
| 41–50 | 370 (22.7) |
| 51–60 | 244 (15.0) |
| 61–70 | 73 (4.5) |
| >70 | 17 (1.0) |
| Total | 1,630 (100) |
Figure 1. Line diagram showing age-wise distribution of cases.
Fatigability/pain in the legs was a complaint of 1,120 (68.7 per cent) patients followed by abdominal pain (46.4 per cent), while nearly one-third of patients complained of nausea, vomiting, and anorexia. Symptoms like malaise and body ache were found among 14.4 per cent of subjects in this study (Table 2).
Table 2. Symptoms-wise distribution of cases (n=1,630).
| Symptoms* | No. of cases | Percent |
|---|---|---|
| Pain in limbs/fatigability | 1,120 | 68.7 |
| Abdominal pain | 756 | 46.4 |
| Nausea, vomiting & anorexia | 500 | 30.7 |
| Malaise & body ache | 234 | 14.4 |
| Flu like symptoms | 86 | 5.3 |
| Jaundice | 30 | 1.8 |
* Multiple responses
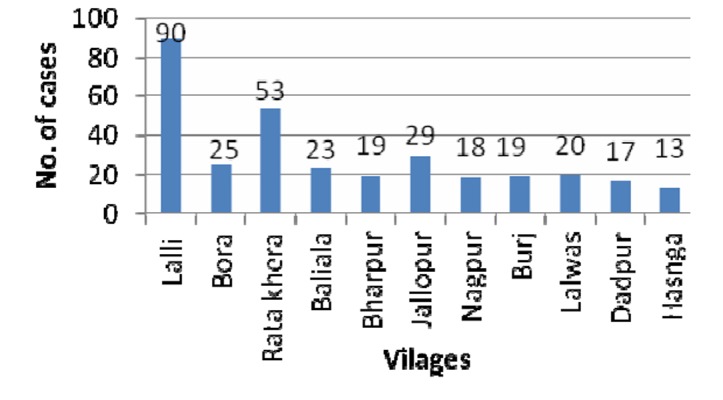
Figure 2 shows the ward-wise distribution of positive cases in the urban area of the block. Wards 7 and 2 showed the maximum number of cases, 45 and 41, respectively. Figure 3 shows village-wise distribution of positive cases. The highest number of positive cases, 90, was found in the village Lalli, while Ratakhera, Jallopur, and Baliala showed 53, 29, and 23 cases, respectively.
Figure 2. Bar diagram showing ward-wise distribution of cases.
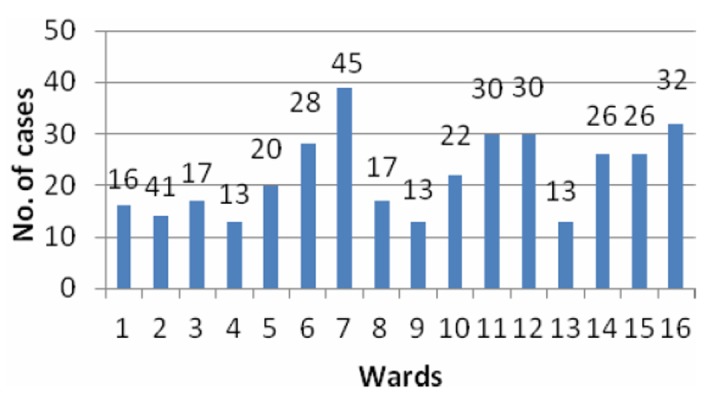
Figure 3. Bar diagram showing village-wise distribution of positive cases.
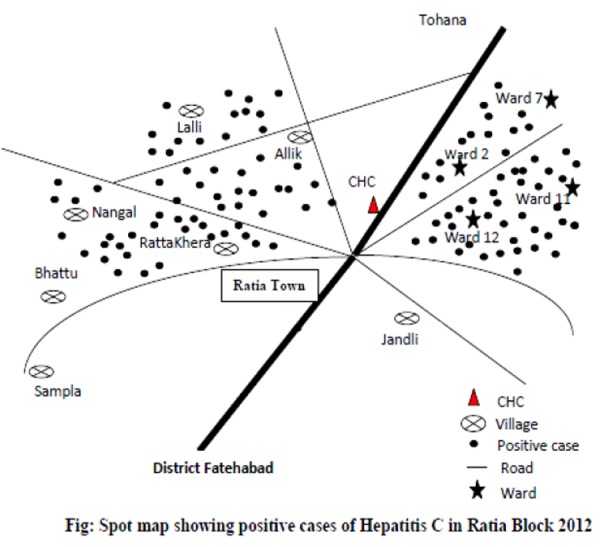
Clustering of positive cases was found around Wards 2, 7, 11, 12, and 16. Similarly, the clustering of positive cases was seen around the villages of Nangal, Ratankhera, Jallopur, and Bora as shown in the spot map (Figure 4).
Figure 4. Spot map, showing distribution of positive cases in Ratia Block.
The study also revealed that 1,146 people (70.3 per cent) had a history of injection as a mode of treatment from the local private practitioner, 1,088 (66.7 per cent) had a history of dental treatment, 5.6 per cent had blood transfusions, and 4 patients had a history of dialysis. None of the patients had organ transplantation.
Discussion
The authors reviewed literature about the prevalence of HCV in the general population of India, but could not find any large community-based epidemiological study carried out in India. In the present study, 22.3 per cent of samples were found to be positive for hepatitis C with ELISA, while 15.5 per cent of patients were previously hepatitis C positive. Previous HCV prevalence data had been derived mostly from blood donors in India.10-11
However, blood donors, as an epidemiologic database, represent a skewed population sample because of the lack of participation by children and senior citizens and underrepresentation of women. Therefore, available data does not effectively represent the general population. There are very few population-based studies conducted in India covering such a huge population.
One of the most systematic population-based studies has been reported from West Bengal, where 3,579 individuals were selected from a population of 10,737 from nine villages. HCV was detected in 26 patients by ELISA among 2,973 patients who were finally willing to participate. A total of 21 patients were finally true positive by PCR (0.71 per cent).12 In the present study, we covered a population of 218,410 of the block, by house-to-house visits to investigate the prevalence of hepatitis C in the block and found a sample positivity rate of 22.3 per cent (1,630/7,533). The researchers observed the practice of local private medical and dental practitioners and found that most of them were using unsterilised needles and syringes. Most of the patients (76 per cent) had a history of treatment/injections from these doctors in the last 1.5–6 months. Thakral et al. highlighted an important fact that percutaneous exposure through minor routes of transmission like multiple use of unsafe injections and procedures by private practitioners and dental surgeons, respectively, sharing of shaving kits, and visiting roadside barbers have played an important role in HCV transmission in our blood donors.13 Contaminated needles and syringes appears to be the major risk factor for HCV infection in the most populous countries in the world. The prevalence study among both pediatric and elderly Taiwanese populations showed the therapeutic injections were also associated with HCV infection.14
In the present study, the most common symptom was fatigue in 68.7 per cent of patients, followed by abdominal pain (46.4 per cent), while nearly half of the patients complained of nausea, vomiting, and anorexia. Symptoms like malaise and body ache were found among 14.4 per cent of patients in this study. Merican et al. 15 conducted a study among 102 patients in a liver unit and found that the most common symptom was fatigability in 35 per cent of subjects. Other complaints like body ache, fever, jaundice, dark urine, nausea, vomiting, loss of appetite, flu-like symptoms, and abdominal pain were also commonly reported by other studies.16-17 Our study also shows that men were infected more than women. Similar observations were also revealed by other studies.18-20
Our study also revealed that the adults in the age group of 21–60 years were maximally affected (70.0 per cent). Similar results have been reported by other studies.4 This could be due to the fact that people in this age group have to earn a living for their families and prefer injectables for faster recovery whereas other age groups avoid injectables as they are afraid of them.
The spot map shows the clustering of cases around Wards 2, 7, 11, 12, and 16, and the villages of Nangal, Khairpur, Jalopur, and Baliala. The study also revealed that 70.3 per cent had a history of injection as a mode of treatment from the local private practitioner, and 66.7 per cent had a history of dental treatment. We observed their practices and found that they were using unsterilised needles, syringes, and equipment. The authors assume a temporal association between use of unsterilised needles and syringes with huge number of hepatitis C cases in this block. In this study, 14.1 per cent (230/1,630) of patients were already diagnosed with hepatitis C and most patients carried genotype 3 of HCV. Based on the available clinical, epidemiological, and laboratory investigation reports, it was confirmed that the outbreak was due to hepatitis C viral infection. Because of constraints of funds, one of the limitations of our study is that these cases could not be investigated for viral load and genotype.
Conclusion
Hepatitis C is an emerging infection and one of the most common causes of chronic liver disease worldwide. The study found that the major mode of HCV transmission was improper sterilization and reuse of needles/syringes. The following recommendations should be followed for the prevention and control of HCV infection in India:
Organise public awareness and health education campaigns targeting healthcare providers, private practitioners, and the public.
Prevent HCV infection by thorough sterilization and disposable injections, and reduce opportunities for percutaneous exposure to blood.
Develop a national curriculum: a general curriculum in schools and colleges to explain and avoid exposure to HCV; and a professional curriculum to upgrade knowledge about prevention of HCV transmission among medical, dental, nursing, and pharmacy students, as well as medical and dental assistants.
Develop and strengthen evaluation methodology for Integrated Disease Surveillance Project (IDSP).
ACKNOWLEDGEMENTS
We sincerely thank the Director General of Health Services, Haryana, India; Vice Chancellor, Pt B D Sharma UHS, Rohtak, Haryana, India; Director, Pt B D Sharma PGIMS, Rohtak, Haryana, India; Sr. Prof and Head, Dr Pardeep Khanna, Department of Community Medicine, Pt B D Sharma PGIMS, Rohtak, Haryana, India, to carry out this difficult task.
Footnotes
PEER REVIEW
Not commissioned. Externally peer reviewed
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
FUNDING
This research was supported by the Director General of Health Services, Haryana, India.
ETHICS COMMITTEE APPROVAL
Approval was obtained from Director General of Health Services, Haryana, India, to carry out this study.
Please cite this paper as: Verma R, Behera B K, Jain R B, Arora V, Chayal V, Gill P S. Hepatitis C: A silent threat to the community of Haryana, India: A community based study. AMJ 2014, 7, 1, 11-16. http//dx.doi.org/10.4066/AMJ.2014.1883
References
- 1.World Health Organisation. Hepatitis C Fact Sheet No.164, July 2013. Available from: http://www.who.int/mediacentre/factsheets/fs164/en/. [Google Scholar]
- 2.World Health Organization, Regional Office for South-East Asia. Viral Hepatitis in the WHO South-East Asia Region, New Delhi; 2011 [Google Scholar]
- 3.Uppal Y, Garg S, Malhotra S, Singh MM, Gupta VK, Mishra B, Singh SV. Hepatitis B and C virus infection in an urban slum of Northern India. J Commun Dis. 2009 Sep;41(3):201–204. [PubMed] [Google Scholar]
- 4.Siddiqui FA, Akhtar K, Sherwani RK, Rehman K, Alam F, Ansar AI. Prevalence of Hepatitis C Virus in Aligarh: A Seven Year Experience. Indian J Community Med. 2009 Jul;34(3):264–5. doi: 10.4103/0970-0218.55299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharya P, Chandra PK, Datta S, Banerjee A, Chakraborty S, Rajendran K, Basu SK, Bhattacharya SK, Chakravarty R. Significant increase in HBV, HCV, HIV and syphilis infections among blood donors in West Bengal, Eastern India 2004–2005: exploratory screening reveals high frequency of occult HBV infection. World J Gastroenterol. 2007 Jul 21;13(27):3730–3. doi: 10.3748/wjg.v13.i27.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acharya SK, Madan K, Dattagupta S, Panda SK. Viral hepatitis in India. Natl Med J India. 2006 Jul-Aug;19(4):203–17. [PubMed] [Google Scholar]
- 7.Gupta N, Kumar V, Kaur A. Seroprevalence of HIV, HBV, HCV and syphilis in voluntary blood donors. Indian J Med Sci. 2004;58:255–7. [PubMed] [Google Scholar]
- 8.Sonwane BR, Birare SD, Kulkarni PV. Prevalence of seroreactivity among blood donors in rural population. Indian J Med Sci. 2003;57:405–7. [PubMed] [Google Scholar]
- 9.Proceedings of the International Workshop on Epidemiology, Diagnosis and Management of Hepatitis C Infection. Medicine and the Community. 1996;6-7:1–32. [Google Scholar]
- 10.Sonwane BR, Birare SD, Kulkarni PV. Prevalence of seroreactivity among blood donors in rural population. Indian J Med Sci. 2003;57:405–7. [PubMed] [Google Scholar]
- 11.Chandrasekaran S, Palaniappan N, Krishnan V, Mohan G, Chandrasekaran N. Relative prevalence of hepatitis B viral markers and hepatitis C virus antibodies (anti HCV) in Madurai, south India. Indian J Med Sci. 2000;54:270–3. [PubMed] [Google Scholar]
- 12.Chowdhury A, Santra A, Chaudhuri S, Dhali GK, Chaudhuri S, Maity SG, Naik TN, Bhattacharya SK, Mazumder DN. Hepatitis C virus infection in the general population: a community-based study in West Bengal, India. Hepatology. 2003;37:802–9. doi: 10.1053/jhep.2003.50157. [DOI] [PubMed] [Google Scholar]
- 13.Thakral B, Marwaha N, Chawla YK, Saluja K, Sharma A, Sharma RR. et al. Prevalence & significance of hepatitis C virus (HCV) seropositivity in blood donors. Indian J Med Res. 2006;124:431–8. [PubMed] [Google Scholar]
- 14.Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis. 2005;9:383–98. doi: 10.1016/j.cld.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Lin CC, Hwang SJ, Chiou ST, Kuan CL, Chen LW, Lee TC, Lee MB, Lee HH, Hsu PS, Tsai ST. The prevalence and risk factors analysis of serum antibody to hepatitis C virus in the elders in northeast Taiwan. J Chin Med Assoc. 2003;66:103–08. [PubMed] [Google Scholar]
- 16.Seeff LB, Hoofnagle JH. Appendix: The National Institutes of Health Consensus Development Conference Management of Hepatitis C, 2002. Clin Liver Dis. 2003;7:261–87. doi: 10.1016/s1089-3261(02)00078-8. [DOI] [PubMed] [Google Scholar]
- 17.Wasley A, Alter MJ. Epidemiology of hepatitis C: Geographic differences and temporal trends. Semin Liver Dis. 2000;20:1–16. doi: 10.1055/s-2000-9506. [DOI] [PubMed] [Google Scholar]
- 18.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436–41. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okayama A, Stuver SO, Tabor E. Incident hepatitis C virus infection in a community-based population in Japan. J Viral Hepat. 2002;9:43–51. doi: 10.1046/j.1365-2893.2002.00331.x. [DOI] [PubMed] [Google Scholar]
- 20.Deshpande A, Kumar A, Khodaiji S, Gupta AD. Prevalence of hepatitis C virus antibody in healthy blood donors. Indian J Hemat Blood Transf. 1998;16:71–2. cited in http://icmr.nic.in/ijmr/2006/october/1010.pdf. [Google Scholar]


