Abstract
Biliary cystadenomas are cystic hepatic tumours of biliary origin. Cystadenomas are often slow-growing benign tumours, but always harbour the risk of malignant transformation. Cystadenomas are often asymptomatic, but may present with abdominal pain and distension. Though suspected with cross-sectional abdominal imaging, definitive diagnosis almost always requires histology. Spontaneous rupture of cystadenoma had been reported three times in the medical literature to date, all presenting with peritonitis. Here we report a case of spontaneous intraperitoneal rupture of biliary cystadenoma presenting as ascites without peritonitis.
Keywords: Nonparasitic hepatic cyst, abdominal pain, cystic neoplasm, leaking cyst
Implications for Practice
-
What is known about this subject?
Biliary cystadenomas are uncommon cystic neoplasms of the liver. Spontaneous rupture is an exceedingly uncommon complication of biliary cystadenoma and has been reported in medical literature only three times to date.
-
What is the key finding in this case report?
Spontaneous rupture of biliary cystadenoma presenting as ascites without evidence of peritonitis.
-
What are the implications for future practice?
Though rare, spontaneous rupture can be a potential complication of biliary cystadenoma. Lack of peritonitis might not always rule out the possibility of spontaneous rupture of nonparasitic hepatic cysts, including biliary cystadenoma.
Background
Biliary cystadenoma is an uncommon hepatic tumour most often reported in middle-aged Caucasian females.1–3 Spontaneous rupture of biliary cystadenoma has been reported in the literature presenting as acute peritonitis. Here we report a case of spontaneous intraperitoneal rupture of biliary cystadenoma presenting as ascites without peritonitis, which to the best of our knowledge is the first to be reported in medical literature.
Case details
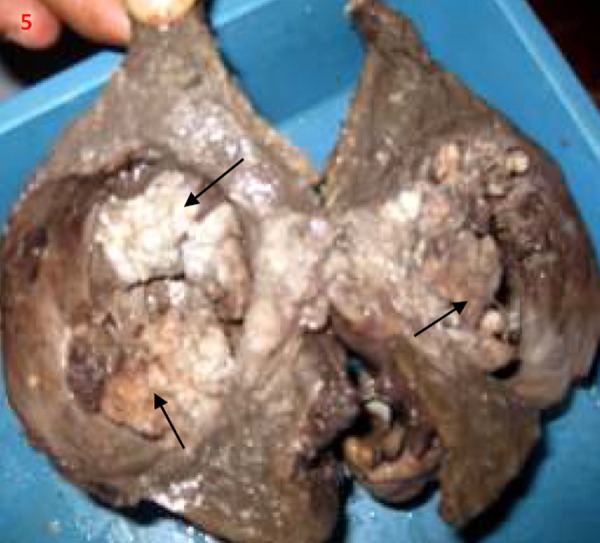
A 46-year-old male presented with dull aching abdominal pain in the right upper quadrant of 1-month duration followed 1 week later by abdominal distension. He had no recent abdominal trauma or falls. Examination was unremarkable except for a palpable liver and ascites. Haemogram, electrolytes, and amylase were within normal limits. Liver function tests; bilirubin 1.1mg% (normal, 0.3-1.3 mg%), albumin 3.5gm% (normal, 4-5 gm%), alanine aminotransferase 52 IU/L (normal, 7-41 IU/L), aspartate aminotransferase 97 IU/L (normal, 12-38 IU/L), and alkaline phosphate 250 IU/L (normal, 20-140 IU/L). Ultrasound (USG) abdomen revealed 2 large cystic lesions in the left lobe of the liver with well-defined walls and internal echoes as well as ascites. Diagnostic paracentesis yielded clear but slightly viscid fluid. Ascitic fluid analysis: protein 4.8gm/dl, albumin 2.8gm/dl, and total lymphocyte count of 80 cells/mm3 lymphocytes only. Ascitic fluid cytology was negative while adenosine deaminase, lactate dehydrogenase, bilirubin, and glucose levels were normal. Amebic and hydatid serology were negative. Contrast-enhanced computerized tomography (CECT) abdomen revealed a predominantly cystic lesion (6.2x7.8cm) with enhancing papillary projections in the left hepatic lobe, another cystic lesion adjacently and ascites with a normal liver as well as pancreas (Figures 1– 4). CA 19.9 was grossly elevated (1,585 U/ml), while alfa fetoprotein was normal. The patient was subjected to laparotomy and left hepatectomy. A large multiloculated hepatic cystic lesion with rupture and free peritoneal fluid was noted at surgery (Figure 5). The rest of the abdominal viscera was normal and there were no peritoneal implants. Histopathology revealed biliary cystadenoma with no evidence of malignant transformation (Figures 6 and 7). The patient improved subsequently, discharged in a stable state and has been on regular followup since then.
Figures 1–4. Contrast-enhanced computerised tomography abdomen transverse (1-3) and coronal images (4); showing predominantly cystic left hepatic lobe lesions (thick arrow) with mural nodules/papillary projections (thin arrow).
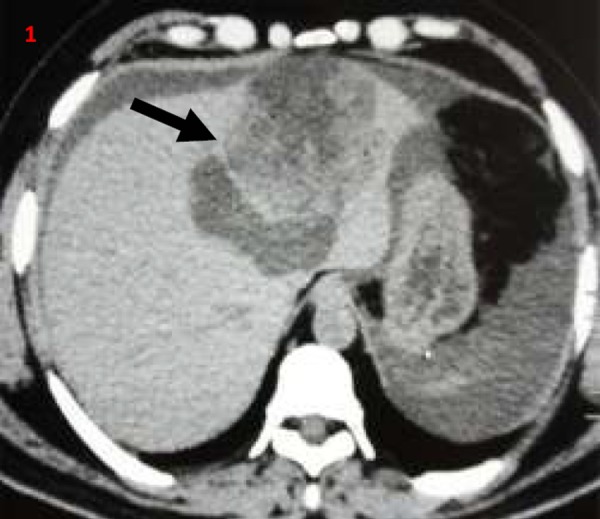
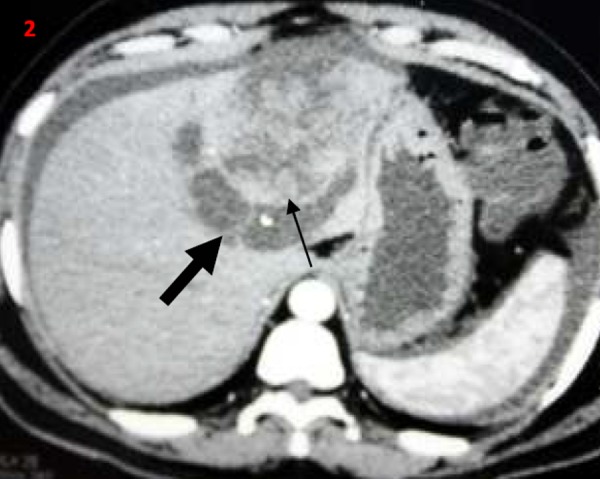
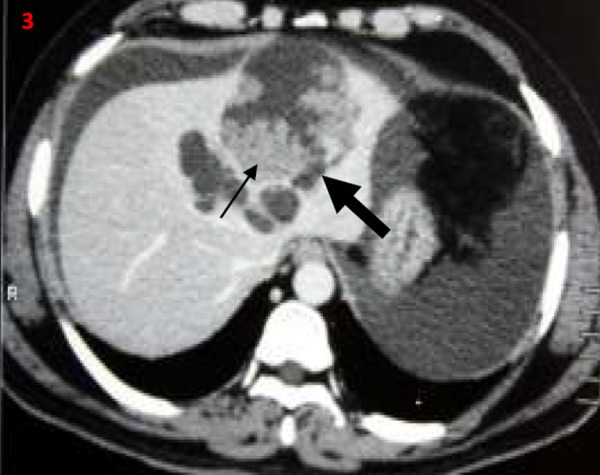
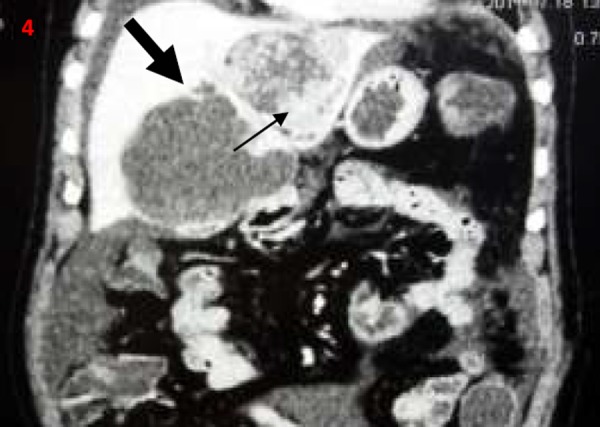
Figure 5. Postoperative gross specimen showing cut section through the cyst with papillary projections/mural nodules (thin arrows).
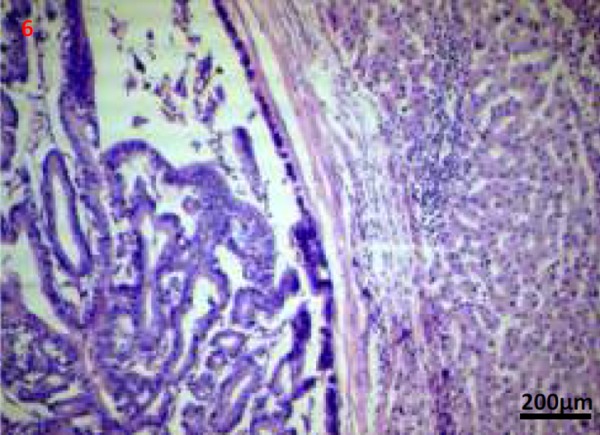
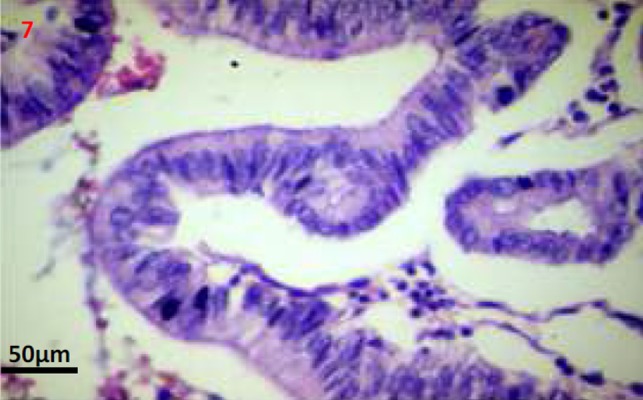
Figures 6, 7. Photomicrographs (Haematoxylin and eosin stain; 50x and 200x images) shows a well encapsulated cystic neoplasm with papillary projections lined by single layered epithelium with basement membrane and mesenchymal stroma. There is no invasion into the underlying stroma, no nuclear atypia and only scant mitosis.
Discussion
Cystic lesions of the liver often pose a diagnostic challenge to the clinician. Broadly, cystic lesions of the liver may be classified as developmental (includes simple hepatic cysts, polycystic liver disease, biliary hamartomas, carolis disease), neoplastic (includes biliary cystadenomas or cystadenocarcinomas, cystic hepatocellular carcinoma, cystic hepatic metastasis), inflammatory (includes abscess, hydatid cyst), and miscellaneous (includes pseudocyst, biloma, haematoma).1
Biliary cystadenomas are rare, slow-growing tumours and constitute around 5 per cent of cystic liver lesions.2 Biliary cystadenomas have a female predilection and are considered premalignant lesions.1–3 The tumour involves the right lobe (55 per cent) more than the left lobe (29 per cent).1,2 The fluid content in the tumour is often proteinaceous or mucinous but occasionally haemorrhagic or purulent.1–3 Although of biliary origin, the tumour rarely communicates with the biliary tree.4 Biliary cystadenomas are often asymptomatic. Abdominal pain is the most common presenting symptom. Other common symptoms include abdominal distension, anorexia, and lump abdomen.4 Jaundice due to compression of biliary tree and ascites due to compression of hepatic veins or inferior vena cava have been described in the literature.4–6 Patients may present with acute abdominal pain in the setting of intracystic bleed, spontaneous cyst rupture, and cyst infection. Spontaneous rupture of biliary cystadenoma is extremely rare.7,8 Initial diagnosis is often made with abdominal imaging, either USG or CECT. Biliary cystadenoma appears as a well-defined cystic hepatic mass with mural nodules, internal septa, and rarely capsular calcification on CECT.2,3,7 Large and prominent mural nodules and papillary projections occur more frequently in cystadenocarcinoma though they have been reported in benign cystadenomas as well.9 Tumour markers alfa feto protein and carcinoembryonic antigen are often normal, while CA19.9 may be elevated. However, a normal CA 19.9 value does not rule out the diagnosis.10 Cross-sectional imaging with USG, CECT, or MRI often helps in differentiating cystadenomas from other hepatic cysts. However, it is often impossible to differentiate biliary cystadenoma from cystadenocarcinoma based on imaging or gross appearance. Histopathology offers the best differentiation between the tumour types. Considering the malignant potential and inability to differentiate between benign and malignant variants preoperatively, radical resection remains the treatment of choice in patients with cystadenoma.
Our patient was a middle-aged male who had presented with abdominal pain and distension. Imaging features were consistent with the diagnosis and elevated CA19.9 level is an inconsistent marker of biliary cystadenoma. The patient was evaluated extensively for the cause of ascites in the background of the cystic neoplasm. The patient had no stigmata of chronic liver disease, imaging did not reveal cirrhosis or portal hypertension, and the ascites was of a low serum ascites albumin gradient type; all features against the possibility of an underlying liver disease. Doppler study had revealed normal venous flow pattern in portal and hepatic veins and inferior venacava negating the possibility of venous compression causing ascites. Also, the patient had normal cardio-respiratory evaluation, renal and thyroid functions, ruling out other causes of ascites. Ascitic fluid cytology was repeatedly negative as was the adenosine deaminase level. Since imaging modalities cannot differentiate the benign versus malignant counterparts with certainty, and since there was no conclusive evidence that the ascites is malignant, the surgeon proceeded with laparotomy. At laparotomy the large multiloculated leaking cyst was identified with ascites. With exudative ascites in the setting of a leaking cyst and lack of any other identifiable aetiology, the diagnosis of ascites secondary to spontaneous cyst rupture was made. The possibility of cystadenocarcinoma was entertained pre- and intraoperatively in view of prominent papillary projections and mural nodules. However, histology revealed no stromal invasion or nuclear atypia, and only scant mitosis, all consistent with a benign lesion suggesting cystadenoma rather than cystadenocarcinoma. A literature search had revealed 15 reported cases of spontaneous nonparasitic hepatic cyst rupture and 3 cases of spontaneous biliary cystadenoma rupture to date. Previously reported cases of biliary cystadenoma rupture had presented with features of peritonitis;7,8 however, our patient had no clinical or laboratory evidence of peritonitis. The mechanism of exudative ascites following cystadenoma rupture is unclear. As hypothesised for pseudomyxoma peritonei might have probably resulted from the irritating effect of some of the chemical constituents in the cyst fluid or the tumourogenic stimulus by the neoplastic cells released into the peritoneum.13 Spontaneous rupture of parasitic and nonparasitic cysts without peritonitis have been reported in the literature.11,12 Probably the ooze through the small rent in the cyst wall, relatively inert chemical contents, and the microbiologically sterile nature of the cyst fluid might not have evoked an intense inflammatory peritoneal response to cause acute peritonitis.
The rarity of biliary cystadenoma, its uncommon presentation as spontaneous intraperitoneal rupture, and the lack of peritonitis following cyst rupture make our case unique. To the best of our knowledge this unique combination is the first to be reported in medical literature. We recommend a systematic approach to aetiological diagnosis of ascites as ascites can be the manifestation of a large variety of local and systemic diseases. Table 1 summarises a simple systematic approach to etiological diagnosis of ascites although the list is not exhaustive.
Table 1. Approach to aetiological diagnosis of ascites.
| SAAG ≥ 1.1 | |
| Ascitic fluid total protein <2.5gm/dl | |
| Uncomplicated cirrhotic ascites | USG abdomen with spleno-portal doppler, liver biopsy |
| Acute fatty liver of pregnancy | USG abdomen, liver biopsy |
| Massive liver metastasis | USG or CT abdomen |
| Ascitic fluid total protein > 2.5gm/dl | |
| Cardiac failure, Constrictive pericarditis | Chest X-ray, echocardiogram |
| Myxedema | Thyroid function tests |
| Budd Chiari syndrome | USG abdomen with hepatic venous doppler, venogram |
| Veno-occlusive disease | Liver biopsy |
| Mixed ascites | |
| Cirrhotic patients on diuretics | |
| SAAG < 1.1 | |
| Ascitic fluid total protein <2.5gm/dl | |
| Nephrotic syndrome | 24 hour urine albumin excretion or urine protein-creatinine ratio |
| Protein losing enteropathy | Stool α1 anti-trypsin quantification, plasma α1 anti-trypsin clearance |
| Ascitic fluid total protein < 2.5gm/dl | |
| Tuberculous ascites | Ascitic fluid ADA (> 30 U/L), mycobacterial culture, diagnostic laparoscopy, peritoneal biopsy |
| Peritoneal carcinomatosis | Ascitic fluid cytology, diagnostic laparoscopy, peritoneal biopsy |
| Secondary peritonitis (includes spontaneous or traumatic or iatrogenic hollow viscus perforation or rupture of intra-abdominal cysts or abscesses) | Ascitic fluid analysis showing atleast two of the following: protein >1g/dl, glucose < 50mg/dl, LDH > upper limit of normal for serum. |
| Ascitic fluid CEA > 5mcg/L | |
| Ascitic fluid ALP > 240 U/L | |
| Polymicrobial ascitic fluid culture | |
| USG or CT abdomen | |
| Free air on plain x-ray abdomen | |
| Pancreatic ascites | Ascitic fluid amylase (~ 2000 U/L), Ascitic fluid/serum amylase ratio(~ 6) |
| Biliary ascites | Ascitic fluid bilirubin (> 6mg/dl), Ascitic fluid/serum bilirubin ratio (> 1) |
| Serositis | Serology for autoantibodies |
| Peritoneal sarcoidosis | Diagnostic laparoscopy, peritoneal biopsy |
| Eosinophilic ascites | Serum and ascitic fluid eosinophil counts, peritoneal biopsy |
| Peritoneal mesothelioma and lymphoma | Peritoneal biopsy |
(USG - ultrasonogram, CT - computerised tomography, TB - tuberculosis, ADA - adenosine deaminase)
Footnotes
PEER REVIEW
Not commissioned. Externally peer reviewed.
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
PATIENT CONSENT
The authors, Abhishek Sasidharan, Jino Thomas, George Sarin Zacharia, Sandesh Kolassery, Valiya Kambarath Prathapan and Thazhath Mavali Ramachandran, declare that:
- They have obtained written, informed consent for the publication of the details relating to the patient(s) in this report.
- All possible steps have been taken to safeguard the identity of the patient(s).
- This submission is compliant with the requirements of local research ethics committees.
Please cite this paper as: Abhishek S, Jino T, Sarin GZ, Sandesh K, Prathapan VK, Ramachandran TM. An uncommon cause of ascites: spontaneous rupture of biliary cystadenoma. AMJ 2014, 7, 1, 6-10. http//dx.doi.org/10.4066/AMJ.2014.1875
References
- 1.Mortelé KJ, Ros PR. Cystic focal liver lesions in the adult: differential CT and MR imaging features. Radiographics. 2001;21:895–910. doi: 10.1148/radiographics.21.4.g01jl16895. [DOI] [PubMed] [Google Scholar]
- 2.Palacios E, Shannon M, Solomon C, Guzman M. Biliary cystadenoma: ultrasound, CT, and MRI. Gastrointest Radiol. 1990;15:313–6. doi: 10.1007/BF01888807. [DOI] [PubMed] [Google Scholar]
- 3.Buetow PC, Midkiff RB. Primary malignant neoplasms in the adult. Magn Reson Imaging Clin N Am. 1997;5:289–318. [PubMed] [Google Scholar]
- 4.Ahanatha Pillai S, Velayutham V, Perumal S, Ulagendra Perumal S, Lakshmanan A, Ramaswami S, Ramasamy R, Sathyanesan J, Palaniappan R, Rajagopal S. Biliary cystadenomas: a case for complete resection. HPB Surg. 2012;501705 doi: 10.1155/2012/501705. doi: 10.1155/2012/501705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preetha M, Chung AY, Lim-Tan SK, Lim DT, Thng CH. Intrahepatic biliary cystadenoma presenting with obstructive jaundice. Asian J Surg. 2004;27:243–5. doi: 10.1016/S1015-9584(09)60043-9. [DOI] [PubMed] [Google Scholar]
- 6.Catinis GE, Frey DJ, Skinner JW, Balart LA. Hepatic cystadenoma: an unusual presentation. Am J Gastroenterol. 1998;93:827–9. doi: 10.1111/j.1572-0241.1998.234_a.x. [DOI] [PubMed] [Google Scholar]
- 7.Elfadili H, Majbar A, Zouaidia F, Elamrani N, Sabbah F, Raiss M, Mahassini N, Hrora A, Ahallat M. Spontaneous rupture of a recurrent hepatic cystadenoma. World J Hepatol. 2010;2:322–4. doi: 10.4254/wjh.v2.i8.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miliadis L, Giannakopoulos T, Boutsikos G, Terzis I, Kyriazanos ID. Spontaneous rupture of a large non-parasitic liver cyst: a case report. J Med Case Rep. 2010;4(2) doi: 10.1186/1752-1947-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powers C, Ros PR, Stoupis C, Johnson WK, Segel KH. Primary liver neoplasms: MR imaging with pathologic correlation. Radiographics. 1994;14:459–82. doi: 10.1148/radiographics.14.3.8066263. [DOI] [PubMed] [Google Scholar]
- 10.Tsepelaki A, Kirkilesis I, Katsiva V, Triantafillidis JK, Vagianos C. Biliary cystadenoma of the liver: case report and systematic review of the literature. Ann Gastroenterol. 2009;22:278–83. [Google Scholar]
- 11.Turkbeyler IH, Babacan T, Dilli I, Balkan A, Dag MS, Kadayifçi A. Ascites as an initial presentation of spontaneously ruptured hydatid cyst. S Afr Med J. 2012;102(664) doi: 10.7196/samj.5935. [DOI] [PubMed] [Google Scholar]
- 12.Ueda J, Yoshida H, Taniai N, Mineta S, Kawano Y, Uchida E. A case of spontaneous rupture of a simple hepatic cyst. J Nippon Med Sch. 2010;77:181–5. doi: 10.1272/jnms.77.181. [DOI] [PubMed] [Google Scholar]
- 13.Shanks HGI. Pseudomyxoma peritonei. J Obstet Gynecol Br Common W. 1961;68:212–224. [Google Scholar]


