Abstract
Cognitive changes in older women receiving chemotherapy are poorly understood. We examined self-reported cognitive function for older women who received adjuvant chemotherapy on Cancer and Leukemia Group B (CALGB) 49907. CALGB 49907 randomized 633 women aged ≥65 with stage I–III breast cancer to standard adjuvant chemotherapy (cyclophosphamide–methotrexate–5-fluoro-uracil or doxorubicin–cyclophosphamide) versus capecitabine. We examined self-reported cognitive function in 297 women (CALGB 361002) who enrolled on the quality of life substudy and had no gross impairment on cognitive screening. Women were evaluated using an 18-item instrument at six time points (baseline through 24 months). At each time point for each patient, we calculated a cognitive function score (CFS) defined as the mean response of items 1–18 and defined impairment as a score >1.5 standard deviations above the overall average baseline score. Differences in scores by patient characteristics were evaluated using a Kruskal–Wallis test. A linear mixed-effects model was used to assess CFSs by treatment over time. Among 297 women, the median age was 71.5 (range 65–85) and 73 % had performance status of 0. Baseline depression and fatigue were reported in 6 and 14 % of patients, respectively. The average CFS at baseline was 2.08 (corresponding to “normal ability”), and baseline cognitive function did not differ by treatment regimen (p = 0.350). Over 24 months, women reported minimal changes at each time point and insignificant differences by treatment arm were observed. In a healthy group of older women, chemotherapy was not associated with longitudinal changes in self-reported cognitive function.
Keywords: Cognitive function, Older women, Breast cancer, Age
Introduction
The majority of breast cancer diagnoses occur in older women [1], many of whom will be recommended to receive chemotherapy to lower their risk of disease recurrence. Although many toxicities of chemotherapy have been well elucidated for younger women, the side effects that older women experience are less defined, largely as a result of the underrepresentation of older women in most clinical trials. There has been some suggestion that older patients experience greater chemotherapy-related toxicity compared with younger women, despite similar risk reductions in relapse-free and overall survival with treatment [2, 3]. However, the extent of cognitive decline in older women who receive chemotherapy remains poorly studied.
The potential for “chemo-brain” and “chemo-fog,” terms often used to describe the cognitive changes women report while receiving chemotherapy, is of great concern to the patient and survivorship community and is a widely discussed entity in the media and cancer literature [4, 5]. Prior studies have reported variable cognitive changes in 15–50 % of cancer patients who undergo chemotherapy [6–13], with observed difficulties in attention, learning, and processing speed during treatment [11] that typically improve over time [9, 14]. The risk factors for developing cognitive changes during cancer-directed therapies are unknown but may include specific treatments (high-dose chemotherapy, bone marrow transplant) [15, 16], genetic predisposition [17], menopausal symptoms [13], inflammatory markers [18], depression [16], fatigue [19], and other potential factors, particularly age [20–22]. However, studying changes in cognitive function is challenging, particularly because the changes observed on objective testing of cognition often do not correlate with subjective report and may not fully reflect patients’ experiences [10, 23–26]. Self-reported changes in cognitive function during and after chemotherapy are associated with psychological distress, quality of life, health status, and fatigue [6, 19, 27].
Small studies in populations of older patients receiving chemotherapy have shown conflicting results on whether cognitive dysfunction is a significant clinical problem, and longitudinal assessments have not been consistently included [21, 28–32]. In the quality of life substudy Cancer and Leukemia Group B (CALGB) 361002, we examined changes in a self-reported cognitive function scale in older women with breast cancer who received adjuvant chemotherapy on CALGB protocol 49907. CALGB 49907 randomized women to receive standard chemotherapy (either cyclophosphamide–methotrexate–5-fluorouracil [CMF] or doxorubicin–cyclophosphamide [AC] by provider choice) or capecitabine [33]. This study, one of the few prospective trials dedicated to older patients, demonstrated superiority in relapse-free survival and overall survival for patients treated with standard chemotherapy compared with capecitabine. Multiple preplanned, prospective, ancillary studies were embedded within 49907, including examination of quality of life (QOL) [34]. For the QOL substudy, the first 368 women who spoke English or Spanish were asked to participate. The longitudinal data collection for the QOL substudy provided a unique opportunity to conduct a secondary analysis of cognitive function for older women who received chemotherapy. We assessed longitudinal, subjective changes in cognitive function for 297 women on the QOL substudy (CALGB 361002).
Patients and methods
Population and data collection
CALGB 49907 enrolled 633 patients aged ≥65 during 2001–2006 at 149 nationwide centers with the objective of evaluating the potential noninferiority of an oral, potentially less toxic adjuvant chemotherapeutic agent (capecitabine) to standard chemotherapy (CMF or AC, by provider choice). Patients were eligible for the parent study if they had at least a 1-cm, operable, invasive breast cancer with negative surgical margins and no distant metastases, Eastern Cooperative Oncology Group performance status of 0–2, adequate organ function, expected survival of more than 5 years, and no medical condition that would make protocol treatments unreasonably hazardous. Women were excluded because of a concurrent malignancy or a previous cancer with a risk of relapse >30 %. The full details of eligibility for CALGB 49907 have been previously published [33]. Treatment arms were assigned randomly in a 1:1 fashion to standard chemotherapy (CMF or AC) or capecitabine.
The QOL companion study simultaneously enrolled 350 of the first 368 patients on CALGB 49907 between 2002 and 2006 randomized to either standard chemotherapy (n = 182) or capecitabine (n = 168) [34]. Women signed an institutional review board–approved informed consent prior to participation. Patients were English- or Spanish-speaking and passed an initial screening to rule out gross cognitive impairment using the Blessed Orientation-Memory-Concentration (BOMC) test [35]. Those scoring 11 or higher on the BOMC (n = 17) were considered to have significant impairment, making them ineligible. Survey data regarding social support, stressful life events, toxicity, changes in physical function, adherence to therapy, and neurobehavioral symptoms were collected on the QOL study at six time points: (1) baseline, (2) mid-treatment, (3) within 1 month post-treatment, (4) 12 months, (5) 18 months, and (6) 24 months. Results of the QOL substudy have been previously published [34].
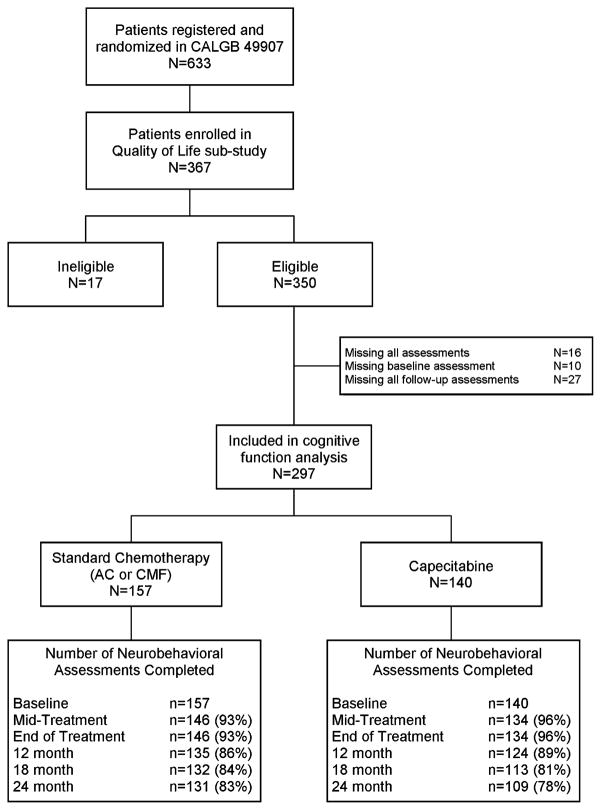
Among the 350 patients who participated in the QOL study, 334 patients completed at least one assessment of cognitive function; 10 missed the baseline evaluation and 27 missed all five follow-up assessments. These 37 patients were excluded from analyses with a final sample size of 297 women (Fig. 1). The reasons for missing questionnaires over time often corresponded to the timing of stopping protocol therapy and included the following: disease progression (n = 1), toxicity (n = 8), death on study (n = 2), patient withdrawal after starting or prior to starting treatment (n = 6), per protocol (n = 7), and other/missing reason (n = 3). There were no withdrawals for cognitive toxicity specifically.
Fig. 1.
Neurobehavioral assessments completed over the study period. *Seventeen patients were deemed ineligible because they did not pass the Blessed Orientation-Memory-Concentration test at screening
Measures
The Neurobehavioral Functioning and Activities of Daily Living (NBFADL) scale is a 60-item scale that has been previously used and validated to assess cognitive issues in daily life that affect functioning [36, 37]. The Memory, Cognitive and Concentration subscales of the NBFADL were selected for the QOL study as a subjective cognitive function measure and were administered as a telephone survey at all six time points along with the other components of the QOL study. Patients who consented to participate were given a packet of QOL questionnaires to complete before the telephone interview, at which time the centralized research interviewer collected their answers [34]. The subscales included 18 questions that evaluated five domains of cognitive function including attention, problem-solving, speed, new learning and prospective memory, and remote memory (Table 1). Patients selected a response for each question ranging from 1 to 7 (1 = “above average” to 7 = “severe problems”).
Table 1.
Questions and instructions from the 18-question measure
| Neurobehavioral Scales form (domain assessed) |
|---|
|
Patients were asked to “mark an X in a single box indicating the appropriate response for each task [marked 1–7]”
Response options for each item ranged from 1 to 7 and included the following: 1 = above average, 2 = normal ability, 3 = mild problem, 4 = mild to moderate problem, 5 = moderate problem, 6 = moderate to severe problem, 7 = severe problem
Variables of interest
The primary variable of interest was cognitive function score (CFS), defined as the mean response of items 1–18 from the Memory, Cognitive and Concentration subscale of the NBFADL scale. Cognitive impairment was defined as a CFS at any time point that was >1.5 standard deviations (SDs) above the overall average baseline score [14].
Patient characteristics evaluated for association with CFS included treatment arm assignment, treatment regimen, age, number of comorbid conditions according to the Older American and Services (OARS) Questionnaire [38, 39], hormonal therapy, education level, and stage of disease. Because of potential impact on cognitive function, we also examined the occurrence of depression, anxiety, and fatigue at baseline according to the European Organization for the Research and Treatment of Cancer (EORTC) Quality of Life (QLQ-C30) questionnaire [40, 41] and the Hospital Anxiety and Depression Scale (HADS) [42]. The QLQ-C30 is a 30-question measure on global health status, function, and symptoms, and it also includes two items on attention and memory. The HADS is a 14-question measure on the frequency of depression- and anxiety-related symptoms (i.e., “I get sudden feelings of panic” and “I look forward with enjoyment to things”). We defined the presence of depression at baseline if participants answered “quite a bit” or “very much” to either of the mood questions on the EORTC QLQ-C30 (“Over the last week, did you feel irritable?…did you feel depressed?”) or had a HADS score ≥11 on the depression subscales. Baseline anxiety was defined as a HADS score ≥11 on anxiety subscales [42]. Fatigue was defined by having answered “quite a bit” or “very much” to a single question on the EORTC measure (“Were you tired?”) [41].
Statistical analysis
Frequency distributions and means were used to describe baseline patient characteristics. Associations among groups for categorical variables were assessed with chi-square and Fisher’s exact tests. The differences in CFSs and baseline characteristics among groups were compared with a Kruskal–Wallis test. For univariate analyses, we used a 0.05 type 1 error rate. A linear mixed-effects model with nominal time to each assessment as the repeated variable was used to assess the differences in CFSs among treatment arms, assuming a compound symmetric covariance structure and assuming that any missing data were missing at random. All available data were used in the analyses of cognitive function over time. Covariates included in the model were age, assessment, treatment arm, and an interaction term between treatment arm and assessment. The type 1 error rate was adjusted to 0.01 to reduce the chance of spurious significant findings. In addition, baseline characteristics for women with<20 % missing data versus those with ≥20 % missing data (i.e., women with 1–4 follow-up assessments missing after baseline) were compared. There were no differences in baseline characteristics for women by the degree of missing data, with the exception of rates of hormone receptor (HR)-positive status (72 % had HR-positive disease in those with <20 % missing data versus 60 % with HR-positive disease in those with ≥20 % missing data, p = 0.041). An exploratory analysis was performed on the women with baseline scores >1.5 SDs from the group baseline mean (n = 19) to determine how they differed from the remaining cohort. Frequency distributions were used to describe cognitive impairment over time.
Lastly, because our 18-item measure has not been validated previously outside of the comprehensive NBFADL scale, we used Pearson correlations to examine whether the EORTC questions for attention and memory impairment (“Have you had difficulty in concentrating on things, like reading a newspaper or watching television?” and “Have you had difficulty remembering things?”) correlated with our results from the NBFADL-based CFS. For the correlation analysis, we averaged the scores between the two EORTC questions and then converted to a 100-point scale as described in the EORTC scoring manual [41]. All statistical analyses were performed by Alliance statisticians using SAS v9.2 (Cary, NC, USA).
Results
There were 367 (350 eligible) women enrolled to the QOL portion of CALGB 49907 from June 2002 through April 2006. Characteristics of the 297 women in this study are displayed in Table 2. The median age of participants was 71.5 years (range 65–85). Over 70 % of women had a performance status of zero, and 30 % reported four to ten comorbidities [38, 39]. Approximately 51 % of women reported having a high school education or less. Baseline anxiety, depression, and fatigue were uncommon. Approximately 21 % of women received AC, 32 % received CMF, and 47 % received capecitabine. Baseline characteristics did not differ for women by treatment regimen received, with the exception of fatigue (i.e., 21 % for CMF, 8 % for AC, and 13 % for capecitabine, p = 0.049). Of note, with the exception of differences in age, patients included in this study were similar to women who enrolled on the treatment component for CALGB 49907 (mean age 71.9 years for women on this study versus 73.0 years on treatment component, p = 0.004).
Table 2.
Baseline cognitive function score by patient characteristics (n = 297)
| Variable | N (%) | Baseline cognitive function scorea (standard deviation) | p valueb |
|---|---|---|---|
| Age | 0.598 | ||
| 65–69 year | 120 (40) | 2.05 (0.43) | |
| 70–79 year | 166 (56) | 2.10 (0.59) | |
| ≥80 year | 11 (4) | 1.95 (0.28) | |
| Race | 0.118 | ||
| White | 259 (87) | 2.05 (0.47) | |
| Black | 32 (11) | 2.27 (0.81) | |
| Other | 3 (0) | 2.07 (0.47) | |
| Unknown | 3 (0) | 2.37 (0.36) | |
| Education | 0.003 | ||
| Grades 1–11 | 35 (12) | 2.33 (0.74) | |
| High school graduate | 115 (39) | 2.09 (0.42) | |
| Some college/junior college | 82 (27) | 2.06 (0.51) | |
| College/post-college/advanced | 59 (20) | 1.96 (0.51) | |
| Degree unknown | 6 (2) | 1.77 (0.60) | |
| Tumor size | 0.787 | ||
| ≤2 cm | 129 (43) | 2.07 (0.44) | |
| 2–5 cm | 156 (53) | 2.10 (0.59) | |
| >5 cm | 11 (4) | 1.96 (0.38) | |
| Number of positive lymph nodes | 0.022 | ||
| 0 | 88 (30) | 2.03 (0.55) | |
| 1–3 | 164 (55) | 2.05 (0.44) | |
| 4+ | 44 (15) | 2.27 (0.69) | |
| Stage | 0.228 | ||
| I | 21 (7) | 1.88 (0.45) | |
| IIA | 117 (39) | 2.11 (0.53) | |
| IIB | 92 (31) | 2.11 (0.58) | |
| IIIA | 20 (7) | 1.96 (0.32) | |
| IIIB | 1 ( <1) | 2.67 (n/a) | |
| Unknown | 46 (15) | 2.05 (0.44) | |
| Hormone receptor statusc | 0.150 | ||
| Negative | 95 (32) | 2.06 (0.65) | |
| Positive | 202 (68) | 2.08 (0.45) | |
| HER2 status | 0.379 | ||
| Negative | 234 (79) | 2.07 (0.45) | |
| Positive | 44 (15) | 2.18 (0.80) | |
| Unknown | 19 (6) | 1.97 (0.51) | |
| ECOG performance status | 0.265 | ||
| 0 (Normal) | 218 (73) | 2.03 (0.42) | |
| 1 (Ambulatory) | 74 (25) | 2.22 (0.72) | |
| 2 ( <50 % time in bed) | 5 (2) | 2.21 (0.53) | |
| Treatment arm | 0.619 | ||
| Standard (AC or CMF) | 157 (53) | 2.10 (0.46) | |
| Capecitabine | 140 (47) | 2.06 (0.58) | |
| Treatment received | 0.350 | ||
| AC | 95 (32) | 2.08 (0.51) | |
| CMF | 62 (21) | 2.11 (0.37) | |
| Capecitabine | 140 (47) | 2.06 (0.58) | |
| Tamoxifen use | 0.527 | ||
| No | 238 (80) | 2.08 (0.54) | |
| Yes | 58 (21) | 2.08 (0.44) | |
| Unknown | 1 (0.4) | 1.22 (not applicable) | |
| OARS number of comorbidities | 0.002 | ||
| 0 | 24(8) | 1.93 (0.40) | |
| 1 | 36 (21) | 1.94 (0.42) | |
| 2–3 | 120 (40) | 2.06 (0.47) | |
| 4–10 | 90 (30) | 2.24 (0.63) | |
| Fatigue | 0.020 | ||
| No | 252 (85) | 2.05 (0.50) | |
| Yes | 40 (13) | 2.30 (0.64) | |
| Unknown | 5 (2) | 1.99 (0.09) | |
| Depression | 0.276 | ||
| No | 280 (94) | 2.07 (0.52) | |
| Yes | 17 (6) | 2.20 (0.43) | |
| Anxiety | 0.010 | ||
| No (score <11) | 271 (91) | 2.05 (0.48) | |
| Yes (score ≥11) | 26 (9) | 2.40 (0.74) |
ER estrogen receptor, PgR progesterone receptor, HER2 human epidermal growth factor receptor 2, ECOG Eastern Cooperative Oncology Group, AC doxorubicin plus cyclophosphamide, CMF cyclophosphamide–methotrexate–5-fluorouracil, OARS Older American Resources and Services, HADS Hospital Anxiety and Depression Scale, EORTC European Organization for the Research and Treatment of Cancer
Possible scores ranged from 1 to 7 (1 = above average and 7 = severe problem)
For differences in mean cognitive function score at baseline, by Kruskal–Wallis tests
Hormone receptor (HR) status defined as the following: negative = ER-negative and PR-negative; positive = ER-positive and PR-positive, ER-negative/missing and PR-positive, or ER-positive and PR-negative/missing
At baseline, the average CFS was 2.08 (range 1.00–5.56), corresponding to nearly “normal ability.” Women with worse cognitive function at baseline were less educated (p = 0.003) and had more positive nodes (p = 0.022), more comorbid conditions (p = 0.002), more fatigue (p = 0.020), and more anxiety (p = 0.010), although numerical differences between subgroups were small (average CFSs range = 1.77–2.37 across all categories, corresponding to “normal ability”) (Table 2). There were no differences in baseline CFSs by treatment regimen (p = 0.350).
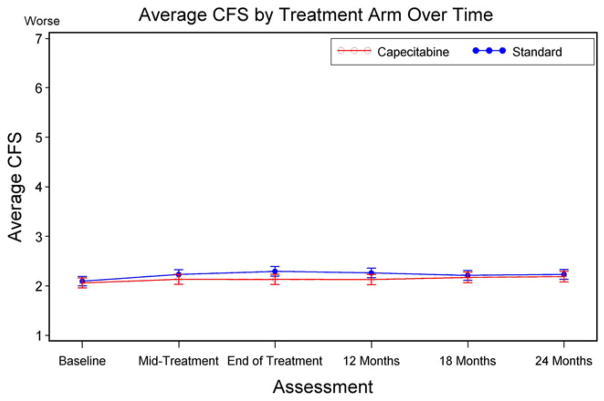
During the study period, the overall average CFSs by treatment arm remained within “normal ability” (Table 3). At mid-treatment, within 1 month post-treatment, and at 12, 18, and 24 months, adjusted CFSs ranged from 2.1 to 2.3. Results from the linear mixed-effects model also showed little variation in self-reported CFSs over time (Table 3). There were no differences by treatment arm (Fig. 2) over time).
Table 3.
Adjusted cognitive function scores by treatment over time (linear mixed-effects model)
| Assessment | Overall
|
Standard chemotherapya
|
Capecitabineb
|
p valuec | |||
|---|---|---|---|---|---|---|---|
| CFS | SE | CFS | SE | CFS | SE | ||
| Baseline | 2.08 | 0.04 | 2.10 | 0.05 | 2.06 | 0.05 | 0.605 |
| Mid-treatment | 2.18 | 0.04 | 2.23 | 0.05 | 2.13 | 0.05 | 0.172 |
| End of the treatment | 2.21 | 0.04 | 2.29 | 0.05 | 2.13 | 0.05 | 0.024 |
| 12 months | 2.19 | 0.04 | 2.21 | 0.05 | 2.17 | 0.05 | 0.573 |
| 18 months | 2.19 | 0.04 | 2.23 | 0.05 | 2.19 | 0.05 | 0.546 |
| 24 months | 2.21 | 0.04 | 2.26 | 0.05 | 2.13 | 0.05 | 0.065 |
Higher score = worse cognitive function. Covariates are age, treatment arm, assessment, and assessment–treatment arm interaction CFS cognitive function score, SE standard error
For standard treatment with doxorubicin and cyclophosphamide (AC), mid-treatment, day 29; end of the treatment, 4–5 months; for treatment with cyclophosphamide, methotrexate, and fluorouracil (CMF): mid-treatment, day 77; end of the treatment, 6–7 months
For treatment with capecitabine: mid-treatment, day 63; end of the treatment, 4–5 months
p value compares standard and capecitabine treatment arms; a type 1 error rate was adjusted to 0.01 to reduce the chance of spurious significant findings
Fig. 2.
Mean cognitive function score (CFS) by treatment arm over time. Mid-treatment, doxorubicin–cyclophosphamide (AC): day 29; end of treatment, AC: 4–5 months. Mid-treatment, cyclophosphamide–methotrexate–5-fluorouracil (CMF): day 77; end of treatment, CMF: 6–7 months. Mid-treatment, capecitabine: day 63; end of treatment, capecitabine: 4–5 months. Error bars represent 95 % CI for the adjusted average cognitive function score (CFS)
Cognitive impairment was observed in 19 (6 %) women at baseline, 19 (6 %) at mid-treatment, 29 (10 %) at the end of the treatment, 31 (12 %) at 12 months post-treatment, 18 (7 %) at 18 months post-treatment, and 22 (9 %) at 24 months post-treatment. At 24 months, 14 of the 19 women had measures completed at both baseline and 24 months. Of these women, nine reported impairment (64 %) at both time points. Although relative impairment was observed in these patients by our definition, the numerical differences between time points for nearly all patients were small.
Finally, the Pearson correlation coefficients for mean self-reported CFSs and the memory and attention components of the EORTC were calculated at each time point and ranged in magnitude from 0.539 to 0.680 (data not shown). These observations indicate that in general, women who reported good memory and attention on the EORTC also reported good cognitive function on the Neurobehavioral Scales form (and vice versa).
Discussion
We examined self-reported cognitive function for a prospective, large cohort of older women with breast cancer who received adjuvant chemotherapy on a clinical trial. In this healthy, older population with primarily normal self-reported cognitive function at baseline, we observed minimal changes in cognitive function over time and minimal differences in subjective cognitive function by treatment received. At 24 months, nearly all patients reported normal cognitive function.
Prior studies on cognitive function changes in older women receiving systemic therapy have shown variable results and have generally included small samples of women without multiple longitudinal assessments or consistent inclusion of subjective report. Hurria et al. [21] examined objective cognitive function in 31 patients aged 65 and older who received adjuvant chemotherapy for breast cancer and demonstrated objective declines in cognitive function in 25 % of patients (defined as a difference in 1 SD in 2 or more domains on neuropsychological testing) from before chemotherapy to 6 months after completion of chemotherapy, and 50 % reported subjective decline in cognitive function, which was most pronounced in patients who reported pre-existing memory problems. Another study assessed cognitive function longitudinally in older women undergoing chemotherapy and found significant changes in processing speed and verbal ability compared with controls. Impairment was worse among older women, although the mean age was 55 years and only 60 women received chemotherapy [20].
A meta-analysis assessing 30 cognitive function studies including over 800 women receiving adjuvant chemotherapy for breast cancer showed only modest changes in verbal and visuospatial ability for women on treatment. In this analysis, no differences by age were observed, although the median age for women included in the analysis was 52.3 years [15]. Yamada et al. performed a focused examination for longer-term effects in 30 older women who received chemotherapy 10 years prior to enrollment. Here, women who received chemotherapy performed worse on objective cognitive function testing compared with matched controls [29], demonstrating the importance of following women over time. Overall, because of the small sample sizes and variable methodological approaches of these studies, the evidence for cognitive function changes remains controversial and definitive data are lacking.
Our study is the largest, prospective study to date of cognitive function in older women receiving chemotherapy and included six time points, allowing for examination of the entire treatment and post-treatment trajectory. We also had individual information on comorbidity, baseline cognitive function, tumor characteristics, and presence of fatigue, depression, and anxiety, which are all factors that may impact cognitive function. Nevertheless, our study has some limitations. First, we studied a highly selected population of older women in a randomized trial who were healthy enough to receive chemotherapy and who passed an initial cognitive function screening test, excluding women with gross cognitive dysfunction. In addition, women had good performance status and organ function. Although our findings cannot be generalized to all older patients, our observations are very relevant to healthy, older women who are typically selected to receive chemotherapy. Second, our analyses did not allow a comparison of cognitive function with a control group of older breast cancer patients not receiving chemotherapy, a control group of older noncancer patients, or a control group of younger women to assess impact of having cancer and/or cancer therapy as well as age. Others have noted that a cancer diagnosis itself may have adverse effects on cognition apart from any anxiety, fatigue, or depression [11, 19, 43, 44]. We also could not evaluate age–treatment interactions since all patients were 65 and older. Ahles et al. [20] have noted such interactions, and these will be important to confirm in future research. Third, assignment to treatment with AC versus CMF within the standard treatment arm was not random but was selected by providers, which may influence findings, although cognitive function changes were rare for all study participants regardless of treatment received. Fourth, our assessments of cognitive function have not been previously validated as a measure independent from the complete NBFADL and are self-reported only. However, arguably, how women feel about their cognitive changes may be more important from a quality of life standpoint than any objective changes observed, and others have documented the poor correlation between objective and subjective cognitive functional decline [10, 23–26]. Lastly, because few women met the criteria for cognitive impairment and because differences in scores over time were small, our ability to perform analyses assessing factors associated with such impairment was limited.
In summary, older women on CALGB 49907 tolerated standard and nonstandard chemotherapy without subjective declines in cognitive function over the first 24 months after starting treatment. Although our study included a selected population of older women, these findings are reassuring because of the concerns for cognitive decline in older women receiving cancer-directed systemic therapy. Future studies should include consistent, prospective, detailed subjective and objective assessments of cognitive and physical function to further expand our knowledge about how our treatments impact older women with breast cancer. Systematic studies on the various effects of chemotherapy on older patients will be necessary to optimally serve and support this growing population of patients.
Acknowledgments
We thank all of the breast cancer patients who shared their experiences while participating in this study. We also thank Dr. Andrew Saykin for his guidance in using his cognitive function measure and Jacqueline Lafky for her administrative and logistical support. We acknowledge support from Celgene provided through the Cancer and Leukemia Group B Foundation Young Investigator Award [RF]. This research was also supported in part by the National Cancer Institute at the National Institutes of Health [Grant # U10 CA 84131, CA 127617, CA096940, and CA129769 to JSM] and by the National Institute on Aging at the National Institutes of Health [Grant #CA85850 to HM]. The research for CALGB 49907 was also supported, in part, by the National Cancer Institute at the National Institutes of Health [Grant # CA31946 to the Cancer and Leukemia Group B (Monica Bernagnoli, MD, Chairman) and Grant # CA33601 to the CALGB Statistical Center (Stephen George, PhD)].
Footnotes
Presented at the San Antonio Breast Cancer Symposium (December 8, 2012).
Disclaimer The content of this manuscript is solely the responsibility of the authors and does not represent the official views of the National Cancer Institute at the National Institutes of Health or the Cancer and Leukemia Group B. Cancer and Leukemia Group B is presently part of the Alliance for Clinical Trials in Oncology. The sponsors had no role in the design, methods, subject recruitment, data collection, analysis, or preparation of this manuscript.
Conflict of interest The authors declare that they have no conflicts of interest.
Contributor Information
Rachel A. Freedman, Email: rafreedman@partners.org, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, MA 02215, USA
Brandelyn Pitcher, Alliance Statistics and Data Center, Duke University, Durham, NC, USA.
Nancy L. Keating, Department of Health Care Policy, Harvard Medical School, Boston, MA, USA. Division of General Internal Medicine, Brigham and Women’s Hospital, Boston, MA, USA
Karla V. Ballman, Alliance Statistics and Data Center, Mayo Clinic, Rochester, MN, USA
Jeanne Mandelblatt, Department of Oncology, Lombardi Comprehensive Cancer Center, Georgetown University, Washington, DC, USA.
Alice B. Kornblith, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, MA 02215, USA
Gretchen G. Kimmick, Duke Medical Oncology, Durham, NC, USA
Arti Hurria, Department of Medical Oncology & Therapeutics Research, City of Hope, Duarte, CA, USA. Department of Population Sciences, City of Hope, Duarte, CA, USA.
Eric P. Winer, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, MA 02215, USA
Clifford A. Hudis, Department of Medical Oncology, Memorial Sloan Kettering, New York, NY, USA
Harvey Jay Cohen, Center for the Study of Aging, Duke Medical Oncology, Durham, NC, USA.
Hyman B. Muss, Department of Medicine and Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC, USA
References
- 1. [Accessed 20 Dec 2009];Surveillance Epidemiology and End Results Cancer Statistics Review 1975–2006. http://www.seer.cancer.gov/csr/1975_2006/index.html.
- 2.Muss HB, Woolf S, Berry D, Cirrincione C, Weiss RB, Budman D, Wood WC, Henderson IC, Hudis C, Winer E, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293(9):1073–1081. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 3.Giordano SH, Duan Z, Kuo YF, Hortobagyi GN, Goodwin JS. Use and outcomes of adjuvant chemotherapy in older women with breast cancer. J Clin Oncol. 2006;24(18):2750–2756. doi: 10.1200/JCO.2005.02.3028. [DOI] [PubMed] [Google Scholar]
- 4. [Accessed 2 Aug 2013]; http://chemobrain.org/
- 5.American Cancer Society. [Accessed 2 Aug 2013];Chemo Brain. http://www.cancer.org/treatment/treatmentsandsideeffects/physicalsideeffects/chemotherapyeffects/chemo-brain.
- 6.Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, Wilson C. Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat Rev. 2012;38(7):926–934. doi: 10.1016/j.ctrv.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: a review of published studies and recommendations for future research. J Clin Oncol. 2007;25(17):2455–2463. doi: 10.1200/JCO.2006.08.1604. [DOI] [PubMed] [Google Scholar]
- 8.Vardy J, Tannock I. Cognitive function after chemotherapy in adults with solid tumours. Crit Rev Oncol Hematol. 2007;63(3):183–202. doi: 10.1016/j.critrevonc.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Fan HG, Houede-Tchen N, Yi QL, Chemerynsky I, Downie FP, Sabate K, Tannock IF. Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. J Clin Oncol. 2005;23(31):8025–8032. doi: 10.1200/JCO.2005.01.6550. [DOI] [PubMed] [Google Scholar]
- 10.van Dam FS, Schagen SB, Muller MJ, Boogerd W, vd Wall E, Droogleever Fortuyn ME, Rodenhuis S. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst. 1998;90(3):210–218. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- 11.Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100(11):2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 12.Brezden CB, Phillips KA, Abdolell M, Bunston T, Tannock IF. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2000;18(14):2695–2701. doi: 10.1200/JCO.2000.18.14.2695. [DOI] [PubMed] [Google Scholar]
- 13.Tchen N, Juffs HG, Downie FP, Yi QL, Hu H, Chemerynsky I, Clemons M, Crump M, Goss PE, Warr D, et al. Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. J Clin Oncol. 2003;21(22):4175–4183. doi: 10.1200/JCO.2003.01.119. [DOI] [PubMed] [Google Scholar]
- 14.Schagen SB, Muller MJ, Boogerd W, Rosenbrand RM, van Rhijn D, Rodenhuis S, van Dam FS. Late effects of adjuvant chemotherapy on cognitive function: a follow-up study in breast cancer patients. Ann Oncol. 2002;13(9):1387–1397. doi: 10.1093/annonc/mdf241. [DOI] [PubMed] [Google Scholar]
- 15.Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE. Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. J Int Neuropsychol Soc. 2003;9(7):967–982. doi: 10.1017/S1355617703970019. [DOI] [PubMed] [Google Scholar]
- 16.McAllister TW, Ahles TA, Saykin AJ, Ferguson RJ, McDonald BC, Lewis LD, Flashman LA, Rhodes CH. Cognitive effects of cytotoxic cancer chemotherapy: predisposing risk factors and potential treatments. Curr Psychiatry Rep. 2004;6(5):364–371. doi: 10.1007/s11920-004-0023-y. [DOI] [PubMed] [Google Scholar]
- 17.Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, Mott LA. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12(6):612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 18.Ganz PA, Bower JE, Kwan L, Castellon SA, Silverman DH, Geist C, Breen EC, Irwin MR, Cole SW. Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun. 2012;(suppl):S99–108. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cimprich B, Hayes DF, Askren MK, Jung MS, Berman MG, Ossher L, Therrien B, Reuter-Lorenz PA, Zhang M, Peltier S, et al. Neurocognitive impact in adjuvant chemotherapy for breast cancer linked to fatigue: a prospective MRI study. San Antonio Breast Cancer Symposium; Presented as oral abstract; December 7, 2012; 2012. Abstract #S6-3. [Google Scholar]
- 20.Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28(29):4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurria A, Rosen C, Hudis C, Zuckerman E, Panageas KS, Lachs MS, Witmer M, van Gorp WG, Fornier M, D’Andrea G, et al. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: a pilot prospective longitudinal study. J Am Geriatr Soc. 2006;54(6):925–931. doi: 10.1111/j.1532-5415.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 22.Hurria A, Goldfarb S, Rosen C, Holland J, Zuckerman E, Lachs MS, Witmer M, van Gorp WG, Fornier M, D’Andrea G, et al. Effect of adjuvant breast cancer chemotherapy on cognitive function from the older patient’s perspective. Breast Cancer Res Treat. 2006;98(3):343–348. doi: 10.1007/s10549-006-9171-6. [DOI] [PubMed] [Google Scholar]
- 23.Downie FP, Mar Fan HG, Houede-Tchen N, Yi Q, Tannock IF. Cognitive function, fatigue, and menopausal symptoms in breast cancer patients receiving adjuvant chemotherapy: evaluation with patient interview after formal assessment. Psychooncology. 2006;15(10):921–930. doi: 10.1002/pon.1035. [DOI] [PubMed] [Google Scholar]
- 24.Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, Whedon MB, Bivens S, Mitchell T, Greenberg ER, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20(2):485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- 25.Schagen SB, van Dam FS, Muller MJ, Boogerd W, Lindeboom J, Bruning PF. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85(3):640–650. doi: 10.1002/(sici)1097-0142(19990201)85:3<640::aid-cncr14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 26.Phillips KA, Bernhard J. Adjuvant breast cancer treatment and cognitive function: current knowledge and research directions. J Natl Cancer Inst. 2003;95(3):190–197. doi: 10.1093/jnci/95.3.190. [DOI] [PubMed] [Google Scholar]
- 27.Pullens MJ, De Vries J, Roukema JA. Subjective cognitive dysfunction in breast cancer patients: a systematic review. Psychooncology. 2010;19(11):1127–1138. doi: 10.1002/pon.1673. [DOI] [PubMed] [Google Scholar]
- 28.Shaffer VA, Merkle EC, Fagerlin A, Griggs JJ, Langa KM, Iwashyna TJ. Chemotherapy was not associated with cognitive decline in older adults with breast and colorectal cancer: findings from a prospective cohort study. Med Care. 2012;50(10):849–855. doi: 10.1097/MLR.0b013e31825a8bb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada TH, Denburg NL, Beglinger LJ, Schultz SK. Neuropsychological outcomes of older breast cancer survivors: cognitive features ten or more years after chemotherapy. J Neuropsychiatry Clin Neurosci. 2010;22(1):48–54. doi: 10.1176/appi.neuropsych.22.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du XL, Xia R, Hardy D. Relationship between chemotherapy use and cognitive impairments in older women with breast cancer: findings from a large population-based cohort. Am J Clin Oncol. 2010;33(6):533–543. doi: 10.1097/COC.0b013e3181b9cf1b. [DOI] [PubMed] [Google Scholar]
- 31.Jim HS, Phillips KM, Chait S, Faul LA, Popa MA, Lee YH, Hussin MG, Jacobsen PB, Small BJ. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol. 2012;30(29):3578–3587. doi: 10.1200/JCO.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips KM, Jim HS, Small BJ, Laronga C, Andrykowski MA, Jacobsen PB. Cognitive functioning after cancer treatment: a 3-year longitudinal comparison of breast cancer survivors treated with chemotherapy or radiation and noncancer controls. Cancer. 2012;118(7):1925–1932. doi: 10.1002/cncr.26432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muss HB, Berry DA, Cirrincione CT, Theodoulou M, Mauer AM, Kornblith AB, Partridge AH, Dressler LG, Cohen HJ, Becker HP, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360(20):2055–2065. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kornblith AB, Lan L, Archer L, Partridge A, Kimmick G, Hudis C, Winer E, Casey R, Bennett S, Cohen HJ, et al. Quality of life of older patients with early-stage breast cancer receiving adjuvant chemotherapy: a companion study to cancer and leukemia group B 49907. J Clin Oncol. 2011;29(8):1022–1028. doi: 10.1200/JCO.2010.29.9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry. 1983;140(6):734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 36.Souder E, Saykin AJ, Alavi A. Multi-modal assessment in Alzheimer’s disease. ADL in relation to PET, MRI and neuropsychology. J Gerontol Nurs. 1995;21(9):7–13. doi: 10.3928/0098-9134-19950901-05. [DOI] [PubMed] [Google Scholar]
- 37.Saykin AJ, Janssen RS, Sprehn GC, Kaplan JE, Spira TJ, O’Connor B. Longitudinal evaluation of neuropsychological function in homosexual men with HIV infection: 18-month follow-up. J Neuropsychiatry Clin Neurosci. 1991;3(3):286–298. doi: 10.1176/jnp.3.3.286. [DOI] [PubMed] [Google Scholar]
- 38.George LK, Fillenbaum GG. OARS methodology. A decade of experience in geriatric assessment. J Am Geriatr Soc. 1985;33(9):607–615. doi: 10.1111/j.1532-5415.1985.tb06317.x. [DOI] [PubMed] [Google Scholar]
- 39.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36(4):428–434. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- 40.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 41.Sprangers MA, Cull A, Bjordal K, Groenvold M, Aaronson NK. The European Organization for Research and Treatment of Cancer. Approach to quality of life assessment: guidelines for developing questionnaire modules. EORTC Study Group on Quality of Life. Qual Life Res. 1993;2(4):287–295. doi: 10.1007/BF00434800. [DOI] [PubMed] [Google Scholar]
- 42.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 43.Cimprich B, Reuter-Lorenz P, Nelson J, Clark PM, Therrien B, Normolle D, Berman MG, Hayes DF, Noll DC, Peltier S, et al. Prechemotherapy alterations in brain function in women with breast cancer. J Clin Exp Neuropsychol. 2010;32(3):324–331. doi: 10.1080/13803390903032537. [DOI] [PubMed] [Google Scholar]
- 44.Cimprich B, So H, Ronis DL, Trask C. Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psychooncology. 2005;14(1):70–78. doi: 10.1002/pon.821. [DOI] [PubMed] [Google Scholar]