Chronic lymphocytic leukemia (CLL), is characterized by the expansion of monoclonal, mature CD5+/CD23+ B-cells.1 In addition, small lymphocytic lymphoma (SLL) shares the biological characteristics of CLL; however, it is distinguished from CLL based on not exceeding 5,000 clonal B-lymphocytes per uL. Here we use the term CLL throughout to denote both CLL and SLL.
A role of antigen signaling in the pathogenesis of CLL has been inferred from the observation that CLL cells use a restricted repertoire of Immunoglobulin Heavy Chain Variable (IGHV) genes, which encode antigen binding domains of the B-cell receptor (BCR),2 and that many CLL cases express virtually identical BCRs, so called “stereotyped BCRs”, that could recognize shared antigens.3 Furthermore, clinical disease progression is predicted by whether the expressed IGHV gene has undergone somatic hypermutation (M-CLL) or not (U-CLL).1 The U-CLL subtype is more rapidly progressive and often expresses ZAP-70, a tyrosine kinase essential for T-cell receptor signaling, which has been shown to enhance BCR signaling in vitro.4 In addition, our recent demonstration of inducible activation of BCR and NF-κB pathways in CLL cells in the lymph node suggests that antigen-dependent BCR signaling continues throughout the disease course.5 While both CLL subtypes showed evidence of antigen signaling in vivo, the more progressive U-CLL subtype had stronger BCR activation, higher expression of MYC and increased proliferation, suggesting that BCR signaling may contribute to clinical progression. These data indicate that BCR signaling likely plays a pivotal role in the pathogenesis of CLL.
Preclinical studies identified inhibition of BCR signaling as a promising strategy in B-malignancies and provided a rationale for the clinical testing of such inhibitors in these diseases.6 Fostamatinib (a prodrug of R406) was developed as a first in class oral inhibitor of SYK, an essential downstream mediator of BCR activation. In vitro, fostamatinib inhibits BCR and integrin signaling, antagonizes the protective effect of stromal cells, reduces migration to chemokines, and induces a moderate degree of apoptosis.7-8 In the TCL1 transgenic mouse model, fostamatinib has been shown to inhibit the growth of malignant B-cells without significant alteration of normal B-cells.9 In the first phase 1/2 clinical trial investigating fostamatinib in relapsed B-cell non-Hodgkin's lymphoma (NHL) and CLL, clinical efficacy was observed in a variety of histologies with the highest response rate in CLL patients (responses and patient characteristics summarized in Supplemental Table S1).10
More recently, additional inhibitors of SYK, as well as inhibitors of PI3Kδ and BTK have shown in vitro and in some cases clinical activity comparable to the effects described for fostamatinib.11 Specifically, all these kinase inhibitors reduce BCR signaling, and inhibit survival and migration of CLL cells in vitro and in mouse models.12-14 However, despite the active clinical development of these agents, no studies have yet reported on treatment-induced changes in tumor biology in patients treated with fostamatinib or other kinase inhibitors targeting the BCR.
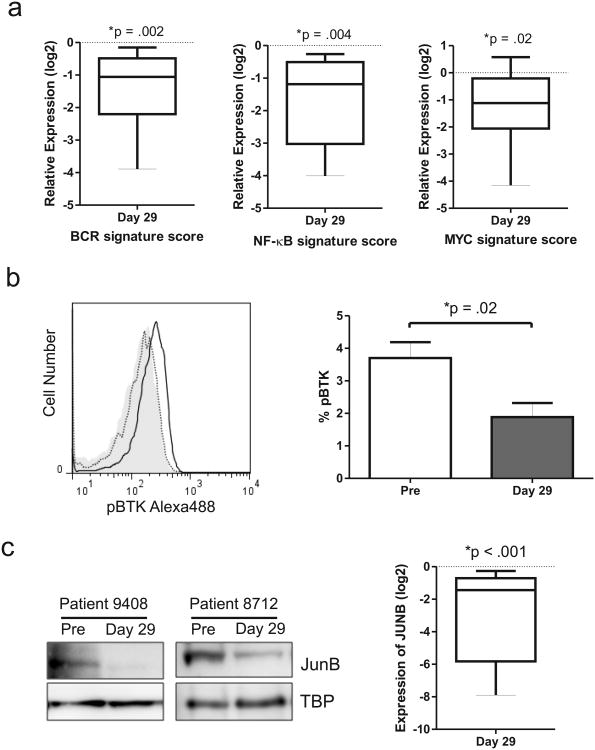
To determine whether fostamatinib inhibits BCR-dependent activation of CLL cells in vivo we measured the expression of representative BCR, NF-κB and MYC target genes in primary tumor samples donated by the eleven CLL patients enrolled in the phase 1/2 study of fostamatinib. Gene expression in purified CLL cells (>90% 19+/CD5+ cells, using CD19+ selection, Miltenyi Biotec, Cambridge, MA) pre-treatment and after one cycle of fostamatinib (Day 29) were compared by quantitative PCR TaqMan assays (Table S2). As a quantitative measure of pathway inhibition in each patient we derived a gene signature score by averaging the mRNA expression level of all target genes. Across all patients, we found that the BCR signature score was significantly reduced (decreased 3-4 fold) by fostamatinib treatment (P =.002; Figure 1a, FigureS1a). Similarly, the NF-κB target and MYC signature scores were also significantly reduced by fostamatinib in all patients evaluated (P =.004 and P =.02, respectively; Figure 1a, Figure S1b-c).
Figure 1.
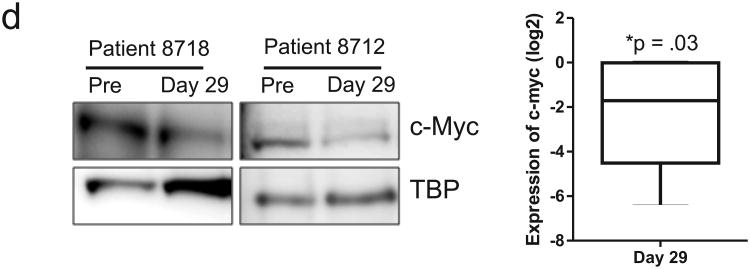
Fostamatinib inhibits BCR signal transduction in vivo. (a) Relative mRNA expression of BCR, NF-κB and MYC signature genes (identified in 5) in purified CLL cells (CD19+) of eleven patients assessed on day 29 compared to pre-treatment and normalized to the housekeeping gene VCP (valosin containing protein). Relative expression of signature scores is summarized for all patients in a boxplot. (b) Inhibition of BTK activation by fostamatinib in CLL cells was assessed by flow cytometry. Shown is the histogram of pBTK staining in CLL cells of a representative patient; isotype control (grey shaded area), pre-treatment sample (solid black line), day 29 (dotted black line) and the mean percentage (± SEM) of pBTK expressing CLL cells pre-treatment and on day 29 of fostamatinib treatment (n=7). Comparison by paired student T-test. (c) Immunoblots showing reduced JUNB expression in CLL cells from two representative patients on day 29 of fostamatinib treatment compared to pre-treatment (TBP is used as loading control) and the mean (± SEM) change in JUNB on fostamatinib treatment (n=5). Comparison by paired student T-test. (d) Immunoblots showing reduced MYC expression in CLL cells from two representative patients on day 29 of fostamatinib treatment compared to pre-treatment and the mean (± SEM) change in MYC expression on fostamatinib treatment (n=5). Comparison by paired student T-test.
We next evaluated the activation state of signal transduction proteins in these pathways. A direct downstream target of SYK is BTK. We therefore evaluated the effect of fostamatinib on BTK phosphorylation by flow cytometry. Figure 1b demonstrates treatment induced reduction of BTK phosphorylation, pBTK(Y551), in a representative sample. By comparing pre-treatment samples to day 29 samples we found that the amount of activated BTK in CLL cells was significantly reduced by fostamatinib in all patients tested (P =.02; Figure 1b). Consistently, we found that levels of JUNB protein, the product of a representative NF-κB target gene, was significantly reduced by fostamatinib in all patients evaluated (P <.001; Figure 1c). Lastly, a reduction in MYC protein expression was seen in all patients having detectable MYC protein at baseline, consistent with the observed reduction in gene expression (P =.03; Figure 1d). Taken together, these data demonstrate that fostamatinib effectively inhibits BCR signal transduction and activation of downstream effector pathways in the tumor cells in vivo.
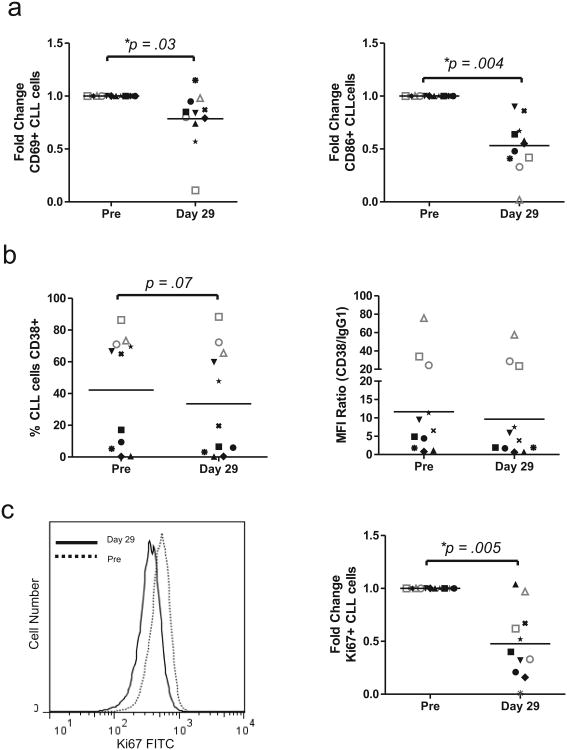
Having observed a significant decrease in BCR mediated signaling on fostamatinib we next assessed the effect on CLL cell activation as reflected in the expression of cell surface markers by flow cytometry. Expression of CD69 and CD86, two activation markers known to be unregulated on CLL cells activated in vivo,5 was decreased on CLL cells expressing on day 29 compared to baseline (P =.03 and P=.004, respectively Figure 2a). We next evaluated the expression of CD38, which is not only a prognostic marker but is upregulated on activated CLL cells in the LN-microenvironment.8, 31 As expected, only in a subset of patients ≥30% of the CLL population expressed CD38 (Figure 2b). Fostamatinib reduced CD38 expression in some patients but not in others and overall this difference was not significant (Figure 2b). Interestingly, in the three patients with the highest percentage of CD38 positive cells, fostamatinib had no effect on CD38 expression and all three failed to achieve a clinical response. CLL cells of these non-responders showed bright CD38 expression with a nearly 9-fold increase in mean fluorescent intensity (MFI) compared to patients achieving a clinical response (P =.002, Figure 2b). If confirmed in larger studies, this suggests that expression of CD38 could serve as a possible biomarker for response.
Figure 2.
Fostamatinib reduces CLL cell activation and proliferation in vivo. (a-b) Expression of cell surface markers on CLL cells evaluated by flow cytometry on day 29 is compared to pre-treatment samples. Patients are coded by different symbols; open symbols with grey lines identify patients with no clinical response to fostamatinib. Relative percentage of cells expressing (a) CD69 or CD86 is depicted. (b) Percentage of CLL cells expressing CD38 (left panel) and the ratio of the mean florescent intensity (MFI) of CD38 to an IgG1 isotype control (right pannel) is shown. Comparisons by paired student T-test. (c) Ki67 expression in CLL cells measured by flow cytometry on day 29 compared to pre-treatment samples. A histogram showing decreased Ki67 staining in CLL cells of a representative patient treated with fostamatinib; dotted black line (pre-treatment), solid black line (day 29 on fostamatinib) and fold change in the number of CLL cells expressing Ki67 on day 29 compared to pre-treatment. Patients are coded by different symbols; open symbols with grey lines identify patients with no clinical response to fostamatinib. Comparison by paired student T-test.
Lastly, we sought to determine whether fostamatinib could disrupt CLL cell proliferation. Tumor proliferation has been correlated with clinical outcome and is likely driven by BCR signaling.5, 12 Using the proliferation marker, Ki67, we found a substantial decrease in the proliferative fraction of CLL cells on day 29 compared to pre-treatment. The percentage of CLL cells positive for Ki67 decreased in nine of the eleven patients and across the whole group was significantly decreased by fostamatinib (P =.005, Figure 2c). The two patients with no detectable decrease in Ki67 expression had so few Ki67+ cells at baseline, that a possible treatment induced reduction could not be assessed. Together this demonstrates that fostamatinib inhibits CLL cell activation and proliferation in vivo.
Although much pre-clinical data on these kinase inhibitors is now available (summarized in 11) herein we present, to our knowledge, the first ex vivo analysis of tumor samples from patients treated with fostamatinib, or any of the kinase inhibitors targeting the BCR pathway. We demonstrate that patients treated in vivo with fostamatinib display inhibition of BCR and NF-κB signaling, reduction in activation and reduced proliferation of the tumor cell population. An additional intriguing novel finding is the correlation of CD38bright expression to inferior response, suggesting CD38 as a possible biomarker to predict treatment response. In immature B-cells CD38 activation leads to activation of SYK and engagement of the PI3K pathway.15 This raises the question whether CD38 activation might be able to overcome inhibition of SYK either through increased activation of the kinase or by directly activating downstream pathways. We suggest that future trials of novel BCR inhibitors correlate clinical activity with CD38 expression to extend our initial findings.
Fostamatinib was the first kinase inhibitor demonstrating clinical activity in relapsed mature B-cell malignancies.10 Recently, several additional inhibitors that target kinases downstream of the BCR including the BTK inhibitor ibrutinib and the PI3Kδ inhibitor GS-1101 have shown promising clinical activity in early stage trials.11 Fostamatinib, ibrutinib, and GS-1101 induce only a moderate degree of apoptosis in vitro,11-14 suggesting that interruption of BCR signaling and possibly other survival pathways activated in the tissue microenvironments may be responsible for the observed clinical response. The ex vivo analysis of peripheral blood derived CLL cells presented here provides an important confirmation of on target inhibition of BCR signaling by fostamatinib and demonstrates inhibition of CLL cell activation and proliferation in vivo. However, given the importance of the microenvironment in CLL pathogenesis it will be important to extend these studies to tissue samples. Given the enthusiasm and ongoing development of novel inhibitors of BCR in CLL, our results could serve as a paradigm for future pharmacodynamic evaluation of these agents, alone, and in rational, targeted therapy combinations.
Supplementary Material
Supplemental Figure S1. (a-c) Relative mRNA expression of BCR (a) NF-κB (b) and MYC (c) signature genes (identified in 5) in purified CLL cells (CD19+) of eleven patients assessed on day 29 compared to pre-treatment and normalized to the VCP housekeeping gene. Shown is the mean change (± SEM) in the expression of each gene in the signature across all patients.
Acknowledgments
This research was funded through the Intramural Research Program of the National, Heart, Lung and Blood Institute. This work was supported in part by the University of Rochester SPORE in lymphoma P50 CA13080503 and the James P. Wilmot Foundation. PMB is a Lymphoma Research Foundation Clinical Investigator. JWF is a Scholar in Clinical Research of the Leukemia and Lymphoma Society.
Research support: This research was supported by the Intramural Research Program of the National, Heart, Lung and Blood Institute
Footnotes
Supplementary information is available at Leukemia's website (http://www.nature.com/leu)
Authorship Contributions: SEMH planned the research, performed experiments, and analyzed data; PMB analyzed patient data; EMM was involved in planning components of the research and supported experiments; DL performed statistical analysis; and AW and JWF planned and supervised the research and analyzed data. All authors assisted in the writing of this paper and approved the final version of the manuscript.
Conflict-of-Interest Disclosure: JWF received research support from Rigel for the clinical trial and subsequent analysis. All other authors declare no competing financial interests.
References
- 1.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352(8):804–15. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 2.Tobin G, Thunberg U, Karlsson K, Murray F, Laurell A, Willander K, et al. Subsets with restricted immunoglobulin gene rearrangement features indicate a role for antigen selection in the development of chronic lymphocytic leukemia. Blood. 2004;104(9):2879–85. doi: 10.1182/blood-2004-01-0132. [DOI] [PubMed] [Google Scholar]
- 3.Messmer BT, Albesiano E, Efremov DG, Ghiotto F, Allen SL, Kolitz J, et al. Multiple distinct sets of stereotyped antigen receptors indicate a role for antigen in promoting chronic lymphocytic leukemia. J Exp Med. 2004;200(4):519–25. doi: 10.1084/jem.20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L, Apgar J, Huynh L, Dicker F, Giago-McGahan T, Rassenti L, et al. ZAP-70 directly enhances IgM signaling in chronic lymphocytic leukemia. Blood. 2005;105(5):2036–41. doi: 10.1182/blood-2004-05-1715. [DOI] [PubMed] [Google Scholar]
- 5.Herishanu Y, Perez-Galan P, Liu D, Biancotto A, Pittaluga S, Vire B, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117(2):563–74. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gobessi S, Laurenti L, Longo PG, Carsetti L, Berno V, Sica S, et al. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia. 2009;23(4):686–97. doi: 10.1038/leu.2008.346. [DOI] [PubMed] [Google Scholar]
- 7.Buchner M, Baer C, Prinz G, Dierks C, Burger M, Zenz T, et al. Spleen tyrosine kinase inhibition prevents chemokine- and integrin-mediated stromal protective effects in chronic lymphocytic leukemia. Blood. 2010;115(22):4497–506. doi: 10.1182/blood-2009-07-233692. [DOI] [PubMed] [Google Scholar]
- 8.Quiroga MP, Balakrishnan K, Kurtova AV, Sivina M, Keating MJ, Wierda WG, et al. B-cell antigen receptor signaling enhances chronic lymphocytic leukemia cell migration and survival: specific targeting with a novel spleen tyrosine kinase inhibitor, R406. Blood. 2009;114(5):1029–37. doi: 10.1182/blood-2009-03-212837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suljagic M, Longo PG, Bennardo S, Perlas E, Leone G, Laurenti L, et al. The Syk inhibitor fostamatinib disodium (R788) inhibits tumor growth in the Emu- TCL1 transgenic mouse model of CLL by blocking antigen-dependent B-cell receptor signaling. Blood. 2010;116(23):4894–905. doi: 10.1182/blood-2010-03-275180. [DOI] [PubMed] [Google Scholar]
- 10.Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115(13):2578–85. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiestner A. Emerging role of kinase-targeted strategies in chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2012;2012:88–96. doi: 10.1182/asheducation-2012.1.88. [DOI] [PubMed] [Google Scholar]
- 12.Herman SEM, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117(23):6287–96. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herman SEM, Gordon AL, Wagner AJ, Heerema NA, Zhao W, Flynn JM, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116(12):2078–88. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoellenriegel J, Coffey GP, Sinha U, Pandey A, Sivina M, Ferrajoli A, et al. Selective, novel spleen tyrosine kinase (Syk) inhibitors suppress chronic lymphocytic leukemia B-cell activation and migration. Leukemia. 2012;26(7):1576–83. doi: 10.1038/leu.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silvennoinen O, Nishigaki H, Kitanaka A, Kumagai M, Ito C, Malavasi F, et al. CD38 signal transduction in human B cell precursors. Rapid induction of tyrosine phosphorylation, activation of syk tyrosine kinase, and phosphorylation of phospholipase C-gamma and phosphatidylinositol 3-kinase. J Immunol. 1996;156(1):100–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. (a-c) Relative mRNA expression of BCR (a) NF-κB (b) and MYC (c) signature genes (identified in 5) in purified CLL cells (CD19+) of eleven patients assessed on day 29 compared to pre-treatment and normalized to the VCP housekeeping gene. Shown is the mean change (± SEM) in the expression of each gene in the signature across all patients.