Abstract
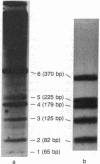
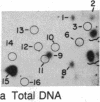
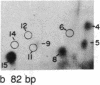
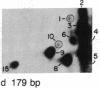
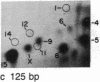
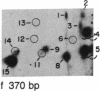
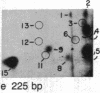
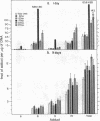
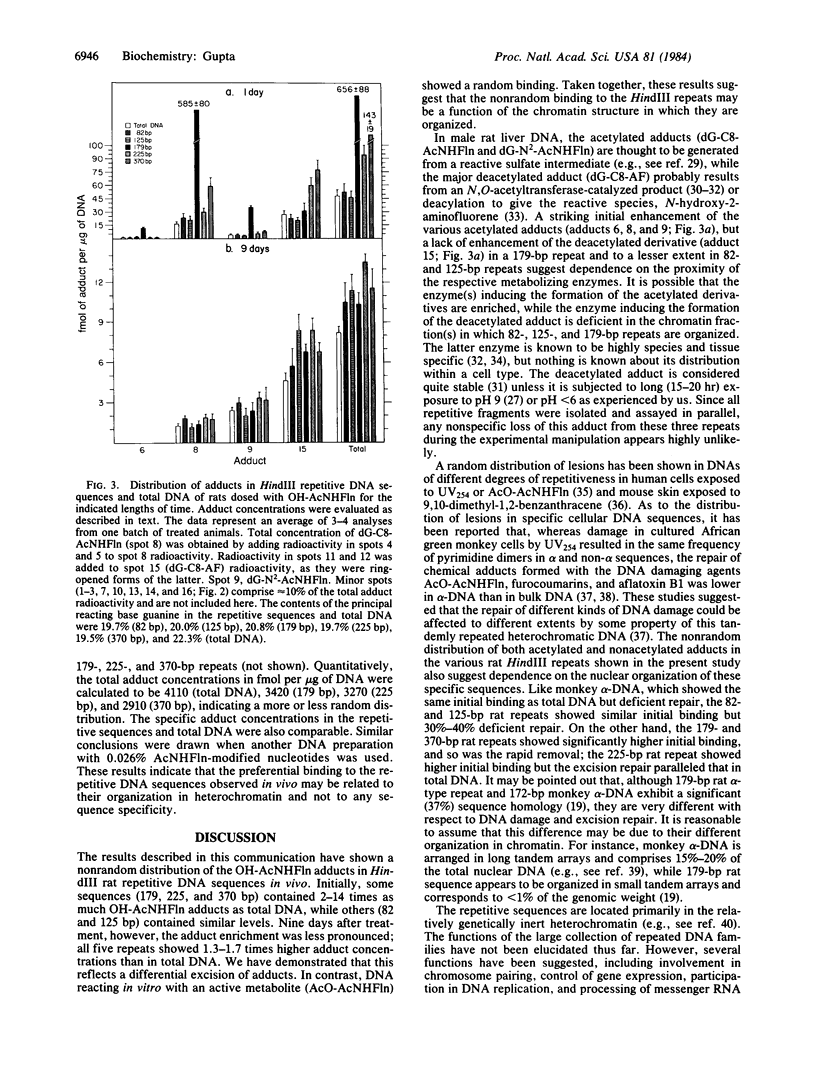
We have examined the distribution of individual adducts in repetitive DNA sequences of rat liver in vivo after a single dose of the carcinogen N-hydroxy-2-acetyl-aminofluorene. Repetitive fragments [82, 125, 179, 225, and 370 base pairs (bp)] were isolated by digestion of hepatic DNA with HindIII restriction endonuclease (EC 3.1.23.21) and gel electrophoresis. As assayed by 32P postlabeling, no qualitative differences were observed between the DNA-bound metabolites in repetitive sequences and total DNA, but preferential binding to these sequences occurred. After 1 day of treatment, the amounts of N-hydroxy-2-acetylaminofluorene-induced adducts were found to be 13.8, 2.0, and 3.0 times higher in 179-, 225-, and 370-bp repeats, respectively, than in total DNA, while 82- and 125-bp repeats showed no differences. The relative distribution of individual adducts varied among the various sequences. After 9 days, all five sequences showed 1.3-1.7 times higher binding as compared to total DNA. In contrast, a random binding was observed when DNA reacted in vitro with an active metabolite, N-acetoxy-2-acetylaminofluorene. Taken together, these results suggest that the enrichment and differential excision of adducts in the repetitive DNA sequences may be a function of the nuclear organization of DNA. This application of the 32P assay constitutes a means to study the DNA damage and excision repair in vivo in chromatin structural components, including transcribed and nontranscribed multiple-copy genes, in a much more sensitive and precise way than has been hitherto possible.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey G. S., Nixon J. E., Hendricks J. D., Sinnhuber R. O., Van Holde K. E. Carcinogen aflatoxin B1 is located preferentially in internucleosomal deoxyribonucleic acid following exposure in vivo in rainbow trout. Biochemistry. 1980 Dec 9;19(25):5836–5842. doi: 10.1021/bi00566a027. [DOI] [PubMed] [Google Scholar]
- Bartsch H., Dworkin M., Miller J. A., Miller E. C. Electrophilic N-acetoxyaminoarenes derived from carcinogenic N-hydroxy-N-acetylaminoarenes by enzymatic deacetylation and transacetylation in liver. Biochim Biophys Acta. 1972 Dec 29;286(2):272–298. doi: 10.1016/0304-4165(72)90265-6. [DOI] [PubMed] [Google Scholar]
- Beland F. A., Allaben W. T., Evans F. E. Acyltransferase-mediated binding of N-hydroxyarylamides to nucleic acids. Cancer Res. 1980 Mar;40(3):834–840. [PubMed] [Google Scholar]
- Bonner J., Garrard W. T., Gottesfeld J., Holmes D. S. Functional organization of the mammalian genome. Cold Spring Harb Symp Quant Biol. 1974;38:303–310. doi: 10.1101/sqb.1974.038.01.034. [DOI] [PubMed] [Google Scholar]
- Brutlag D. L. Molecular arrangement and evolution of heterochromatic DNA. Annu Rev Genet. 1980;14:121–144. doi: 10.1146/annurev.ge.14.120180.001005. [DOI] [PubMed] [Google Scholar]
- Fuke M., Busch H. HindIII-sensitive sites present once in every four repeats of EcoRI-sensitive sites in Novikoff rat hepatoma DNA. FEBS Lett. 1979 Mar 1;99(1):136–140. doi: 10.1016/0014-5793(79)80265-3. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Dighe N. R. Formation and removal of DNA adducts in rat liver treated with N-hydroxy derivatives of 2-acetylaminofluorene, 4-acetylaminobiphenyl, and 2-acetylaminophenanthrene. Carcinogenesis. 1984 Mar;5(3):343–349. doi: 10.1093/carcin/5.3.343. [DOI] [PubMed] [Google Scholar]
- Gupta R. C. Nucleotide sequence of a reiterated rat DNA fragment. FEBS Lett. 1983 Nov 28;164(1):175–180. doi: 10.1016/0014-5793(83)80044-1. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Reddy M. V., Randerath K. 32P-postlabeling analysis of non-radioactive aromatic carcinogen--DNA adducts. Carcinogenesis. 1982;3(9):1081–1092. doi: 10.1093/carcin/3.9.1081. [DOI] [PubMed] [Google Scholar]
- Hemminki K. Nucleic acid adducts of chemical carcinogens and mutagens. Arch Toxicol. 1983 Apr;52(4):249–285. doi: 10.1007/BF00316495. [DOI] [PubMed] [Google Scholar]
- Hemminki K., Vainio H. Preferential binding of benzo[a]pyrene into nuclear matrix fraction. Cancer Lett. 1979 Mar;6(3):167–173. doi: 10.1016/s0304-3835(79)80028-2. [DOI] [PubMed] [Google Scholar]
- Irvin T. R., Wogan G. N. Quantitation of aflatoxin B1 adduction within the ribosomal RNA gene sequences of rat liver DNA. Proc Natl Acad Sci U S A. 1984 Feb;81(3):664–668. doi: 10.1073/pnas.81.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack P. L., Brookes P. The distribution of benzo(a)pyrene DNA adducts in mammalian chromatin. Nucleic Acids Res. 1981 Nov 11;9(21):5533–5552. doi: 10.1093/nar/9.21.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W. R., Schmid C. W. Repetitive sequences in eukaryotic DNA and their expression. Annu Rev Biochem. 1982;51:813–844. doi: 10.1146/annurev.bi.51.070182.004121. [DOI] [PubMed] [Google Scholar]
- KIRBY K. S. A new method for the isolation of deoxyribonucleic acids; evidence on the nature of bonds between deoxyribonucleic acid and protein. Biochem J. 1957 Jul;66(3):495–504. doi: 10.1042/bj0660495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M., Cerutti P. A. Excision of benzo[a]pyrene diol epoxide I adducts from nucleosomal DNA of confluent normal human fibroblasts. Chem Biol Interact. 1982 Feb;38(3):261–274. doi: 10.1016/0009-2797(82)90057-6. [DOI] [PubMed] [Google Scholar]
- King C. M. Mechanism of reaction, tissue distribution, and inhibition of arylhydroxamic acid acyltransferase. Cancer Res. 1974 Jun;34(6):1503–1515. [PubMed] [Google Scholar]
- King C. M., Olive C. W. Comparative effects of strain, species, and sex on the acyltransferase- and sulfotransferase-catalyzed activations of N-hydroxy-N-2-fluorenylacetamide. Cancer Res. 1975 Apr;35(4):906–912. [PubMed] [Google Scholar]
- Kootstra A., Slaga T. J. Binding of isomers of benzo[a]pyrene diol-epoxide to chromatin. Biochem Biophys Res Commun. 1980 Apr 14;93(3):954–959. doi: 10.1016/0006-291x(80)91168-7. [DOI] [PubMed] [Google Scholar]
- Lapeyre J. N., Becker F. F. Analysis of highly repeated DNA sequences of rat with EcoR1 endonuclease. Biochim Biophys Acta. 1980 Mar 28;607(1):23–35. doi: 10.1016/0005-2787(80)90217-8. [DOI] [PubMed] [Google Scholar]
- Leadon S. A., Zolan M. E., Hanawalt P. C. Restricted repair of aflatoxin B1 induced damage in alpha DNA of monkey cells. Nucleic Acids Res. 1983 Aug 25;11(16):5675–5689. doi: 10.1093/nar/11.16.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin R. Repeated DNA still in search of a function. Science. 1982 Aug 13;217(4560):621–623. doi: 10.1126/science.6283639. [DOI] [PubMed] [Google Scholar]
- Lieberman M. W., Poirier M. C. Distribution of deoxyribonucleic acid repair synthesis among repetitive and unique sequences in the human diploid genome. Biochemistry. 1974 Jul 16;13(15):3018–3023. doi: 10.1021/bi00712a003. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Meerman J. H., Beland F. A., Mulder G. J. Role of sulfation in the formation of DNA adducts from N-hydroxy-2-acetylaminofluorene in rat liver in vivo. Inhibition of N-acetylated aminofluorene adduct formation by pentachlorophenol. Carcinogenesis. 1981;2(5):413–416. doi: 10.1093/carcin/2.5.413. [DOI] [PubMed] [Google Scholar]
- Mironov N. M., Grover P. L., Sims P. Preferential binding of polycyclic hydrocarbons to matrix-bound DNA in rat-liver nuclei. Carcinogenesis. 1983;4(2):189–193. doi: 10.1093/carcin/4.2.189. [DOI] [PubMed] [Google Scholar]
- Moyer G. H., Gumbiner B., Austin G. E. Binding of N-hydroxy acetylaminofluorene to eu- and heterochromatin fractions of rat liver in vivo. Cancer Lett. 1977 Mar;2(4-5):259–265. doi: 10.1016/s0304-3835(77)80030-x. [DOI] [PubMed] [Google Scholar]
- Müller R., Rajewsky M. F. Immunological quantification by high-affinity antibodies of O6-ethyldeoxyguanosine in DNA exposed to N-ethyl-N-nitrosourea. Cancer Res. 1980 Mar;40(3):887–896. [PubMed] [Google Scholar]
- Pech M., Igo-Kemenes T., Zachau H. G. Nucleotide sequence of a highly repetitive component of rat DNA. Nucleic Acids Res. 1979 Sep 25;7(2):417–432. doi: 10.1093/nar/7.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippsen P., Streeck R. E., Zachau H. G. Defined fragments of calf, human, and rat DNA produced by restriction nucleases. Eur J Biochem. 1974 Jun 15;45(2):479–488. doi: 10.1111/j.1432-1033.1974.tb03573.x. [DOI] [PubMed] [Google Scholar]
- Ramanathan R., Rajalakshmi S., Sarma D. S., Farber E. Nonrandom nature of in vivo methylation of dimethylnitrosamine and the subsequent removal of methylated products from rat liver chromatin DNA. Cancer Res. 1976 Jun;36(6):2073–2079. [PubMed] [Google Scholar]
- Schut H. A., Wirth P. J., Thorgeirsson S. S. Mutagenic activation of N-hydroxy-2-acetylaminofluorene in the Salmonella test system: the role of deacetylation by liver and kidney fractions from mouse and rat. Mol Pharmacol. 1978 Jul;14(4):682–692. [PubMed] [Google Scholar]
- Schwartz E. L., Braselton W. E., Jr, Goodman J. I. Factors influencing the binding of N-2-fluorenylacetamide to specific regions of the hepatic genome in vivo in rats. J Natl Cancer Inst. 1981 Apr;66(4):667–672. [PubMed] [Google Scholar]
- Singer D. S. Arrangement of a highly repeated DNA sequence in the genome and chromatin of the African green monkey. J Biol Chem. 1979 Jun 25;254(12):5506–5514. [PubMed] [Google Scholar]
- Ueyama H., Matsuura T., Nomi S., Nakayasu H., Ueda K. Binding of benzo(a)pyrene to rat liver nuclear matrix. Life Sci. 1981 Aug 17;29(7):655–661. doi: 10.1016/0024-3205(81)90017-5. [DOI] [PubMed] [Google Scholar]
- Yu F. L. Preferential binding of aflatoxin B1 to the transcriptionally active regions of rat liver nucleolar chromatin in vivo and in vitro. Carcinogenesis. 1983;4(7):889–893. doi: 10.1093/carcin/4.7.889. [DOI] [PubMed] [Google Scholar]
- Zeiger R. S., Salomon R., Kinoshita N., Peacock A. C. The binding of 9,10-dimethyl-1,2-benzanthracene to mouse epidermal satellite DNA in vivo. Cancer Res. 1972 Mar;32(3):643–647. [PubMed] [Google Scholar]
- Zolan M. E., Cortopassi G. A., Smith C. A., Hanawalt P. C. Deficient repair of chemical adducts in alpha DNA of monkey cells. Cell. 1982 Mar;28(3):613–619. doi: 10.1016/0092-8674(82)90216-1. [DOI] [PubMed] [Google Scholar]