Abstract
Removing the bacteria, including Enterococcus faecalis, from the root canal is one of the important aims in endodontic treatment.We aimed to compare the antibacterial activity of Chlorhexidine with two natural drugs. The antibacterial activities of three different propolis extracts (alcohol concentrations: 0, 15, 40%) and Aloe vera gel on E. faecalis were compared using three methods: disk diffusion, microdilution and direct contact test. In addition to the above bacterium, the Aloe vera gel effect on Staphylococcus aureus and Streptococcus mutans was evaluated. Disk diffusion test revealed that propolis ethanolic extracts (the alcohol concentration of 15 and 40%) and Aloe vera gel have antibacterial activities but aqueous extract of propolis did not show any effect in this test. The MICs for propolis ethanolic extracts, Aloe vera gel and aqueous extract of propolis (0% alcohol) were 313 µg/ml, 750 µg/ml, 2250 µg/ml, and ≥ 500 µg/ml respectively, much higher than the Chlorhexidine one. In direct contact test, contrary to Aloe vera, all three propolis extracts showed antibacterial effects on E. faecalis. The Aloe vera gel also showed significant antibacterial effect on S.aureus and S.mutans. The hydroalcoholic extracts of propolis and Aloe vera gel had antibacterial effects on E. faecalis, however, propolis is more potent than Aloe vera. The antibacterial effect of Aloe vera on S. aureus and S. mutans is low (MIC ≥ 2250 µg/ml). Appropriate concentrations of alcoholic extracts of propolis and some fractions of Aloe vera gel might be good choices for disinfecting the root canal in endodontic treatments.
Key Words: Chlorhexidine, root canal, antiseptic, S. aureus, S. mutans
One of the main goals in endodontic treatments is removing the bacteria from the root canal system. Although chemo - mechanical preparation of root canal is able to decrease the bacterial load, the resistant microorganisms usually remain in the canal space even after the instrumentation and washing processes. The main reasons behind this contamination are: the complex anatomy of pulp system, existence of the secondary canals, and ability of microorganisms to survive in harsh conditions (1-2). E. faecalis is an anaerobic gram-positive bacterium which is found in periapical lesions. It is able to attack dentinal tubules and easily copes with hard condition of root canal which make it a resistant microorganisms (3). Some studies on root treated teeth have shown that E. faecalis bacteria are prevalent up to 77% in the periradicular lesions. In fact, the involvement of this bacterium in root canal treatment failure is more likely than the primary endodontic lesions (4). Sodium hypochlorite has been used as an intracanal irrigant, however, due to its adverse effects including damage to tissues and inducing emph-ysema, its used has been restricted. Chlorhexidine 2% solution is used as an intracanal irrigant with antibacterial properties and great ability to disinfect the dentinal tubules against E. faecalis, however its use has been restricted due to: discoloration of the teeth and tongue, decreasing the sense of taste, irritation of oral mucosa and mouth dryness. Nowadays, due to its antibacterial properties, calcium hydroxide is highly used as the intracanal medication. But again, because of its high pH, this subtance is so toxic to the tissues which can lead to chronic inflammation and cell necrosis (5-6). Because of the cytotoxicity induced by common intracanal drugs, their inability to remove some bacteria from the dentinal tubules, and the microorganisms’ resistance phenomenon, looking for new intracanal drugs especially among natural resources are highly recommended (7).
Propolis is a dense yellow-brown resin-like material which its solubility is low in water, but high in ethanol (8). This material is made from resin, bud and other parts of the plants by bees. It is used for protecting the hive against the outside pollutions and blocking the slots and cracks. Propolis has antibacterial, antifungal, antiviral, antiinflammation, antioxidant and anti-tumor effects (8-9) and many applications for this substance in dentistry has been recently reported (7). Aloe vera, along with other 360 species, belongs to liliaceae family. This plant can grow in hot and dry weather due to its high capacity in maintaining water. Aloe vera has antibacterial, anti-fungal, antivirus, antiinflammation, and anti-tumor properties which make it useful in broadrange of ailments including: arthritis, asthma, gastrointestinal diseases, and skin problems (e.g. psoriasis, burning and wounds).
In dentistry, Aloe vera has been used in recurrent aphthous ulcers, alveolar osteitis, and lichen planus lesions (10-12).
The aim of this study was to determine the antibacterial potency of Aloe vera compared to propolis and Chlorhexidine. Also, the effect of ethanol concentration on antibacterial activity of hydroalcoholic extracts of propolis was investigated.
Materials and Methods
Propolis quality control assays
About 150 grams of propolis was freshly collected from Amirkola’s (Mazadaran-Iran) honey bees’ nests during the 2012 winter. Standard microbiological and chemical assays were performed on the sample by Suren Tak Toos Lab. Co. (Mashhad-Iran).
Propolis hydroalcoholic extraction
Propolis was dispersed in absolute ethanol (500 mg in 50 ml) at 37ºC using magnet stirring for 1.5 hours. The obtained opaque yellow liquid passed through filter (Whatman#1) and centrifuged at 22ºC for 10 minutes (800 g). The clear supernatant was diluted with appropriate amounts of sterile distilled water to give ethanol concentration of either 15%. or 40%. To make aqueous extract, propolis was dispersed in sterile distilled water (500 mg in 50 ml) at 22ºC using magnet stirring for 4 hours. The obtained opaque liquid was filtered and centrifuged at 22ºC for 10 minutes (800g). These extracts were kept in the fridge (less than 1 week) and by warming up to 37ºC any precipitate was dissolved before use.
Aloe vera physicochemical analyses
Aloe vera gel was kindly gifted by Barij Essence (Kashan-Iran). Standard physicochemical assays including carbohydrates content, dry substance, ash weight, and capillary viscometry were performed.
The test microorganisms
The sample of standard strains of E. faecalis PTCC 1394, S. mutans ATCC 1601 and S. aureus ATCC 25923, were obtained from the Scientific-Industrial Research Center of Asre-Enghelab (Tehran-Iran) and were inoculated in Brain Heart Infusion (BHI) culture medium.
Disk diffusion test
The method of Kirby-Bauer disk-diffusion was performed for this assay. Briefly sterile paper disks (6.4 mm) were soaked in the test material solutions for 10 minutes. Ethanol (15, 40%) and distilled water were used as negative control. The impregnated paper disks were placed on the surface of blood agar culture plates previously inoculated by the test microorganism (E. faecalis, S. mutans, S. aureous). The inhibition zone was measured for each test material.
Direct contact test
The test material solutions (500 µL each) were dried on the bottom of a 24-well plate. Then 50 µL of the test bacterial suspension (1.5×107 CFU/ml) was poured into each well and left to dry in a laminar airflow. After that, 500 µL of BHI was added to each well and the plate was incubated at 37ºC. After 24 hours, the colony count of 5 µL of each well’s solution was measured.
The microdilution test
Broth microdilution test was performed as described in M27-A2 (CLSI) with minor modi-fications. The test material solutions was firstly diluted 50:50 in 2X BHI medium then serial dilutions were made using (100 µL) 1X BHI in each well, then 10 µL of microbial suspension (1.5×107 CFU/ml) was added. After 24 hours incubating at 37ºC, the last well without opacity was considered as minimum inhibitory concen-tration (MIC). The well with lowest concentration of the tested material, which could not lead to microbial growth (99.9% inhibition) after inocu-lating the blood agar plate, was considered as the minimum bactericidal concentration (MBC). Also the microdilution test was performed on Aloe vera using two additional microorganisms (S.aureus and S.mutans).
Statistical analyses
The data are presented as mean±SD and analyzed by ANOVA. In case of significance, the multi fold Scheffe comparisons and t-test were used for two by two comparisons. P < 0.05 was consid-ered significant.
Results
The antibacterial activity of propolis hydroalcoholic extracts (with 0, 15, 40% ethanol), Aloe vera gel, and Chlorhexidine 2% on E. faecalis bacteria are compared using three methods: disk diffusion, direct contact and microdilution. In regards to Aleo vera, disk diffusion and micro-dilution tests, have been performed usingtwo additional bacteria (S. aureus, S. mutans) to investigate more its antimicrobial spectrum.
Propolis and Aloe vera quality control assays
The results of some quality control tests on propolis are shown in table 1. The physicochemical analysis data of Aloe vera are shown in table 2.
Table 1.
The quality control assays on propolis sample and its extracts
| Result (unit) | Conducted assay |
|---|---|
| brown | Sample color |
| 26.2 (%) | Total polyphenol content |
| Negative (cfu/g) | E. coli growth |
| Negative (cfu/g) | Staphylococcus aureus growth |
| Negative (cfu/g) | Pseudomonas Sp. growth |
| Negative (cfu/g) | Aspergillus growth |
| 55.8 (%) | Dried mass |
| 16.3 (%) | Total carbohydrate content |
| 0.5 (%) | Total protein content |
| Positive | Free amino acid (detected by TLC) |
| Positive | Free sugars (4 and 5 carbon detected by TLC) |
| 2.35 (%) | Insoluble substances in 10% alcohol |
| 2.87 (%) | Reduced sugar |
| 0.1 (%) | Dry substance of saturated aqueous extract (0% ethanol) |
| 0.5 (%) | Dry substance of propolis hydroalcoholic extract (40% ethanol) |
| 0.3 (%) | Dry substance of propolis hydroalcoholic extract (15% ethanol) |
Table 2.
The physicochemical analysis of Aloe vera sample
| Color | Colorless |
|---|---|
| pH | 4.45 |
| Density | 0.9739 (g/ml) |
| Dry weight | 0.9 % |
| Ash weight | 0.29% |
| Viscosity | 2.0575 )cP( |
| Glucomannan | 0.049% |
| Carbohydrate | 0.43% |
Disk diffusion test
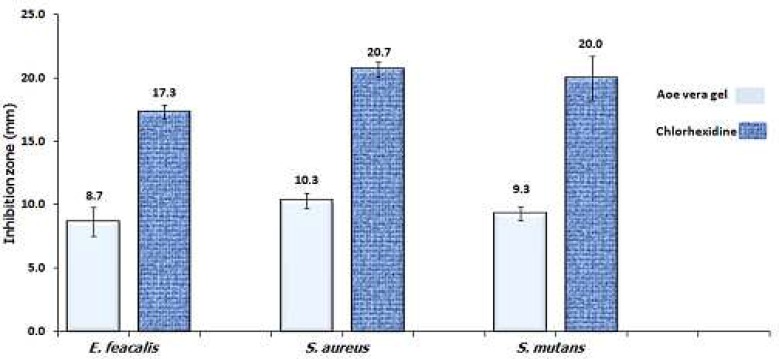
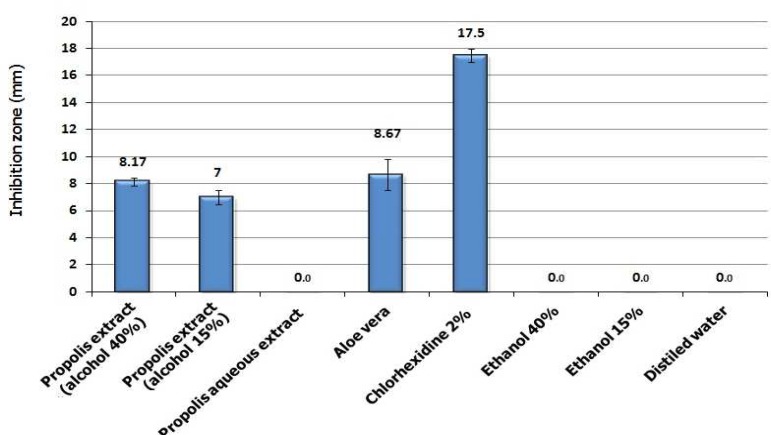
Propolis hydroalcoholic extract (with 15 and 40% ethanol) and Aloe vera gel showed antibacterial effect with no significant difference among them. However, no inhibition zone was observed with propolis aqueous extract (0% ethanol). Chlorhexidine 2% produced significantly higher inhibition zone compared to the other extracts (P< 0.001) (Fig. 1). The Aloe vera gel was less effective than Chlorhexidine 2% not only against E. faecalis but also against S. aureus and S. mutans (Fig. 2).
Fig 1.
Growth inhibition zone (mean ±SD) induced by different propolis hydroalcoholic extracts (with 0, 15, 40% ethanol), Aloe vera gel and chlorohexidine 2% in the method of disk diffusion with E. faecalis
Fig 2.
A comparison between Aloe vera and chlorohexidine 2% antibacterial activity against 3 test microorganisms using disk diffusion test. The label numbers are the mean of inhibition zone for three replicate disks
Microdilution test
The MIC results for propolis hydroalcoholic extracts, Aloe vera gel and Chlorhexidine 2% solution have been presented below (Table 3).The propolis aqueous extract (0% ethanol) did not show any inhibition in microdilution test (MIC> propolis solubility). Chlorhexidine showed the lowest MIC (2 µg/ml) compared to the other tested materials. In addition to E. faecalis, Aloe vera showed antibacterial activity against two gram positive cocci (S. aureus, S. mutans) in this test (Table 3).
Table 3.
MIC and MBC in hydroalcoholic extract of propolis, Aleo vera and Chlorohexidine 2 % using the microdilution test on E. faecalis
| MBC(µg/ml) | MIC(µg/ml) | Groups |
|---|---|---|
| 625 | 313 | Propolos hydroalcoholic extract (40% ethanol) |
| 1500 | 750 | Propolos hydroalcoholic extract (15% ethanol) |
| NA | NA | Propolis aqueous extract (0% ethanol) |
| 4500 NA (1) 4500 (2) |
2250 4500 (1) 2250 (2) |
Aloe vera |
| 4 | 2 | Chlorohexidine 2% |
| NA | NA | Ethanol40 % |
| NA | NA | Ethanol 15% |
| NA | NA | Distilled water |
NA: without antibacterial inhibitory effect (1) The test microorganism was S. aureous (2) The test microorganism was S. mutans
Direct contact test
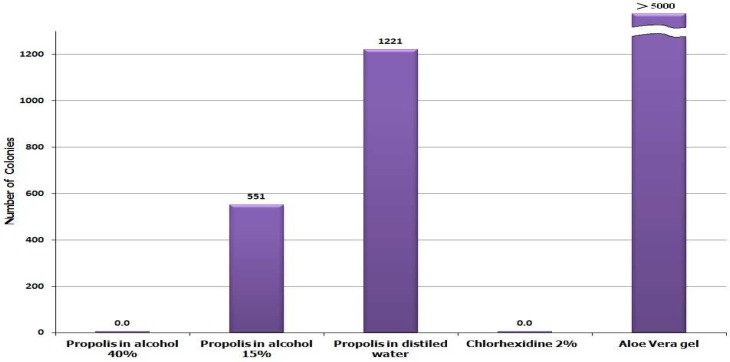
The number of colonies of bacteria grown after 24 hours is shown in fig. 3. The hydroalcoholic extract of propolis with 40% alcohol showed significant antibacterial effect against E. faecalis (similar to Chlorhexidine 2% solution). The aqueous extract of propolis showed a lesser extent in this antibacterial effect. However, Aloe vera showed no antibacterial effect in this method and the resulting colonies were practically uncountable same as the negative controls (because of countless resulting colonies, the negative controls are not depicted in this figure).
Fig. 3.
The number of grown colonies of E. faecalis after 24 hour contact with propolis hydroalcoholic extracts, Aloe vera, and Chlorohexidine in direct contact test
Discussion
In this study, we showed that Aloe vera gel and propolis ethanolic extracts have antibacterial activity against E. faecalis in in vitro. However, both these naturally available substances showed lower potency compared to Chlorhexidine in either disk diffusion and microdilution assays (Table 3, Fig. 1, 2). On the other hand, propolis ethanolic extract showed high antibacterial activity against E. faecalis comparable to that of Chlorhexidine in direct contact test (Fig. 3) which signifies the importance of solubility issue. Some gram positive bacteria such as E. faecalis resist the cleaning and shaping of root canal, and potentially can lead to endodontic failure (13-15).
Aloe vera gel and propolis are two naturally occurring substances which have been long used in the treatment of inflammation and infectious diseases of the mouth (13, 16-17). The physicochemical assays conducted on both Aloe vera and propolis samples confirm their standard characteristics (Tables 1, 2). Since the solubility of propolis components in alcohol is different, the concentration of ethanol used for extraction is critical. The dry weight of each propolis alcoholic extract is correlated to its ethanol concentration (Table 2). In this study, we used high speed centrifugation following filtration to omit any dispersed solid material off the extract. Colloidal particles in the extract might exert direct antibacterial effects. The noticeable difference in antibacterial activity results obtained by the three test procedures, especially with propolis aqueous extract, indicates that ethanol soluble constituents of propolis are responsible for its antibacterial effect (8, 18). These components show quite high antibacterial activity in direct contact test against E. faecalis (Fig. 3).
The anti-microbial effect of hydroalcoholic extracts of propolis in disk diffusion was less than that in microdilution, this issue might be aroused by low diffusion ability of alcohol soluble components in agar. On the other hand, since Aloe vera gel is aqueous, no such a difference was observable between its microdilution and its disk diffusion test (Fig. 1, Table 3)
In direct contact test the microorganism gets in touch with the surface of the dried material directly, hence, there is no problem with insolubility of antimicrobial components. For this reason, the aqueous extract of propolis, which contains the least amount of ethanol soluble antimicrobial components, only shows its weak antibacterial activity in direct contact test (Fig. 3).
These substances have low solubility in water but they are highly soluble in ethanol. Some components in propolis, which have been suggested as its active agents, include flavonoids, phenolic and aromatic compounds like caffeic acid (19). Our results are in concordance with a study conducted by Mattigatti et al. (2012) who investigated the effects of propolis on three microorganisms (E. faecalis, S. aureus and Candida albicans) using agar diffusion test (20). They have shown (same to our results) that Chlorhexidine along with MTAD® (a mixture of tetracycline, citric acid and a detergent) has superior activity against the tested micro-organisms.
On the other hand, Aloe vera gel which showed weak antibacterial activity in disk diffusion and microdilution tests, failed to show any activity in direct contact test (Fig. 3). This might be the result of low concentration of its antibacterial components compared to nutrient polysaccharides which could prevent the microorganism to be fully in touch with the Aloe vera active components.
Aloe vera’s pharmacotherapeutic and cosmetic properties have been studied since long time ago (16-17). However, studies about its antibacterial effect on E. faecalis and its comparison to intra canal drug like Chlorhexidine 2% has not yet been done. The leaf of Aloe vera contains some active substances like acemanan, anthraquinone, anthracine, cinnamonic acid with anti inflamma-tory/antimicrobial properties (17, 21).
As a comparison between Aloe vera and propolis, the antimicrobial effect of Aloe vera gel in microdilution was less than hydroalcoholic extracts of propolis and its obtained MIC on all tested microorganisms (E. feaclais, S. aureus and S. mutans) was more than 2250 µg/ml. Recently, conducted studies with other test organisms or methods of antibacterial activity assyas, have shown similar results in our study. In the study by Anuj Bhardwaj et al. in 2012, the antimicrobial effect of some natural extracts and Aloe vera with Chlorhexidine 2% on E. faecalis was compared which similar to the present study (22-23).
Conclusion
Aloe vera gel has mild antibacterial effect against E. faecalis, S. aureus and S. mutans. It seems that Aloe vera gel has low antibacterial potency compared to propolis, hence its subfractionation may be a good choice to make a better antibacterial compound for root canal treatments. On the other hand, the hydroalcoholic extract of propolis could be a good anti-microbial agent against E. faecalis especially following direct contact to this germ. Both tested natural substances have less antibacterial activity compared to Chlorhexidine , however their potency could be significantly increased by improvement in the extraction techniques. This could potentially lead to root canal antibacterials with fewer side effects.
Acknowledgment
This research project has been funded by a research grant from Babol University of Medical Sciences (Grant No:9133725). The authors wish to thank: Barij Essence company (Kashan-Iran) and Behsa Pharmaceutical Company (Tehran-Iran) for honoring the Aloe vera gel and Chlorhexidine solution respectively.
References
- 1.Gomes BP, Souza SF, Ferraz CC, et al. Effectiveness of 2% Chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro. Int Endod J. 2003;36:267–75. doi: 10.1046/j.1365-2591.2003.00634.x. [DOI] [PubMed] [Google Scholar]
- 2.Victorino FR, Bramante CM, Zapata RO, et al. Removal efficiency of propolis paste dressing from the root canal. J Appl Oral Sci. 2010;18:621–4. doi: 10.1590/S1678-77572010000600014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siqueira JF, Rocas IN. Polymerase chain reaction-based analysis of microorganisms associated with failed endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:85–94. doi: 10.1016/s1079-2104(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 4.Molander A, Reit C, Dahlen G, et al. Microbiological status of root - filled teeth with apical periodontitis. Int Endod J. 1998;31:1–7. [PubMed] [Google Scholar]
- 5.Pujar M, Makandar S. Herbal Usage In Endodontics- A Review. Int J contem dentis. 2011;2:34–7. [Google Scholar]
- 6.Kayaoglu G, Omurlu H, Akca G, et al. Antibacterial activity of Propolis versus conventional endodontic disinfectants against Enterococcus faecalis in infected dentinal tubules. J Endod. 2011;37:376–81. doi: 10.1016/j.joen.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Sforcin JM, Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol. 2011;133:253–60. doi: 10.1016/j.jep.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 8.Viuda-Martos M, Ruiz-Navajas Y, Fernandez-Lopez J, et al. Functional properties of honey, propolis, and royal jelly. J Food Sci. 2008;73:R117–24. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- 9.Sforcin JM. Propolis and the immune system: a review. J Ethnopharmacol. 2007;113:1–14. doi: 10.1016/j.jep.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Cera LM, Heggers JP, Robson MC, et al. The therapeutic efficacy of aloe vera ceramin thermal injuries. two case report. J Am Animal Hosp Assoc. 1980;16:768–72. [Google Scholar]
- 11.West DP, Zhu YF. Evaluation of aloe vera gel gloves in the treatment of dry skin associated with occupational exposure. Am J Infect Control. 2003;31:40–2. doi: 10.1067/mic.2003.12. [DOI] [PubMed] [Google Scholar]
- 12.Babaee N, Zabihi E, Mohseni S, et al. Evaluation of the therapeutic effects of Aloe vera gel on minor recurrent aphthous stomatitis. Dent Res J. 2012;9:381–5. [PMC free article] [PubMed] [Google Scholar]
- 13.Chavez de Paz LE, Molander A, Dahlen G. Gram-positive rods prevailing in teeth with apical periodontitis undergoing root canal treatment. Int Endod J. 2004;37:579–87. doi: 10.1111/j.1365-2591.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- 14.Chu FC, Leung WK, Tsang PC, et al. Identification of cultivable microorganisms from root canals with apical periodontitis following two-visit endodontic treatment with antibiotics/steroid or calcium hydroxide dressings. J Endod. 2006;32:17–23. doi: 10.1016/j.joen.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Chávez de Paz LE, Molander A, Dahlén G. Gram-positive rods prevailing in teeth with apical periodontitis undergoing root canal treatment. Int Endod J. 2004;37:579–87. doi: 10.1111/j.1365-2591.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds T, Dweck AC. Aloe vera leaf gel: a review update. J Ethnopharmacol. 1999;68:3–37. doi: 10.1016/s0378-8741(99)00085-9. [DOI] [PubMed] [Google Scholar]
- 17.Wynn RL. Aloe vera gel: update for dentistry. Gen Dent. 2005;53:6–9. [PubMed] [Google Scholar]
- 18.Miguel MG, Antunes MD. Is propolis safe as an alternative medicine? J Pharm Bioallied Sci. 2011;3:479–95. doi: 10.4103/0975-7406.90101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salomao K, Dantas AP, Borba CM, et al. Chemical composition and microbicidal activity of extracts from Brazilian and Bulgarian propolis. Lett Appl Microbiol. 2004;38:87–92. doi: 10.1111/j.1472-765x.2003.01458.x. [DOI] [PubMed] [Google Scholar]
- 20.Mattigatti S, Ratnakar P, Moturi S, et al. Antimicrobial effect of conventional root canal medicaments vs propolis against Enterococcus faecalis, Staphylococcus aureus and Candida albicans. J Contemp Dent Pract. 2012;13:305–9. doi: 10.5005/jp-journals-10024-1142. [DOI] [PubMed] [Google Scholar]
- 21.Surjushe A, Vasani R, Saple DG. Aloe vera: a short review. Indian J Dermatol. 2008;53:163–6. doi: 10.4103/0019-5154.44785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhardwaj A, Ballal S, Velmurugan N. Comparative evaluation of the antimicrobial activity of natural extracts of Morinda citrifolia, papain and aloe vera (all in gel formulation), 2% Chlorhexidine gel and calcium hydroxide, against Enterococcus faecalis: An in vitro study. J Conserv Dent. 2012;15:293–7. doi: 10.4103/0972-0707.97964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaithwath G, Kumar A, Pandey H, et al. Investigation of comparative antimicrobial activity of aloe vera gel and juice. Pharmacologyonline. 2008;1:239–43. [Google Scholar]