Abstract
The purpose of this study was to determine: 1) the extent to which an acute session of high-intensity interval training (HIIT) increases systemic inflammatory cytokines and chemokines, and 2) whether 2 weeks of HIIT training alters the inflammatory response. Eight recreationally active males (aged 22±2 years) performed 2 weeks of HIIT on a cycle ergometer (six HIIT sessions at 8–12 intervals; 60-second intervals, 75-second active rest) at a power output equivalent to 100% of their predetermined peak oxygen uptake (VO2max). Serum samples were collected during the first and sixth HIIT sessions at rest and immediately, 15, 30, and 45 minutes post-exercise. An acute session of HIIT induced significant increases in interleukin (IL)-6, IL-8, IL-10, tumor necrosis factor-α, and monocyte chemotactic protein-1 compared with rest. The concentrations of interferon-γ, granulocyte macrophage-colony-stimulating factor, and IL-1β were unaltered with an acute session of HIIT Two weeks of training did not alter the inflammatory response to an acute bout of HIIT exercise. Maximal power achieved during a VO2max test significantly increased 4.6%, despite no improvements in VO2max after 2 weeks of HIIT. These data suggest that HIIT exercise induces a small inflammatory response in young, recreationally active men; however, 2 weeks of HIIT does not alter this response.
Keywords: cycle ergometer, inflammatory cytokines, exercise training
Introduction
High-intensity interval training (HIIT) is characterized by repeated sessions of relatively brief (30–60 seconds), intermittent exercise, often performed with an “all out” effort or at an intensity close to that which elicits peak oxygen uptake (ie, ≥90% of VO2max) and is generally performed on a cycle ergometer.1 An HIIT exercise regimen requires as little as 75 minutes/week of training, compared with the American College of Sports Medicine (ACSM)-recommended 150 minutes/week of moderate intensity exercise;2 yet provides a stimulus sufficient to improve fitness in a variety of populations.3–6 Evidence suggests that HIIT is a time-efficient exercise that may be better than longer duration moderate-intensity exercise training (ie, jogging or brisk walking) for improving fitness and inducing beneficial metabolic adaptations.7
Initial HIIT training protocols consisted of repeated Wingate tests (30-second “all out” sprints) over a period of 2 weeks;1,3 however, this mode of HIIT utilizes supramaximal intensities (ie, > 100% VO2max) that may not be suitable for all subject populations. Recently Gibala’s laboratory developed a lower-intensity 2-week HIIT protocol consisting of repeated intervals at or near maximal oxygen uptake (ie, 90%–100% VO2max) that may be safer for both healthy and clinical (eg, diabetic) cohorts.8,9
Prolonged, continuous aerobic exercise (eg, 2–3 hours at 60%–70% VO2max) induces a large systemic inflammatory response, marked by substantial increases in several inflammatory cytokines and chemokines.10–16 Exercise of this nature can transiently suppress immune function12 and increase susceptibility to infections.17 Furthermore, it appears that the longer and more intense the exercise bout, the greater and more prolonged the immune response.18 However, repeated bouts of the same prolonged, continuous exercise stimulus appear to attenuate the inflammatory response,19 suggesting an adaptive immune response to exercise.
Less is known about the inflammatory response to HIIT exercise. It has been reported that a single bout of high-intensity interval exercise increases circulating levels of the inflammatory cytokine interleukin (IL)-6;20,21 however, the inflammatory response to a short duration HIIT regimen has not been examined. Therefore, the purposes of this study were to determine: 1) the extent to which an acute session of HIIT increases systemic inflammatory cytokines and chemokines, and 2) whether 2 weeks of HIIT training alters the systemic inflammatory response in moderately active, young men.
Methods
Study participants
The Appalachian State University Institutional Review Board approved all procedures in this study. Eight healthy, recreationally active young males (aged 22±2 years, height 180.9±7.9 cm, weight 75.7±6.6 kg, self-reported physical activity 4±2 days/week) completed all components of the study. All participants were nonsmokers, with no history of cardiovascular, pulmonary, or neuromuscular diseases, and no lower body musculoskeletal injury in the previous 6 months. Upon completion of initial testing, participants with a VO2max less than 42.2 mL/kg/min were excluded from the study; this exclusion criterion was based on ACSM recommendations for individuals performing high-intensity exercise (40th percentile of normative values according to ACSM’s Guidelines for Exercise Testing – 8th Edition). Participants were instructed to refrain from consuming non-steroidal anti-inflammatory drugs or any other nutritional supplements that may possess anti-inflammatory or antioxidant properties (ie, daily multivitamins) and to refrain from any other aerobic activities for the duration of the study. Participants performed testing and training sessions at approximately the same time of day for the duration of the study. Participants were also instructed to report to the lab well hydrated and to consume the same diet on each of the two exercise testing visits.
Experimental design
This study consisted of eight total visits: one initial testing and familiarization session, six HIIT exercise sessions, and one post-training testing session (Figure 1). The entire study lasted approximately 3 weeks, and all testing and exercise sessions took place at approximately the same time of the day.
Figure 1.
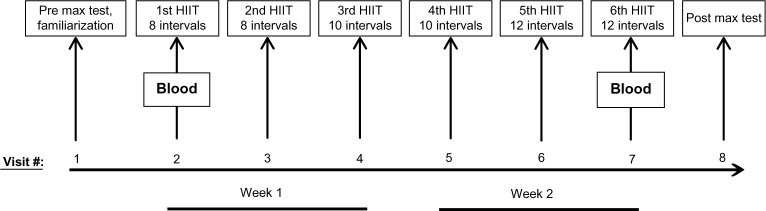
Protocol schematic. Subjects completed eight total visits over a 3-week period, including a pre-training testing session and HIIT familiarization session, six HIIT sessions, and a post-training testing session. Blood was collected during HIIT session 1 and HIIT session 6 for the analysis of inflammatory cytokines and chemokines.
Abbreviation: HIIT, high-intensity interval training.
Visit 1: initial testing and familiarization session
On the first visit, participants completed the informed consent and health history questionnaire. Height (cm) and weight (kg) were recorded. Participants then completed a maximal graded exercise test on a cycle ergometer (Lode; Groningen, the Netherlands). The maximal graded exercise testing protocol, modified from the protocol used by Gibala’s laboratory,9 started with a warm-up of 2 minutes at 50 watts (W) at a self-selected cadence above 60 revolutions per minute. After the warm-up, the workload increased 15 W every 30 seconds until volitional fatigue. During the maximal graded exercise test, metabolic parameters (oxygen consumption [VO2], carbon dioxide production [VCO2], respiratory exchange ratio [RER], and minute ventilation [VE]) were measured with a metabolic cart (Parvo True2400, ParvoMedics, Sandy, UT, USA) and workload (in Watts) was recorded. Maximal oxygen consumption was determined using the 15-second averaging analysis setting. During all exercise bouts, heart rate (HR) was monitored using a telemetric HR monitor (Polar, Lake Success, NY, USA), and ratings of perceived exertion (RPE) were recorded every minute on the Borg scale of 6–20.
After completing the maximal graded exercise test, participants rested for 20 minutes and then completed an HIIT familiarization session of four cycling HIIT intervals (60 seconds each) at a workload (in Watts) equivalent to 100% of their VO2max, with 75-second active rest periods (50 W) between intervals. The protocol used in the current study was modeled after a previously published protocol.9
Visits 2–7: HIIT training sessions
The HIIT training protocol utilized for this study was modeled after a previously published 2-week HIIT training protocol.9 At least 48 hours following the first visit (4.25±3.96 days), participants began the 2-week HIIT training regimen: three sessions per week (eg, Monday, Wednesday, and Friday) for a total of six HIIT training sessions. Each HIIT training session consisted of repeated 60-second intervals of cycling on the Lode cycle ergometer at a workload equivalent to each participant’s 100% VO2max (according to the initial maximal graded exercise test), with 75 seconds of active recovery at 50 W between intervals. All participants completed a 3-minute warm-up and cool-down at 50 W prior to and following each training session. During each session, RPE and HR were recorded at the end of each interval. For HIIT sessions 1 and 2, participants performed eight HIIT intervals; for sessions 3 and 4, participants performed ten HIIT intervals; and for sessions 5 and 6, participants performed 12 HIIT intervals.
Visit 8: post-training testing
At least 48 hours following the last HIIT training session (2.74±0.46 days), participants performed the post-training maximal graded exercise test using the same protocol as stated above. During the post-training maximal graded exercise test, metabolic parameters (VO2, VCO2, RER, and VE) were measured with a metabolic cart (Parvo True2400) and workload HR, and RPE were recorded as stated above.
Blood collection procedures
During the first and last (sixth) HIIT training sessions, blood samples were collected for the analysis of inflammatory cytokines and chemokines. Venous blood samples were collected via an intravenous catheter at the following time points: before exercise, immediately after, and at 15, 30, and 45 minutes after completion of the HIIT exercise. These sampling time points were based on findings from preliminary HIIT studies performed in our laboratory. Approximately 10 mL of blood was collected at each time point in individual serum collection tubes (BD Vacutainer, Franklin Lakes, NJ, USA), separated by centrifugation, aliquoted into cryovials, and stored at −80°C until further analysis.
Analysis of circulating inflammatory cytokine and chemokine concentrations
Serum samples were analyzed for inflammatory cytokines and chemokines with a bead-based multiplex assay using the MAGPIX instrument and xPONENT® analysis software (Luminex, Austin, TX, USA). The concentration (pg/mL) of the inflammatory cytokines IL-1β, IL-6, IL-10, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ), and the inflammatory chemokines IL-8, monocyte chemotactic protein-1 (MCP-1), and granulocyte macrophage-colony-stimulating factor (GM-CSF) were measured using a commercially available assay kit (Millipore, Billerica, MA, USA) according to manufacturer’s specifications. The inter-assay % coefficient of variability (%CV) for this panel of analytes was: GM-CSF =13.9%; IFN-γ=5.9%; IL-10 =8.7%; IL-1β=8.3%; IL-6 =5.1%; IL-8 =4.3%; MCP-1 =11.0%; and TNF-α =6.0%. The intra-assay %CV for this panel of analytes was: GM-CSF =8.8%; IFN-γ=9.8%; IL-10 =3.9%; IL-1β=1.6%; IL-6 =2.1%; IL-8 =1.6%; MCP-1 =9.6%; and TNF-α =3.0%. Of note, eight subjects completed this study, however one participant’s inflammatory cytokine/chemokine values were excluded from analysis because his cytokine concentrations were abnormally high (ie, >2 standard deviations [SD] from the mean) at rest and in response to exercise, compared with all other participants. Therefore, statistical analyses and all reported cytokine/chemokine data are from n=7.
Statistical analyses
To improve normality and variance homogeneity, the values for cytokine concentrations were log transformed prior to statistical analysis. Two-way repeated measures analysis of variance (training × time point) was used to determine whether differences existed in inflammatory cytokine and chemokine concentrations in serum samples. Statistical significance was set a priori at P≤0.05. Following a significant F-ratio, Bonferroni post-hoc analyses were used to determine differences between time points within an acute bout of HIIT, and after 2 weeks of exercise training. Weight, VO2max, cycling power (in Watts), HR, and RPE data were analyzed using paired Student’s t-test to determine whether significant differences existed before and after 2 weeks of training. All data are reported as mean ± SD. Statistical analyses were performed using statistical analysis software (Sigma Plot 12.0; Systat Software, Inc., San Jose, CA, USA).
Results
Inflammatory response to an acute session of HIIT
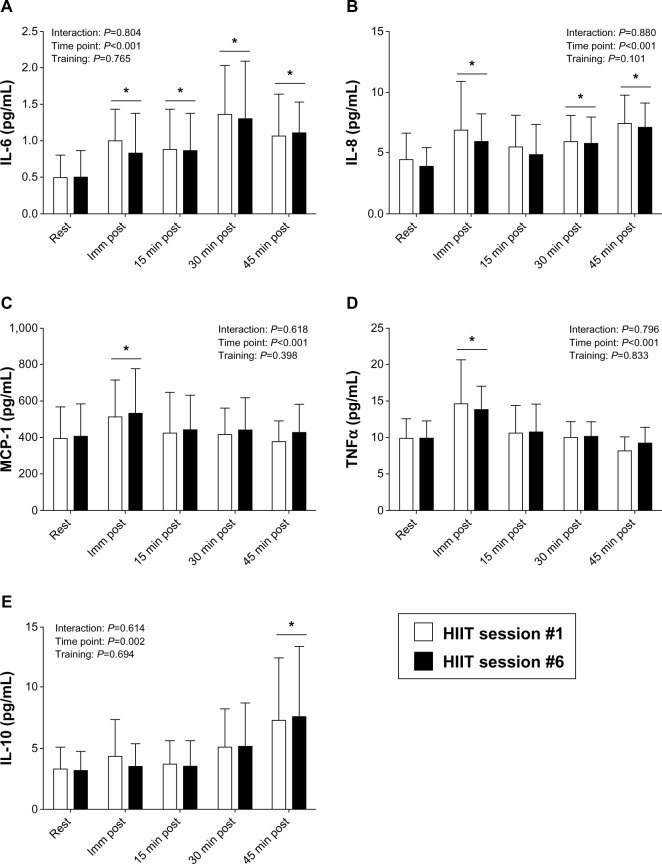
An acute bout of HIIT, ie, eight cycling intervals at a workload equivalent to 100% of VO2max (~20 minutes of exercise), resulted in small, but significant increases in serum concentrations of several inflammatory cytokines (IL-6, TNF-α, and IL-10) and chemokines (IL-8 and MCP-1), compared with resting concentrations. Specifically, IL-6 increased immediately after a single bout of HIIT (P=0.001; effect size =1.0), and remained elevated at 15 minutes post (P=0.003; effect size =0.85), 30 minutes post (P<0.001; effect size =2.6), and 45 minutes post exercise (P<0.001; effect size =1.4), compared with rest (Figure 2A). Similar increases were observed for IL-8, but to a lesser extent than for IL-6. An acute bout of HIIT increased IL-8 concentrations immediately after (P<0.001; effect size =0.85), and at 30 minutes post (P=0.004; effect size =0.83), and 45 minutes post exercise (P<0.001; effect size =1.5), compared with rest (Figure 2B). IL-8 concentrations at 15 minutes post exercise were not significantly different than rest (P=0.140; effect size =0.45). MCP-1 increased immediately after exercise (P<0.001; effect size =0.58; Figure 2C). TNF-α increased immediately after exercise as well (P<0.001; effect size =1.1; Figure 2D). As shown in Figure 2E, IL-10 increased 45 minutes after the completion of exercise, compared with resting values (P=0.001; effect size =1.0). The concentrations of IFN-γ, GM-CSF, and IL-1β were not significantly altered at any time point after an acute bout of HIIT (Table 1).
Figure 2.
Serum inflammatory cytokines in response to an acute bout of HIIT. IL-6 (A), IL-8 (B), MCP-1 (C), TNF-α (D), and IL-10 (E) concentrations were measured before and immediately (Imm), 15, 30, and 45 minutes after completion of an acute bout of HIIT exercise (at a workload equivalent to 100% of VO2max) on a cycle ergometer during the first and sixth HIIT exercise sessions.
Notes: Open bars, first HIIT session; filled bars, sixth HIIT session. Data are reported as mean ± SD; n=7.*indicates significantly different from at rest.
Abbreviations: HIIT, high-intensity interval training; IL, interleukin; MCP, monocyte chemotactic protein; SD, standard deviation; TNF, tumor necrosis factor; VO2max, peak oxygen uptake.
Table 1.
Inflammatory cytokines not significantly altered by HIIT
| Rest
|
Immediately post
|
15 min post
|
30 min post
|
45 min post
|
P-value
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIIT #1 | HIIT #6 | HIIT #1 | HIIT #6 | HIIT #1 | HIIT #6 | HIIT #1 | HIIT #6 | HIIT #1 | HIIT #6 | Interaction | Time point | Training | |
| GM-CSF (pg/mL) | 1.01±1.20 | 1.05±1.12 | 1.76±2.47 | 1.10±1.91 | 0.98±1.13 | 0.38±0.37 | 0.61±0.38 | 0.68±0.61 | 0.31±0.15 | 0.77±0.91 | 0.382 | 0.172 | 0.484 |
| IFNγ(pg/mL) | 1.49±1.52 | 1.63±2.11 | 3.51±5.85 | 3.87±7.86 | 2.74±5.22 | 0.84±0.35 | 1.18±0.92 | 1.42±1.74 | 1.00±0.86 | 1.76±2.76 | 0.518 | 0.286 | 0.266 |
| IL-1β(pg/mL) | 0.57±0.40 | 0.46±0.32 | 0.65±0.48 | 0.56±0.43 | 0.78±1.10 | 0.43±0.27 | 0.43±0.33 | 0.43±0.33 | 0.38±0.21 | 0.41±0.22 | 0.425 | 0.205 | 0.195 |
Note: Values are mean ± SD (n=7).
Abbreviations: GM-CSF, granulocyte macrophage-colony-stimulating factor; HIIT, high-intensity interval training; IFNγ, interferon-γ, IL-1β, interleukin-1β; HIIT #1, HIIT session #1; HIIT #6, HIIT session #6.
Inflammatory response to HIIT with 2 weeks of training
While it appears that an acute bout of HIIT exercise induces modest increases in several inflammatory cytokines, we found no difference in the inflammatory response to HIIT after 2 weeks of training, compared with the first HIIT session (ie, no main effect of training). The pattern of changes in inflammatory cytokine concentrations was almost identical in the first HIIT session as it was in the last (sixth) HIIT session (Figure 2A–E), despite a 50% increase in exercise volume during the sixth HIIT session.
Alterations in physiological variables with 2 weeks of training
Although 2 weeks of HIIT training did not increase VO2max (P=0.481) (Table 2), it did result in a significant increase in peak power output (P=0.007). As alternate indicators of exercise adaptation over this 2-week HIIT regimen, we analyzed HR and RPE after the eighth interval at the beginning and end of training and found that HR and RPE were significantly lower in the last (sixth) HIIT session, compared with the first HIIT session (P=0.014 and P=0.028, respectively; Table 2).
Table 2.
Physiological variables before and after two weeks of HIIT exercise training
| PRE | POST | % Change | P-value | |
|---|---|---|---|---|
| VO2max (mL/kg/min) | 49.2±2.6 | 49.6±2.0 | 0.8% | 0.481 |
| Peak power (Watts) | 324±40 | 339±39* | 4.6% | 0.007 |
| Watts at 100% VO2max | 314±45 | 326±35 | 3.8% | 0.190 |
| RPE after interval 8 | 20±1 | 17±3* | −15.0% | 0.028 |
| HR after interval 8 (bpm) | 190±10 | 184±12* | −3.2% | 0.014 |
Notes: Values are mean ± SD (n=8).
indicates significantly different from PRE.
Abbreviations: HIIT, high-intensity interval training; PRE, before two weeks of HIIT exercise training; POST, after two weeks of HIIT exercise training; VO2max, peak oxygen uptake.
Discussion
The current study investigated the systemic inflammatory response to an acute bout of HIIT at the beginning and end of 2 weeks of training in young, recreationally active men. Herein we report modest increases in several inflammatory cytokines and chemokines, namely IL-6, IL-8, IL-10, TNF-α, and MCP-1, to an acute bout of HIIT We also report novel findings of increased TNF-α, MCP-1, and IL-10 in response to an acute bout of HIIT; however, these inflammatory cytokine/chemokine responses to HIIT are much lower than those previously measured in response to prolonged continuous aerobic exercise (eg, marathon).11,14,16,22 This is the first study to demonstrate these perturbations in inflammatory cytokines/chemokines with a HIIT protocol consisting of 60-second cycling intervals (8–12 intervals per session) at a power output equivalent to 100% VO2max. Furthermore, we demonstrate that there is no difference in the inflammatory response to an acute bout of HIIT exercise after 2 weeks of training, despite a 50% increase in exercise volume. In addition, we report that maximal cycling power, but not VO2max, increases with 2 weeks of HIIT training.
Inflammatory responses to exercise
Cytokines are proteins that modulate immune function and/or inflammation. Pro-inflammatory cytokines promote inflammation, anti-inflammatory cytokines attenuate inflammation, and chemokines and colony-stimulating factors attract immune cells to the sight of inflammation. It is commonly held that the inflammatory cytokine response to exercise is a result of muscle tissue damage incurred during exercise, with eccentric contractions generally inducing larger changes in systemic cytokine concentrations than concentric contractions.18,23 While there is a minimal eccentric component during normal cycling (including HIIT exercise), prolonged, aerobic cycling exercise still results in a significant inflammatory response.18,24 A recent report indicates that the intensity of prolonged exercise is a determinant of the inflammatory response to exercise.15
Several previous studies have reported substantial inflammatory responses to various forms of prolonged, continuous aerobic exercise10,14–16,19,22 (also refer to a review by Suzuki et al18); however, much less is known about the inflammatory response to high-intensity interval exercise. The current study analyzed inflammatory cytokine/chemokine concentrations in the blood at multiple time points during the acute recovery period after exercise (immediately post, and 15-, 30-, and 45-minutes post). The timing of the inflammatory cytokine/chemokine responses to an acute bout of HIIT observed in this study are consistent with the typical inflammatory response to prolonged, continuous aerobic exercise previously reported in the literature.10,11,13–16,22
In the current study, we demonstrate modest increases in IL-6, IL-8, IL-10, TNF-α, and MCP-1 in the short time after completing a single bout of HIIT. IL-6 is a multifunctional cytokine that inhibits the release of the pro-inflammatory cytokines, IL-1β and TNF-α, but also stimulates the secretion of the anti-inflammatory cytokines, IL-1ra, IL-10, and soluble TNF receptor.25 IL-8 is a potent neutrophil chemotactic chemokine that also elicits chemotactic properties on other immune cells, such as basophils, eosinophils, and T-lymphocytes.26 TNF-α is a pro-inflammatory cytokine that mediates muscle proteolysis.27 MCP-1 elicits chemotactic properties on monocytes, macrophages, and lymphocytes;26 thus, together with IL-8, these chemokines facilitate infiltration of immune cells to the site of inflammation. IL-10, on the other hand, is an anti-inflammatory cytokine that suppresses IL-1β and TNF-α secretion, while stimulating IL-1ra production.28
Previous studies have reported 2.0–2.5-fold increases in IL-6 immediately after interval running21,29 and interval cycling20 in trained athletes. Compared with prolonged, continuous aerobic exercise, in which 4–40-fold increases in IL-6 concentrations are observed for up to 1.5 hours after exercise,11,13–16,22 the IL-6 response to HIIT appears to be much lower. Similar to our findings, Arent et al20 reported increased IL-8 in response to a single Wingate, plus eight 10-second cycling intervals at maximal effort, but this is still lower than the 3–10-fold increases in IL-8 reported in response to prolonged, continuous aerobic exercise.11,13–16 Furthermore, we report increases in TNF-α (43%), MCP-1 (29%), and IL-10 (130%) following an acute bout of HIIT exercise. Others have reported 20%–30% increases in TNF-α10,13,15 and approximately threefold increases in both MCP-1 and IL-1011,16,19 after prolonged, continuous aerobic exercise. We did not observe significant changes in GM-CSF, IL-1β, or IFN-γ following an acute bout of HIIT exercise. Previous studies have reported up to 30% increases in both GM-CSF and IL-1 β immediately after ~2 hours of high-intensity cycling15 or running,10 as well as after a marathon race,16 while prolonged continuous aerobic exercise induces ~10% increase in IFN-γ.10,15 Our time course design is within the confines to be able to detect changes in GM-CSF, IL-1β, and IFN-γ with HIIT exercise, but it is possible that our HIIT exercise protocol may not provide enough of a stimulus to induce significant changes in these inflammatory cytokines/chemokines in relatively fit males. Taken together, HIIT exercise appears to elicit a much lower magnitude inflammatory response compared with prolonged continuous aerobic exercise. These differences are likely due to the relative time spent exercising between HIIT (~20 minutes) and prolonged exercise (≥2 hours).
It also appears that rest influences the inflammatory response during the exercise bout. Our study and previous HIIT studies have shown that high intensity, intermittent exercise for relatively brief overall exercise time elicits a small inflammatory cytokine response;20,30 however, continuous, prolonged aerobic exercise induces a much more exaggerated inflammatory cytokine response than that of short duration HIIT exercise.11,15,16,22 Recently, Knab et al31 reported that prolonged, high-intensity interval swimming (2 hours of 1:1 and 1:2 swim-to-rest ratio) also induces small increases in IL-6 (87%) and IL-10 (1.5-fold). This cytokine response is similar to ours and other HIIT studies; suggesting that rest intervals, separating intense work intervals, have a profound attenuating effect on the inflammatory response to high-intensity exercise. We posit that the massive inflammatory response observed with prolonged, continuous aerobic exercise may be reduced if rest intervals are incorporated into intense bouts of exercise.
Possible adaptive responses to HIIT exercise
The other aim of this study was to determine whether 2 weeks of HIIT alters the inflammatory response to an acute bout of HIIT exercise. We observed no significant alterations in the pattern of responses, in any of the cytokines/chemokines analyzed, between HIIT session 1 and HIIT session 6. Previously, Croft et al30 reported that 6 weeks of high-intensity interval running (five 3-minute intervals at 90% VO2max) significantly attenuated the IL-6 response to an acute interval bout by ~40%. While the Croft et al study increased the interval intensity by 5% every 2 weeks of training, the exercise intensity was reduced back to starting levels for their final interval exercise bout and blood draws for determination of IL-6 concentrations. On the contrary, our study design was modeled after a previously published protocol9 that increased exercise volume by 50% (from 8 to 12 intervals) over the 2-week training period. Therefore, inflammatory cytokine/chemokine concentrations at HIIT session 6 were measured when participants were performing 50% more exercise compared with HIIT session 1. Nieman et al13 reported that prolonged, high-intensity cycling on three consecutive days significantly attenuates the inflammatory response to exercise; a response the authors attributed to an adaptation in immune system function. A potential limitation in our study is that subjects performed 50% more exercise in HIIT session 6 compared with HIIT session 1 (12 intervals versus 8 intervals). We believe this directly contributed to why we did not measure differences in the inflammatory response between the first and last acute bout of HIIT. Although we did not measure differences between HIIT sessions 1 and 6, the 50% increase in exercise volume did not increase the inflammatory response. This could be interpreted as an adaptation in immune function to 2 weeks of HIIT; further investigation is warranted to test this hypothesis.
Previous research investigating whether HIIT improves VO2max is inconclusive. While it has been reported that four-to six-week HIIT exercise regimens increase VO2max ~9%,30,32 we observed no significant improvements in VO2max after 2 weeks of HIIT exercise, which is consistent with previous HIIT studies of this duration.3,4 Others have demonstrated significant increases in oxidative enzyme capacity with short-term HIIT regimens.3,4,8,9 The studies demonstrating increased VO2max following 2 weeks of HIIT utilized higher training volumes and less fit subjects than our study.5,6 Although we did not demonstrate improvements in VO2max after just 2 weeks of training, we did observe significant increases in peak cycling power achieved during the VO2max test, as well as significant decreases in HR and RPE after the eighth interval between session 1 and session 6. The changes in these physiological variables suggest that, despite the lack of change in VO2max, exercise tolerance or exercise capacity increased in response to 2 weeks of HIIT in our study.
In conclusion, the inflammatory response to an acute bout of HIIT exercise appears to be substantially lower than that of prolonged, continuous aerobic exercise. We believe these novel findings provide valuable insight into the inflammatory response to HIIT exercise and extend the previous literature suggesting that HIIT exercise may be safe and effective for at-risk populations.9,33,34 Since HIIT exercise does not induce substantial elevations in inflammatory cytokine/chemokine levels in the blood of healthy young men, further research should investigate the inflammatory response to HIIT in clinical populations to determine whether HIIT exercise is a viable alternative mode of exercise for clinical populations and immunocompromised individuals, such as the elderly or individuals with type II diabetes, cancer, or AIDS (acquired immunodeficiency syndrome). While exercise mode, duration, intensity, and rest intervals each contribute to the inflammatory response to exercise, further research is needed to fully elucidate the physiological ramifications of elevated inflammatory cytokines and chemokines on the muscle tissue acutely after exercise.
Acknowledgments
We would like to thank all of the subjects who participated in this study. We also thank James Johnson, Thomas Jurrissen, and Chelsea Zemmin for their assistance during the study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gibala MJ, Little JP, van Essen M, et al. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol. 2006;575(Pt 3):901–911. doi: 10.1113/jphysiol.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American College of Sports M. Thompson WR, Gordon NF, Pescatello LS. ACSM’s Guidelines for Exercise Testing and Prescription. Philadelphia: Lippincott Williams and Wilkins; 2010. [Google Scholar]
- 3.Burgomaster KA, Heigenhauser GJ, Gibala MJ. Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance. J Appl Physiol. 2006;100(6):2041–2047. doi: 10.1152/japplphysiol.01220.2005. [DOI] [PubMed] [Google Scholar]
- 4.Burgomaster KA, Hughes SC, Heigenhauser GJ, Bradwell SN, Gibala MJ. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol. 2005;98(6):1985–1990. doi: 10.1152/japplphysiol.01095.2004. [DOI] [PubMed] [Google Scholar]
- 5.Rodas G, Ventura JL, Cadefau JA, Cusso R, Parra J. A short training programme for the rapid improvement of both aerobic and anaerobic metabolism. Eur J Appl Physiol. 2000;82(5–6):480–486. doi: 10.1007/s004210000223. [DOI] [PubMed] [Google Scholar]
- 6.Talanian JL, Galloway SD, Heigenhauser GJ, Bonen A, Spriet LL. Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. J Appl Physiol. 2007;102(4):1439–1447. doi: 10.1152/japplphysiol.01098.2006. [DOI] [PubMed] [Google Scholar]
- 7.Gaesser GA, Angadi SS. High-intensity interval training for health and fitness: can less be more? J Appl Physiol. 2011;111(6):1540–1541. doi: 10.1152/japplphysiol.01237.2011. [DOI] [PubMed] [Google Scholar]
- 8.Little JP, Gillen JB, Percival ME, et al. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol. 2011;111(6):1554–1560. doi: 10.1152/japplphysiol.00921.2011. [DOI] [PubMed] [Google Scholar]
- 9.Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol. 2010;588(Pt 6):1011–1022. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konrad M, Nieman DC, Henson DA, Kennerly KM, Jin F, Wallner-Liebmann SJ. The acute effect of ingesting a quercetin-based supplement on exercise-induced inflammation and immune changes in runners. Int J Sport Nutr Exerc Metab. 2011;21(4):338–346. doi: 10.1123/ijsnem.21.4.338. [DOI] [PubMed] [Google Scholar]
- 11.Shanely RA, Nieman DC, Zwetsloot KA, et al. Evaluation of Rhodiola rosea supplementation on skeletal muscle damage and inflammation in runners following a competitive marathon. Brain Behav Immun. 2013 Sep 18; doi: 10.1016/j.bbi.2013.09.005. Epub. [DOI] [PubMed] [Google Scholar]
- 12.Nieman DC. Immune response to heavy exertion. J Appl Physiol. 1997;82(5):1385–1394. doi: 10.1152/jappl.1997.82.5.1385. [DOI] [PubMed] [Google Scholar]
- 13.Nieman DC, Henson DA, Davis JM, et al. Quercetin’s influence on exercise-induced changes in plasma cytokines and muscle and leukocyte cytokine mRNA. J Appl Physiol. 2007;103(5):1728–1735. doi: 10.1152/japplphysiol.00707.2007. [DOI] [PubMed] [Google Scholar]
- 14.Nieman DC, Henson DA, Smith LL, et al. Cytokine changes after a marathon race. J Appl Physiol. 2001;91(1):109–114. doi: 10.1152/jappl.2001.91.1.109. [DOI] [PubMed] [Google Scholar]
- 15.Nieman DC, Konrad M, Henson DA, Kennerly K, Shanely RA, Wallner-Liebmann SJ. Variance in the acute inflammatory response to prolonged cycling is linked to exercise intensity. J Interferon Cytokine Res. 2012;32(1):12–17. doi: 10.1089/jir.2011.0038. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki K, Nakaji S, Yamada M, et al. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med Sci Sports Exerc. 2003;35(2):348–355. doi: 10.1249/01.MSS.0000048861.57899.04. [DOI] [PubMed] [Google Scholar]
- 17.Nieman DC. Immunonutrition support for athletes. Nutr Rev. 2008;66(6):310–320. doi: 10.1111/j.1753-4887.2008.00038.x. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki K, Nakaji S, Yamada M, Totsuka M, Sato K, Sugawara K. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc Immunol Rev. 2002;8:6–48. [PubMed] [Google Scholar]
- 19.Nieman DC, Henson DA, Gross SJ, et al. Quercetin reduces illness but not immune perturbations after intensive exercise. Med Sci Sports Exerc. 2007;39(9):1561–1569. doi: 10.1249/mss.0b013e318076b566. [DOI] [PubMed] [Google Scholar]
- 20.Arent SM, Senso M, Golem DL, McKeever KH. The effects of theaflavin-enriched black tea extract on muscle soreness, oxidative stress, inflammation, and endocrine responses to acute anaerobic interval training: a randomized, double-blind, crossover study. J Int Soc Sports Nutr. 2010;7(1):11. doi: 10.1186/1550-2783-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meckel Y, Eliakim A, Seraev M, et al. The effect of a brief sprint interval exercise on growth factors and inflammatory mediators. J Strength Cond Res. 2009;23(1):225–230. doi: 10.1519/JSC.0b013e3181876a9a. [DOI] [PubMed] [Google Scholar]
- 22.Nehlsen-Cannarella SL, Fagoaga OR, Nieman DC, et al. Carbohydrate and the cytokine response to 2.5 h of running. J Appl Physiol. 1997;82(5):1662–1667. doi: 10.1152/jappl.1997.82.5.1662. [DOI] [PubMed] [Google Scholar]
- 23.Peake J, Nosaka K, Suzuki K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc Immunol Rev. 2005;11:64–85. [PubMed] [Google Scholar]
- 24.Nieman DC, Davis JM, Henson DA, et al. Muscle cytokine mRNA changes after 2.5 h of cycling: influence of carbohydrate. Med Sci Sports Exerc. 2005;37(8):1283–1290. doi: 10.1249/01.mss.0000175054.99588.b1. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen BK, Steensberg A, Schjerling P. Muscle-derived interleukin-6: possible biological effects. J Physiol. 2001;536(Pt 2):329–337. doi: 10.1111/j.1469-7793.2001.0329c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukaida N, Harada A, Matsushima K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998;9(1):9–23. doi: 10.1016/s1359-6101(97)00022-1. [DOI] [PubMed] [Google Scholar]
- 27.Cannon JG, St Pierre BA. Cytokines in exertion-induced skeletal muscle injury. Mol Cell Biochem. 1998;179(1–2):159–167. doi: 10.1023/a:1006828425418. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins JK, Malyak M, Arend WP. The effects of interleukin-10 on interleukin-1 receptor antagonist and interleukin-1 beta production in human monocytes and neutrophils. Lymphokine Cytokine Res. 1994;13(1):47–54. [PubMed] [Google Scholar]
- 29.Meckel Y, Nemet D, Bar-Sela S, et al. Hormonal and inflammatory responses to different types of sprint interval training. J Strength Cond Res. 2011;25(8):2161–2169. doi: 10.1519/JSC.0b013e3181dc4571. [DOI] [PubMed] [Google Scholar]
- 30.Croft L, Bartlett JD, MacLaren DP, et al. High-intensity interval training attenuates the exercise-induced increase in plasma IL-6 in response to acute exercise. Appl Physiol Nutr Metab. 2009;34(6):1098–1107. doi: 10.1139/H09-117. [DOI] [PubMed] [Google Scholar]
- 31.Knab AM, Nieman DC, Gillitt ND, et al. Effects of a flavonoid-rich juice on inflammation, oxidative stress, and immunity in elite swimmers: a metabolomics-based approach. Int J Sport Nutr Exerc Metab. 2013;23(2):150–160. doi: 10.1123/ijsnem.23.2.150. [DOI] [PubMed] [Google Scholar]
- 32.Bayati M, Farzad B, Gharakhanlou R, Agha-Alinejad H. A practical model of low-volume high-intensity interval training induces performance and metabolic adaptations that resemble ‘all-out’ sprint interval training. J Sports Sci Med. 2011;10(3):571–576. [PMC free article] [PubMed] [Google Scholar]
- 33.Gauldie J. Inflammation and the aging process: devil or angel. Nutr Rev. 2007;65(12 Pt 2):S167–S169. doi: 10.1111/j.1753-4887.2007.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 34.Moholdt T, Aamot IL, Granoien I, et al. Aerobic interval training increases peak oxygen uptake more than usual care exercise training in myocardial infarction patients: a randomized controlled study. Clin Rehabil. 2012;26(1):33–44. doi: 10.1177/0269215511405229. [DOI] [PubMed] [Google Scholar]