Abstract
Toll-like receptor 3 (TLR3) recognizes dsRNA and initiates an innate immune response through the formation of a signaling unit (SU) composed of one double-stranded RNA (dsRNA) and two TLR3 molecules. We report the crystal structure of human TLR3 ectodomain (TLR3ecd) in a quaternary complex with three neutralizing Fab fragments. Fab15 binds an epitope that overlaps the C-terminal dsRNA binding site and, in biochemical assays, blocks the interaction of TLR3ecd with dsRNA, thus directly antagonizing TLR3 signaling through inhibition of SU formation. In contrast, Fab12 and Fab1068 bind TLR3ecd at sites distinct from the N- and C-terminal regions that interact with dsRNA and do not inhibit minimal SU formation with short dsRNA. Molecular modeling based on the co-structure rationalizes these observations by showing that both Fab12 and Fab1068 prevent lateral clustering of SUs along the length of the dsRNA ligand. This model is further supported by cell-based assay results using dsRNA ligands of lengths that support single and multiple SUs. Thus, their antagonism of TLR3 signaling indicates that lateral clustering of SUs is required for TLR3 signal transduction.
Keywords: Toll-like receptor 3, TLR3, innate immunity, lateral TLR3 clustering, quaternary complex
Introduction
Innate immunity provides the first line of defense against invading pathogens.1 Toll-like receptors (TLRs), a major component of this system, sense a variety of microbial components such as lipopolysaccharides, lipids, and nucleic acids2 and trigger immunological responses including proinflammatory cytokine production by the host.3 When the TLR response is unchecked or dysregulated, these receptors and their signaling pathways have been associated with several human diseases such as inflammatory disorders,4 autoimmune diseases,5 cancer,6 and atherosclerosis.7
Receptor:ligand complexes mediate receptor dimerization to initiate TLR signaling. When TLR3 recognizes its ligand, double-stranded RNA (dsRNA),8,9 a signaling unit (SU) composed of one dsRNA and two TLR3 molecules forms.10,11 The dimerization of TLR3 leads to an intracellular TLR3 TIR dimer that engages its adaptor, TRIF, to initiate the downstream activation of NF-κB, AP-1, and IRF3, leading to pro-inflammatory and antiviral responses.12,13
The TLR3 ectodomain (TLR3ecd), containing 23 leucine-rich repeats (LRRs), adopts the overall shape of a horseshoe solenoid decorated by glycans.11,14,15 The molecular structure of an SU shows that the dsRNA ligand binds two regions, one near the N-terminus (LRR-NT to LRR3) and the other at the C-terminus (LRR19 to LRR21).11 This structure provides the basis for dsRNA-mediated cooperative TLR3 dimerization that is required for the downstream signaling cascade.10 However, the functional differences observed between short (∼48 bp) dsRNA ligands that form a single SU and longer (>∼90 bp) dsRNA ligands with multiple SUs docked to the ligand10,16 cannot be addressed by this single SU structure. Furthermore, it cannot explain how short dsRNA ligands (20–40 bp), which are too short to support the SU formation, activate TLR3 signaling.9,10,17,18
Previous studies of the TLR3:RNA complex showed that a minimum of two SUs were required to initiate endosomal TLR3 signaling, suggesting that, in addition to receptor dimerization for ligand recognition, clustering of receptor:ligand complexes may be necessary for competent signaling.10 Receptor clustering appears to be a common molecular feature among TLRs that signal through the adaptor molecule MyD88, which interacts with downstream signaling components to form a 14-subunit left-handed helical oligomer. Formation of this Myddosome would require multiple, clustered receptor: adaptor complexes.19 TLR3 is independent of MyD88 and signals exclusively through the adaptor TRIF. To elucidate the potential role of receptor clustering in TLR3 signal transduction, we generated and characterized three TLR3ecd-specific monoclonal antibodies (mAb1068, mAb12, and mAb15). These antibodies down-regulate dsRNA-induced pro-inflammatory mediators (mAb106820) or ablate dsRNA-induced TLR3 activity (mAb12/15, Fig. S1). Here, we describe the structure of a quaternary complex of human TLR3ecd with the Fab fragments of these three antibodies. The structure revealed the binding epitopes and their spatial orientation with respect to the SU. Rationalization of the activity of these antibodies with their binding sites provides structural evidence that lateral SU clustering is necessary for productive TLR3 signaling.
Results
A 49-bp dsRNA (single SU) is not sufficient to induce TLR3 signaling in HEK293 cells transiently expressing TLR3
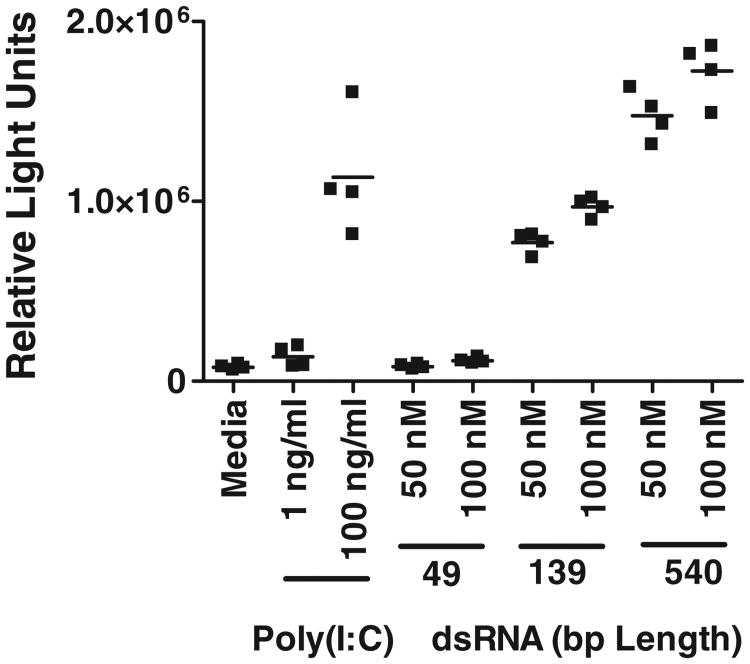
As reported earlier,20 in an NF-κB-driven luciferase reporter gene assay (RGA), poly(I:C) as well as long dsRNA (139 and 540 bp) ligands induced robust signaling mediated through TLR3 in HEK293 cells transiently expressing human TLR3 (Fig. 1). In contrast, a 49-bp dsRNA that supports formation of a single SU did not induce detectable activation in this assay (Fig. 1). These data are similar to the reported size dependence for poly(I:C) ligands,16 suggesting that single SUs are not sufficient to induce TLR3 signaling in some assays. This result would not be expected based upon the dsRNA-mediated TLR3 dimerization alone, suggesting that multiple SUs are needed for TLR3 signaling.
Fig. 1.
Length-dependent induction of NF-κB-driven TLR3 signaling RGA by dsRNA and poly(I:C) in HEK293T cells transiently expressing human TLR3. The 49-bp dsRNA did not induce a detectable signal whereas longer dsRNA and poly(I:C) generated robust activity.
Three high-affinity antibodies appear to neutralize by different mechanisms
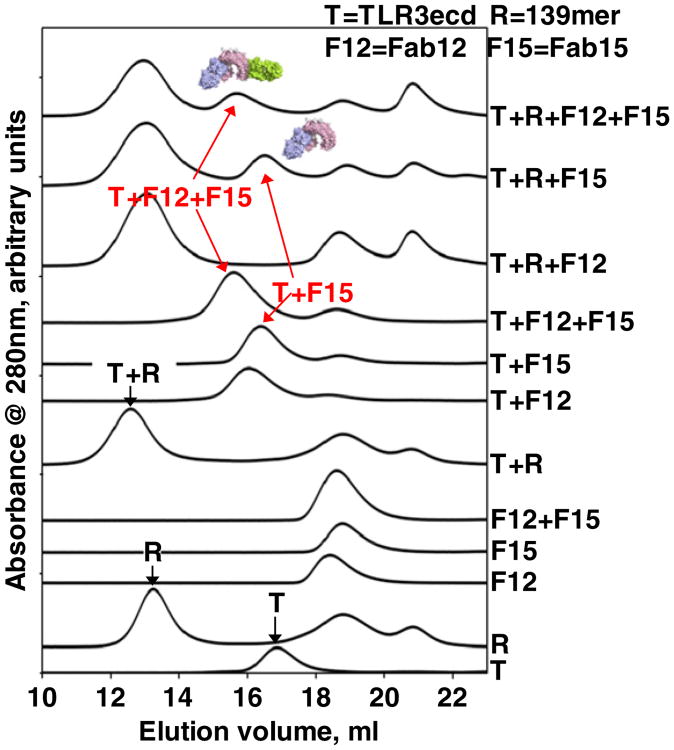
Three high-affinity anti-TLR3ecd mAbs (mAb1068, mAb12, and mAb15) were developed in order to probe TLR3 function. The affinities of the Fab fragments for TLR3ecd are in the low picomolar range (Table 1). All three mAbs dose-dependently inhibit the TLR3-mediated poly(I:C)-induced signals in the luciferase RGA (Fig. S1). MAb1068 only exhibited partial neutralization of poly(I:C)-induced TLR3 signaling (Fig. S1) as opposed to complete inhibition by mAb12 and mAb15. For the latter two antibodies, we also probed whether they blocked TLR3ecd binding to dsRNA in a size-exclusion chromatography (SEC) experiment using the Fab fragments to avoid potential complications of the bivalency of mAbs. The data are shown in Fig. 2. TLR3ecd and a 139-bp dsRNA form an apparent binary complex as shown by the absence of free RNA and TLR3 peaks and appearance of an earlier migrating peak (T+R, Fig. 2). Fab15 and Fab12 each form an apparent binary complex with TLR3ecd (T+F15, T+F12, respectively). Fab15, Fab12, and TLR3ecd can form a ternary complex (T+F12+F15). When Fab15 is combined with T+R (T+R+F15), a peak corresponding to T+F15 is apparent. In contrast, Fab12 does not form a T+F12 peak when combined with T+R (T+R+12). When all four components are mixed, a peak corresponding to T+ F12+F15 ternary complex is apparent. These data indicate that Fab15 prevents TLR3ecd from binding to dsRNA, whereas Fab12 can bind the TLR3ecd that is bound to dsRNA. We conclude that Fab15 inhibits TLR3 signaling by blocking TLR3ecd binding to the ligand dsRNA. However, how Fab12 neutralizes TLR3 function is not obvious. We crystallized and determined the structure of the quaternary complex of TL3ecd with Fab15, Fab12, and Fab1068. This structure confirmed direct site competition of Fab15 with RNA and indicated how the non-RNA competitive Fab12 and Fab1068 could nonetheless inhibit TLR signaling, leading to further experimental investigation.
Table 1. Fab binding affinity and buried surface area (SA) upon TLR3 binding.
| Fab | ka×106 (M−1s−1) | kd×10−4 (s−1) | Kd (pM) | SAAg (Å2) | SAAb (Å2) |
|---|---|---|---|---|---|
| Fab12 | 4.11 (1) | 2.83 (0) | 68.8 (4) | 928 | 896 |
| Fab1068 | 3.54 (1) | 1.03 (1) | 29.1 (4) | 914 | 963 |
| Fab15 | 3.33 (1) | 1.02 (3) | 3.1 (1) | 1064 | 1080 |
Fig. 2.
Fab15 inhibits TLR3ecd:dsRNA binding. Size-exclusion chromatograms of various combinations of TLR3ecd (T), Fab15 (F15), Fab12 (F12), and 139 bp dsRNA (R). Fab15 forms a dsRNA-free TLR3+Fab15 complex (T+R+F15), indicating that it blocks TLR3ecd from binding to dsRNA. The excess TLR3ecd was loaded onto dsRNA. Fab12 molecules were completely bound by the TLR3ecd:dsRNA complex (T+R+F12). In the presence of Fab15, Fab12 can form a dsRNA-free TLR3ecd+Fab15 +Fab12 ternary complex (T+F12+F15).
Overall structure and antibody:antigen interactions
The structures of Fab12 and Fab1068 were determined in the absence of TLR3 (Table 2 and Supplementary Results). The structure of Fab15 alone was reported previously.22 The overall molecular structure of the quaternary complex, determined in two crystal forms, is shown in Fig. 3a (Table 2). The asymmetric unit contained one TLR3ecd and one molecule of each Fab. In the quaternary complex, the Fabs retained their respective unbound structures (Fab1068 and Fab12, Table 2; Fab1522), except for the CDR-L3 of Fab12 due to antigen interactions, as well as a conformational change in the Fab elbow regions. The structural model for TLR3ecd included residues 30–687 in Form 1 and residues 29–696 in Form 2 and 11 glycans, which were visible in the electron density unbiased by the models used for molecular replacement (MR) (Fig. S2). The TLR3ecd molecule is similar to previously reported TLR3ecd structures in overall topology (rmsd of 0.79 Å for 1ZIW,15 613 Cα atoms, and 1.37 Å for 2A0Z,14 595 Cα atoms). In the quaternary structure, each Fab binds a unique TLR3ecd surface (Fig. 3a and b), burying between 896 and 1080 Å2 at the Fab:antigen interface. The buried surface areas roughly correlated with their respective binding affinities (Tables 1 and 2).
Table 2. Crystal data and structure refinement statistics.
| Fab12 | Fab1068a | TLR3:3Fabs (Form 1)b | TLR3:3Fabs (Form 2)b | |
|---|---|---|---|---|
| Data collection | ||||
| Space group | P21 | P21 | C2 | C2 |
| Cell dimensions | ||||
| a, b, c (Å) | 75.80, 80.21, 83.05 | 82.48, 136.94, 83.25 | 215.28, 142.32, 125.34 | 363.53, 131.08, 153.82 |
| α, β, γ (°) | 90, 115.60, 90 | 90, 114.95, 90 | 90, 103.32, 90 | 90, 91.23, 90 |
| Mol/asymmetric unit | 2 | 4 | 1 quat. complex | 1 quat. complex |
| Resolution (Å) | 50–2.5 (2.56–2.50) | 50–1.9 (2.0–1.9) | 50–3.5 (3.7–3.5) | 50–3.5 (3.7–3.5) |
| Unique reflections | 30,263 (1818) | 117,490 (5916) | 34,487 (892) | 48,050 (1626) |
| Completeness (%) | 96.1 (79.3) | 89.3 (45.2) | 74.1 (12.6) | 52.8 (11.7) |
| Redundancy | 3.7 (3.3) | 3.2 (2.0) | 5.3 (5.0) | 5.5 (4.3) |
| Rmergec | 0.093 (0.504) | 0.065 (0.264) | 0.202 (0.682) | 0.208 (0.672) |
| (I/σ) (unaveraged) | 11.5 (2.4) | 5.7 (1.6) | 8.4 (2.2) | 8.9 (2.3) |
| Structure refinement | ||||
| Resolution (Å) | 34.40–2.50 (2.56–2.50) | 44.55–1.90 (1.92–1.90) | 45.08–3.52 (3.63–2.52) | 48.43–3.52 (3.57–3.52) |
| Rcryst/Rfree(%)d | 21.2/26.7 (27.7/37.2) | 20.3/25.8 (42.7/45.9) | 27.4/31.3 (33.6/41.9) | 23.0/25.3 (31.7/46.7) |
| Number of reflections | ||||
| Working/test set | 28,248/1999 | 111,413/5914 | 34,185/1722 | 48,256/2396 |
| Number of atoms | ||||
| Proteins | 6567 | 13,532 | 15,496 | 15,602 |
| Solvent (water, etc.) | 350 | 1627 | ||
| RMSD bond lengths (Å) | 0.003 | 0.007 | 0.002 | 0.002 |
| RMSD bond angles (°) | 0.72 | 1.11 | 0.62 | 0.61 |
| Mean B-factors (Å2) | ||||
| Protein | 41.4 | 29.8 | 117.0 | 70.0 |
| Solvent | 36.5 | 39.8 | ||
| Ramachandran plote | ||||
| Favored regions (%) | 93.9 | 97.5 | 93.9 | 93.1 |
| Outliers (%) | 0.9 | 0.2 | 0.5 | 0.8 |
Values for highest-resolution shell are in parentheses.
Part of this column was included in the supplementary material of Ref. 21.
The anisotropic resolution limits in a*, b*, and c* are 3.5, 4.4, and 3.5 Å, respectively, for Form 1, and 3.5, 6.4, and 3.5 Å, respectively for Form 2, according to the diffraction anisotropy scale server (http://services.mbi.ucla.edu/anisoscale/). Diffraction data statistics are for the data sets after anisotropic truncation using these limits.
Rmerge=Σ|I−<I>|/ΣI, where I is the intensity of the measured reflection and <I> is the mean intensity of all measurements of this reflection.
Rcryst=Σ‖Fobs|−|Fcalc ‖/Σ|Fobs|, where Fobs and Fcalc are observed and calculated structure factors and Rfree is calculated for a set of randomly chosen 5% of reflections prior to refinement.
The Ramachandran plot was calculated with MolProbity.
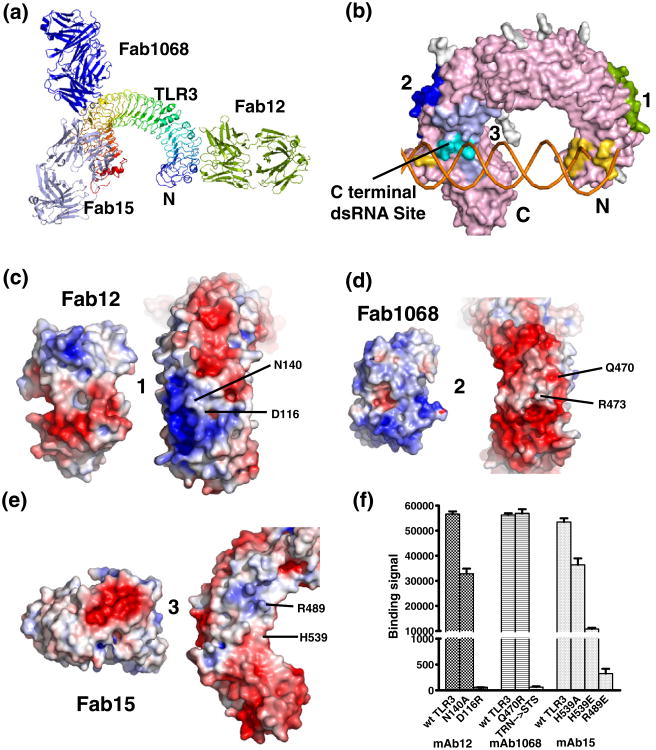
Fig. 3.
Molecular structure of the quaternary complex of TLR3ecd/3Fabs, binding epitopes, and paratopes. Overall structure of the complex in ribbon (a) and surface (b) representations. TLR3 colored in rainbow scheme (blue-to-red for N-to-C), Fab12 in yellow-green, Fab15 in light blue, and Fab1068 in blue. Carbohydrates are omitted for clarity. (b) Distinct binding epitopes are highlighted on TLR3. The epitopes are colored on the TLR3ecd as for the Fabs in (a). The C-terminal dsRNA binding site is indicated. The overlap of the Fab15 epitope and dsRNA binding site is indicated in cyan. (c–e) Open-book views of epitope and paratope pairs (Fab12, Fab1068, and Fab15, respectively) with electrostatic potentials (blue, positive; red, negative) mapped on the molecular surfaces. (f) Mutational analysis confirms importance of structural epitope residues.
Fab12 binds near the N-terminus of the TLR3ecd on the convex surface of the TLR3ecd solenoid (Fig. 3a and b). The conformational epitope is composed of residues from the LRRs 3–7 and isdistinct from the N-terminal dsRNA binding site. The epitope/paratope surface is predominated by mirrored patches of complementary electrostatic charge (Fig. 3c). When this surface was mutated to reverse the electrostatic properties, D116R, or to remove side-chain interactions, N140A, mAb12 binding was reduced to 2% and ∼60% binding, respectively (Fig. 3f).
Fab1068 and Fab15 bind non-overlapping epitopes spanning LRRs 15–23 near the C-terminus (Fig. 3a and b). Fab1068 targets the convex surface of the solenoid from LRRs 16–20 and presents a basic paratope surface to the negatively charged TLR3ecd epitope surface (Fig. 3d). The triple mutant 472TRN474→STS (human TLR3 to mouse TLR3 sequence), altering both shape and charge of the interacting surface, completely abrogated mAb1068 binding, whereas a non-epitope mutation, Q470R, retained full antibody binding (Fig. 3f).
The Fab15 epitope lies on the glycan-free lateral surface of TLR3ecd covering LRRs 15–23 (Fig. 3a and b). The paratope of Fab15 consists of a concave crater with a strongly negative well (Fig. 3e, left) that is highly complementary to a small cluster of positively charged residues on TLR3ecd (Fig. 3e, right). Removing a partial positive charge from the epitope surface, H539A, reduced mAb15 binding by ∼32%, while charge reversal mutations H539E and R489E led to ∼80% and 99% loss, respectively, in antibody binding (Fig. 3f).
Fab15 neutralizes by blocking TLR3:dsRNA binding
The Fab15 epitope contains amino acid residues N517, H539, and N541 that are part of the C-terminal dsRNA binding site and were shown to be critical for ligand binding11,23 (Fig. 3b). Binding of Fab15 would prevent TLR3ecd from binding dsRNA. This is consistent with the SEC data showing that Fab15 forms dsRNA-free TLR3ecd: Fab15 complex in the TLR3ecd:dsRNA:Fab15 mixture (Fig. 2). Thus, Fab15 competes with dsRNA for TLR3 binding and prevents ligand-induced receptor dimerization, which is required for the formation of the SU.11 The TLR3ecd:Fab15 interaction is consistent with mAb15's complete inhibition of poly(I:C)-induced TLR3 activation (Fig. S1).
Fab12 and Fab1068 are compatible with TLR3ecd:dsRNA SU
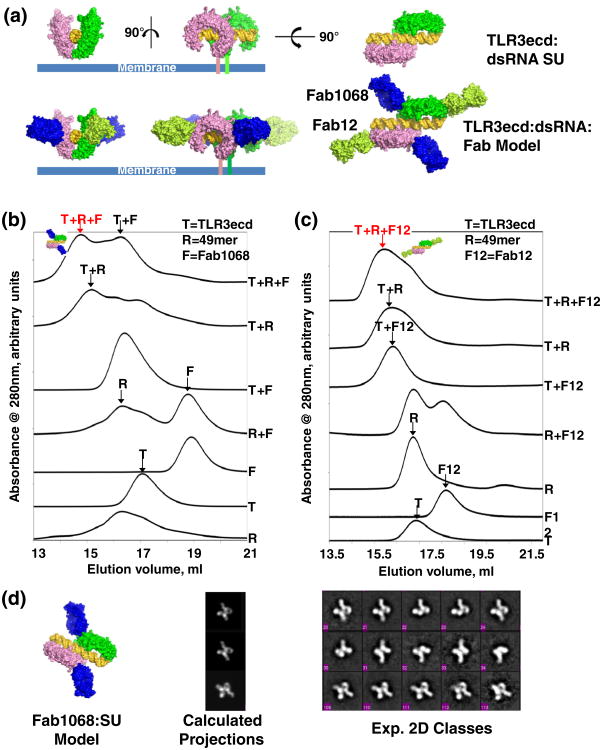
The Fab1068 and Fab12 binding sites do not overlap any of the amino acid residues involved in dsRNA binding. Based upon the structures of TLR3ecd:3Fabs and the TLR3ecd:dsRNA,11 we generated composite molecular models of Fab12 and/or Fab1068 bound to the TLR3ecd:dsRNA dimer (Fig. 4a). The bound Fab1068 or Fab12 does not sterically clash with the dsRNA ligand or the TLR3ecd dimer partner in the SU (Fig. 4a, bottom). Additionally, both bound Fabs are nearly parallel to the cell membrane surface (Fig. 4a, bottom) and would therefore be unlikely to disrupt the orientation of the TLR3:dsRNA complex with respect to the membrane surface and subsequent TIR domain dimerization. Thus, Fab1068 and Fab12 are predicted to bind to an SU without disrupting its function. SEC data show that Fab12 and Fab1068 can bind to the dsRNA-bound TLR3ecd (Figs. 2 and 4b and c). The Fab1068:TLR3ecd:dsRNA species was also examined by negative stain electron microscopy (EM). Representative two-dimensional (2D) class averages show that molecular assemblies composed of 2TLR3ecd:dsRNA:2Fab1068 and 2TLR3ecd: dsRNA:Fab1068 are present (Fig. 4d, right), similar to composites generated from the Fab1068:TLR3ecd: dsRNA model (Fig. 4d, middle). These data indicate that Fab1068, and similarly Fab12, can bind simultaneously to two TLR3 molecules of an SU without preventing dsRNA binding or TLR3 dimerization (Fig. 4a). Thus, Fab12 and Fab1068 are unlikely to neutralize TLR3 function by direct inhibition of SU formation.
Fig. 4.
Fab12 and Fab1068 bind TLR3ecd in the TLR3ecd:dsRNA SU. (a) Model of Fab1068 and Fab12 binding to a single SU. Fab binding does not inhibit dsRNA binding, TLR3 dimerization, or membrane association of TLR3. (b) SEC of various combinations of TLR3ecd (T), Fab1068 (F), and 49 bp dsRNA (R) indicate the presence of the R+T+F ternary complex. The R+T+F peak was collected and concentrated for EM. (c) SEC of various combinations of TLR3ecd (T), Fab12 (F12), and 49 bp dsRNA (R) indicate formation of R+T+F12 ternary complex. (d) EM 2D class averages of the ternary dsRNA:TLR3ecd:Fab1068 complex. 2D projections (middle panel) of a composite model complex (left panel) and selected class averages showing molecular complexes of dsRNA:2TLR3ecd:2Fab and dsRNA:2TLR3ecd:1Fab (right panel).
Lateral clustering model of TLR3 signaling
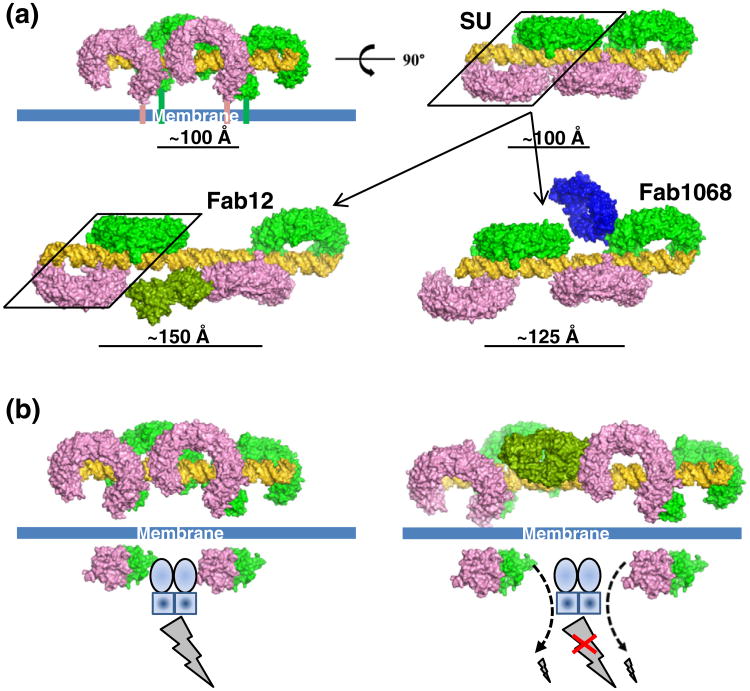
Here, we propose a modified model of TLR3 signaling following ligand-induced dimerization (Fig. 5a and b). Longer ligands support the formation of multiple SUs on a single dsRNA molecule10 and the length of poly(I:C) is important for the induction of TLR3-dependent activity.16 The apparent affinities of TLR3ecd for longer dsRNA ligands are much higher than for 49 bp dsRNA,10 potentially indicating the occurrence of lateral SU interactions and avidity effects of multiple binding sites on the longer dsRNA. A model of two adjacent SUs assembled on dsRNA is shown in Fig. 5a. Binding of Fab12 and Fab1068 laterally separates but does not disrupt individual SUs along the dsRNA molecules (Fig. 5a, bottom panel). The Fabinduced SU separation likely provides the neutralizing activity of these antibodies (Fig. S1) and indicates that maintaining a short distance between the SUs, termed “lateral clustering”, is required for TLR3 signaling. From the molecular model of multiple SUs on a dsRNA molecule (Fig. 5a), no additional interactions between individual ectodomains of an SU have been identified, suggesting that lateral clustering is not required for cooperative assembly of TLR3ecd:dsRNA SUs. Additionally, the periodic nature of dsRNA only allows TLR3ecd binding in discrete steps and few interactions exist between neighboring SUs based upon our molecular modeling (Fig. 5a), indicating that additional TLR3ecd intermolecular interactions are unlikely to mediate SU lateral clustering. With respect to the receptor's cytosolic domain, the TLR3 TIR domain dimer of a single SU cannot contact a neighboring SU TIR dimer directly (Fig. 5b, left panel). However, the lateral clustering between SUs could position the TIR dimers to coordinate an intracellular factor, which strengthens interactions between TLR3 TIR dimers and downstream factors due to avidity effects of two-site binding (Fig. 5b).
Fig. 5.
Lateral SU clustering mediated TLR3 signaling. (a) Model of a two-SUs clustered on a dsRNA of about 76 bp (top panel) and separation of SUs (bottom panel) on long dsRNA by Fab1068 and Fab12 due to steric clashes between the antibodies and neighboring SUs. The separation distances were calculated using the center of gravity of a group of TLR3ecd C-terminal atoms. (b) Model of TLR3 signaling enhanced by lateral SU clustering (left panel) and inhibition of signaling by Fab12 (right panel). The broken line and arrow denote binding of the bridging factor to one side of the TLR3 TIR dimer with weaker signaling strength. The clustering is likely mediated by an intracellular dimeric factor, postulated to be TRIF (TICAM-1). The composite model was generated based upon this work and PDB code 3CIY for the dsRNA: TLR3ecd:Fabs and PDB code 2J67 (TLR10 TIR dimer) for the TLR3 TIR dimer.
Structural modeling thus indicates that both Fab12 and Fab1068 neutralize the lateral clustering by separating the SUs to avoid steric clashes between bound antibody and a neighboring SU (Fig. 5b, right panel). This model suggests that increasing neutralization effects of antibodies are dependent upon the distance between SUs resulting from antibody binding. This separation should not inhibit the basal activity of a single SU. As shown in Fig. 5a, Fab12 causes a larger SU separation than Fab1068. Fab12's larger SU separation correlates with mAb12's ability to consistently exhibit greater neutralizing activity than mAb1068 (Fig. S1) even though Fab12 has a slightly lower binding affinity and smaller buried antibody:antigen surface area than Fab1068 (Table 1). These data suggest that disruption of lateral clustering is key to antibody inhibitory function.
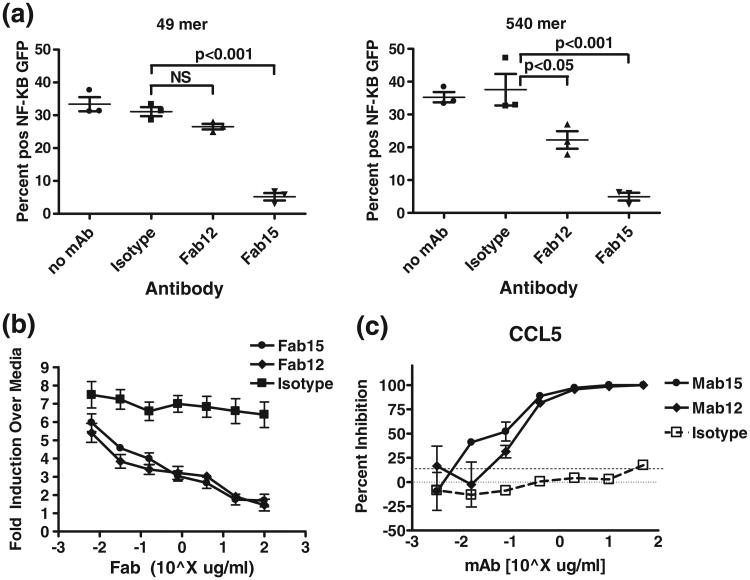
To test the validity of this model, we carried out neutralization assays in TLR3hi cells that stably express TLR3 and a GFP reporter gene driven by NF-κB.10 As opposed to the transient TLR3 RGA, short dsRNA ligands were able to generate robust TLR3 activation in this cell line.10 Cells were stimulated with a 49-bp (single SU binding) or 540- bp (multiple SU binding) dsRNA in the presence of saturating amounts of Fab. Fab15 completely inhibited GFP fluorescence induced by both ligands (Fig. 6a), indicating that dsRNA-mediated TLR3 dimerization is necessary for downstream signaling. In contrast, Fab12 did not significantly inhibit 49 bp dsRNA (single SU)-induced fluorescence, but did partially inhibit 540-bpinduced fluorescence (Fig. 6a). These results are consistent with the model above in which Fab12 (and Fab1068 by inference) reduce TLR3 signaling via disruption of SU–SU interactions but have little impact on signaling from the single SU.
Fig. 6.
Antibody inhibition of TLR3 activation induced by dsRNA and poly(I:C). (a) Fab15 completely inhibits fluorescence of TLR3hi cells.14 Fab12 partially inhibits signal induced by 540 bp dsRNAs and has no significant impact on that of 49 bp dsRNA. Percentage of GFP-positive cells after 48 h treatment with dsRNA (49 bp or 540 bp)±antibody treatment as indicated. Error bars indicate mean and SEM from three independent experiments. Tests for significant differences were performed by Bonferroni posttests of one-way ANOVA (GraphPad Prism). ns, not significant. (b) Fab15 and Fab12 nearly completely inhibit poly(I:C)-induced TLR3 signaling in NF-kB RGA in HEK293 cells with transient TLR3 expression. (c) mAb15 and mAb12 nearly completely inhibit poly(I:C)-induced chemokine (CCL5) production in NHBE cells.
We further evaluated the relative contributions of dimerization and SU–SU interactions to TLR3 signaling. In an NF-κB-driven luciferase RGA in HEK293 cells transiently expressing human TLR3,20 where 49 bp dsRNA does not induce detectable levels of TLR3-mediated signals, both Fab15 and Fab12 strongly inhibited the RGA luciferase activity induced by poly(I:C) in a dose-dependent manner (Fig. 6b). Similarly, in normal human bronchial epithelial (NHBE) cells that endogenously express TLR3, poly(I:C)-induced chemokine secretion was dose-dependently inhibited by both antibodies (Fig. 6c). These data indicate that blocking the SU lateral clustering by Fab12/mAb12 is nearly as effective as blocking dsRNA-mediated dimerization by Fab15/mAb15. Thus, SU–SU lateral clustering is essential for efficient TLR3 signaling induced by its ligand.
Discussion
Our structural and functional studies of TLR3 with neutralizing antibodies provide evidence for a new TLR3 signaling model in which dsRNA:TLR3 SUs laterally cluster to achieve efficient signaling (Fig. 5b). Furthermore, in cell lines transiently expressing TLR3 or endogenous TLR3 in normal human epithelial cells, this cluster-mediated TLR3 signaling appears equally important to ligand-mediated dimerization. While previous data that TLR3ecd binds longer dsRNA with much higher affinity could potentially indicate weak lateral interactions between SUs,10 our molecular modeling results do not provide strong support for that hypothesis. Thus, as speculated above, intracellular factors likely play a role in bridging TIR dimers for SU lateral clustering. To link clustered SU, this internal protein assembly would contain two binding sites to interact with two different TIR dimers. A likely candidate to mediate this intracellular assembly is TRIF (TICAM-1), which mediates TLR3 signaling.13 Recent data indicate that TRIF dimerization is required for TLR3 function and that the TIR dimer of TRIF interacts with the TLR3 TIR dimer,24 consistent with a role in assembling/stabilizing clustered receptors. Lateral clustering of TLR3 SUs appears to be a unique feature that capitalizes upon the recognition of the repeating pattern of dsRNA ligand.
Short dsRNA ligands (21–40 bp) have been shown to activate TLR3.9,10,17,18 Due to the short length, these ligands are not capable of forming the TLR3:dsRNA complex as in the crystal structure.11 It was proposed that TLR3 may form a different dsRNA:TLR3 complex in which the two TLR3 monomers slide along the dsRNA by one turn with respect to each other, thus requiring a shorter ligand.25 While this is an attractive model and would also be compatible with Fab1068 and Fab12 binding, it is not supported by our EM studies of dsRNA:TLR3ecd:Fab1068 ternary complex (Fig. 4d), which provide support that the solution dsRNA:TLR3 SU is very similar to the complex revealed by the crystal structure.11 Furthermore, the Pirher model still requires dsRNA of ∼36 bp. Short dsRNA ligands (∼21 bp) are long enoughtoformacomplex with TLR3 with only the C-terminal binding sites engaged. Normally, due to weak interactions, this complex will bequite unstable. However, under certain conditions, neighboring complexes may be linked by the cytosolic bridging factor speculated above and thus stabilized, in a fashion very analogous to the lateral clustering for long dsRNA ligands. Analogously, the observations that TLR3hi cells are responsive to 49 bp dsRNA whereas HEK293 cells transiently expressing TLR3 are not could be a result of the TLR3 density in the endosomes due to different levels of TLR3 expression in these cells. At high TLR3 density, the speculated lateral SU clustering could be bridged by the proposed intracellular TRIF dimers without the repeating patterns of long dsRNA. The validity of this hypothesis certainly requires additional experimental verification. Nevertheless, lateral clustering seems to be a mechanism that could unify the TLR3 activation by both short and long dsRNA.
Materials and Methods
Generation of neutralizing antibodies mAb12 and mAb15
The parent antigen binding fragments (Fab12 and Fab15) of these antibodies were selected against human TLR3ecd from the HuCAL Gold synthetic human antibody library26 using standard protocols. The Fv fragments for Fab12 and Fab15 were transferred onto human IgG4λ and IgG4κ constant domains to generate the final mAb12 and mAb15, respectively.
Expression and purification of proteins
TLR3ecd
The coding sequence for TLR3ecd (26–697) with a C-terminal 6× His tag was inserted into pAcGP67B (Clontech, California) and expressed in Sf9 cells. The His-tagged TLR3ecd was then purified by HisTrap chromatography (GE Healthcare).
TLR3ecd mutants
All mutants were generated by site-directed mutagenesis using the Stratagene QuikChange II XL kit (Stratagene, San Diego, CA) according to the manufacturer's protocol. The mutant proteins with changes in the Fab1068 epitope were produced as described for the wild-type protein above in Sf9 cells. The mutant proteins in Fab12 and Fab15 epitopes were transiently expressed under a CMV promoter in HEK293 cells. TLR3ecd mutants within the Fab15 and Fab12 epitopes were co-transfected with mAb12 and mAb15, respectively. Mutant TLR3ecd:mAb complexes were purified and used for ELISA. This co-expression of mAbs with TLR3ecd, a poorly expressed protein in mammalian cells, enhanced TLR3ecd protein levels and facilitated binding assays, indicating that high-affinity antibodies can function as specific chaperones. This approach may find application in structural biology and other areas of biological research.
Fabs
The expression, purification, and the crystal structure determination of Fab15 have been previously described.22 Fab1068, which is composed of the Fv of CNTO242420 with a human IgG4 CH1 and κ CL, and Fab12 were expressed in HEK293 cells and purified as previously described.27 The Fab12 and Fab1068 proteins were concentrated to 11.3 mg/mL (10 mM Tris, pH 7.1, and 50 mM NaCl) and 8.8 mg/mL (20 mM 4-morpholineetha-nesulfonic acid, pH 6.0, and 10% glycerol), respectively.
Fab affinity determination
Human TLR3ecd was minimally biotinylated and captured at three densities (40, 70, and 160 RU) onto a streptavidin-coated sensor chip. The three Fabs were all tested in duplicate in a threefold dilution series starting at 16.7 nM. Studies were performed at 25 °C in Dulbecco's phosphate-buffered saline (D-PBS) (Invitrogen, California), pH 7.4, with 0.005% P20 using Biacore 2000 (GE Healthcare, NJ). Each Fab was injected for 1 min. The dissociation was monitored for 10 min for Fab1068 and Fab12, but dissociation for Fab 15 was monitored for 30 min. Between binding cycles, the antigen surface was regenerated with two 12-s pulses of 11 mM phosphoric acid (for Fab1068 and Fab12) or 33 mM phosphoric acid (for Fab15). For all interactions, the responses from the three antigen surfaces were globally fit to a 1:1 interaction model. The rate and binding constants are listed in Table 1.
Crystallization
The details of crystallization of the TLR3:Fab12:Fab15: Fab1068 quaternary complex were described elsewhere.28 Crystallization of the Fab12 and Fab1068 was carried out by the vapor-diffusion hanging-drop method at 20 °C. Crystals of Fab12 (11.3 mg/mL) were grown from drops mixed 1:1 with a well solution of 18% polyethylene glycol (PEG) 8000 in 0.1 M sodium acetate buffer, pH 4.5. Crystals of Fab1068 (8.8 mg/mL) were grown from drops mixed 1:1 with well solution of 20–22% PEG 8000 and 0.2 M magnesium chloride in 0.1 M Tris buffer, pH 8.5, after microseeding.
X-ray data collection and structure determination
Fab12
One crystal of Fab12 and Fab1068 each was soaked for a few seconds in a cryo-protectant solution (Fab12: 20% PEG 8000, 0.1 M Na acetate, pH 4.5, and 20% glycerol; Fab1068: 0.2 M MgCl2, 28% PEG 8000, 20% glycerol, and Tris–HCl, pH 8.5) and flash frozen in the stream of nitrogen at 121 K. For the quaternary complex, one crystal of each form was soaked for a few seconds in synthetic mother liquors (Form 1: 0.1 M Na acetate, pH 4.5, 28% PEG 3350,1 M LiCl, and 16% glycerol; Form 2: 0.1 M Na acetate, pH 4.5, 2.5 M (NH4)2SO4, 5% PEG 400, and 16% glycerol) and flash frozen in the stream of nitrogen at 100 K. X-ray diffraction data were collected using the Rigaku MicroMax™-007HF microfocus X-ray generator equipped with an Osmic™ VariMax™ confocal optics, Saturn 944 CCD detector, and an X-stream™ 2000 cryocooling system (Rigaku, Woodlands, TX). The data were processed with XDS29 (D*TREK30 for Fab1068). The diffraction data for the quaternary complex exhibited severe anisotropy and were further anisotropically truncated and scaled using the Diffraction Anisotropy Server† as previously described.31
All structures were determined by MR using the program Phaser.32 Structure refinement was carried out with REFMAC33 or PHENIX.34 Model adjustments were carried out using the program Coot35 with integrated MolProbity clash checking.36 Other crystallographic calculations were performed with the CCP4 suite of programs.37 Molecular graphics was done with PyMOL.38 The structure refinement statistics are shown in Table 2.
Fab12
The MR models were derived from Fab607.39 Refinement with PHENIX converged at Rcryst =21.2% and Rfree =26.7% for all data in the resolution range 50–2.5 Å.
Fab1068
The Fab1068 crystal contained strong pseudo translational symmetry with four Fabs in the asymmetric unit. The MR search models were derived from Fab15.22 The final refinement with PHENIX converged at Rcryst =20.3% and Rfree =25.8% for all reflections to 1.9 Å resolution.
Quaternary complex
The structures were determined by MR using the program Phaser.32 The MR search models were TLR3ecd [Protein Data Bank (PDB) ID 1ZIW or 2A0Z]14,15 with all glycans removed and the structures of Fab15,22 Fab12, and Fab1068 (Supplementary Table 1). Form 1 was first solved and refined using PHENIX34 with restraints derived from TLR3ecd (2A0Z) and the three Fabs using a procedure similar to that described40 against anisotropically processed data to 3.5 Å. The Form 2 structure was solved with the Form 1 model and refined similarly. Model adjustments were carried out using the program Coot35 with integrated MolProbity clash checking.36 Other crystallographic calculations were performed with the CCP4 suite of programs.37 Molecular graphics was done with PyMOL.38 The X-ray data and structure refinement statistics are shown in Table 2.
ELISA binding of TLR3 mutants with mAbs
Wild-type TLR3ecd or mutant proteins (100 ng) in PBS were coated on an MSD HighBind plate (Meso Scale Discovery, Gaithersburg, MD). The plates were incubated at room temperature for 60 min and blocked overnight in MSD Blocker A buffer (Meso Scale Discovery) at 4 °C. The plates were then washed, and the MSD Sulfo-tag-labeled mAb was added at concentrations from 500 pM to 1 pM for 1.5 h. After washing, the labeled antibody was detected using MSD Read Buffer T and the plates were read using a SECTOR Imager 6000.
SEC of TLR3ecd:dsRNA:Fab complexes
Individual components or complexes were separated on a Superose 6 Tricorn column (GE Healthcare) equilibrated in 0.02 M Pipes, pH 6, 150 mM NaCl, and 1 mM beta mercaptoethanol prepared with diethylpyrocarbonatetreated water. Component or complexes were incubated at least 1 h at 4 °C before separation. TLR3ecd and/or Fab were mixed with dsRNA in a 2:1 molar ratio. Separation was monitored by absorbance at 280 nm and components in peak fractions confirmed by SDS-PAGE separation and Coomassie/silver staining.
EM 2D averages of TLR3:dsRNA:Fab1068 ternary complex
Aliquots (2.5 μL) of concentrated complex from the R+T+F peak were applied to carbon grids, blotted and stained by 1% uranyl formate for 2 min. The stained sample was sandwiched with another layer of carbon and air dried. CCD fields (F415 4K×4 K CCD, TVIPS GmbH) were taken at 29,000× nominal magnification in an FEI Tecnai F20 electron microscope (FEI, Hillsboro, OR) operated at 200 kV acceleration. The actual magnification on CCD is 40,600×, and the pixel size is 3.69 Å. Particles were then semiautomatically boxed by EMAN,41 and the resulting particles were subjected to EMAN refine2d.py module for 2D reference free classification. A total of 17,831 particles were classified into 200 classes in 18 iterations.
For comparison with experimental 2D averages, a composite model of TLR3ecd:49 bp dsRNA:Fab1068 was constructed by combining the TLR3ecd/Fab1068 part of the crystal structure of the TLR3ecd:3Fab complex (this work) with the TLR3ecd:dsRNA complex structure11 with the TLR3ecds superimposed. This model was then blurred to 25 Å resolution to generate a 3D volume and 2D projections were generated according to the orientations of select 2D averages of R+T+F.
Inhibition of TLR3 activity induced by dsRNA of different lengths (49 bp and 540 bp) in TLR3hi cells using NF-kB-driven GFP reporter assay
TLR3hi cells10 were stimulated with media, media+ dsRNA (25 μg/mL), media+Fab or control mAb (100 μg/mL), or media+dsRNA+Fab/control mAb for 48 h. Cells were harvested by washing wells with PBS+ 20 mM ethylenediaminetetraacetic acid. Cells were pelleted, washed three times with FACS buffer (1× Hank's buffered salt solution, 0.1% bovine serum albumin, and 0.1% sodium azide), and resuspended in FACS buffer. GFP expression was analyzed via flow cytometry on an FC500 using CXP v.1 acquisition/analysis software (Beckman Coulter). For each sample, 10,000 events were recorded. Mean of three separate experiments±SEM is reported. The 49-bp dsRNA, as described in Ref. 28, was purchased from Dharmacon. The 540-bp dsRNA was prepared as previously described.28
Inhibition of poly(I:C)-induced TLR3 activity by anti-TLR3 mAbs/Fabs
The NF-κB-driven luciferase RGA experiments were carried out essentially as described in Ref. 20 with the 100-μg/mL mAb/Fab15, mAb/Fab12, and mAb/Fab1068 instead of mAb CNTO2424.
Primary NHBE cells (Lonza, CC-2540) were expanded for one passage in modified Bronchial Epithelial Basal Medium plus supplements in the SingleQuot Kit (Lonza, CC-4175), harvested, aliquoted, and stored in liquid nitrogen. After thawing, 1 million passage 2 cells were transferred to a collagen-coated flask and incubated at 37 °C, 5% CO2 for 24 h. The cells were harvested and resuspended at 5 × 104 cells/mL and distributed at 0.2 mL/well in collagen-coated 96-well plates. The NHBE cells were cultured at 37 °C, 5% CO2 for 48 h (wells were 80–90% confluent) after which the media were removed and replenished with 0.2 mL of fresh media. The cells were incubated for 1 h at 37 °C, 5% CO2, with or without anti-human TLR3 mAbs prior to the addition of poly(I:C) at 62.5 ng/mL. Human IgG4κ (Sigma, I4369) or IgG4λ (Sigma, I4764) was used as negative controls. All conditions were run in duplicate. Supernatants were collected after 24 h and stored at −20 °C or assayed immediately for cytokine secretion using a custom multiplex bead assay (Invitrogen) for detection of cytokines including CCL5. Sample acquisition and analysis were performed using the Luminex 100™ IS (Luminex, Austin, TX) with StarStation software (Applied Cytometry Systems, Sacramento, CA).
Supplementary Material
Acknowledgments
The authors wish to thank Mark Cunningham, Bethany Swenckie-Underwood, and Juliane Mills for protein expression and purification; David Myszka of Biosensors, Inc. (UT) for Biacore analysis; and the VCU Flow Cytometry core laboratory, which is supported, in part, by funding from the National Institutes of Health–National Cancer Institute Cancer Center Support Grant (P30 CA016059).
Abbreviations used
- TLR
Toll-like receptor
- SU
signaling unit
- dsRNA
double-stranded RNA
- TLR3ecd
TLR3 ectodomain
- LRR
leucine-rich repeat
- mAb
monoclonal antibody
- RGA
reporter gene assay
- SEC
size-exclusion chromatography
- EM
electron microscopy
- 2D
two-dimensional
- NHBE
normal human bronchial epithelial
- PBS
phosphate-buffered saline
- PEG
polyethylene glycol
- MR
molecular replacement
- PDB
Protein Data Bank
Footnotes
Accession codes: The coordinates and the structure factors for Fab12, Fab1068, and TLR3ecd:3Fabs Forms 1 and 2 have been deposited at the PDB under accession numbers 3ULS, 3QPQ, 3ULU, and 3ULV, respectively.
Supplementary Data: Supplementary data to this article can be found online at doi:10.1016/j.jmb.2012.05.006
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5:975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev, Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 4.Keogh B, Parker AE. Toll-like receptors as targets for immune disorders. Trends Pharmacol Sci. 2011;32:435–442. doi: 10.1016/j.tips.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Clanchy FI, Sacre SM. Modulation of tolllike receptor function has therapeutic potential in autoimmune disease. Expert Opin Biol Ther. 2010;10:1703–1716. doi: 10.1517/14712598.2010.534080. [DOI] [PubMed] [Google Scholar]
- 6.Matijevic T, Pavelic J. Toll-like receptors: cost or benefit for cancer? Curr Pharm Des. 2010;16:1081–1090. doi: 10.2174/138161210790963779. [DOI] [PubMed] [Google Scholar]
- 7.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 9.Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 10.Leonard JN, Ghirlando R, Askins J, Bell JK, Margulies DH, Davies DR, Segal DM. The TLR3 signaling complex forms by cooperative receptor dimerization. Proc Natl Acad Sci USA. 2008;105:258–263. doi: 10.1073/pnas.0710779105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, Botos I, Wang Y, Leonard JN, Shiloach J, Segal DM, Davies DR. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science. 2008;320:379–381. doi: 10.1126/science.1155406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawai T, Akira S. The role of patternrecognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 14.Bell JK, Botos I, Hall PR, Askins J, Shiloach J, Segal DM, Davies DR. The molecular structure of the Toll-like receptor 3 ligand-binding domain. Proc Natl Acad Sci USA. 2005;102:10976–10980. doi: 10.1073/pnas.0505077102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choe J, Kelker MS, Wilson IA. Crystal structure of human toll-like receptor 3 (TLR3) ectodomain. Science. 2005;309:581–585. doi: 10.1126/science.1115253. [DOI] [PubMed] [Google Scholar]
- 16.Ranjith-Kumar CT, Murali A, Dong W, Srisathiyanarayanan D, Vaughan R, Ortiz-Alacantara J, et al. Agonist and antagonist recognition by RIG-I, a cytoplasmic innate immunity receptor. J Biol Chem. 2009;284:1155–1165. doi: 10.1074/jbc.M806219200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kariko K, Bhuyan P, Capodici J, Weissman D. Small interfering RNAs mediate sequence-independent gene suppression and induce immune activation by signaling through toll-like receptor 3. J Immunol. 2004;172:6545–6549. doi: 10.4049/jimmunol.172.11.6545. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds A, Anderson EM, Vermeulen A, Fedorov Y, Robinson K, Leake D, et al. Induction of the interferon response by siRNA is cell type- and duplex length-dependent. RNA. 2006;12:988–993. doi: 10.1261/rna.2340906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin SC, Lo YC, Wu H. Helical assembly in the MyD88–IRAK4–IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465:885–890. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duffy KE, Lamb RJ, San Mateo LR, Jordan JL, Canziani G, Brigham-Burke M, et al. Down modulation of human TLR3 function by a monoclonal antibody. Cell Immunol. 2007;248:103–114. doi: 10.1016/j.cellimm.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Almagro JC, Beavers MP, Hernandez-Guzman F, Maier J, Shaulsky J, Butenhof K, et al. Antibody modeling assessment. Proteins. 2011;79:3050–3066. doi: 10.1002/prot.23130. [DOI] [PubMed] [Google Scholar]
- 22.Luo J, Obmolova G, Huang A, Strake B, Teplyakov A, Malia T, et al. Coevolution of antibody stability and Vkappa CDR-L3 canonical structure. J Mol Biol. 2010;402:708–719. doi: 10.1016/j.jmb.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Bell JK, Askins J, Hall PR, Davies DR, Segal DM. The dsRNA binding site of human Tolllike receptor 3. Proc Natl Acad Sci USA. 2006;103:8792–8797. doi: 10.1073/pnas.0603245103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funami K, Sasai M, Oshiumi H, Seya T, Matsumoto M. Homo-oligomerization is essential for Toll/interleukin-1 receptor domain-containing adaptor molecule-1-mediated NF-kappaB and interferon regulatory factor-3 activation. J Biol Chem. 2008;283:18283–18291. doi: 10.1074/jbc.M801013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pirher N, Ivicak K, Pohar J, Bencina M, Jerala R. A second binding site for double-stranded RNA in TLR3 and consequences for interferon activation. Nat Struct Mol Biol. 2008;15:761–763. doi: 10.1038/nsmb.1453. [DOI] [PubMed] [Google Scholar]
- 26.Rothe C, Urlinger S, Lohning C, Prassler J, Stark Y, Jager U, et al. The human combinatorial antibody library HuCAL GOLD combines diversification of all six CDRs according to the natural immune system with a novel display method for efficient selection of high-affinity antibodies. J Mol Biol. 2008;376:1182–1200. doi: 10.1016/j.jmb.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y, Gutshall L, Jiang H, Baker A, Beil E, Obmolova G, et al. Two routes for production and purification of Fab fragments in biopharmaceutical discovery research: papain digestion of mAb and transient expression in mammalian cells. Protein Expr Purif. 2009;67:182–189. doi: 10.1016/j.pep.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Malia TJ, Obmolova G, Luo J, Teplyakov A, Sweet RM, Gilliland GL. Crystallization of a challenging antigen/antibody complex-TLR3 ECD with three non-competing Fabs. Acta Crystallogr, Sect F. 2011;67:1290–1295. doi: 10.1107/S1744309111030983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kabsch W. Xds. Acta Crystallogr, Sect D: Biol Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pflugrath J. The finer things in X-ray diffraction data collection. Acta Crystallogr, Sect D. 1999;55:1718–1725. doi: 10.1107/s090744499900935x. [DOI] [PubMed] [Google Scholar]
- 31.Strong M, Sawaya MR, Wang S, Phillips M, Cascio D, Eisenberg D. Toward the structural genomics of complexes: crystal structure of a PE/PPE protein complex from Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2006;103:8060–8065. doi: 10.1073/pnas.0602606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Read RJ. Pushing the boundaries of molecular replacement with maximum likelihood. Acta Crystallogr, Sect D: Biol Crystallogr. 2001;57:1373–1382. doi: 10.1107/s0907444901012471. [DOI] [PubMed] [Google Scholar]
- 33.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr, Sect D: Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 34.Adams PD, Gopal K, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, et al. Recent developments in the PHENIX software for automated crystallographic structure determination. J Synchrotron Radiat. 2004;11:53–55. doi: 10.1107/s0909049503024130. [DOI] [PubMed] [Google Scholar]
- 35.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr, Sect D: Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 36.Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, Wang X, et al. MolProbity: allatom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007;35:W375–383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collaborative Computational Project, No. 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr, Sect D: Biol Crystallogr. 1994;53:240–255. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 38.DeLano W. The PyMOL Molecular Graphics System. Delano Scientific; Palo Alto, CA: 2002. [Google Scholar]
- 39.Teplyakov A, Obmolova G, Wu SJ, Luo J, Kang J, O'Neil K, Gilliland GL. Epitope mapping of anti-interleukin-13 neutralizing antibody CNTO607. J Mol Biol. 2009;389:115–123. doi: 10.1016/j.jmb.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 40.Schroder GF, Levitt M, Brunger AT. Super-resolution biomolecular crystallography with low-resolution data. Nature. 2010;464:1218–1222. doi: 10.1038/nature08892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ludtke SJ, Baldwin PR, Chiu W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J Struct Biol. 1999;128:82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.