Abstract
Deubiquitinating enzymes (DUBs) appear to be critical regulators of a multitude of processes such as proliferation, apoptosis, differentiation, and inflammation. We have recently demonstrated that a DUB of ubiquitin carboxyl terminal hydrolyase L1 (UCH-L1) inhibits vascular lesion formation via suppressing inflammatory responses in vasculature. However, the precise underlying mechanism remains to be defined. Herein, we report that a posttranscriptional up-regulation of UCH-L1 provides a negative feedback to tumor necrosis factor alpha (TNFα)-mediated activation of extracellular signal-regulated kinases (ERK) and proliferation in vascular smooth muscle cells (VSMCs). In rat adult VSMCs, adenoviral over-expression of UCH-L1 inhibited TNFα-induced activation of ERK and DNA synthesis. In contrast, over-expression of UCH-L1 did not affect platelet derived growth factor (PDGF)-induced VSMC proliferation and activation of growth stimulating cascades including ERK. TNFα hardly altered UCH-L1 mRNA expression and stability; however, up-regulated UCH-L1 protein expression via increasing UCH-L1 translation. These results uncover a novel mechanism by which UCH-L1 suppresses vascular inflammation.
Keywords: UCH-L1, Vascular smooth muscle cells, TNFα, Inflammation, Proliferation, Posttranscriptional regulation
Introduction
Cardiovascular diseases continue to be a leading cause of disability and mortality in the United States, and the majority of cardiovascular disorders results from complications of vascular diseases [1]. While it is still far from a comprehensive understanding of molecular and cellular mechanisms leading to vascular diseases, a preponderance of evidence supports a notion that inflammation plays a critical role in a wide range of vascular complications and dysfunctions [2–6].
Vascular inflammation has been characterized as a complex process involving endothelial dysfunction, leukocyte recruitment, VSMC activation, and malfunction of inflammatory mediators including both anti-inflammatory and pro-inflammatory cytokines [2–7]. Recently, TNFα, a pro-inflammatory cytokine, has emerged as a key factor in the pathogenesis of vascular diseases [7, 8]. TNFα triggers myriads of pro-inflammatory effects on vascular cells such as VSMC migration and proliferation, thereby contributing to maladaptive vascular modeling. It has been demonstrated that nuclear factor (NF)-κB and mitogen-activated protein kinase (MAPK) cascades are major component of TNFα signal transduction [9]; however, the precise signaling mechanisms responsible for the pathological TNFα activity in vasculature remain to be defined.
We have recently demonstrated that a DUB of ubiquitin carboxyl terminal hydrolyase L1 (UCH-L1) inhibits vascular lesion formation via suppressing inflammatory responses in vasculature [10]. However, cellular and signaling mechanisms by which UCH-L1 suppresses vascular inflammatory responses remain to be further investigated. In the present study, we explored role of UCH-L1 in the regulation of TNFα-mediated VSMC proliferation in vitro. Our results uncovered for the first time that UCH-L1 negatively regulates TNFα-mediated VSMC proliferation via suppressing ERK activity.
Material and methods
Cell culture and adenoviral infection
Vascular smooth muscle cells (VSMCs) were isolated from thoracic aorta of adult Spague-Dawley rats as previously described [11], and cultured in low glucose (1 g/L) Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen) supplemented with 10% fetal bovine serum. Sub-confluent rat aortic smooth muscle cells (RASMCs) were infected with adenovirus of control beta-galactoside (Ad-βGal) and human UCH-L1 (Ad-hUCH-L1) (Welgen Inc.) in serum free DMEM for 48 hours.
[3H]thymidine uptake
RASMCs were cultured in serum free DMEM for 24 hours to induce a quiescent status, and then stimulated with or without TNFα (Sigma-Aldrich) for 40 hours. [3H]thymidine (MP Biomedicals) was added to the media (final concentration 1 µCi/ml) during the last 24 hours. [3H]thymidine uptake was measured by a Beckman LS6000 scintillation counter (Beckman Coulter, Inc.) as previously described [11]. [3H]thymidine incorporation was normalized by the amount of cellular protein counted.
Reverse transcription-polymerase chain reaction (RT-PCR) and quantitative real time (Q-PCR)
Total RNA purification, RT reaction, and Q-PCR were performed as described previously [12]. Expression levels of target genes were normalized by concurrent measurement of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels. Primers that were used for Q-PCR are as follows: Forward primer (5'-CCCCGAGATGCTGAACAAAGT-3') and reverse primer (5'-ATGGTCTGCTTCATGAAGTA-3') were used for PCR amplification of rat UCH-L1 (NM_017237). Forward primer (5'-ACCACAGTCCATGCCATCAC-3') and reverse primer (5'-TCCACCACCCTGTTGCTGTA-3') were used for PCR amplification of rat GAPDH (NM_017008).
UCH-L1 mRNA stability assessment
Quiescent RASMCs were pretreated with or without actinomysin D, an inhibitor of gene transcription, for 1 hour, and then stimulated with or without TNFα (5 ng/ml) as indicated. As actinomysin D at a dose of 1 µg/ml has been demonstrated not to inhibit GAPDH but other genes’ transcription in RASMCs [13], we applied actinomysin D (1 µg/ml) in the present study. UCH-L1 mRNA expression was quantified by Q-PCR as described above. Expression UCH-L1 mRNA levels in RASMCs treated with vehicle alone was considered as 100%.
Western blot
Cell lysates were subjected to immunoblot analysis as previously describe [11] using antibodies of phosphor-ERK (Cat# 9101, Cell Signaling Technology), anti-UCH-L1 (AB1761, Millipore) and anti-GAPDH (FL-335, sc-25778, Santa Cruz Biotechnology). Densitometric analysis was performed using an image scanner (EPSON GT-8000) and NIH image software.
Statistical analysis
Data are shown as mean ± s.d.. Means were compared by ANOVA, followed by Bonferroni test for multiple comparisons. Differences were considered significant at p<0.05.
Results and discussion
Over-expression of UCH-L1 inhibits TNFα-mediated VSMC proliferation
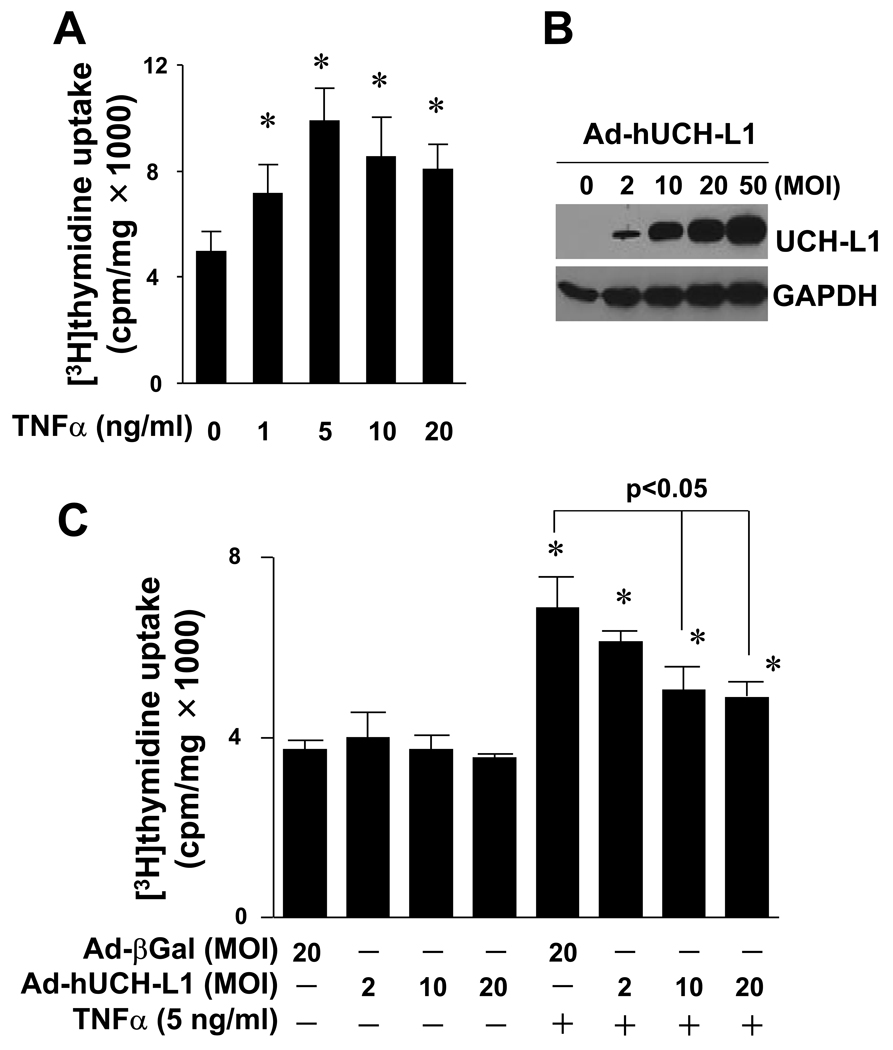
We have observed that UCH-L1 is up-regulated in injured arteries and local gene delivery of UCH-L1 inhibited vascular lesion formation with suppression of inflammatory responses in vasculature [10]. To establish a direct link between the up-regulation of UCH-L1 expression and the inhibition of inflammatory vascular remodeling, we first explored an effect of over-expression of UCH-L1 on TNFα-mediated VSMC proliferation, a key feature of vascular diseases including atherosclerosis, restenosis and hypertension [2, 6, 14, 15]. Because of the controversial reports on TNFα-mediated rat VSMC proliferation [16, 17], we carefully determined experimental conditions that TNFα stimulates RASMC proliferation. We established that TNFα dose-dependently stimulated RASMC proliferation with a maximum effect at a dose of 5 ng/ml (Fig. 1A). In addition, up-regulation of UCH-L1 expression was achieved by adenoviral over-expression of hUCH-L1. Adenovirus of hUCH-L1 at 20 MOI led to a substantial increase in UCH-L1 protein expression without any observable cytotoxic effects in RASMCs (Fig. 1B). Thus, we used TNFα at a dose of 5 ng/ml and adenovirus of control or hUCH-L1 at doses up to 20 MOI for the subsequent studies. Importantly, adenoviral over-expression of UCH-L1 dose-dependently inhibited TNFα-mediated RASMC proliferation (Fig. 1C). These results provide direct evidence that UCH-L1 suppresses vascular lesion formation via inhibiting proinflammatory cytokine TNFα-mediated VSMC proliferation.
Fig. 1.
Effect of UCH-L1 over-expression on TNFα-induced VSMC proliferation. (A) TNFα-induced proliferation of rat aortic smooth muscle cells (RASMCs). Cell proliferation was assessed by measuring [3H]thymidine update as described in “Methods”. * p<0.05 vs TNFα (−), n=4. (B) Adenoviral over-expression of UCH-L1 in RASMCs. Results are representative of three independent Western blot analysis of UCH-L1 in RASMCs infected with or without adenovirus of UCH-L1. (C) Effect of over-expression of UCH-L1 on TNFα-induced RASMC proliferation. Cells infected with Ad-UCH-L1 or Ad-βGal were stimulated with or without TNFα (5 ng/ml) as indicated for 40 hours. * p<0.05 vs TNFα (−), n=4.
Over-expression of UCH-L1 suppresses TNFα-induced ERK activation in VSMCs
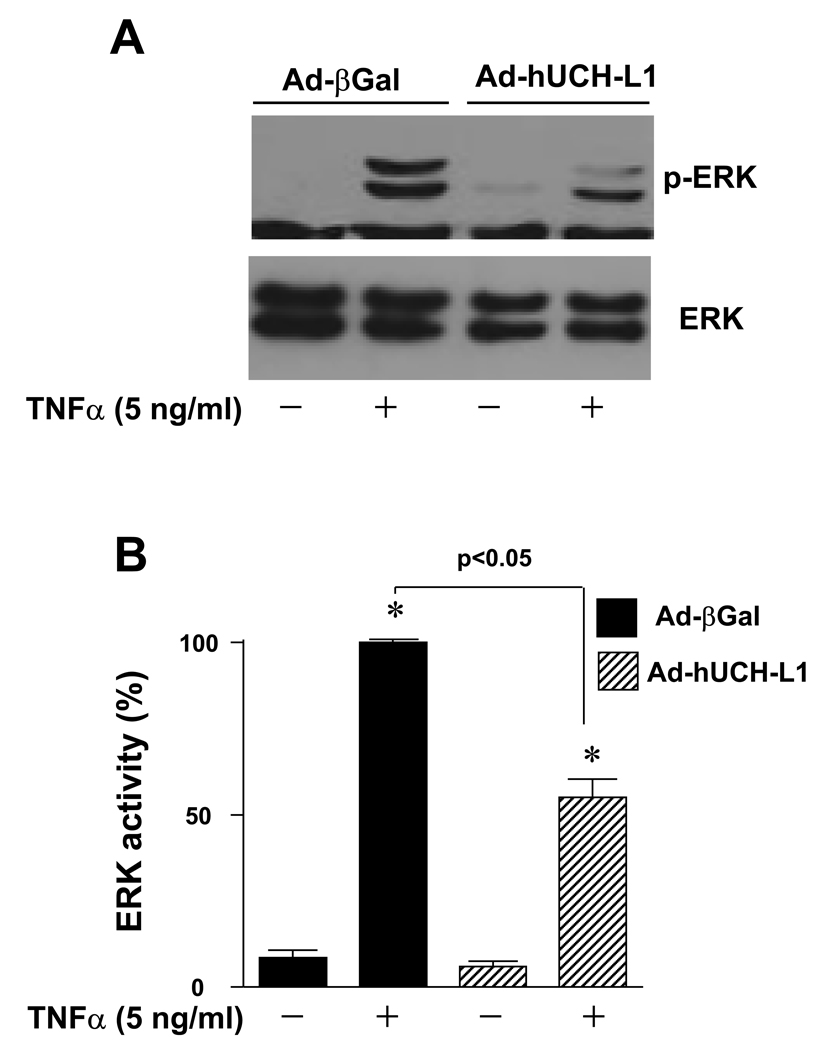
It has been well documented that TNFα activates MAPKs including ERK, c-Jun N-terminal kinases (JNK) and p38, phosphoinositide 3-kinase (PI3-K), as well as NF-κB, contributing to VSMC growth [18, 19]. As we have demonstrated that over-expression of UCH-L1 suppresses NF-κB p65 transcriptional activity in VSMCs [10], it is conceivable that UCH-L1 inhibits VSMC proliferation via at least partly suppressing NF-κB pathway. However, it still remains unclear whether UCH-L1 regulates the TNFα-mediated activation of other signaling cascades leading to VSMC proliferation. In our pilot experiments, we observed that TNFα (5 ng/ml) induced phosphorylation of ERK without any detectable phosphorylation of JNK, p38, and Akt, a downstream kinase of PI3-K in RASMCs (data not shown). Interestingly, over-expression of UCH-L1 attenuated TNFα-induced activation of ERK in RASMCs (Fig. 2), suggesting that suppression of ERK activation also contributes to the growth inhibitory effect of UCH-L1 in TNFα-inflamed VSMCs.
Fig. 2.
Effect of UCH-L1 expression on ERK activity in VSMCs. Cells infected with Ad-βGal (20 MOI) or Ad-hUCH-L1 (20 MOI) were treated with TNFα (5 ng/ml) for 10 minutes, and then subjected to Western blot analysis for ERK phosphorylation. (A) Representatives of ERK phosphorlyation from 4 separate experiments. (B) Densitometric analysis of ERK phosphorylation. The density of ERK phosphorylation induced by TNFα in RASMCs infected with Ad-βGal was set as the maximal increase (100%). *p<0.05 vs Ad-βGal (TNFα −); #p<0.05 vs Ad-βGal (TNFα +), n=4.
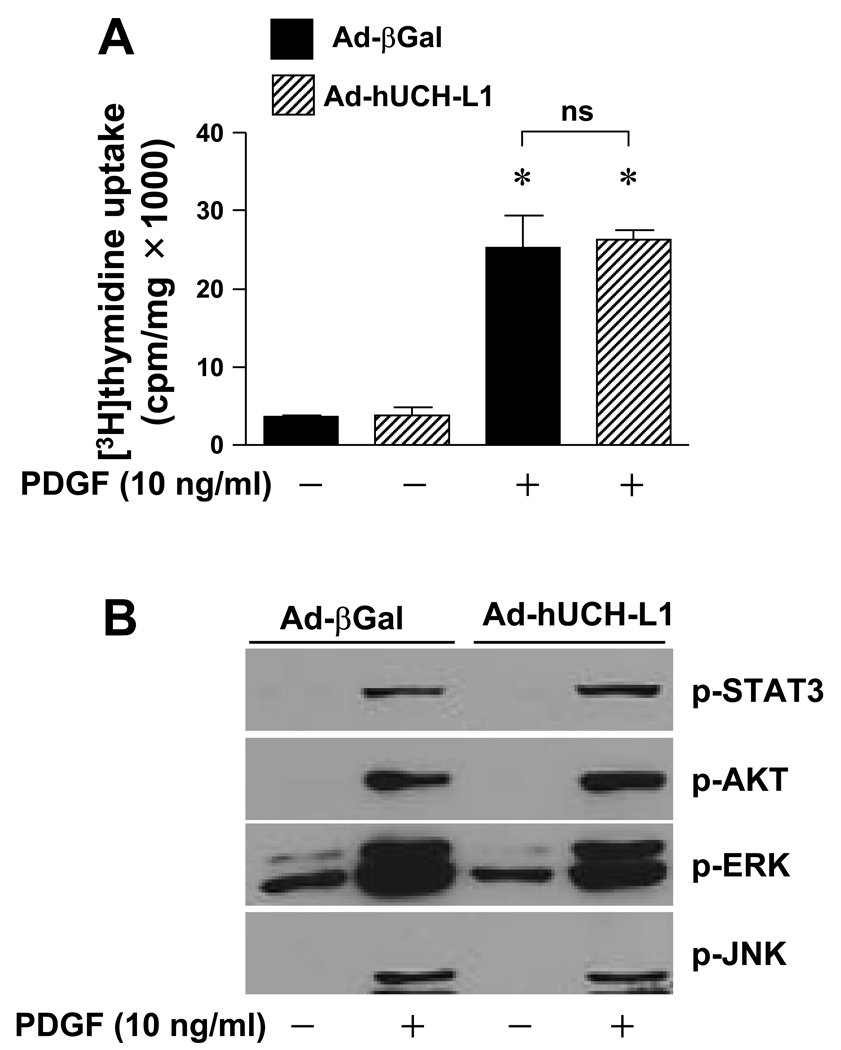
To study a specificity of the UCH-L1-mediated growth inhibitory effect in VSMCs, we examined the effect of UCH-L1 over-expression on the PDGF-mediated RASMC proliferation. Notably, adenoviral over-expression of UCH-L1 hardly affected the PDGF-induced activation of ERK, JNK, Akt, and signal transducer and activator of transcription 3 (STAT3), as well as DNA synthesis in RASMCs (Fig. 3). Moreover, PDGF did not regulate UCH-L1 expression at either mRNA or protein levels (data not shown). These results demonstrate a unique growth inhibitory role of UCH-L1 preferentially in pro-inflammatory cytokines such as TNFα-inflamed VSMCs.
Fig. 3.
Effect of UCH-L1 on PDGF-induced proliferation and activation of ERK in VSMCs. (A) PDGF-induced RASMC proliferation. Cells infected with Ad-βGal (20 MOI) or Ad-hUCH-L1 (20 MOI) were treated with or with out PGDF (10 ng/ml) for 40 hours. Cell proliferation was assessed by measuring [3H]thymidine update as described in “Methods”. * p<0.05 vs PDGF (−), n=4. (B) Infected cells were treated with PDGF (10 ng/ml) for 10 minutes, and then subjected to Western blot analysis as indicated. Results are representative of three independent experiments.
Up-regulation of UCH-L1 in TNFα-inflamed VSMCs via a posttranscriptional regulation
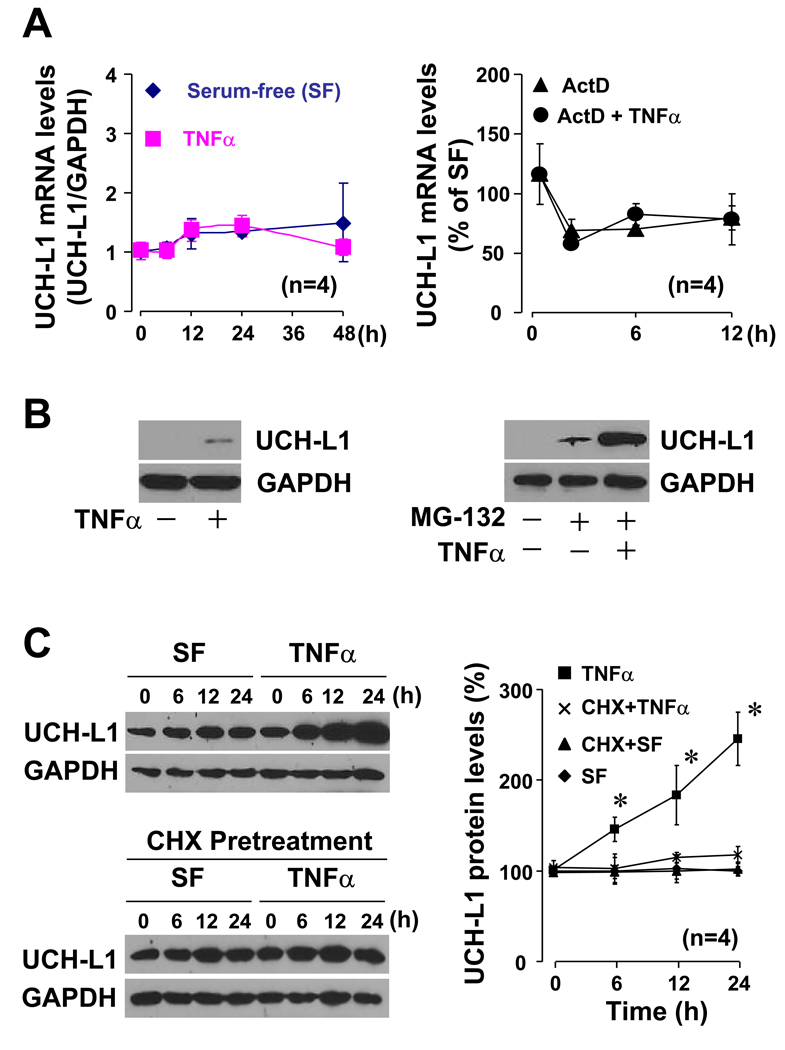
To gain mechanistic insight into the anti-inflammatory role of UCH-L1 in VSMCs, we characterized expression profile of UCH-L1 in TNFα-inflamed RASMCs. Consistent with our previous observation that TNFα did not regulate UCH-L1 mRNA expression in human VSMCs [10], TNFα stimulation for 48 hours had no effect on UCH-L1 mRNA expression in RASMCs (Fig. 4A). While actinomycin D, an inhibitor of gene transcription, suppressed UCH-L1 mRNA expression, TNFα did not affect actinomysin D-induced suppression of UCH-L1 mRNA in RASMCs (Fig. 4A). These results demonstrate that TNFα could not regulate either UCH-L1 transcription or its mRNA stability. However, Western blot analysis with a long time exposure revealed that TNFα stimulation slightly increased UCH-L1 protein expression (Fig. 1B). Immunochemistry with biotin-labeled secondary antibodies to enhance UCH-L1 staining signal confirmed that TNFα did up-regulate UCH-L1 protein expression in RASMCs (data not shown). These results suggest that TNFα up-regulates UCH-L1 protein via a posttranscriptional regulation in VSMCs. To explore the underlying mechanism, we determined effect of MG-132, a proteasome inhibitor, on UCH-L1 protein expression in RASMCs. We observed that MG-132 at concentration over 10 µM exhibited cytotoxic effects in RASMCs (data not shown). MG-132 (0–5 µM) alone dose-dependently up-regulated UCH-L1 protein expression in RASMCs (data not shown). Therefore, we treated the cells with MG-132 at a non-cytotoxic dose of 5 µM. As shown in Fig. 4C, MG-132 treatment for 24 hours significantly increased basal UCH-L1 protein levels, and the MG-132-induced up-regulation of UCH-L1 protein expression was further enhanced by TNFα in RASMCs. These results suggest that TNFα up-regulates UCH-L1 protein levels by inhibiting UCH-L1 degradation and/or increasing UCH-L1 translation. To clarify this issue, we used cycloheximide, an inhibitor of protein synthesis. Presumably, TNFα is able to up-regulate UCH-L1 protein levels in RASMCs that UCH-L1 gene translation is blocked by cycloheximine if TNFα inhibits UCH-L1 degradation. While TNFα dramatically enhanced UCH-L1 protein expression in vehicle treated RASMCs over-expressed with UCH-L1, it could not up-regulate UCH-L1 protein expression in cycloheximide treated cells (Fig. 4C). We used cycloheximide (5 µg/ml) that has been established not to suppress house keeping GAPDH protein synthesis but significantly suppress other protein synthesis in RASMCs [20]. These results indicate that TNFα up-regulates UCH-L1 via a translational regulation. Cycloheximide alone up to 24 hours did not change the level of UCH-L1 protein expression in RASMCs (Fig. 4C), indicating that the turnover of UCH-L1 protein is slow in VSMCs. Taken together, we demonstrate that TNFα upregulates UCH-L1 protein expression by enhancing UCH-L1 translation rather than inhibiting its degradation in VSMCs. Because UCH-L1 protein is quite stable, the up-regulated UCH-L1 might provide a powerful negative feed back on TNFα-mediated pro-inflammatory signaling in VSMCs.
Fig. 4.
UCH-L1 expression in TNFα-inflamed VSMCs. (A) Effect of TNFα on UCH-L1 mRNA expression (Left panel) and mRNA stability (Right panel) in RASMCs. Left; Quiescent RASMCs were treated with or with TNFα (5 ng/ml) as indicated, and then subjected to Q-PCR analysis for UCH-L1 mRNA expression. Right, Quiescent RASMCs were pretreated with or without actinomysin D (ActD, 1 µg/ml) for 1 hour. The cells pretreated with ActD were future stimulated with or with TNFα (5 ng/ml) as indicated while the cells without pretreatment of ActD were cultured with vehicle by the end points as indicated. The expression levels of UCH-L1 mRNA in the cells treated with vehicle alone were set as 100%. UCH-L1 mRNA expression was quantified by Q-PCR. (B) Western blot analysis of UCH-L1 protein expression in RASMCs. Left panel; cells were treated with or with TNFa (5 ng/ml) for 24 hours. Right panel; cells were pretreated with or without MG-132 (5 µM) for 2 hours, and then stimulated with or without TNFα (5 ng/ml) for additional 24 hours. Results are representative of three separated experiments. (C) Effect of TNFα on UCH-L1 protein synthesis in RASMCs. Quiescent RASMCs infected with Ad-hUCH-L1 (20 MOI) were treated TNFα (5 ng/ml) and/ or cycloheximide (CHX, 5 µg/ml) as indicated. CHX was pretreated for 1 hour. Left panel is representative of 4 separated experiments. Right panel is densitometric analysis of UCH-L1 protein expression. *p<0.05 vs SF (0), n=4.
Overall, our data demonstrate that TNFα up-regulates UCH-L1 via a translational regulation to inhibit ERK activity, thereby providing a negative feedback to control its growth promoting signaling in VSMCs. Recently, UCH-L1 has been shown to inhibit α2-adrenergic receptor (AR) agonist-mediated activation of ERK via a direct association with α2A-AR receptor, implicating a role of UCH-L1 in certain tumor suppression and neuro-protection [21]. In contrast, other studies have documented that UCH-L1 up-regulates β-catenin/TCF via a positive feedback mechanism or exerts anti-apoptotic and growth stimulating effects, supporting an oncogenic potential of UCH-L1 [22, 23]. These results suggest that UCH-L1 appears to be a multifunctional protein and exerts cell type and/or tissue specific actions. To further address precise mechanism by which UCH-L1 suppresses TNFα-mediated activation of ERK will provide novel insight into the understanding of TNFα-mediated inflammatory responses in VSMCs, facilitating development of new therapeutic approaches for the treatment of vascular diseases.
Acknowledgement
This work was supported by AHA BGIA (0865101E) and COBRE (5P20 RR016434-09)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8:1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 4.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 5.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 6.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008;79:360–376. doi: 10.1093/cvr/cvn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKellar GE, McCarey DW, Sattar N, McInnes IB. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat Rev Cardiol. 2009;6:410–417. doi: 10.1038/nrcardio.2009.57. [DOI] [PubMed] [Google Scholar]
- 9.Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214:149–160. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 10.Takami Y, Nakagami H, Morishita R, Katsuya T, Cui TX, Ichikawa T, Saito Y, Hayashi H, Kikuchi Y, Nishikawa T, Baba Y, Yasuda O, Rakugi H, Ogihara T, Kaneda Y. Ubiquitin carboxyl-terminal hydrolase L1, a novel deubiquitinating enzyme in the vasculature, attenuates NF-kappaB activation. Arterioscler Thromb Vasc Biol. 2007;27:2184–2190. doi: 10.1161/ATVBAHA.107.142505. [DOI] [PubMed] [Google Scholar]
- 11.Cui T, Nakagami H, Iwai M, Takeda Y, Shiuchi T, Tamura K, Daviet L, Horiuchi M. ATRAP, novel AT1 receptor associated protein, enhances internalization of AT1 receptor and inhibits vascular smooth muscle cell growth. Biochem Biophys Res Commun. 2000;279:938–941. doi: 10.1006/bbrc.2000.4055. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M, Cui T. Nrf2 Protects Against Maladaptive Cardiac Responses to Hemodynamic Stress. Arterioscler Thromb Vasc Biol. 2009;29:1843–1850. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]
- 13.Minamino N, Shoji H, Sugo S, Kangawa K, Matsuo H. Adrenocortical steroids, thyroid hormones and retinoic acid augment the production of adrenomedullin in vascular smooth muscle cells. Biochem Biophys Res Commun. 1995;211:686–693. doi: 10.1006/bbrc.1995.1866. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz SM, Campbell GR, Campbell JH. Replication of smooth muscle cells in vascular disease. Circ Res. 1986;58:427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- 15.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. 2000;190:300–309. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 16.Goetze S, Xi XP, Kawano Y, Kawano H, Fleck E, Hsueh WA, Law RE. TNF-alpha-induced migration of vascular smooth muscle cells is MAPK dependent. Hypertension. 1999;33:183–189. doi: 10.1161/01.hyp.33.1.183. [DOI] [PubMed] [Google Scholar]
- 17.Haider A, Lee I, Grabarek J, Darzynkiewicz Z, Ferreri NR. Dual functionality of cyclooxygenase-2 as a regulator of tumor necrosis factor-mediated G1 shortening and nitric oxide-mediated inhibition of vascular smooth muscle cell proliferation. Circulation. 2003;108:1015–1021. doi: 10.1161/01.CIR.0000085211.97972.2C. [DOI] [PubMed] [Google Scholar]
- 18.Li M, Mossman BT, Kolpa E, Timblin CR, Shukla A, Taatjes DJ, Fukagawa NK. Age-related differences in MAP kinase activity in VSMC in response to glucose or TNF-alpha. J Cell Physiol. 2003;197:418–425. doi: 10.1002/jcp.10384. [DOI] [PubMed] [Google Scholar]
- 19.Peppel K, Zhang L, Orman ES, Hagen PO, Amalfitano A, Brian L, Freedman NJ. Activation of vascular smooth muscle cells by TNF and PDGF: overlapping and complementary signal transduction mechanisms. Cardiovasc Res. 2005;65:674–682. doi: 10.1016/j.cardiores.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 20.Peyton KJ, Reyna SV, Chapman GB, Ensenat D, Liu XM, Wang H, Schafer AI, Durante W. Heme oxygenase-1-derived carbon monoxide is an autocrine inhibitor of vascular smooth muscle cell growth. Blood. 2002;99:4443–4448. doi: 10.1182/blood.v99.12.4443. [DOI] [PubMed] [Google Scholar]
- 21.Weber B, Schaper C, Wang Y, Scholz J, Bein B. Interaction of the ubiquitin carboxyl terminal esterase L1 with alpha(2)-adrenergic receptors inhibits agonist-mediated p44/42 MAP kinase activation. Cell Signal. 2009;21:1513–1521. doi: 10.1016/j.cellsig.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Bheda A, Yue W, Gullapalli A, Whitehurst C, Liu R, Pagano JS, Shackelford J. Positive reciprocal regulation of ubiquitin C-terminal hydrolase L1 and beta-catenin/TCF signaling. PLoS One. 2009;4:e5955. doi: 10.1371/journal.pone.0005955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bheda A, Shackelford J, Pagano JS. Expression and functional studies of ubiquitin C-terminal hydrolase L1 regulated genes. PLoS One. 2009;4:e6764. doi: 10.1371/journal.pone.0006764. [DOI] [PMC free article] [PubMed] [Google Scholar]