Abstract
Disseminated non-tuberculous mycobacterium (dNTM) infection is rare in humans without human immunodeficiency virus (HIV) infection. Previous reports have shown autoantibodies to human interferon-gamma (IFN-γ), which play important roles in mycobacterium infection, in the sera of patients with non-HIV dNTM disease. Herein, we describe a 53-year-old male who was strongly suspected to have multicentric Castleman disease (MCD) based on bone marrow study and chest radiological findings. However, Mycobacterium kansasii was detected in respiratory samples including pleural effusion. We initiated anti-mycobacterial therapy under intensive care; he died on the 48th hospital day. We detected no hematological disorders, ruling out MCD postmortem. However, we detected M. kansasii in pulmonary, liver, spleen and bone marrow tissues. Moreover, anti-IFN-γ autoantibody was detected with strong neutralizing capacity for IFN-γ. We consider our present report to contribute to understanding of the relationship between anti-IFN-γ autoantibody and disease development.
Keywords: Disseminated non-tuberculous mycobacterium disease, Anti-IFN-γ autoantibody, Non-HIV patient
1. Introduction
Non-tuberculous mycobacteria (NTM) are environmental acid-fast bacilli (AFB) commonly found worldwide. NTM usually cause infections of pulmonary bronchi (pulmonary NTM; pNTM).1 Over 90% of pNTM disease is caused by Mycobacterium avium complex (MAC), about 5% by Mycobacterium kansasii. In immunocompent patients, pNTM disease is usually limited to the bronchi and lungs, but patients with human immunodeficiency virus (HIV) infection may progress to systemic disseminated disease.2 Disseminated NTM (dNTM) disease is regarded as a sign of acquired immunodeficiency syndrome (AIDS) due to HIV infection, whereas dNTM disease without HIV infection is very rare. Recently, the existence of autoantibodies against interferon-gamma (IFN-γ) has been shown to be closely related to dNTM disease without HIV infection.3–5 A few reports on dNTM disease without HIV infection noted the existence of anti-IFN-γ autoantibody with strong neutralizing capacity.5 We describe herein our dNTM patient who was HIV negative but was infected with M. kansasii. This patient did not have MAC infection but anti-IFN-γ autoantibody, with strong neutralizing capacity, was detected.
2. Case report
2.1. Patient profile and clinical course
A 53-year-old male was referred to our hospital because of dyspnea, decreased hemoglobin, and white blood cell (WBC) counts over 30,000/μL. He was admitted and his laboratory data and radiological findings were evaluated. Clinical laboratory data on admission confirmed high WBC counts and decreased hemoglobin. A high serum total immunoglobulin-G level was also demonstrated. Biochemical data showed elevated alkaline phosphatase and globulins and a low level of serum albumin. No serum M protein was detected. Serum C-reactive protein was elevated, indicating an inflammatory reaction. Soluble interleukin-2 receptor (sIL-2R) was elevated to 11,470 U/ml. Both anti-HIV and anti-human T-cell leukemia virus (HTLV) antibody were negative. Radiological findings on admission showed non-segmental and patchy consolidation along bronchi in the bilateral lung parenchyma. Multiple mediastinal lymph nodes were enlarged (Fig. 1A–C).
Fig. 1.
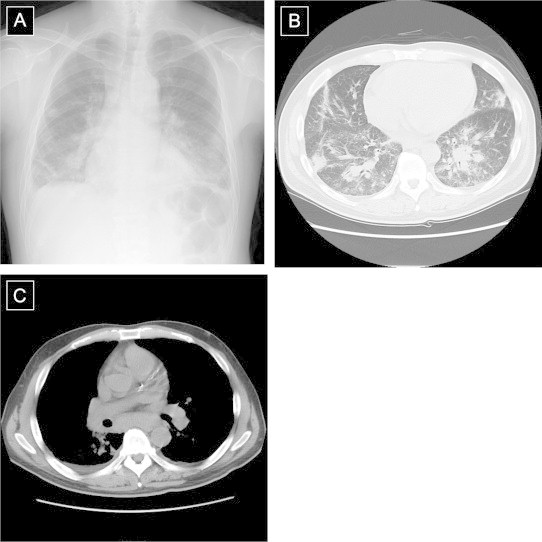
(A) Chest roentgenography (CR) and (B) and (C): thoracic computed tomography (TCT) findings on 1st hospital day. CR showed consolidation in bilateral lung fields and TCT showed non-segmental consolidation along bronchi with bronchovascular septal thickening and bulky mediastinal lymphadenopathy.
We initially suspected an abnormality of hematopoietic organs and thus evaluated his bone marrow. The specimens revealed mild hyperplasia with markedly increased atypical plasma cells. Several further studies, including flowcytometric analysis, were conducted to assess whether the abnormal cells showed any signs of malignancy. However, these atypical cells were ultimately determined to represent benign reactive changes. To further evaluate the mediastinal lymphadenopathy, transbronchial aspiration cytology using endobronchial ultrasonography was performed, but no significant findings were obtained. Based on polyclonal hypergammmopathy by serology and plasma cells collected for the bone marrow analysis, MCD was highly suspected. To confirm this diagnosis, surgical biopsy specimens of mediastinal lymph nodes and the lung parenchyma sample were obtained on the 31st hospital day. However, a pulmonary specimen was obtained only from the right lung, and adequate mediastinal lymph node specimens could not be collected due to severe adhesion.
After surgical biopsy, we initiated tocilizumab and steroid administration, because his general condition was deteriorating. However, AFB were detected in a bronchial lavage specimen obtained before surgical biopsy and pleural effusion collected at surgical biopsy. The organism was shown to be M. kansasii by DNA–DNA hybridization methods. We immediately began isoniazid, rifampicin and ethambutol treatment. However, despite these anti-tuberculous agents, his condition continued to deteriorate. On the 40th hospital day, his laboratory data showed ongoing disseminated intravascular coagulaton and multiple organ failure. An anticoagulant agent and continuous hemodiafiltration were started. Despite intensive care, he died on the 48th hospital day.
With permission from his family, a postmortem anatomical evaluation was performed. M. kansasii was detected in tissues from the lungs, liver, spleen and bone marrow, as well as blood cultures (on the 44th, 45th and 46th hospital days).
2.2. Detection and neutralizing capacity of anti-IFN-γ autoantibody
To assess the binding avidity of immunoglobulins to IFN-γ, we assayed anti-IFN-γ autoantibody with an antigen capture assay by ELISA. Briefly, serum from the present patient, other serum samples from patients with pNTM disease or pulmonary tuberculosis, and from healthy controls were each diluted 2000-fold with 0.1% bovine serum albumin/0.1% Tween-T™/phosphate buffered saline (PBS). A 50 μL volume of diluted serum was applied to a 96-well transparent flat-bottom plate (Nunc, Roskilde, Denmark) coated with 100 ng/mL of rhIFN-γ (Escherichia coli derived; R&D systems, Minneapolis, MN, US) after blocking with adequate agents. The plate was kept at room temperature for 60 min. After washing, autoantibodies captured by rhIFN-γ were detected by peroxidase-labeled anti-human Fab autoantibody (Santa Cruz, CA, US). After washing, color was developed using tetramethylbenzidine (TMB). Anti-IFN-γ autoantibody was detected only in diluted serum derived from the patient's blood (Fig. 2A).
Fig. 2.
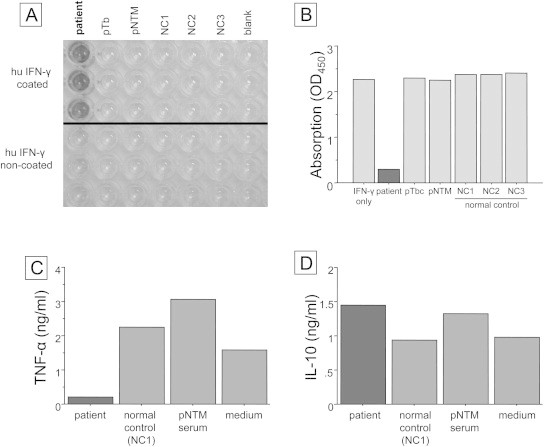
(A) Antigen capture assays of immunoglobulins against IFN-γ in our patient's serum (left lane), a pulmonary tuberculosis (pTb) patient's serum (2nd lane from the left), a pulmonary NTM (pNTM) patient's serum (3rd lane from the left), and normal control (NC) sera (4th–6th lanes from the left). In each lane, the upper 3 wells were coated with recombinant human (rh) IFN-γ and the lower 3 wells were not. The bound immunoglobulins were detected using horseradish peroxidase conjugated anti-human Fab specific antibody. (B) Inhibition binding activity of anti-IFN-γ autoantibody was as described in the “Case report” section. All samples include 100 pg of IFN-γ and sera from patients, patients with pTb and pNTM, and normal controls except the 1st lane from the left. The sample of the 1st lane contained 100 pg of IFN-γ only with PBS. All added sera were the same as those used for the antigen capture assays. The vertical axis is absorption of IFN-γ, as shown by optical density at 450 nm (OD450). (C) and (D) Productions of TNF-α and IL-10 by peripheral blood mononuclear cells (PBMNCs) were measured using sera from a normal individual, as described in the “Case report” section. In total, 1.0 × 106 PBMNCs in a total of 2 mL of complete medium (RPMI1640 and 10%/v fetal calf serum) were incubated with LPS (200 ng/ml) and IFN-γ (1 ng/ml) in the presence of 100 μl of our present patient's serum, as well as sera from other previous pNTM patients and normal controls. All added sera were the same as those used for the antigen capture assays.
Inhibition binding activity for IFN-γ was assayed using an IFN-γ assay ELISA plate (R&D systems). Briefly, the same samples as those used for the antigen capture assays were diluted 1,000-fold with 0.1% BSA/0.1% Tween-T™/PBS containing 100 pg/mL rhIFN-γ and incubated at 37 °C for 1 h. A 50 μL volume of the incubated sample was applied to the IFN-γ assay plate, for measurement according to the manufacturer's instructions. TMB development was inhibited in an incubated sample containing only the patient's serum (Fig. 2B).
Neutralizing activity was measured as in previous reports.5,6 Briefly, one million peripheral blood mononuclear cells (PBMNCs) in a total of 1 mL of complete medium (10% fetal calf serum/RPMI 1640) were stimulated with LPS (200 ng) and IFN-γ (1000 IU/mL) in the presence of 100 μL of normal control serum, the pNTM patient's serum, or our patient's serum (final volume 1.1 mL), for 48 h at 37 °C in 5%CO2. All added sera were the same as those used for the antigen capture assays. Supernatants were harvested and frozen at −20 °C until the TNF-α and IL-10 measurements by Quantikine™ (R&D systems) plate ELISA. Production of TNF-α was inhibited in the patient's serum, whereas IL-10 production was not (Fig. 2C and D).
3. Discussion
Disseminated NTM disease usually occurs in patients who are severely immunocompromised, such as those with HIV disease or AIDS. However, some dNTM disease cases do not have HIV infection. There are two retrospective clinical analyses of dNTM disease without HIV infection.7,8 Chou et al. reported that their 18 patients without HIV infection were usually febrile with elevated WBC counts.7 NTM was mainly isolated from not only the lung but also blood or bone marrow, and MAC was the most frequently detected pathogen in their study.7 Chetchotisakd et al. reported that NTM was most frequently detected in lymph nodes in their 129 dNTM patients without HIV infection.8 It is noteworthy that in both of these studies, dNTM patients had other opportunistic or severe infections in addition to the NTM. These included Salmonellosis, severe systemic intravascular infection, fungal infections including Aspergillus spp, Candida spp and Penicillium marneffei, and viral infections including varicella zoster and cytomegalovirus. Furthermore, the cases with dNTM disease, confirmed by detection of NTM in multiple specimens, often did not have HIV. With adequate anti-AFB treatment, the survival rate was better than without such treatment. Female gender and co-infection with other bacterial pathogens were associated with poor outcomes. However, HIV infection had no significant prognostic impact.
In 2005, two important studies detected autoantibodies against IFN-γ in plasma from dNTM patients without HIV infection.4,5 IFN-γ has a crucial role in the control and regulation of NTM disease because some mutations in the genes for Th1 cytokines and their receptors are associated with severe conditions.9 Watanabe et al. reported that anti-IFN-γ autoantibody is detected even in sera from healthy individuals.10 However, the reason for the existence of an autoantibody against IFN-γ remains unclear. Evaluating anti-IFN-γ autoantibody in healthy individuals is important for elucidating the autoantibody differences between dNTM patients and healthy individuals. There must be an underlying factor triggering the onset of dNTM disease in the absence of HIV-infection.
Regarding these previous reports, we considered it to be important for understanding the present patient's pathophysiology to assay his serum. We wanted to know whether his serum contained specific immunoglobulins which bound and neutralized IFN-γ. To assay anti-IFN-γ autoantibody, we applied a protocol allowing comparison with bioassay results obtained using normal PBMNCs, as described in previous reports.5 In our view, mediastinal lymphadenopathy with elevated WBC counts and leukocytosis, of unknown origin, should prompt assay of the patient's serum to detect anti-IFN-γ autoantibody. In addition, we suggest that infectious organisms such as P. marneffei, which are rare in immunocompetent patients without HIV infection, should be cultured along with conducting the anti-IFN-γ autoantibody assay.
Our present case showed no specific signs or symptoms pointing to an accurate diagnosis. His noteworthy signs were elevated WBC counts, mediastinal lymphadenopathy and general malaise. According to previous reports, symptoms in the present case might support a diagnosis of dNTM disease without HIV infection.
On the other hand, this patient was first suspected to have MCD, and was treated with tocilizumab, a monoclonal antibody against the human interleukin-6 receptor (IL-6R). However, after tocilizumab administration, his serum IL-6 level (normal range: <4.0 pg/ml) increased from 108 pg/ml to 12,987 pg/ml (the 44th hospital day). We thus considered tocilizumab administration to have had no influence on his deteriorating state.
No standard control for anti-IFN-γ autoantibody detection is yet available. Although dNTM disease with anti-IFN-γ autoantibody is still rare, the relationships between the quantity and neutralizing capacity of anti-IFN-γ autoantibody and disease development warrant evaluation in the near future. Whether anti-IFN-γ autoantibody is monoclonal or polyclonal is another important issue in dNTM disease cases without HIV infection.
In conclusion, we have reported herein a case of dNTM caused by M. kansasii with anti-IFN-γ autoantibody. In our view, patients who have dNTM disease without HIV infection should be evaluated for the presence of anti-IFN-γ autoantibody. In addition, we advocate that anti-IFN-γ autoantibody be measured in non-HIV patients.
Conflict of interest statement
We have no conflicts of interest to disclose.
None of the authors have any financial relationship with a commercial entity with an interest in the subject of this manuscript.
Acknowledgment
We thank Bierta Barfod for editing manuscript, Toshie Sekine for secretarial assistance, and Kana Kadota, Yoko Hori and Izumi Matsuo (Department of Intensive Care Unit, Nippon Medical School) for the patient's care, Junichi Okamoto and Shuji Haraguchi (Division of Thoracic Surgery, Nippon Medical School) for surgical biopsy assistance. We appreciate Mika Terasaki and Yuh Fukuda (Department of Analytic Human Pathology, Nippon Medical School) for postmortem pathological and anatomical evaluations, and Akihiro Shinoyama, Kazunari Sonobe, Akiko Watanabe and Yoshiko Kojima (Department of Central Laboratory, Nippon Medical School Hospital) for microbiological advice. We also thank Dr. Takefumi Saito (Department of Internal Medicine, National Hospital Institute, Ibaraki-higashi Hospital) for help with the present report.
Contributor Information
Takahito Nei, Email: takahitonei@gmail.com.
Masahiro Okabe, Email: s7018@nms.ac.jp.
Iwao Mikami, Email: iwikami@nms.ac.jp.
Yumika Koizumi, Email: yumika@nms.ac.jp.
Hiroshi Mase, Email: hiroshi-m@nms.ac.jp.
Kuniko Matsuda, Email: kuniko-m@nms.ac.jp.
Takeshi Yamamoto, Email: yamamoto56@nms.ac.jp.
Shinhiro Takeda, Email: shinhiro@nms.ac.jp.
Keiji Tanaka, Email: k-tanaka@nms.ac.jp.
Kazuo Dan, Email: dan@nms.ac.jp.
References
- 1.Cosma C., Sherman D., Ramakrishnan L. The secret lives of the pathogenic mycobacteria. Ann Rev Microbiol. 2003;57:641–676. doi: 10.1146/annurev.micro.57.030502.091033. [DOI] [PubMed] [Google Scholar]
- 2.Prince D.S., Peterson D.D., Steiner R.M., Gottlieb J.E., Scott R., Israel H.L. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989;321:863–868. doi: 10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- 3.Höflich C., Sabat R., Rosseau S., Temmesfeld B., Slevogt H., Döcke W.D. Naturally occurring anti-IFN-γ autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenans. Blood. 2004;103:673–675. doi: 10.1182/blood-2003-04-1065. [DOI] [PubMed] [Google Scholar]
- 4.Kampmann B., Hemingway C., Stephens A., Davidson R., Goodsall A., Anderson S. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-γ. J Clin Invest. 2005;115:2480–2488. doi: 10.1172/JCI19316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel S.Y., Ding L., Brown M.R., Lantz L., Gay T., Cohen S. Anti-IFN-γ autoantibodies in disseminated nontuberculous mycobacterial infections. J Immunol. 2005;175:4769–4776. doi: 10.4049/jimmunol.175.7.4769. [DOI] [PubMed] [Google Scholar]
- 6.Koya T., Tsubata C., Kagamu H., Koyama K., Hayashi M., Kuwabara K. Anti-interferron-γ autoantibody in a patient with disseminated Mycobacterium avium complex. J Infect Chemother. 2009;15:118–122. doi: 10.1007/s10156-008-0662-8. [DOI] [PubMed] [Google Scholar]
- 7.Chou C.H., Chen H.Y., Chen C.Y., Huang C.T., Lai C.C., Hsueh P.R. Clinical features and outcomes of disseminated infections caused by non-tuberculous mycobacteria in university hospital in Taiwan. Scand J Infect Dis. 2011;43:8–14. doi: 10.3109/00365548.2010.519345. [DOI] [PubMed] [Google Scholar]
- 8.Chetchotisakd P., Kiertiburanakul S., Mootsikapun P., Assanasen S., Chaiwarith R., Anunnatsiri S. Disseminated nontuberculous mycobacterial infection in patients who are not infected with HIV in Thailand. Clin Infect Dis. 2007;45:421–427. doi: 10.1086/520030. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka Y., Hori T., Ito K., Fujita T., Ishikawa T., Uchiyama T. Disseminated Mycobacterium avium complex infection in a patient with autoantibody to interferon-γ. Intern Med. 2007;46:1005–1009. doi: 10.2169/internalmedicine.46.6452. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe M., Uchida K., Nakagaki K., Kanazawa H., Trapnell B.C., Hoshino Y. Anti-cytokine autoantibodies are ubiquitous in healthy individuals. FEBS Lett. 2007;581:2017–2021. doi: 10.1016/j.febslet.2007.04.029. [DOI] [PubMed] [Google Scholar]


