Abstract
Chronic migraine is a disabling condition that affects hundreds of millions of individuals worldwide. The development of novel migraine treatments has been slow, in part due to a lack of predicative animal models. We have developed a new model of chronic migraine involving the use of nitroglycerin, a known migraine trigger in humans. Chronic intermittent administration of nitroglycerin to mice resulted in acute mechanical hyperalgesia with each exposure as well as a progressive and sustained basal hyperalgesia. This chronic basal hyperalgesia occurred in a dose-dependent fashion and persisted for days following cessation of NTG administration. NTG-evoked hyperalgesia was exacerbated by the phosphodiesterase 5 inhibitor sildenafil, also a human migraine trigger, consistent with nitric oxide as a primary mediator of this hyperalgesia. The acute but not the chronic basal hyperalgesia was significantly reduced by the acute migraine therapy sumatriptan, whereas both the acute and chronic hyperalgesia was significantly attenuated by the migraine preventive therapy topiramate. Chronic NTG-induced hyperalgesia is a mouse model that may be useful for the study of mechanisms underlying progression of migraine from an episodic to a chronic disorder, and for the identification and characterization of novel acute and preventive migraine therapies.
INTRODUCTION
Migraine is one of the most common disorders affecting the general population, resulting in a staggering amount of episodic disability and lost productivity worldwide. For a significant percentage of patients it results in chronic disability [1–4]. Despite the extraordinarily high prevalence of migraine, our understanding of its pathophysiology is incomplete. Moreover, while there has been significant progress in the acute treatment of migraine attacks, the ability to treat frequent and chronically disabling migraine, remains severely limited. There continue to be millions of individuals for whom currently available migraine therapies are either ineffective or poorly tolerated [2; 3].
A significant obstacle to the identification of new migraine therapies has been the lack of predictive animal models. There are a number of promising models for acute migraine attack [5– 8], but it has been particularly difficult to study the progression of migraine from an episodic to a chronic disorder. One approach to modeling acute migraine is the quantification of increased sensory sensitivity in response to known migraine triggers. Nitroglycerin (NTG) reliably triggers headache in normal subjects, and migraine without aura in migraine susceptible patients [9–12]. NTG-evoked migraine is a commonly used experimental paradigm in humans (for review see [12–14]). Nitroglycerin-evoked hyperalgesia in rodents has been developed as a model for sensory hypersensitivity associated with migraine [8; 15]. Acute nitroglycerin was previously shown to produce thermal and mechanical allodynia in mice that was reversed by the antimigraine therapy sumatriptan [8]. In addition, in a transgenic mouse model of familial migraine, animals expressing a human migraine gene showed an even greater sensitivity to NTG-evoked hyperalgesia [16]. Further, NTG has also been shown to produce light-aversive behavior [15], and increased meningeal blood flow in mice [15; 17]. Taken together, these results indicate that the effects of NTG may effectively model migraine-like symptoms in rodents. Here we have extended the NTG-evoked hypersensitivity assays to model the progression of migraine from an acute to a chronic state.
METHODS AND MATERIALS
Animals
Subjects were male and female C57BL6/J mice, weighing 20–30g. Animals were housed in a 12-h light–dark cycle, and food was available ad libitum. All experiments were approved by the University Of California Los Angeles Office Of Animal Research, in accordance with AALAC guidelines. These experiments adhered to the guidelines of the Committee for Research and Ethical Issues of IASP [18].
Drug administration
Nitroglycerin (NTG) was prepared from a stock solution of 5.0 mg/ml nitroglycerin in 30% alcohol, 30% propylene glycol, and water (American Regent). NTG was freshly diluted in 0.9% saline to a dose of 10 mg/kg. The vehicle control used in these experiments was 0.9% saline. We found that there was no significant difference in mechanical thresholds between those observed when 0.9% saline was used vs. those observed with 6% propylene glycol, 6% alcohol, 0.9% saline. All injections were administered as a 10 ml/kg volume. Unless otherwise noted, animals were tested for baseline responses immediately prior to intraperitoneal (ip) injection with nitroglycerin (NTG). Animals were injected with subsequent drugs (ip unless otherwise noted), 1h 15 min following NTG injection, and were tested for mechanical or thermal sensitivity 45 min later (2h post NTG). For chronic experiments, testing occurred every second day over 9 days (5 test days total). For the topiramate experiment, mice were injected with topiramate or vehicle every day for 11 days. On days 3,5,7,9, and 11 of this treatment, basal mechanical sensitivity was determined and mice received an injection of NTG or vehicle followed by an injection of topiramate/vehicle, and post-drug responses were determined 2h later. For experiments testing localized intrathecal injections of sumatriptan into the central nervous system, drug (0.06 µg) or 0.9% saline was injected in a final volume of 5.0 µl [19]. Intrathecal injections were performed with a 30 gauge, 1/2-inch needle at the L4-5 lumbar interspace on lightly restrained, unanesthetized mice.
CFA-induced Inflammatory Pain
Inflammatory pain was induced by injecting Complete Freund’s Adjuvant (CFA, 1 mg Mycobacterium tuberculosis (H37Ra, ATCC 25177)/ml of emulsion in 85% paraffin oil and 15% mannide manooleate - Sigma) into the paw. Prior to the injection of CFA baseline mechanical responses were determined. Inflammation was induced by injecting 15 µl of CFA into the plantar surface of the paw, and animals were subsequently tested 72h later.
Sensory Sensitivity Testing
To determine mechanical sensitivity, the threshold for responses to punctate mechanical stimuli (mechanical hyperalgesia) was tested according to the up-and-down method [20]. In brief, the plantar surface of the animal hindpaw was stimulated with a series of eight von Frey filaments (bending force ranging from 0.01 to 2 g). A response was defined as a lifting or shaking of the paw upon stimulation. The first filament tested was 0.4g. In the absence of a response a heavier filament (up) was tried, and in the presence of a response a lighter filament (down) was tested. This pattern was followed for a maximum of 4 filaments following the first response.
Statistical Analysis
Data are expressed as mean ± s.e.m. All statistical analyses were performed by Sigmastat software. For all acute pain experiments, one-way ANOVAs were performed, and for chronic pain experiments a two-way repeated measures ANOVA was performed. Unless otherwise noted, all experiments were further analyzed using Holm-Sidak post-hoc analysis. A significance level of p < 0.05 was used.
RESULTS
Repeated administration of systemic nitroglycerin produces acute and chronic hyperalgesia
Acute administration of nitroglycerin (NTG) has been shown previously to evoke severe mechanical and thermal hyperalgesia [8]. To model the progression to chronic migraine, we administered varying doses of NTG every second day for 9 days, resulting in a total of 5 NTG injection/test days. Mechanical thresholds were tested before and 2 hours after NTG administration on each test day. Repetitive intermittent NTG administration over 9 days produced a significant time and dose-dependent chronic basal mechanical hyperalgesia as assessed by testing prior to each administration of NTG (Figure 1A). In addition, nitroglycerin evoked significant acute mechanical hyperalgesia in a dose-dependent manner on each test day (Figure 1B). The greatest decrease in basal and post-treatment responses were observed with 10 mg/kg, the dose of NTG previously characterized in an acute study [8]. We chose to further characterize this dose (Figure 2). Progressively increasing basal hyperalgesia was observed with repeated administration of 10 mg/kg NTG (Figure 2A, TREATMENT). Following the final treatment day (day 9), mechanical responses in mice were assessed daily to determine the recovery time for this basal hypersensitivity. Sensory responses returned to the level of naïve mechanical thresholds (day 1) by day 7 post-NTG (Figure 2A, day 16, RECOVERY). Female mice showed greater basal hyperalgesia in response to chronic NTG, and unlike males, basal sensitization was observed after a single NTG injection (Figure 2B, Day 3 NTG). In addition, significant acute/post-treatment hyperalgesia was again observed with each administration (Figure 2C). To determine if the associative learning of repeated testing contributed to the progression of basal hyperalgesia, we examined basal mechanical thresholds in mice that had received identical intermittent NTG treatment over 9 days, but were only tested on the first day and the 9th day (novice). These novice mice did not show significantly different hyperalgesia on the final day of NTG (novice, 0.15 ± 0.06 vs. repeatedly tested, 0.05 ± 0.03).
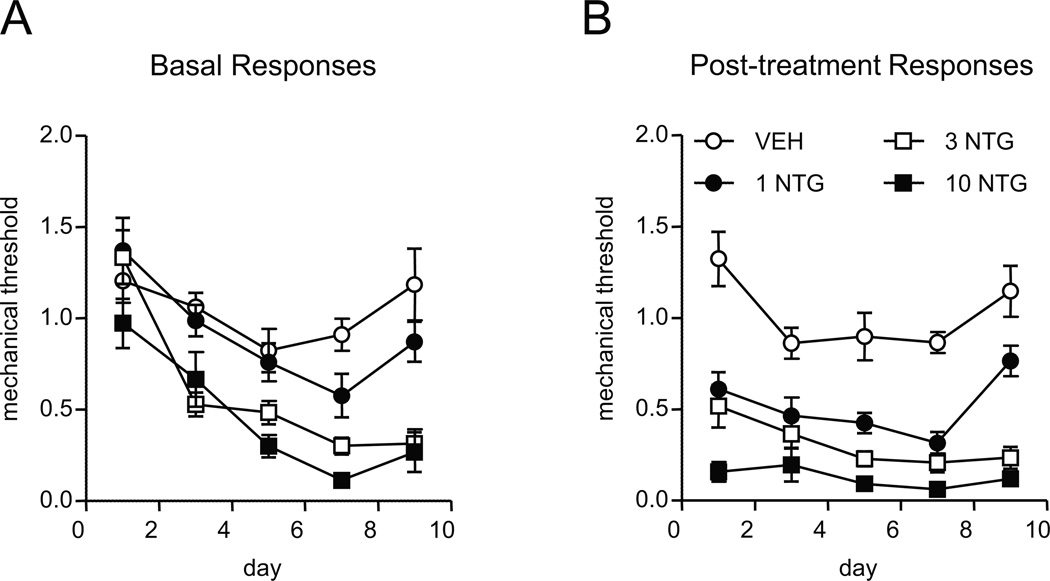
FIGURE 1.
Chronic NTG evokes and sustains mechanical hyperalgesia in a dose-dependent manner. C57Bl/6J mice were treated every second day with varying doses of NTG (0–10 mg/kg, ip) for 9 days. A) Basal mechanical responses, as assessed prior to vehicle or NTG administration, significantly decreased in the 3 and 10 mg/kg NTG group during the treatment period. n=8/group, p < 0.001 effect of dose and time two-way RM ANOVA; the 3 and 10 mg/kg doses were significantly different from vehicle and 1 mg/kg (p < 0.001). (B) Increasing doses of NTG produced increasing levels of mechanical hyperalgesia, in mice tested 2 hours post-NTG or vehicle administration. n=8/group, two-way RM ANOVA with Holm-Sidak multiple comparisons. Each dose was significantly different from vehicle (p < 0.001), and each dose was also significantly different from each other (p < 0.01). Chronic NTG in rodents produces a dose-dependent persistent hypersensitivity.
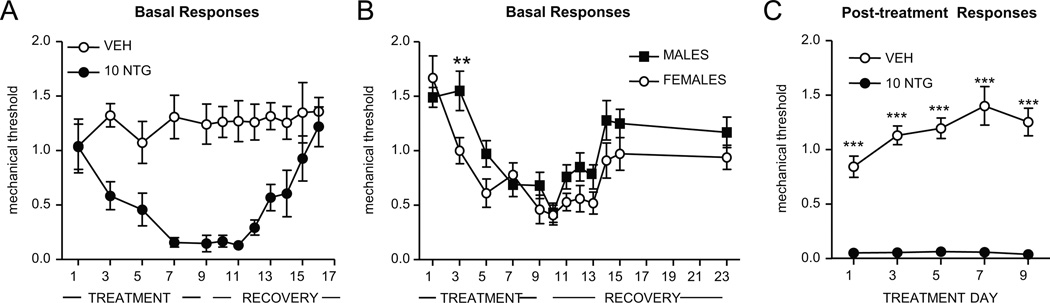
FIGURE 2.
Chronic NTG evokes severe and sustained mechanical hyperalgesia. C57Bl/6J mice were treated every second day with vehicle (VEH) or NTG (10 mg/kg, ip) for 9 days. (A) Basal mechanical responses, as assessed prior to vehicle or NTG administration, significantly decreased in the NTG group during the treatment period, and took 7 days to recover following the final NTG injection. n=7–8/group. (B) Females developed basal mechanical hyperalgesia more quickly than males following chronic NTG treatment. n=12/group, **p < 0.01 two-way RM ANOVA. (C) NTG consistently produced severe mechanical hyperalgesia, in mice tested 2 hours post-NTG or vehicle administration. n=7–8/group, ***p < 0.001 two-way RM ANOVA. Chronic NTG in rodents appears to model the persistent hypersensitivity observed in migraine patients.
The Phosphodiesterase 5 inhibitor sildenafil increases acute and chronic mechanical hyperalgesia
To investigate the role of cGMP in NTG-induced basal and evoked hyperalgesia we examined the effects of the phosphodiesterase 5 inhibitor sildenafil, which increases levels of cGMP and augments the effects of nitric oxide (NO) as an activator of guanylate cyclase. We treated mice every second day for 9 days (5 test days) with a low dose of NTG (1 mg/kg ip), and sildenafil (3 mg/kg ip, Figure 3). On each day of testing, chronic treatment with this low dose of NTG alone did not result in chronic basal hyperalgesia (Figure 3A). However, the combination of this low dose NTG with sildenafil produced significant basal hypersensitivity. Interestingly, by the final day of testing, sildenafil alone had also produced a significant decrease in basal mechanical threshold (Figure 3A). In addition, the combination of these two compounds produced a significantly greater acute hyperalgesia (Figure 3B), than either compound alone. These results indicate changes in cGMP levels play a role in the hyperalgesia induced by chronic intermittent NTG treatment.
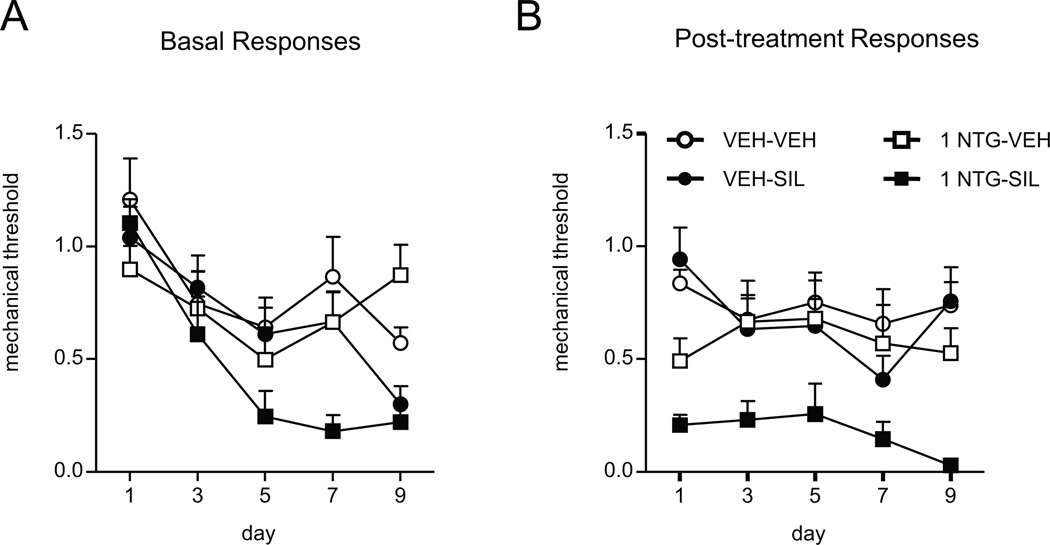
FIGURE 3.
Sildenafil, a phosphodiesterase 5 inhibitor, potentiates hyperalgesia induced by a low dose of NTG. C57Bl/6J mice were treated every second day with vehicle (VEH) or a low dose of NTG (1 mg/kg, ip) for 9 days. (A) Basal mechanical responses were assessed prior to NTG administration, n=8/group p < 0.05 effect of drug and p < 0.001 effect of time and interaction, two-way RM ANOVA; NTG-SIL mice were significantly different from all other groups (p < 0.01) (B) Mice were injected ip with either vehicle (VEH) or 3 mg/kg sildenafil (SIL) 1h15min post-NTG administration, and tested 45min later (2h post-NTG). n=8/group, p < 0.001 effect of drug, two-way RM ANOVA; NTG-SIL mice were significantly different from all other groups (p < 0.001). Animals treated with the combination of NTG and sildenafil showed significantly reduced basal and post-treatment responses.
Sumatriptan alleviates acute NTG-evoked, but not chronic basal hyperalgesia
We also investigated the effects of the migraine-selective acute therapy sumatriptan in the chronic NTG model. Consistent with previous studies [8], we found that sumatriptan (600 µg/kg, IP) reversed the acute hyperalgesia evoked by NTG injection. Sumatriptan continued to effectively ameliorate acute NTG-evoked hyperalgesia with each injection over 9 days. It did not, however, alter the chronic basal hyperalgesia that occurred over time with chronic intermittent NTG exposure (Figure 4A and B). These results indicate that while sumatriptan can reverse the acute effects of NTG, it does not reduce the progression of hyperalgesia that occurs with repeated exposure. To address the possibility that sumatriptan non-specifically inhibited nociception, we tested this same dose of sumatriptan within the Complete Freunds’ Adjuvant (CFA) model of peripheral inflammatory pain. Sumatriptan (600 µg/kg ip) was ineffective at reversing CFA-induced mechanical hyperalgesia (Figure 4C). To investigate the possibility that sumatriptan was acting to inhibit NTG-evoked mechanical hyperalgesia at the spinal level, we injected sumatriptan 0.06 µg (a dose previously shown to have anti-hyperalgesic effects in certain peripheral and visceral pain models [19]) or vehicle intrathecally into the spinal L4-5 region. In contrast to systemic intraperitoneal administration, intrathecal injection of sumatriptan did not result in any inhibition of NTG-evoked hyperalgesia (Figure 4D).
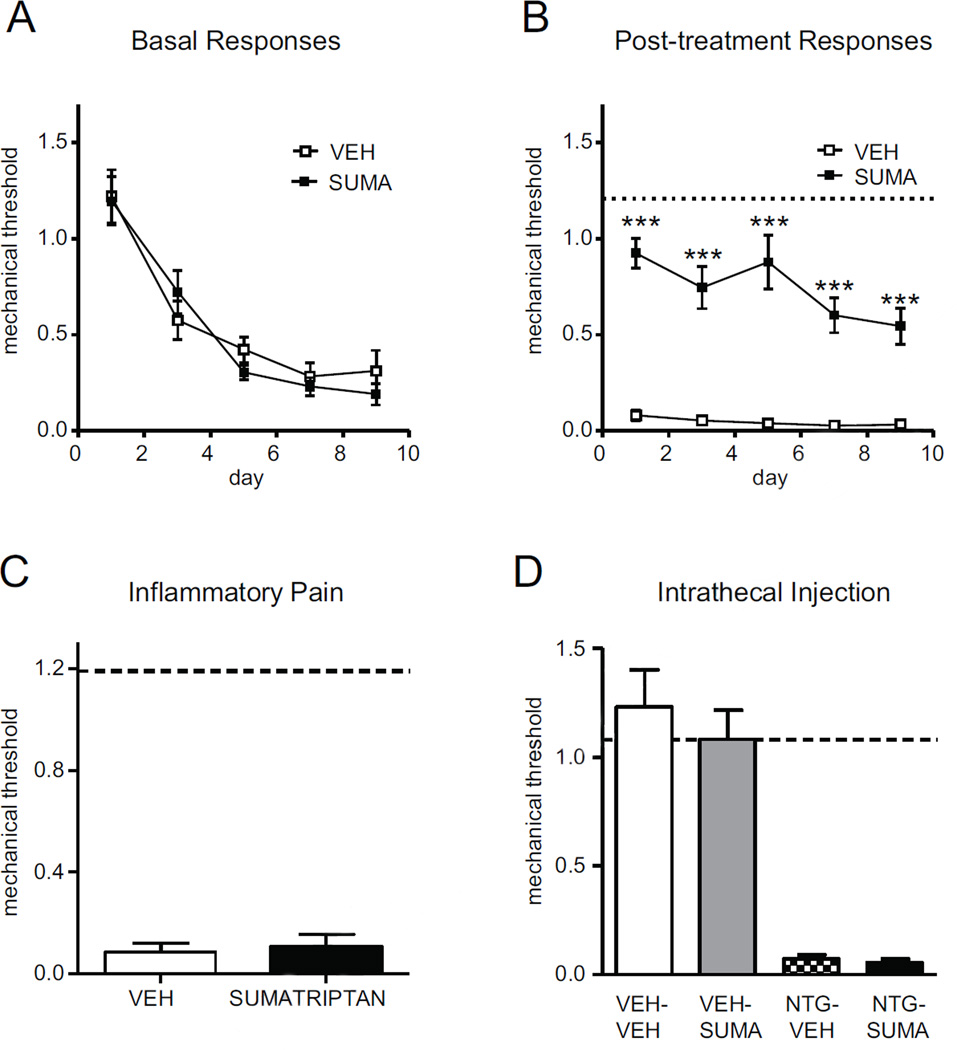
FIGURE 4.
Chronic NTG-evoked hyperalgesia is attenuated by systemic administration of sumatriptan. C57Bl/6J mice were treated every second day with NTG (10 mg/kg, ip) for 9 days. (A,B) Basal mechanical responses were assessed prior to NTG administration, and significantly and progressively decreased following the first treatment day. NTG injection produced further mechanical hyperalgesia (NTG-VEH), which was significantly attenuated by sumatriptan (B, 600 µg/kg, ip). Dashed line represents basal responses on day 1, n=11/group, ***p < 0.001, 2-way RM ANOVA. (C) Intraplantar injection of CFA into the hindpaw produced significant hyperalgesia which was not inhibited by sumatriptan (600 µg/kg, ip). Dashed line represents baseline naïve mechanical responses before CFA injection, n=6/group. (D) Mice were injected with vehicle (VEH) or NTG (10 mg/kg, ip) and after 1h30min injected intrathecally with sumatriptan (0.06 µg in 5 µl) and tested 30min later. Dashed line represents basal responses prior to NTG injection. Pain produced by chronic NTG is alleviated by a systemically administered prototypic anti-migraine treatment.
Topiramate inhibited NTG-induced chronic basal hyperalgesia
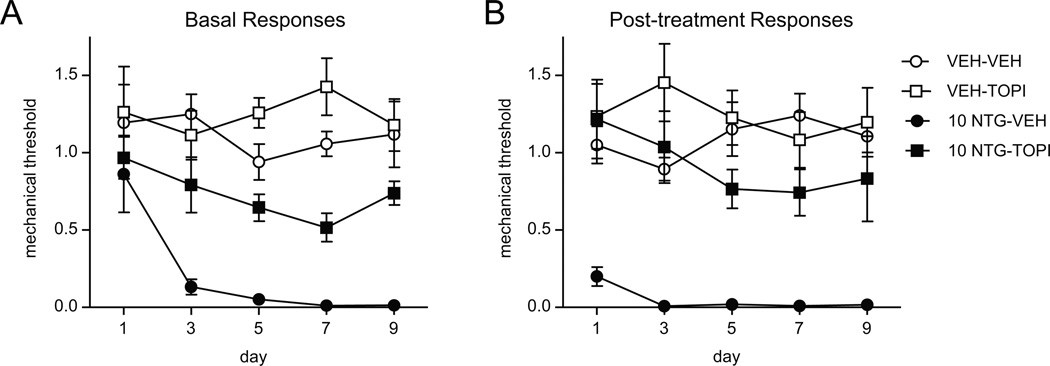
We then examined the effect of topiramate, a migraine preventive therapy, on acute and chronic basal hyperalgesia evoked by NTG. Mice were treated once daily with topiramate (30 mg/kg, IP) for 11 days. On days 3, 5, 7, 9, and 11 basal and acute NTG-evoked mechanical responses were determine. Daily treatment with topiramate significantly inhibited the development of chronic basal hyperalgesia induced by chronic intermittent NTG treatment (Figure 5A), and it also reduced acute NTG-evoked hyperalgesia (Figure 5B).
FIGURE 5.
Chronic treatment with the migraine prophylactic, topiramate, significantly attenuated NTG-induced basal and post-treatment hyperalgesia. C57Bl/6J mice were treated with vehicle (VEH) or topiramate (30 mg/kg ip) daily for 11 days. On days 3,5,7,9, and 11 baseline responses were determined (A), and mice were injected with either vehicle (VEH) or NTG (10 mg/kg, ip). Post-treatment responses were assessed 2h following NTG administration. n=8/group, p < 0.001 effect of NTG, two-way RM ANOVA. Compared to the NTG-VEH group, NTG-TOPI showed significantly higher basal post-treatment response (p < 0.001). Both basal and acute hyperalgesia induced by chronic NTG can be alleviated by a well-characterized migraine preventative therapy.
DISCUSSION
Our results indicate that chronic intermittent treatment with nitroglycerin produces an acute and chronic hypersensitivity which represents a translationally significant model of chronic migraine. Repeated injection of NTG evoked mechanical hyperalgesia, which was blocked by the anti-migraine medication sumatriptan. We also found that chronic intermittent treatment with NTG produced a severe and long-lasting basal hyperalgesia, which was slightly more pronounced in female mice. In addition, this basal hypersensitivity was alleviated by treatment with the migraine prophylactic, topiramate. Furthermore, the effects of concomitant treatment with another human migraine trigger, sildenafil, confirmed that this basal hypersensitivity was mediated by increased levels of cGMP.
Several lines of evidence indicate that nitroglycerin treatment in rodents could be a predictive model of migraine in humans. Nitroglycerin is a reliable trigger of migraine in susceptible patients [9; 10; 12]. Interestingly, migraine attacks do not occur immediately after NTG administration with the vasodilatory effects of the drug, but rather after 2–6 hours [9; 10; 12], consistent with a delayed response to nitric oxide not mediated by vasodilation [21]. In mice, the timing of NTG-induced hyperalgesia also shows a delayed response, similar to the time course of headache observed in humans [8]. Systemic administration of NTG has been reported to cause cellular activation in nociceptive pathways, including trigeminal nociceptive pathways [8; 22–24], providing additional support for relevance to migraine. In addition, NTG has been shown previously to cause light allodynia [15], and to increase meningeal blood flow [15; 17] in mice, two hallmarks of migraine. Further, the amelioration of NTG-evoked hyperalgesia by the prototypic anti-migraine medication sumatriptan also validates this model for the study of migraine mechanisms. Additionally, acute NTG-induced hyperalgesia has recently been shown to be increased in mice expressing a gene associated with familial migraine [16].
Migraine typically starts as an episodic disorder, but commonly progresses to a frequent or even daily condition [3]. The pathophysiological mechanisms underlying this change are poorly understood. We have extended the previously reported studies on acute NTG-evoked hyperalgesia to show that repetitive intermittent administration of NTG produces a progressive and sustained basal hyperalgesia that persists for days after the last exposure. These results are consistent with clinical observations of patients with chronic migraine in whom allodynia may occur both between and during migraine attacks. In addition, women are far more likely to suffer from chronic migraine than men, and our results suggest that female mice may be more sensitive to chronic NTG. Previous studies have also shown that there are estrogen-related discrepancies in the brain regions activated with NTG administration in rats [25], indicating that the chronic NTG model could potentially be a tool to specifically study sex differences in migraine.
The enhancement of the effects of NTG by sildenafil, also an established human migraine trigger [13; 26], provides evidence that NO-mediated increases in cGMP play a primary role in the hyperalgesia evoked by NTG. We found that by the final test day, sildenafil also independently produced basal hyperalgesia with repeated intermittent dosing, suggesting that potentiation of the endogenous activity of guanylate cylase may be a mechanism for producing chronic hyperalgesia. Drugs that target different aspects of the nitric oxide-cGMP pathway are currently being developed for the treatment of headache [13], and our preclinical data further support the role of this signaling cascade in the development of chronic migraine.
We found that sumatriptan inhibits acute hyperalgesia in response to NTG, similar to the results of previous rodent [8] and human [27] studies. We also found that sumatriptan continued to have this effect with each NTG exposure over time, but that it did not inhibit the resulting progressive chronic basal hyperalgesia. This result is consistent with the observation that while sumatriptan can be consistently effective as an acute migraine therapy, its use does not prevent progression of migraine over time [28]. Since this model tests hyperalgesia in the hindpaw, it is possible that the effects of sumatriptan could be mediated by spinal mechanisms. However, we found that intrathecal administration of sumatriptan did not inhibit NTG-evoked mechanical hyperalgesia, in contrast to systemically administered sumatriptan; indicating that within this model sumatriptan is not acting solely through the lumbar spinal cord. In addition, groups studying the dural inflammation model of migraine have also found that inflammatory mediators applied to the dura [6] or dural TRPA1 activation [29] produced mechanical hyperalgesia in both facial and hindpaw regions, likely through brainstem relays. Cutaneous allodynia following NTG likely reflects the development of central sensitization which is observed during primary headache, which may be mediated through sensitization of neurons within the thalamus [30].
Topiramate inhibited acute NTG-evoked hyperalgesia, as well as chronic basal hyperalgesia, consistent with its actions as a prophylactic migraine therapy. Topiramate has multiple mechanisms of actions that could be involved in its therapeutic efficacy for migraine, including inhibition of voltage gated sodium channels, enhancement of GABAergic signaling, inhibition of glutamatergic signaling, and inhibition of carbonic anhydrase [31]. It has been proven to be an effective preventive therapy even in the setting of chronic migraine [32]. Moreover, based on limited studies, topiramate has also been suggested to inhibit the progression of migraine to a chronic disorder in patients [33].
In this study we used a dose of NTG (10 mg/kg), which is substantially higher than the equivalent dose used to provoke migraine in humans [12]. It is therefore possible that this dose of NTG in mice could have additional effects apart from those associated with migraine induction in humans. The significant inhibition of NTG-induced hyperalgesia by systemic sumatriptan and topiramate, however, indicates that even if this dose of NTG represents a “supra-physiological” stimulus, it can nonetheless be effectively reversed by standard doses of a proven migraine-specific therapy. Similarly, the ability of topiramate to inhibit the chronic basal hyperalgesia induced by chronic NTG indicates that this model may also be used to test potential migraine prophylactics.
Nitroglycerin evoked acute and chronic basal hyperalgesia is a promising mouse model for the investigation of migraine mechanisms and therapies. The enhancement of acute NTG-evoked hyperalgesia by a human migraine-associated gene expressed in mice, and the inhibition of this hyperalgesia by both acute and preventive migraine therapies provide strong support for the translational value of this model. Ongoing studies of the effects of other human migraine genes, as well as other established acute and preventive therapies, will help to further validate the model as a useful tool for enhancing our ability to understand and treat this frequently disabling disorder.
Acknowledgments
This research was supported by the Department of Defense PR100085, The Migraine Research Foundation, NIH-NIDA Grants DA031243 and DA05010, and the Shirley and Stefan Hatos Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia. 2010;30:1065–1072. doi: 10.1177/0333102409355601. [DOI] [PubMed] [Google Scholar]
- 2.Stovner L, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, Steiner T, Zwart JA. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27:193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 3.Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71:559–566. doi: 10.1212/01.wnl.0000323925.29520.e7. [DOI] [PubMed] [Google Scholar]
- 4.Stewart WF, Wood C, Reed ML, Roy J, Lipton RB. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28:1170–1178. doi: 10.1111/j.1468-2982.2008.01666.x. [DOI] [PubMed] [Google Scholar]
- 5.Andreou AP, Summ O, Charbit AR, Romero-Reyes M, Goadsby PJ. Animal models of headache: from bedside to bench and back to bedside. Expert review of neurotherapeutics. 2010;10:389–411. doi: 10.1586/ern.10.16. [DOI] [PubMed] [Google Scholar]
- 6.Edelmayer RM, Ossipov MH, Porreca F. An experimental model of headache-related pain. Methods in molecular biology (Clifton, NJ) 2012;851:109–120. doi: 10.1007/978-1-61779-561-9_7. [DOI] [PubMed] [Google Scholar]
- 7.Oshinsky ML, Sanghvi MM, Maxwell CR, Gonzalez D, Spangenberg RJ, Cooper M, Silberstein SD. Spontaneous trigeminal allodynia in rats: a model of primary headache. Headache. 2012;52:1336–1349. doi: 10.1111/j.1526-4610.2012.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bates EA, Nikai T, Brennan KC, Fu YH, Charles AC, Basbaum AI, Ptacek LJ, Ahn AH. Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia. 2010;30:170–178. doi: 10.1111/j.1468-2982.2009.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iversen HK, Olesen J, Tfelt-Hansen P. Intravenous nitroglycerin as an experimental model of vascular headache. Basic characteristics. Pain. 1989;38:17–24. doi: 10.1016/0304-3959(89)90067-5. [DOI] [PubMed] [Google Scholar]
- 10.Christiansen I, Thomsen LL, Daugaard D, Ulrich V, Olesen J. Glyceryl trinitrate induces attacks of migraine without aura in sufferers of migraine with aura. Cephalalgia. 1999;19:660–667. doi: 10.1046/j.1468-2982.1999.019007660.x. [DOI] [PubMed] [Google Scholar]
- 11.Afridi SK, Matharu MS, Lee L, Kaube H, Friston KJ, Frackowiak RS, Goadsby PJ. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain. 2005;128:932–939. doi: 10.1093/brain/awh416. [DOI] [PubMed] [Google Scholar]
- 12.Olesen J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol Ther. 2008;120:157–171. doi: 10.1016/j.pharmthera.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Olesen J. Nitric oxide-related drug targets in headache. Neurotherapeutics : the journal of the American Society for Experimental Neuro Therapeutics. 2010;7:183–190. doi: 10.1016/j.nurt.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iversen HK. Human migraine models. Cephalalgia. 2001;21:781–785. doi: 10.1111/j.1468-2982.2001.00250.x. [DOI] [PubMed] [Google Scholar]
- 15.Markovics A, Kormos V, Gaszner B, Lashgarara A, Szoke E, Sandor K, Szabadfi K, Tuka B, Tajti J, Szolcsanyi J, Pinter E, Hashimoto H, Kun J, Reglodi D, Helyes Z. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. NeurobiolDis. 2012;45:633–644. doi: 10.1016/j.nbd.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Brennan KC, Bates EA, Shapiro RE, Zyuzin J, Hallows WC, Huang Y, Lee HY, Jones CR, Fu YH, Charles AC, Ptacek LJ. Casein kinase idelta mutations in familial migraine and advanced sleep phase. Science translational medicine. 2013;5 doi: 10.1126/scitranslmed.3005784. 183ra156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greco R, Meazza C, Mangione AS, Allena M, Bolla M, Amantea D, Mizoguchi H, Sandrini G, Nappi G, Tassorelli C. Temporal profile of vascular changes induced by systemic nitroglycerin in the meningeal and cortical districts. Cephalalgia. 2011;31:190–198. doi: 10.1177/0333102410379887. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 19.Nikai T, Basbaum AI, Ahn AH. Profound reduction of somatic and visceral pain in mice by intrathecal administration of the anti-migraine drug, sumatriptan. Pain. 2008;139:533–540. doi: 10.1016/j.pain.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. JNeurosciMethods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 21.Schoonman GG, van der GJ, Kortmann C, van der Geest RJ, Terwindt GM, Ferrari MD. Migraine headache is not associated with cerebral or meningeal vasodilatation--a 3T magnetic resonance angiography study. Brain. 2008;131:2192–2200. doi: 10.1093/brain/awn094. [DOI] [PubMed] [Google Scholar]
- 22.Tassorelli C, Joseph SA. NADPH-diaphorase activity and Fos expression in brain nuclei following nitroglycerin administration. Brain research. 1995;695:37–44. doi: 10.1016/0006-8993(95)00732-6. [DOI] [PubMed] [Google Scholar]
- 23.Ramachandran R, Bhatt DK, Ploug KB, Olesen J, Jansen-Olesen I, Hay-Schmidt A, Gupta S. A naturalistic glyceryl trinitrate infusion migraine model in the rat. Cephalalgia. 2012;32:73–84. doi: 10.1177/0333102411430855. [DOI] [PubMed] [Google Scholar]
- 24.Tassorelli C, Joseph SA. Systemic nitroglycerin induces Fos immunoreactivity in brainstem and forebrain structures of the rat. Brain research. 1995;682:167–181. doi: 10.1016/0006-8993(95)00348-t. [DOI] [PubMed] [Google Scholar]
- 25.Greco R, Tassorelli C, Mangione AS, Smeraldi A, Allena M, Sandrini G, Nappi G, Nappi RE. Effect of sex and estrogens on neuronal activation in an animal model of migraine. Headache. 2013;53:288–296. doi: 10.1111/j.1526-4610.2012.02249.x. [DOI] [PubMed] [Google Scholar]
- 26.Kruuse C, Thomsen LL, Birk S, Olesen J. Migraine can be induced by sildenafil without changes in middle cerebral artery diameter. Brain. 2003;126:241–247. doi: 10.1093/brain/awg009. [DOI] [PubMed] [Google Scholar]
- 27.Iversen HK, Olesen J. Headache induced by a nitric oxide donor (nitroglycerin) responds to sumatriptan. A human model for development of migraine drugs. Cephalalgia. 1996;16:412–418. doi: 10.1046/j.1468-2982.1996.1606412.x. [DOI] [PubMed] [Google Scholar]
- 28.Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology. 2008;71:1821–1828. doi: 10.1212/01.wnl.0000335946.53860.1d. [DOI] [PubMed] [Google Scholar]
- 29.Edelmayer RM, Le LN, Yan J, Wei X, Nassini R, Materazzi S, Preti D, Appendino G, Geppetti P, Dodick DW, Vanderah TW, Porreca F, Dussor G. Activation of TRPA1 on dural afferents: a potential mechanism of headache pain. Pain. 2012;153:1949–1958. doi: 10.1016/j.pain.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, Becerra L, Borsook D. Thalamic sensitization transforms localized pain into widespread allodynia. Annals of neurology. 2010;68:81–91. doi: 10.1002/ana.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulleners WM, Chronicle EP. Anticonvulsants in migraine prophylaxis: a Cochrane review. Cephalalgia. 2008;28:585–597. doi: 10.1111/j.1468-2982.2008.01571.x. [DOI] [PubMed] [Google Scholar]
- 32.Diener HC, Bussone G, Van Oene JC, Lahaye M, Schwalen S, Goadsby PJ. Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia. 2007;27:814–823. doi: 10.1111/j.1468-2982.2007.01326.x. [DOI] [PubMed] [Google Scholar]
- 33.Limmroth V, Biondi D, Pfeil J, Schwalen S. Topiramate in patients with episodic migraine: reducing the risk for chronic forms of headache. Headache. 2007;47:13–21. doi: 10.1111/j.1526-4610.2007.00648.x. [DOI] [PubMed] [Google Scholar]