Abstract
Importance
A focal lesion detected by use of magnetic resonance imaging (MRI) is a favorable prognostic finding for epilepsy surgery. Patients with normal MRI findings and extratemporal lobe epilepsy have less favorable outcomes. Most studies investigating the outcomes of patients with normal MRI findings who underwent (nonlesional) extratemporal epilepsy surgery are confined to a highly select group of patients with limited follow-up.
Objective
To evaluate noninvasive diagnostic test results and their association with excellent surgical outcomes (defined using Engel classes I–IIA of surgical outcomes) in a group of patients with medically resistant nonlesional extratemporal epilepsy.
Design
A retrospective study.
Setting
Mayo Clinic, Rochester, Minnesota.
Participants
From 1997 through 2002, we identified 85 patients with medically resistant extratemporal lobe epilepsy who had normal MRI findings. Based on a standardized presurgical evaluation and review at a multi-disciplinary epilepsy surgery conference, some of these patients were selected for intracranial electroencephalographic (EEG) monitoring and epilepsy surgery.
Exposure
Nonlesional extratemporal lobe epilepsy surgery.
Main Outcomes and Measures
The results of non-invasive diagnostic tests and the clinical variables potentially associated with excellent surgical outcome were examined in patients with a minimum follow-up of 1 year (mean follow-up, 9 years).
Results
Based on the noninvasive diagnostic test results, a clear hypothesis for seizure origin was possible for 47 of the 85 patients (55%), and 31 of these 47 patients (66%) proceeded to intracranial EEG monitoring. For 24 of these 31 patients undergoing long-term intracranial EEG (77%), a seizure focus was identified and surgically resected. Of these 24 patients, 9 (38%) had an excellent outcome after resective epilepsy surgery. All patients with an excellent surgical outcome had at least 10 years of follow-up. Univariate analysis showed that localized interictal epileptiform discharges on scalp EEGs were associated with an excellent surgical outcome.
Conclusions and Relevance
Scalp EEG was the most useful test for identifying patients with normal MRI findings and extratemporal lobe epilepsy who were likely to have excellent outcomes after epilepsy surgery. Extending outcome analysis beyond the resective surgery group to the entire group of patients who were evaluated further highlights the challenge that these patients pose. Although 9 of 24 patients undergoing resective surgery (38%) had excellent outcomes, only 9 of 31 patients undergoing intracranial EEG (29%) and only 9 of 85 patient with nonlesional extratemporal lobe epilepsy (11%) had long-term excellent outcomes.
Surgery for medically resistant epilepsy can be highly effective for patients with a focal epileptogenic lesion identified on a magnetic resonance imaging (MRI) scan.1 When MRI fails to detect a potentially epileptogenic lesion, the chances of an excellent surgical outcome are significantly lower.2–6 Surgical outcomes for patients with neocortical epilepsy who have normal preoperative MRI findings are particularly poor, which reflects the difficulty in localizing and resecting the epileptogenic zone. The reported rates of excellent surgical outcome for nonlesional partial epilepsy range from 41% to 65% for the temporal lobe,2,7–9 37% for mixed mesial temporal and neocortical sites,10 and 29% to 56% for extratemporal epilepsy.3,5,11–13 Reports of outcome in nonlesional extratemporal epilepsy, however, are from relatively small numbers of highly select patients3 with 1-year follow-up (17 patients in Smith et al,13 26 patients in Mosewich et al,12 43 patients in Siegel et al,14 43 patients in Dorward et al,15 10 patients in Chapman et al,11 18 patients in Jeha et al,5 and 7 patients in Bien et al3). Except for Bein et al,3 none of these studies provide data on the total cohort of patients evaluated in order to arrive at the subset considered for resective surgery. In addition, these studies do not report the number of patients who underwent long-term intracranial electroencephalographic (EEG) monitoring but who were not candidates for resection. Thus, the outcomes after resective surgery are from a highly select group of patients, and they do not reflect the probability of an excellent outcome prior to intracranial EEG monitoring. This information would be very useful for counseling patients in the clinic.
Absent a clear anatomical lesion detected on an MRI scan, the noninvasive localization of an epileptic brain relies on seizure semiology, scalp EEG, and functional neuroimaging tests such as single-photon emission computed tomography (SPECT) and positron emission tomography. How these tests help us identify patients who are likely to have an excellent surgical outcome remains an area of active investigation. Factors previously reported to predict excellent surgical outcome in nonlesional extratemporal epilepsy (NLETE) include unifocal interictal epileptiform discharges (IEDs),16 a focal β-frequency ictal discharge on either a scalp or an intracranial EEG,17–19 and a localized SPECT abnormality.20 However, other series11,21,22 have failed to identify any predictive factors. The objective of the present study was to evaluate the noninvasive diagnostic test results and their association with excellent surgical outcome (defined using Engel classes I–IIA of surgical outcomes) in a group of patients with medically resistant NLETE.
METHODS
Following Mayo Clinic investigational review board approval, we retrospectively identified 85 consecutive patients who underwent a standardized epilepsy presurgical evaluation for medically resistant NLETE between January 1997 and December 2002. Medical charts were reviewed to determine patient characteristics, including age at surgery, duration of epilepsy, sex, history of febrile seizures, significant head trauma, meningitis, encephalitis, and family history of epilepsy (Table 1).
Table 1.
Univariate Analysis for Phase II Selection and Excellent Surgical Outcomea
| Feature | No. (%)
|
P Value | OR (95% CI) | No. (%)
|
P Value | OR (95% CI) | ||
|---|---|---|---|---|---|---|---|---|
| Candidates (n = 47) | Noncandidates (n = 38) | Excellent Outcome (n = 9) | Nonexcellent Outcome (n = 15) | |||||
| Female sex | 20 (43) | 20 (53) | .17b | 0.49 (0.18–1.31) | 3 (33) | 7 (47) | .68b | 0.59 (0.07–4.15) |
| Children (<18 y) | 22 (47) | 11 (29) | .12b | 0.50 (0.17–1.25) | 4 (44) | 9 (60) | .68b | 0.54 (0.07–3.79) |
| Risk factors | 5 (11) | 5 (13) | .75b | 0.79 (0.17–3.74) | 0 (0) | 3 (20) | .27b | 0.00 (0.00–3.99) |
| Age at onset of epilepsy, mean (SD), y | 8 (6.0) | 11 (12.6) | .11c | 7 (4.6) | 8 (5.4) | .78c | ||
| Age at presurgical assessment, mean (SD), y | 21 (12.3) | 26 (15.7) | .13c | 20 (10.6) | 20 (14.1) | .96c | ||
| Interictal EEG (localizing vs nonlocalizing) | 16 (34) | 6 (16) | .08b | 2.72 (0.87–9.64) | 7 (78) | 5 (33) | .09b | 6.39 (0.81–85.67) |
| Ictal EEG (localizing vs nonlocalizing) | 26 (55) | 14 (37) | .13b | 2.10 (0.81–5.60) | 7 (78) | 8 (53) | .39b | 2.92 (0.37–38.08) |
| SISCOM (localizing vs nonlocalizing) | 35 (74) | 23 (61) | .24b | 1.89 (0.68–5.32) | 7 (78) | 12 (80) | .99b | 0.88 (0.08–12.98) |
| Semiology (lateralized vs nonlateralized) | 30 (64) | 21 (55) | .51b | 1.42 (0.54–3.74) | 4 (44) | 13 (87) | .06b | 0.14 (0.01–1.22) |
| Mean concordance of presurgical evaluationd | 2.36 (0.92) | 1.87 (1.02) | .03e | 2.78 (0.67) | 2.60 (0.91) | .40e | ||
Abbreviations: EEG, electroencephalogram; OR, odds ratio; SISCOM, subtraction ictal SPECT coregistered with magnetic resonance imaging; SPECT, single-photon emission computed tomography.
Statistical analysis was performed with R version 2.15 (R Foundation for Statistical Computing).
Determined by use of the 2-tailed Fisher exact test.
Determined by use of the Welch t test.
Ictal and interictal EEG, SPECT, and semiology.
Determined by use of the Mann-Whitney U test.
A standardized noninvasive presurgical evaluation of all cases included a neuropsychological evaluation, seizure protocol MRI,23 interictal and ictal scalp EEG, and SPECT. All patients had prolonged, video-EEG monitoring using 31 scalp electrodes to record their habitual seizures. The 1.5-T seizure protocol brain MRI included 1.6-mm T1-weighted spoiled gradient echo coronal slices and 4-mm fluid-attenuated inversion recovery coronal slices.23 Patients with a history of epilepsy surgery, video-EEG–detected temporal lobe epilepsy, or generalized epilepsy were excluded. The seizure protocol MRI, SPECT, and EEG findings were classified on the basis of staff neuroradiologist and epileptologist reviews. Patients with abnormalities detected on MRI scans were excluded, except those with non-specific white matter changes. The IEDs were classified as localizing if they occurred in a single lobar brain region. An ictal EEG was defined as localizing if the onset was from a single lobar location. Patients with exclusively temporal lobe IEDs or a temporal lobe ictal electrographic discharge were excluded. Visual analysis of SPECT was defined as localizing when SISCOM (subtraction ictal SPECT coregistered with MRI) images were visually interpreted to show a single or dominant focus of hyperperfusion.20 The Engel classification (Table 2) was used to identify excellent (classes IA–D and IIA) and nonexcellent (classes IIB–D, III, and IV) surgical outcomes.24
Table 2.
Engel Classification of Surgical Outcomes
| Engel Classification |
|---|
| Class I: free of disabling seizures |
| A: Completely seizure free |
| B: Nondisabling simple partial seizures only |
| C: Some disabling seizures, but free of disabling seizures for at least 2 y |
| D: Generalized convulsion with antiepileptic drug withdrawal only |
| Class II: rare disabling seizures (“almost seizure free”) |
| A: Initially free of disabling seizures but rare seizures now |
| B: Rare disabling seizures since surgery |
| C: More than rare disabling seizures, but rare seizures for at least 2 y |
| D: Nocturnal seizures only |
| Class III: worthwhile improvement |
| A: Worthwhile seizure reduction |
| B: Prolonged seizure-free intervals amounting to more than half the follow-up period, but not less than 2 y |
| Class IV: no worthwhile improvement |
| A: Significant seizure reduction |
| B: No appreciable change |
| C: Seizure worse |
To evaluate the potential confounder that patients with focal abnormalities detected by scalp EEG and SISCOM would be preferentially selected for intracranial EEG, we performed 2 analyses. First, the entire nonselected cohort of 85 patients was analyzed to determine the correlation of variables in the noninvasive presurgical evaluation of candidacy for intracranial EEG. A similar univariate analysis was also performed for the 24 patients who had resective epilepsy surgery to determine clinical and diagnostic testing variables associated with surgical outcome.
In particular, the presurgical evaluation included the potential importance of (1) seizure semiology, (2) localized IEDs, (3) localized ictal scalp EEG discharge, and (4) SISCOM localized to a single lobe. We also investigated multiple clinical variables, including age (adult vs pediatric) and risk factors (head trauma with loss of consciousness, family history of epilepsy, and febrile seizures). In the surgical group, we also investigated the potential importance of the location of surgical resection and histopathology. The statistical significance of these variables was evaluated using the Fisher exact test and the Mann-Whitney U test. Statistical significance was defined as P < .05.
RESULTS
PATIENT CHARACTERISTICS
Of the 85 patients who met the inclusion criteria, 52 (61%) were adults (≥18 years of age). The mean age at the time of presurgical evaluation was 23 years (range, 1–62 years). The mean duration of epilepsy was 13.7 years, with a mean age at seizure onset of 9.3 years. The mean preoperative frequency of seizures was 1 to 6 seizures per week, with 35 patients (41%) experiencing 1 or more seizures daily (Table 1).
INTERICTAL SCALP EEG
Interictal scalp EEGs (both routine studies and as part of prolonged video-EEG monitoring) showed that 26 of 85 patients (31%) had no IEDs. Four patients (5%) were reported to have exclusively generalized IEDs, 19 (22%) had a combination of generalized and focal IEDs, and 14 (16%) had multifocal IEDs without a clear dominant focus. The other 22 patients (26%) had a single or dominant interictal focus.
ICTAL SEMIOLOGY AND SCALP EEG
Video-EEG–detected seizures were reported to demonstrate lateralizing semiology in 51 patients (60%). Ictal semiology suggested onset in the left hemisphere for 28 patients and onset in the right hemisphere for 23 patients. The ictal EEG demonstrated a generalized EEG discharge at onset for 10 patients (12%). For 33 patients (39%), the ictal EEG changes, although not generalized, could not be clearly lateralized to a single hemisphere (eg, bifrontal or central midline ictal discharge). For 42 patients (49%), the ictal EEG activity could be clearly lateralized. Of these 42 patients, 17 (40%) had an ictal onset localized to the right frontocentral head region, 5 (12%) had an ictal onset localized to the right parietooccipital head region, 13 (31%) had an ictal onset localized to the left frontocentral head region, 5 (12%) had an ictal onset localized to the left parietooccipital head region, and 2 (5%) had an ictal onset localized to the left hemisphere without more specific localization.
SINGLE-PHOTON EMISSION COMPUTED TOMOGRAPHY
The SISCOM was reported as localizing in 58 patients (68%). The frontal lobe was the predominant site of SPECT localization in 40 patients (47%). Of the other 18 patients, SPECT studies demonstrated a hyperperfusion focus in the parietal lobe of 10 patients, in the insular region of 4 patients, and in the occipital lobe of 4 patients.
SELECTION FOR INTRACRANIAL EEG MONITORING AND OUTCOMES
Candidacy for intracranial EEG was highly associated with concordance of multiple diagnostic test results. No single test reached statistical significance, but when analyzed together, a higher concordance of variables from presurgical evaluation (seizure semiology, interictal and ictal scalp localizing EEG, and SISCOM) was associated with patients who were offered intracranial EEG (P = .03) (Table 1).
Of the 61 patients who did not undergo resective surgery, 23 (38%) had a follow-up time of longer than 1 year (mean follow-up time, 5 years [range, 1–12 years]). Five patients (8%) were seizure free or only had auras after initiation of a new medication. Unfortunately, 3 patients (5%) died before their last scheduled follow-up.
INTRACRANIAL MONITORING, SURGICAL RESECTION, AND PATHOLOGY
At the conclusion of the noninvasive presurgical evaluation (phase I), 47 patients (55%) were considered candidates for an invasive phase II surgical evaluation. Of these 47 patients, 31 (66%; 36% of original cohort) elected to proceed to intracranial EEG monitoring with implantation of subdural electrodes (Figure 1). Of the 31 patients implanted with subdural electrodes, 25 (81% of patients undergoing invasive phase II evaluation) had intracranial EEGs that demonstrated a clearly localized seizure onset. For the patients with nonlocalizing intracranial EEGs, the subdural electrodes were explanted without cortical resection. One of the patients with a focal ictal discharge on a subdural electrode recording did not undergo resection because the seizure onset involved the motor cortex. The remaining patients with localized seizures recorded by intracranial EEG had surgical resections. Three patients had partial frontal lobectomies, and the rest had focal cortical resections guided by the results of intracranial EEGs. Of the 24 patients who underwent a surgical resection, 18 (75%) underwent a resection in the frontal lobe, evenly divided between the left and right hemispheres, and 6 (25%) underwent a resection in the parietal or occipital lobe. There were no major surgical complications.
Figure 1.
Summary of patient cohort. Eighty-five consecutive patients with normal magnetic resonance imaging (MRI) findings and medically resistant extratemporal lobe epilepsy were retrospectively identified. The only noninvasive studies associated with excellent surgical outcome were focal interictal epileptiform discharges (IEDs). EEG indicates electroencephalogram; iEEG, intracranial EEG; and SPECT, single-photon emission computed tomography.
A pathology examination of resected tissue revealed only nonspecific, primarily subpial gliosis in 16 of the 24 patients’ resected tissue samples (67%). Seven patients (29%) had findings of microscopic cortical dysplasia, and the pathology of 1 patient (4%) was consistent with a dysembryoplastic neuroepithelial tumor.
SURGICAL OUTCOMES
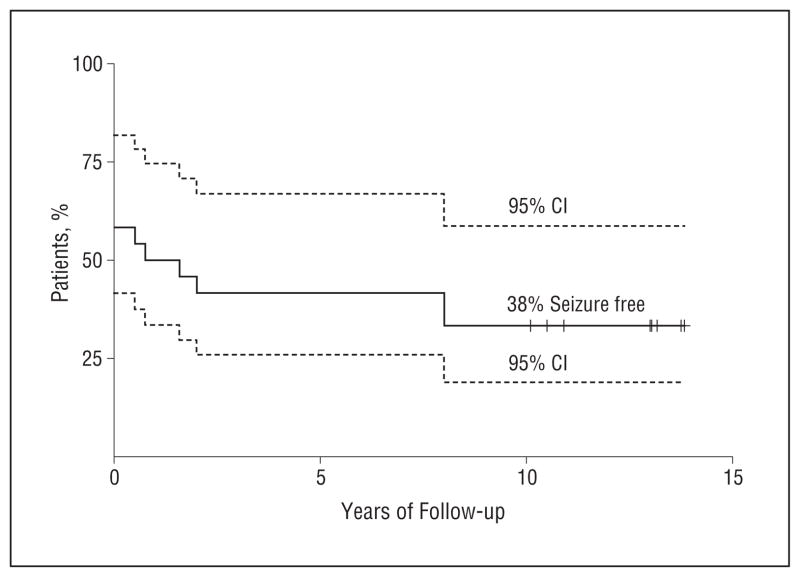
Surgical outcome was classified on the basis of the most recent available follow-up data. All surgical patients had a follow-up time of longer than 1 year (mean follow-up time, 9 years [range, 2.5–13 years]) (Figure 2). Of the 24 patients who had resective surgery, 9 (38%) had an excellent outcome. All 9 patients were Engel class I at the time of their last follow-up, and all of these patients had more than 10 years of follow-up. There were no Engel class IIA patients. The other 15 patients had Engel classes IIB–D, III, or IV outcomes; however, 3 of these patients had only nocturnal seizures (Engel class IIB), 6 had significant improvement in seizure frequency (Engel classes IIIA and IIIB), and 1 had a subsequent surgical extension of the first resection and was then rendered seizure free (Table 3).
Figure 2.
Kaplan-Meier survival curves. Of 24 patients who underwent epilepsy surgery, 10 had a recurrence of seizures within 3 months. Of the remaining 14 patients, 5 had a recurrence of medically resistant seizures over the next 8 years.
Table 3.
Final Surgical Outcomes for 24 Patients
| Engel Classification | No. (%) of Patients | Subclassification |
|---|---|---|
| Class I | 9 (38%) | 8 patients class IA, 1 patient class ID |
| Class II | 3 (13%) | 3 patients class IID |
| Class III | 6 (25%) | 4 patients class IIIA, 2 patients class IIIB |
| Class IV | 6 (25%) | 4 patients class IVA, 2 patients class IVB |
When considering the entire cohort of 85 patients using univariate analysis with a 2-tailed Fisher exact test, we found that the presence of IEDs in a single focus (P = .02) was associated with an excellent surgical outcome (Table 4). No significant relationship was shown between surgical outcome and any clinical variable or diagnostic test, including, age (adult vs pediatric), risk factors, lateralizing ictal semiology, ictal scalp EEG localization, or SPECT. Furthermore, surgical outcomes did not differ significantly according to histopathologic finding or location of resection. When considering only the 24 patients who underwent resective epilepsy surgery, we found that univariate analysis only showed a weak association between IEDs in a single focus (P = .09) and excellent surgical outcome (Table 1).
Table 4.
Univariate Analysis for Excellent Outcomea
| Feature | OR (95% CI) | P Valueb |
|---|---|---|
| Seizure semiology (lateralized vs nonlateralized) | 0.50 (0.09–2.52) | .47 |
| Interictal localized EEG discharges | 6.97 (1.21–73.37) | .02 |
| Ictal localized EEG discharge | 4.48 (0.78–47.00) | .08 |
| SISCOM (localized vs nonlocalized) | 1.63 (0.28–17.33) | .71 |
| Histopathology (dysplasia/tumor vs nonspecific gliosis) | 0.44 (0.03–3.60) | .66 |
| Location of surgical resection | ||
| Frontal vs parietooccipital | 3.80 (0.32–212.14) | .35 |
| Left vs right | 0.68 (0.24–14.74) | .68 |
Abbreviations: EEG, electroencephalographic; OR, odds ratio; SISCOM, subtraction ictal SPECT coregistered with magnetic resonance imaging.
For a total of 85 patients with nonlesional extratemporal epilepsy.
Determined by use of the 2-tailed Fisher exact test; statistical analysis was performed with R version 2.15 (R Foundation for Statistical Computing).
DISCUSSION
The absence of an MRI-detected lesion during the pre-surgical evaluation of a patient with medically resistant epilepsy complicates clinical decision making and results in lower surgical efficacy compared with the presence of a surgically resectable epileptogenic MRI-detected lesion.1,4,24,25 In particular, patients with NLETE represent some of the most challenging patients evaluated at epilepsy centers.3,5,26 To identify results from non-invasive testing and clinical variables associated with excellent surgical outcome in NLETE, we evaluated a standardized presurgical protocol. In univariate analysis, the only noninvasive test associated with excellent surgical outcome was the presence of localized IEDs. This finding is in agreement with a study by Holmes et al7 that combined lesional and nonlesional extratemporal lobe epilepsy surgical procedures, although other studies27,28 did not find an association. The lack of agreement may reflect the variability of IEDs in frontal lobe epilepsy or the small number of patients.29,30 In the present study, the presence of a localized SISCOM did not predict surgical outcome. Previous studies that included lesional extratemporal lobe epilepsy surgery and smaller numbers of nonlesional patients with extratemporal epilepsy found an association between outcome and complete resection of a SISCOM abnormality.20 Again, a possible explanation is the relatively small number of patients, but a more likely explanation is the bias introduced in NLETE because patients with positive SISCOM studies are more likely to have invasive monitoring and resective surgery. Our analysis of presurgical evaluation shows that positive SISCOM studies and a higher concordance of non-invasive studies are associated with a greater likelihood of selection for invasive phase II evaluation with intracranial EEG (Table 1).
Consistent with the challenge that these patients present, only 47 of the 85 patients (55%) had sufficiently localizing noninvasive findings to justify implantation of intracranial electrodes. Of these 47 patients, 31 (66%) proceeded to long-term intracranial EEG, which is an invasive procedure associated with potential morbidity.31 For 24 of these 31 patients undergoing long-term intracranial EEG (77%), a seizure focus was identified and surgically resected. Of these 24 patients, 9 (38%) had an excellent outcome after resective epilepsy surgery, which represents only 11% of the original cohort of 85 patients (Figure 1).
For the group of 61 patients who did not undergo resective surgery, we were able to achieve a minimum 1-year follow-up for 21 patients (34%). At the time of their last follow-up, 5 patients (8%) in this group achieved extended periods without seizures, which is consistent with the rates reported in this refractory population.32 Unfortunately, there were 3 deaths in this group, with one that met criteria for probable sudden unexpected death in epilepsy.33
Nonlesional extratemporal lobe epilepsy surgery is less successful (29%–56% success rate3,5,11–13) than nonlesional temporal lobe epilepsy surgery (41%–65% success rate2,7–9). Approximately 60% of patients with nonlesional temporal lobe epilepsy are rendered seizure free after an anterior temporal lobectomy.2,8 The efficacy of temporal lobe epilepsy surgery is likely related to the fact that temporal lobe epilepsy most commonly originates from the anteriomesial temporal lobe (anterior temporal neocortex, amygdala, and hippocampus), a network commonly involved in epileptogenesis that is entirely resected in a standard anterior temporal lobectomy.1 The networks involved in epileptogenesis and seizure generation in nonlesional extratemporal lobe epilepsy are less clearly defined, and creating a surgical plan for these patients presents a significant challenge.
The optimal approach to patients with medically resistant NLETE cannot be established from our retrospective study, but the data should be useful for counseling patients about invasive intracranial EEG monitoring, resective surgery, and long-term seizure outcome. From the perspective of the patients ultimately undergoing cortical resection, 38% had excellent outcomes spanning a decade of follow-up. Notably, an additional 3 patients had only nocturnal seizures (Engel class IID), and 6 patients (ie, 25% of patients) had worthwhile improvements in seizure frequency (Engel classes IIIA and IIIB). However, it should be recognized that this is a highly select group of patients undergoing surgery. Only 29% (9 of 31) of patients undergoing long-term intracranial EEG and only 11% (9 of 85) of the original study population were candidates for surgery and had excellent outcomes. The large number of patients who must undergo an extensive and costly evaluation to identify good NLETE surgery candidates is in marked contrast to temporal epilepsy, for which the number needed to treat to achieve an excellent outcome is 2.34
Acknowledgments
Funding/Support: This research was supported by the National Institutes of Health (grant R01-NS63039 to Dr Worrell), the European Regional Development Fund (Project FNUSA-ICRC [registration no. CZ.1.05/1.1.00/02.0123]), and the European Social Fund within the project Young Talent Incubator II (registration no. CZ.1.07/2.3.00/20.0117).
Footnotes
Conflict of Interest Disclosures: None reported.
Author Contributions: Drs Noe, Sulc, and Worrell contributed equally to this article. Study concept and design: Noe, Wetjen, So, Cascino, Marsh, Horinek, and Worrell. Acquisition of data: Noe, Wirrell, Cascino, Meyer, Giannini, Watson, Brinkmann, and Worrell. Analysis and interpretation of data: Noe, Sulc, Wong-Kisiel, Wirrell, Van Gompel, Britton, So, Cascino, Watson, Brinkmann, Stead, and Worrell. Drafting of the manuscript: Sulc, Wong-Kisiel, Wirrell, Van Gompel, Cascino, Brinkmann, and Worrell. Critical revision of the manuscript for important intellectual content: Noe, Wong-Kisiel, Wirrell, Van Gompel, Wetjen, Britton, So, Cascino, Marsh, Meyer, Horinek, Giannini, Watson, Brinkmann, Stead, and Worrell. Statistical analysis: Noe, Sulc, and Worrell. Obtained funding: Horinek and Worrell. Administrative, technical, and material support: So, Meyer, Brinkmann, Stead, and Worrell. Study supervision: Wetjen, So, Cascino, Marsh, Giannini, and Worrell.
Additional Contributions: We thank Karla Crockett and Cindy Nelson for providing technical support.
References
- 1.Engel J., Jr . Principle of epilepsy surgery. In: Shorvon SD, Dreifuss F, Fish DR, editors. The Treatment of Epilepsy. Oxford, England: Blackwell; 1996. pp. 519–529. [Google Scholar]
- 2.Bell ML, Rao S, So EL, et al. Epilepsy surgery outcomes in temporal lobe epilepsy with a normal MRI. Epilepsia. 2009;50(9):2053–2060. doi: 10.1111/j.1528-1167.2009.02079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bien CG, Szinay M, Wagner J, Clusmann H, Becker AJ, Urbach H. Characteristics and surgical outcomes of patients with refractory magnetic resonance imaging-negative epilepsies. Arch Neurol. 2009;66(12):1491–1499. doi: 10.1001/archneurol.2009.283. [DOI] [PubMed] [Google Scholar]
- 4.Cascino GD, Jack CR, Jr, Parisi JE, et al. MRI in the presurgical evaluation of patients with frontal lobe epilepsy and children with temporal lobe epilepsy: pathologic correlation and prognostic importance. Epilepsy Res. 1992;11(1):51–59. doi: 10.1016/0920-1211(92)90021-k. [DOI] [PubMed] [Google Scholar]
- 5.Jeha LE, Najm I, Bingaman W, Dinner D, Widdess-Walsh P, Lüders H. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain. 2007;130(pt 2):574–584. doi: 10.1093/brain/awl364. [DOI] [PubMed] [Google Scholar]
- 6.Téllez-Zenteno JF, Hernández Ronquillo L, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2010;89(2–3):310–318. doi: 10.1016/j.eplepsyres.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Holmes MD, Born DE, Kutsy RL, Wilensky AJ, Ojemann GA, Ojemann LM. Outcome after surgery in patients with refractory temporal lobe epilepsy and normal MRI. Seizure. 2000;9(6):407–411. doi: 10.1053/seiz.2000.0423. [DOI] [PubMed] [Google Scholar]
- 8.Radhakrishnan K, So EL, Silbert PL, et al. Predictors of outcome of anterior temporal lobectomy for intractable epilepsy: a multivariate study. Neurology. 1998;51(2):465–471. doi: 10.1212/wnl.51.2.465. [DOI] [PubMed] [Google Scholar]
- 9.Sylaja PN, Radhakrishnan K, Kesavadas C, Sarma PS. Seizure outcome after anterior temporal lobectomy and its predictors in patients with apparent temporal lobe epilepsy and normal MRI. Epilepsia. 2004;45(7):803–808. doi: 10.1111/j.0013-9580.2004.48503.x. [DOI] [PubMed] [Google Scholar]
- 10.Blume WT, Ganapathy GR, Munoz D, Lee DH. Indices of resective surgery effectiveness for intractable nonlesional focal epilepsy. Epilepsia. 2004;45(1):46–53. doi: 10.1111/j.0013-9580.2004.11203.x. [DOI] [PubMed] [Google Scholar]
- 11.Chapman K, Wyllie E, Najm I, et al. Seizure outcome after epilepsy surgery in patients with normal preoperative MRI. J Neurol Neurosurg Psychiatry. 2005;76(5):710–713. doi: 10.1136/jnnp.2003.026757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosewich RK, So EL, O’Brien TJ, et al. Factors predictive of the outcome of frontal lobe epilepsy surgery. Epilepsia. 2000;41(7):843–849. doi: 10.1111/j.1528-1157.2000.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 13.Smith JR, Lee MR, King DW, et al. Results of lesional vs. nonlesional frontal lobe epilepsy surgery. Stereotact Funct Neurosurg. 1997;69(1–4 pt 2):202–209. doi: 10.1159/000099875. [DOI] [PubMed] [Google Scholar]
- 14.Siegel AM, Jobst BC, Thadani VM, et al. Medically intractable, localization-related epilepsy with normal MRI: presurgical evaluation and surgical outcome in 43 patients. Epilepsia. 2001;42(7):883–888. doi: 10.1046/j.1528-1157.2001.042007883.x. [DOI] [PubMed] [Google Scholar]
- 15.Dorward IG, Titus JB, Limbrick DD, Johnston JM, Bertrand ME, Smyth MD. Extratemporal, nonlesional epilepsy in children: postsurgical clinical and neuro-cognitive outcomes. J Neurosurg Pediatr. 2011;7(2):179–188. doi: 10.3171/2010.11.PEDS10265. [DOI] [PubMed] [Google Scholar]
- 16.Holmes MD, Kutsy RL, Ojemann GA, Wilensky AJ, Ojemann LM. Interictal, unifocal spikes in refractory extratemporal epilepsy predict ictal origin and post-surgical outcome. Clin Neurophysiol. 2000;111(10):1802–1808. doi: 10.1016/s1388-2457(00)00389-8. [DOI] [PubMed] [Google Scholar]
- 17.Park SA, Lim SR, Kim GS, et al. Ictal electrocorticographic findings related with surgical outcomes in nonlesional neocortical epilepsy. Epilepsy Res. 2002;48(3):199–206. doi: 10.1016/s0920-1211(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 18.Worrell GA, So EL, Kazemi J, et al. Focal ictal beta discharge on scalp EEG predicts excellent outcome of frontal lobe epilepsy surgery. Epilepsia. 2002;43 (3):277–282. doi: 10.1046/j.1528-1157.2002.37501.x. [DOI] [PubMed] [Google Scholar]
- 19.Zakaria T, Noe K, So E, et al. Scalp and intracranial EEG in medically intractable extratemporal epilepsy with normal MRI. ISRN Neurol. 2012;2012:942849. doi: 10.5402/2012/942849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Brien TJ, So EL, Mullan BP, et al. Subtraction peri-ictal SPECT is predictive of extratemporal epilepsy surgery outcome. Neurology. 2000;55(11):1668–1677. doi: 10.1212/wnl.55.11.1668. [DOI] [PubMed] [Google Scholar]
- 21.Schiller Y, Cascino GD, Busacker NE, Sharbrough FW. Characterization and comparison of local onset and remote propagated electrographic seizures recorded with intracranial electrodes. Epilepsia. 1998;39(4):380–388. doi: 10.1111/j.1528-1157.1998.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 22.Schiller Y, Cascino GD, Sharbrough FW. Chronic intracranial EEG monitoring for localizing the epileptogenic zone: an electroclinical correlation. Epilepsia. 1998;39(12):1302–1308. doi: 10.1111/j.1528-1157.1998.tb01328.x. [DOI] [PubMed] [Google Scholar]
- 23.Jack CR., Jr Magnetic resonance imaging. Neuroimaging and anatomy. Neuroimaging Clin N Am. 1995;5(4):597–622. [PubMed] [Google Scholar]
- 24.Engel J., Jr . Surgical Treatment of the Epilepsies. New York, NY: Raven Press; 1987. [Google Scholar]
- 25.Engel J., Jr Surgical treatment for epilepsy: too little, too late? JAMA. 2008;300 (21):2548–2550. doi: 10.1001/jama.2008.756. [DOI] [PubMed] [Google Scholar]
- 26.Cascino GD, Kelly PJ, Sharbrough FW, Hulihan JF, Hirschorn KA, Trenerry MR. Long-term follow-up of stereotactic lesionectomy in partial epilepsy: predictive factors and electroencephalographic results. Epilepsia. 1992;33(4):639–644. doi: 10.1111/j.1528-1157.1992.tb02340.x. [DOI] [PubMed] [Google Scholar]
- 27.Ferrier CH, Engelsman J, Alarcón G, Binnie CD, Polkey CE. Prognostic factors in presurgical assessment of frontal lobe epilepsy. J Neurol Neurosurg Psychiatry. 1999;66(3):350–356. doi: 10.1136/jnnp.66.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quesney LF, Constain M, Rasmussen T, Olivier A, Palmini A. Presurgical EEG investigation in frontal lobe epilepsy. Epilepsy Res Suppl. 1992;5:55–69. [PubMed] [Google Scholar]
- 29.Quesney LF, Constain M, Rasmussen T, Stefan H, Olivier A. How large are frontal lobe epileptogenic zones? EEG, ECoG, and SEEG evidence. Adv Neurol. 1992;57:311–323. [PubMed] [Google Scholar]
- 30.Vadlamudi L, So EL, Worrell GA, et al. Factors underlying scalp-EEG interictal epileptiform discharges in intractable frontal lobe epilepsy. Epileptic Disord. 2004;6(2):89–95. [PubMed] [Google Scholar]
- 31.Van Gompel JJ, Worrell GA, Bell ML, et al. Intracranial electroencephalography with subdural grid electrodes: techniques, complications, and outcomes. Neurosurgery. 2008;63(3):498–505. doi: 10.1227/01.NEU.0000324996.37228.F8. discussion 505–506. [DOI] [PubMed] [Google Scholar]
- 32.Callaghan BC, Anand K, Hesdorffer D, Hauser WA, French JA. Likelihood of seizure remission in an adult population with refractory epilepsy. Ann Neurol. 2007;62(4):382–389. doi: 10.1002/ana.21166. [DOI] [PubMed] [Google Scholar]
- 33.Nashef L. Sudden unexpected death in epilepsy: terminology and definitions. Epilepsia. 1997;38(11 suppl):S6–S8. doi: 10.1111/j.1528-1157.1997.tb06130.x. [DOI] [PubMed] [Google Scholar]
- 34.Wiebe S, Blume WT, Girvin JP, Eliasziw M. Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]