Abstract
Synthetic oligonucleotide probes were used to identify a cloned DNA fragment from the cyanobacterium Agmenellum quadruplicatum that contains the genes for the alpha and beta subunits of C-phycocyanin. The coding region for the alpha-subunit gene begins 108 base pairs downstream from the 3' end of the beta-subunit structural gene. The sequences of the coding regions for both genes have been determined as well as 379 base pairs of 5' flanking region, 204 base pairs of 3' flanking region, and the 108 base pairs between the two genes. The site of transcriptional initiation is located approximately equal to 325 base pairs upstream from the beta-subunit gene, and an open reading frame 114 base pairs long is found within this region. The significance of this additional open reading frame is not yet known. The derived amino acid sequences for both C-phycocyanin subunits were compared with other known C-phycocyanin sequences for homology. Homologies between the A. quadruplicatum alpha subunit and alpha subunits from other species were approximately equal to 70%, as were homologies between the A. quadruplicatum beta subunit and other beta subunits. Homologies between the various alpha and beta subunits were 21%-27%. Codon usage for both the C-phycocyanin alpha- and beta-subunit genes shows asymmetries for many amino acids that correspond closely to those seen in highly expressed Escherichia coli genes.
Full text
PDF

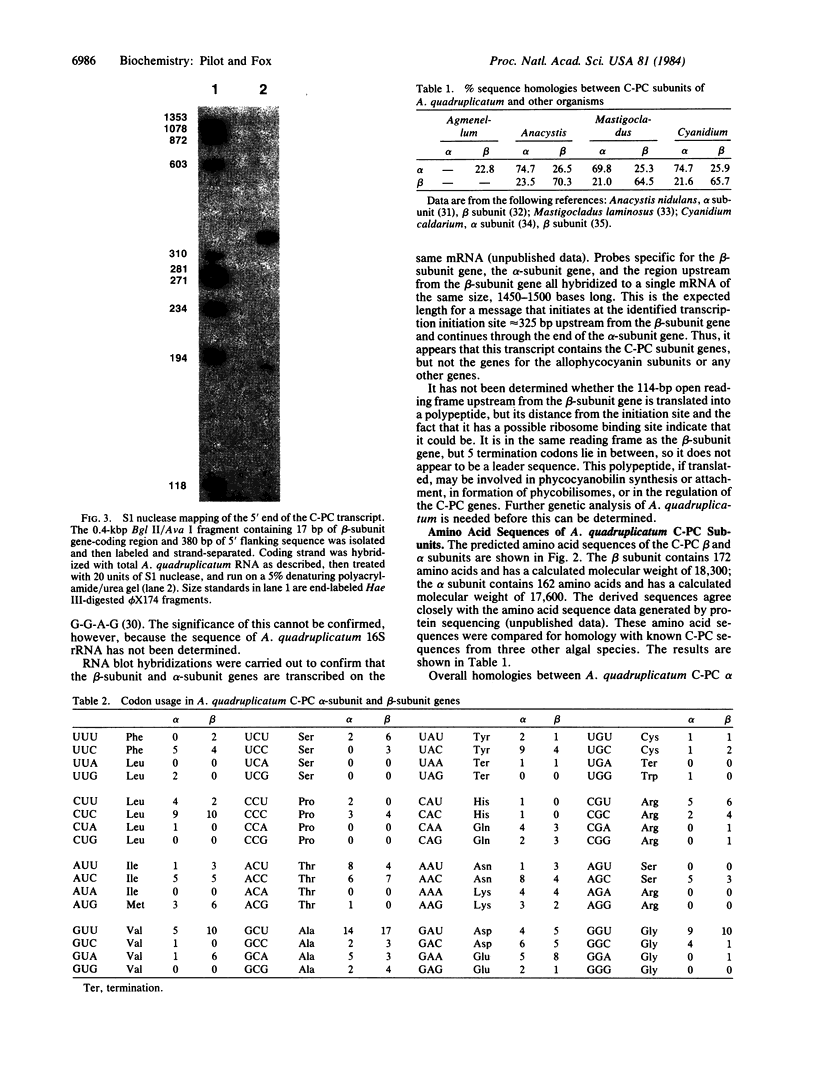

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A., Bogorad L. Properties of subunits and aggregates of blue-green algal biliproteins. Biochemistry. 1971 Sep 14;10(19):3625–3634. doi: 10.1021/bi00795a022. [DOI] [PubMed] [Google Scholar]
- Bryant D. A., Hixson C. S., Glazer A. N. Structural studies on phycobiliproteins III. Comparison of bilin-containing peptides from the beta subunits of C-phycocyanin, R-phycocyanin, and phycoerythrocyanin. J Biol Chem. 1978 Jan 10;253(1):220–225. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cohen J., Cheng M. F. Role of vocalizations in the reproductive cycle of ring doves (Streptopelia risoria): effects of hypoglossal nerve section on the reproductive behavior and physiology of the female. Horm Behav. 1979 Oct;13(2):113–127. doi: 10.1016/0018-506x(79)90051-5. [DOI] [PubMed] [Google Scholar]
- Csatorday K. Fluorescence from sensitizing phycobilin chromophores in the blue-green alga Anacystis nidulans. Biochim Biophys Acta. 1978 Nov 9;504(2):341–343. doi: 10.1016/0005-2728(78)90181-0. [DOI] [PubMed] [Google Scholar]
- Frank G., Sidler W., Widmer H., Zuber H. The complete amino acid sequence of both subunits of C-phycocyanin from the cyanobacterium Mastigocladus laminosus. Hoppe Seylers Z Physiol Chem. 1978 Nov;359(11):1491–1507. doi: 10.1515/bchm2.1978.359.2.1491. [DOI] [PubMed] [Google Scholar]
- Freidenreich P., Apell G. S., Glazer A. N. Structural studies on phycobiliproteins II. C-phycocyanin: amino acid sequence of the beta subunit. Specific cleavage of the alpha subunit. J Biol Chem. 1978 Jan 10;253(1):212–219. [PubMed] [Google Scholar]
- Gardner E. E., Stevens S. E., Jr, Fox J. L. Purification and characterization of the C-phycocyanin from Agmenellum quadruplicatum. Biochim Biophys Acta. 1980 Jul 24;624(1):187–195. doi: 10.1016/0005-2795(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Fang S., Brown D. M. Spectroscopic properties of C-phycocyanin and of its alpha and beta subunits. J Biol Chem. 1973 Aug 25;248(16):5679–5685. [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Gouy M., Gautier C. Codon usage in bacteria: correlation with gene expressivity. Nucleic Acids Res. 1982 Nov 25;10(22):7055–7074. doi: 10.1093/nar/10.22.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley A. C., Butler W. L. Efficiency of energy transfer from photosystem II to photosystem I in Porphyridium cruentum. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3957–3960. doi: 10.1073/pnas.73.11.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundell D. J., Williams R. C., Glazer A. N. Molecular architecture of a light-harvesting antenna. In vitro assembly of the rod substructures of Synechococcus 6301 phycobilisomes. J Biol Chem. 1981 Apr 10;256(7):3580–3592. [PubMed] [Google Scholar]
- MYERS J., KRATZ W. A. Relation between pigment content and photosynthetic characteristics in a blue-green algae. J Gen Physiol. 1955 Sep 20;39(1):11–22. doi: 10.1085/jgp.39.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Myers D. M. Procedure for drying leptospiral antibody on sand and sugar for serological studies in leptospirosis. Appl Microbiol. 1973 Mar;25(3):427–430. doi: 10.1128/am.25.3.427-430.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner G. D., Brown-Mason A. S., Ehrhardt M. M., Troxler R. F. Primary structure of phycocyanin from the unicellular rhodophyte Cyanidium caldarium. I. Complete amino acid sequence of the alpha subunit. J Biol Chem. 1981 Dec 10;256(23):12167–12175. [PubMed] [Google Scholar]
- Porter G., Tredwell C. J., Searle G. F., Barber J. Picosecond time-resolved energy transfer in Porphyridium cruentum. Part I. In the intact alga. Biochim Biophys Acta. 1978 Feb 9;501(2):232–245. doi: 10.1016/0005-2728(78)90029-4. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Troxler R. F., Ehrhardt M. M., Brown-Mason A. S., Offner G. D. Primary structure of phycocyanin from the unicellular rhodophyte Cyanidium caldarium. II. Complete amino acid sequence of the beta subunit. J Biol Chem. 1981 Dec 10;256(23):12176–12184. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams V. P., Glazer A. N. Structural studies on phycobiliproteins. I. Bilin-containing peptides of C-phycocyanin. J Biol Chem. 1978 Jan 10;253(1):202–211. [PubMed] [Google Scholar]