Abstract
Vitamin D and its analogues are widely used as treatments by clinical nephrologists, especially when treating chronic kidney disease (CKD) patients with secondary hyperparathyroidism. As CKD progresses, the ability to compensate for elevations in parathyroid hormone (PTH) and fibroblast growth factor-23 and for decreases in 1,25(OH)2D3 becomes inadequate, which results in hyperphosphatemia, abnormal bone disorders, and extra-skeletal calcification. In addition to its calciotropic effect on the regulation of calcium, phosphate, and parathyroid hormone, vitamin D has many other noncalciotropic effects, including controlling cell differentiation/proliferation and having immunomodulatory effects. There are several immune dysregulations that can be noted when renal function declines. Physicians need to know well both the classical and nonclassical functions of vitamin D. This review is an analysis from the nephrologist's viewpoint and focuses on the relationship between the vitamin D and the immune system, together with vitamin's clinical use to treat kidney diseases.
1. Introduction
Chronic kidney disease (CKD) and end-stage renal disease (ESRD) are diseases that are increasing in the 21st century. Preventing progressive deterioration in renal function and its complications remains the main challenge that nephrology needs to fulfill. CKD is defined according to the glomerular filtration rate (GFR) and/or the presence of pathological damage to the kidneys or the presence of kidney damage markers, such as proteinuria or hematuria, for 3 months [1]. Many complications are found in these patients as the GFR decline; these include fluid overload, anemia, cardiovascular disease, malnutrition, protein energy-wasting, and mineral bone disorders (MBD). In the case of MBD, hyperphosphatemia, hypercalcemia, and hyperparathyroidism contribute to the development of vascular calcification and cardiovascular disease. As CKD progresses, compensation for the elevations in parathyroid hormone (PTH) and fibroblast growth factor-23 (FGF-23) and for reduced levels of 1,25(OH)2D3 becomes inadequate, resulting in hyperphosphatemia, abnormal bone disorders, and extra-skeletal calcification. In the Kidney Disease Outcomes and Quality Initiative (KDOQI) guideline [2] and the Kidney Disease: Improving Global Outcomes (KDIGO) guideline [3], activated vitamin D or its analogues are frequently used to treat patients with secondary hyperparathyroidism and to prevent the renal osteodystrophy. Therefore, how to use vitamin D and its analogues is an important aspect of clinical nephrology.
The classical actions of vitamin D are related to mineral metabolism and skeletal health. Vitamin D regulates blood calcium, phosphate, and parathyroid hormone concentrations by actions targeting the intestines, bone, parathyroid glands, and kidneys. In addition, nonclassical roles for vitamin D, including anticell differentiation and anticell proliferative activity with respect to various cell types, have become more and more important. The anticell differentiation effect has been correlated with cancer epidemiology. Recently, serum vitamin D levels have been found to be inversely associated with many malignancies, including breast cancer [4], head and neck cancer [5], colon cancer [6], prostate cancer [7], and pancreatic cancer [8]. In a systemic review and meta-analysis, it was found that there was a moderate inverse association between 25-hydroxy vitamin D [25(OH)D] concentrations and total cancer incidence and mortality [9]. The antiproliferative properties of vitamin D have been clinically applied to the treatment of psoriasis. Using a vitamin D analogue together with steroid [10] or ultraviolet B (UVB) treatment [11] is useful when treating psoriasis.
In addition to the above, vitamin D has another important role in terms of noncalciotropic activity, its immunomodulatory effect. This immunomodulatory effect is based on the widely expressed vitamin D receptor (VDR) that is present in the immune system. This review will focus on the relationship between the vitamin D and immunity and explore current treatments using vitamin D in the clinical nephrology with the exception of mineral bone disorders.
2. Vitamin D Metabolism and Deficiency in Chronic Kidney Disease
Most people derive the bulk of their vitamin D from the exposure of their skin to UVB light, which is present in sunshine. The process starts with cholesterol in the skin, which is enzymatically converted to 7-dehydrocholesterol and then converted to an unstable compound, previtamin D, by the action of UVB. Nutritional sources, such as fatty fish and some types of mushrooms, also contain major forms of vitamin D, namely, cholecalciferol (vitamin D3) or ergocalciferol (vitamin D2) [12]. These are subsequently activated during a sequential 2-step process that first involves 25-hydroxylation in the liver to produce 25(OH)D and then 1-hydroxylation, which until recently was thought to occur primarily in the kidney, to produce the active product 1,25(OH)2D3 or calcitriol [13–15]. The key enzyme in this process is 1α-hydroxylase (CYP27B1), which is expressed primarily in proximal tubular epithelial cells of the kidney [16]. This enzyme is expressed in other parts of the kidney and in extra-renal tissues and cells as well [17]. An individual's serum 25(OH)D level is widely accepted to determine a person's vitamin D status [13, 18]. The main plasma carrier for vitamin D metabolites is vitamin D-binding protein (VBP) [19]. VBP has the highest affinity for 25(OH)D, and virtually all plasma 25(OH)D is bound to VBP [20]. The 25(OH)D-VBP complex is taken up by the proximal convoluted tubule via an endocytic receptor, megalin. The final step in the vitamin D metabolic pathway is its inactivation, a process catalyzed by 24-hydroxylase (CYP24A1) that catabolizes the conversion of both 1,25(OH)2D3 and 25(OH)D into 1,24,25(OH)3D and ultimately into water-soluble calcitroic acid and the inactive blood metabolite 24,25(OH)2D [21, 22].
In patients with CKD, serum 1,25(OH)2D3 levels decline early in the course of kidney dysfunction, even before any changes in serum calcium or phosphorus concentrations occur and prior to any rise in serum PTH levels [23, 24]. Rising FGF-23 levels may play an even greater role in controlling 1α-hydroxylase activity [25, 26]. Serum values of FGF-23 are regulated by circulating phosphorus levels and values increase as CKD progresses, becoming markedly elevated in individuals with end-stage kidney disease [27]. In patients with CKD, calcitriol levels are inversely related to levels of circulating FGF-23, suggesting that the hormone may play a significant role in mineral metabolism. In total, 70% to 85% patients of CKD have low levels of 25(OH)D [28–30]. As a result of the substrate-dependent process that forms 1,25(OH)2D3, a low 25(OH)D level contributes to vitamin D deficiency [31]. Many other factors may also contribute to vitamin D deficiency, including a lack of sunlight exposure, a low protein diet (lack of vitamin-D rich food), reduced 1α-hydroxylase activity resulting from a reduction in renal mass and tubular dysfunction [32], decreased skin synthesis of 1,25(OH)2D3 in response to sunlight compared with an individual with normal kidney function [13], loss of 25(OH)D-VBP due to heavy proteinuria [33, 34], chronic illness, diabetes [35], and various other unknown factors [36]. On the other hand, an increase in 24-hydroxylase gene expression and an increase in the clearance of 1,25(OH)2D3 with aging have also been reported [37, 38]. These findings suggest that the combined effect of a decline in the ability of the kidney to synthesize 1,25(OH)2D3 and an increase in renal metabolism of 1,25(OH)2D3 may contribute to the high prevalence of vitamin D deficiency among CKD patients.
3. Immune Dysregulation in CKD Patients
CKD patients and ESRD on the replacement therapy patients have significant immune dysregulation as compared with the general population and, subsequently, have a high susceptibility to infection and a high incidence of malignancy, a poorer response to vaccination, and increased levels of cardiovascular disease [39–42]. Uremia and its treatment cause immune alterations in hemodialysis patients [43]. Several factors influence the immunity of these patients, such as uremic toxin, malnutrition, chronic inflammation, vitamin D-parathyroid hormone axis alternation, and therapeutic dialysis [44–46]. Many studies have shown that both the naïve and the acquired immune systems are impaired in these patients. Due to their immunity dysregulation, these patients have more vascular calcification, accelerated atherosclerosis, a loss of appetite, increased insulin resistance, increased muscle catabolism, renal osteodystrophy, and a high prevalence of depression [47, 48]. They also have coexisting chronic immune activation (persisted hypercytokinemia and acute-phase protein response) and chronic immune suppression (a poor vaccination response and a high incidence of infection and malignancy).
Monocytes and monocyte-derived dendritic cells of CKD patients are impaired with respect to endocytosis and maturation [49], while, in parallel, uremia suppresses immune signal-induced CYP27B1 (encoding for 1α-hydroxylase) expression in human monocytes [50]. CKD patients have a lower percentages of peripheral CD4+ T lymphocytes, CD8+ T lymphocytes, and B lymphocytes in the blood [51]. Further, soluble B lymphocyte markers are increased in CKD patients [52], while other studies have also shown that there is an increased incidence of B cell apoptosis in these patients [53]. ESRD patients show increased apoptosis and a diminished populations of naïve and central memory T cells [54], together with impaired antigen-specific memory CD4+ T cells [55]. In dialysis patients, Th1 lymphocytes show decreased expression of the antiapoptotic molecule Bcl-2, which makes the Th1 cells more susceptible to apoptosis [56]. A similar decline in Th1 cell population and the enhancement in Th2 differentiation have also been noted in CKD and dialysis patients [30, 57, 58]. In addition, we have recently shown that Th17 cells are increased in chronic HD patients, whereas Treg cells are decreased (submitted). This Th17/Treg functional imbalance exists in uremic patients and is associated with the development of acute cardiovascular events including myocardial injury and microinflammation [59, 60].
Preactivated monocytes overproduce cytokines such as tumor necrosis factor-α (TNF-α), interleukin- (IL-)1, IL-6, and IL-10 [61, 62]. TNF-α and IL-1 are the major cytokines produced by activation of the Toll-like receptor (TLR) signaling pathway; this is the key receptor that recognizes lipopolysaccharides (LPS) [63]. In addition, IL-6, the proinflammatory cytokine, which has been shown to play a key role in atherosclerosis and protein-energy wasting, is elevated in the CKD patients [64–66]. Serum levels of IL-12 and IL-18 are both increased in CKD patients, and both of them are correlated with the inflammatory process [67, 68]. Moreover, high proinflammatory cytokine (IL-1, IL-6, and TNF-α) levels and low anti-inflammatory cytokine (IL-4, IL-5, and CH50) levels have also been found in hemodialysis patients [69].
In addition to uremic toxin, dialysis-related factors such as bioincompatibility of the hemodialysis dialyzer, the presence of endotoxins in the water, access-related infection, the presence of glucose degradation products in peritoneal dialysis solution, and the presence of advanced glycation end products are important; all of the above are able to induce chronic inflammation and will activate the immune response. Together, these findings indicate that, in general, CKD patients have immune dysregulation that includes both the cellular part and hypercytokinemia (Figure 1).
Figure 1.
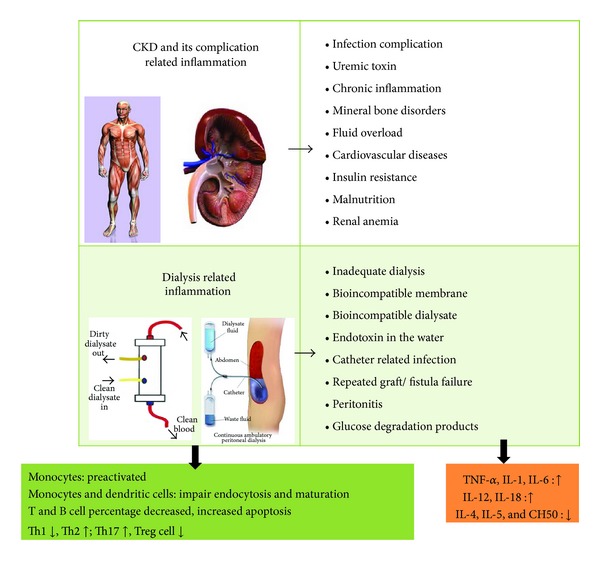
Several factors are related to immune dysregulation when renal function declines and when a patient is on renal replacement therapy.
4. Vitamin D and the Innate Immune System
The innate immune response, which includes natural killer cells, macrophages, and their monocyte precursors, plays a central role in initial responses to pathogenic organisms and/or tissue damage. Their role is to engulf pathogens and cell debris by phagocytosis and then eliminate or assimilate the resulting waste material. The earliest evidence of vitamin D effect on innate immunity came from the treatment of tuberculosis treatment with cod liver oil, which is a major source of vitamin D [70]. The action of vitamin D on macrophages includes the ability to stimulate the differentiation of precursor monocytes into more mature phagocytic macrophages [71–73]. Macrophages have their own 1α-hydroxylase and require sufficient ambient levels of 25(OH)D substrate in order to generate internal 1,25(OH)2D3. Striking evidence of macrophage 1α-hydroxylase activity is found in granulomatous conditions such as tuberculosis, sarcoidosis, and inflammatory bowel disease, where 1,25(OH)2D3 levels may be markedly elevated [74]. In sarcoidosis patients there is increased production of 1,25(OH)2D3 despite hypercalcemia. The disordered calcium homeostasis in sarcoidosis is due to dysregulation of the production of 1,25(OH)2D3 by activated macrophages [75]. Unlike renal 1α-hydroxylase, the 1α-hydroxylase produced by macrophages is not suppressed by elevated calcium or by 1,25(OH)2D3 and is upregulated by immune stimuli such as interferon gamma (IFN-γ) and LPS [76, 77].
Vitamin D, vitamin D receptor, and retinoid X receptor directly activate the transcription of antimicrobial peptides such as defensin β2 and cathelicidin [78–80]. When monocytes are exposed to a pathogen, this will induce 1α-hydroxylase and the vitamin D receptor after the pathogen is recognized by the TLR, which results in production of cathelicidin [81]. This cathelicidin will cleave microbial membranes and is upregulated in response to infections in humans; it acts against bacteria, viruses, and fungi [82–84]. In some critical sepsis patients, significantly lower serum 25(OH)D and cathelicidin levels have been identified [85]. The association between a low level of cathelicidin and death from an infectious cause has also been observed in hemodialysis patients [86]. In addition, our previous study also indicated that the presence of the C allele of −1237T/C in the TLR-9 gene increases susceptibility towards development of ESRD. Thus, patients with this functional TLR-9 promoter polymorphism had a higher mean plasma IL-6 level than those carrying −1237TT [87]. In macrophages, vitamin D suppresses nuclear factor- (NF-)κB activity by upregulating expression of IκB through stabilization of IκB-mRNA and a reduction in its phosphorylation [88, 89]. Decreased macrophage function under conditions of vitamin D deficiency has been noted in sera from patients who are vitamin-D deficient; this resulted in a lower bactericidal response compared to vitamin-D replete individuals [85]. Although vitamin D has an antimicrobial effect, it also provides feedback regulation of the immune activation pathways. 1,25(OH)2D3 has been shown to potently downregulate expression of monocytes TLR2 and TLR4, thereby suppressing inflammatory responses that are normally activated by these receptors [90].
Apart from macrophages/monocytes, some other antigen presenting cells, such as dendritic cells (DCs), also express VDR and the vitamin D metabolizing enzymes, 1α-hydroxylase and 24-hydroxylase. Vitamin D may have an important role in promoting dendritic cell tolerogenicity via alterations in their function and morphology [91, 92]. In the presence of 1,25(OH)2D3, DCs exhibit reduced expression of major histocompatibility complex (MHC) class II molecules and various adhesion molecules (CD40, CD80, and CD86) [93–95]. This leads to reduced antigen presentation that is accompanied by a lower IL-12 secretion but an increased production of the tolerogenic IL-10; this then promotes development of Th2 lymphocyte differentiation [91]. Therefore, vitamin D inhibits the maturation and differentiation of dendritic cells; thus it might be expected that treatment with vitamin D or its analogues may reduce the immune response. Overall, 1,25(OH)2D3 is able to enhance the innate antibacterial defense capacity and create a more tolerogenic profile toward autoimmune phenomena (Figure 2).
Figure 2.
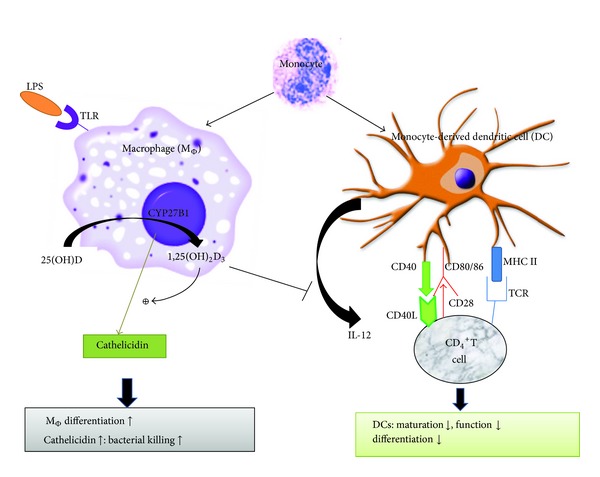
Vitamin D and innate immune system. 1,25(OH)2D3 promotes innate immunity when macrophage (MΦ) is activated by TLRs; CYP27B1 is induced enabling the macrophage to produce 1,25(OH)2D3, which subsequently gives rise to cathelicidin. On the other hand, 1,25(OH)2D3 inhibits the expression of costimulatory molecules (DC40, CD80/86) and major histocompatibility complex class II (MHC II) on the surface of monocyte-derived dendritic cell (DC) and inhibits the production of inflammatory cytokines, such as interleukin-12 (IL-12).
5. Vitamin D and the Adaptive Immune System
Early studies demonstrated that there is expression of VDR in both T and B cells [96]. VDR expression by these cells is very low in resting conditions, but upon activation and proliferation, T cells and B cells upregulate VDR expression significantly, which influences the differentiation and proliferation of these cells [12]. Vitamin D exerts an inhibitory action on this area of the adaptive immune system.
In the T cells, 1,25(OH)2D3 plays an important role in proliferation and differentiation. Currently, four potential mechanisms by which vitamin D influences T cell function have been proposed. These are, firstly, direct endocrine effects via systemic 1,25(OH)2D3, secondly, direct intracrine conversion of 25(OH)D to 1,25(OH)2D3 by T cells itself, thirdly, direct paracrine effects following conversion of 25(OH)D to 1,25(OH)2D3 by local monocytes or dendritic cells, and finally, an indirect effect on antigen presentation to T cells which is mediated via localized APC and is affected by calcitriol [97]. Vitamin D promotes a T cell shift from Th1 to Th2, which might help to limit potential tissue damage associated with Th1 cellular immune responses. Treatment of T cells with calcitriol or analogues inhibits the secretion of the proinflammatory Th1 (IL-2, IFN-γ, and TNF-α), Th9 (IL-9), and Th22 (IL-22) cytokines [98–101] but promotes the production of more anti-inflammatory Th2 cytokines (IL-3, IL-4, IL-5, and IL-10) [30, 102, 103]. Active vitamin D can modulate Th2-cell responses both indirectly, through suppression of IFN-γ and IL-2 in Th1 cells, and directly by influencing expression of Th2 cytokines such as IL-4.
1,25(OH)2D3 reduces expression of IL-17 [104]. IL-17-producing Th17 cells play a crucial role in the induction of autoimmune disease and inflammation [105]. T cell exposed to 1,25(OH)2D3 produced significantly decreased levels of IL-17, IFN-γ, and IL-21 and has significantly increased expression of genes typical for regulatory T cells (Tregs) [3]. The Treg cells have an anti-inflammatory role and control autoimmune diseases by releasing IL-10 and TGF-β [106]; in addition, Treg cells are able to be induced and stimulated by 1,25(OH)2D3 though an indirect pathway, via APCs and DCs, or through a direct pathway, via an endocrine effect or the intracrine conversion of 25(OH)D to 1,25(OH)2D3 by Treg cells themselves [107–109]. Thus, 1,25(OH)2D3 exerts a broad range of effects on inflammation and autoimmune disease by reducing Th17 cell numbers and by having effects that are beneficial in terms of autoimmune and host-graft rejection; these events occur by enhancing Treg cell numbers. However, the regulation of T cells may come at a price because it leads to a decreased response to pathogens and to a reduction in immune surveillance. 1,25(OH)2D3 is able to significantly alter the behavior of the T cells, favoring the development of tolerance via an increase in Th2 and Treg cell activity and a reduction in proinflammatory Th1 and Th17 cell activity (Figure 3).
Figure 3.
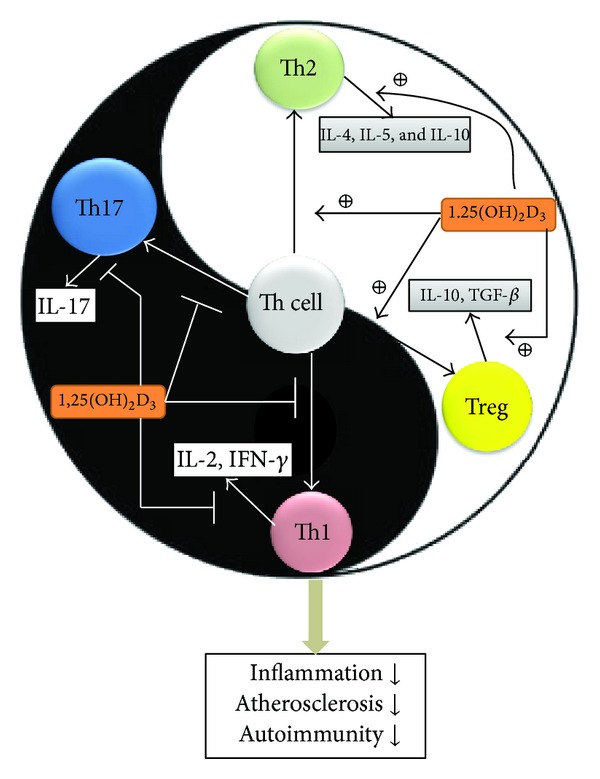
Vitamin D and adaptive immune system. The adaptive immune system is like Tai-Chi, namely, that it separates the Yin and the Yang. 1,25(OH)2D3 directly modulates T cell responses and polarization related to the inflammatory molecules Th1 and Th17 in order to give rise to protective Th2 and Treg cells. In addition, 1,25(OH)2D3 also inhibits the inflammatory Th1 and Th17 cytokines and upregulates the protective Th2 and Treg cytokines. When these effects are integrated, the adaptive immune system may produce lower levels of inflammation, atherosclerosis, and autoimmunity.
VDR is also expressed in inactivated B cells [110]. In B cells, 1,25(OH)2D3 plays an antiproliferative role involving an inhibition of cell differentiation, an inhibition of cell proliferation, reduced initiation of apoptosis, and decreased immunoglobulin production. These effects are probably indirectly mediated by T cells [111, 112]. This control of B cell activation and proliferation is important in autoimmune diseases due to the fact that B cells producing autoantibodies that play a major role in the pathophysiology of autoimmune disease, such as systemic lupus nephritis, type 1 diabetes, inflammatory bowel disease, and multiple sclerosis.
6. Vitamin D Alters Immunity in Clinical Nephrology Patients
The calciotropic action of vitamin D is its major use in clinical nephrology. As CKD progresses, compensation for the elevations of PTH and FGF-23 as well as the decreased levels of 1,25(OH)2D3 becomes inadequate, resulting in hyperphosphatemia, hypocalcemia, abnormal bone disorders, and extra-skeletal calcification. Recent studies have unraveled some of the complications that are present in ESRD patients, including anemia [113, 114], lipid and insulin abnormalities, cardiovascular risk [115, 116], and overall mortality [117–119]; these are able to be improved by correcting for the patient's vitamin D deficiency. 1,25(OH)2D3 has been proven to have an antiproteinuric effect and to interfere with the renin-angiotensin-aldosterone system (RAAS). 1,25(OH)2D3 is able to maintain the structural and functional integrity of podocytes [120–123] and also suppresses directly renin expression at the transcriptional level [124–126]. Studies have shown that a combination of vitamin D or its analogue with RAAS blockade agents is able to ameliorate renal fibrosis [127]. The renoprotective effects of vitamin D and its analogues include suppression of the RAAS and a reduction in proteinuria; these may occur either directly through the protection of podocytes or via negative regulation of the RAAS. The anti-inflammatory properties of 1,25(OH)2D3 may be attributed to a suppression of the NF-κB pathway. The NF-κB pathway plays an important role in the progression of renal disease because it promotes both inflammation and fibrogenesis via regulation of various inflammatory cytokines (MCP-1, TNF-α, and PAI-1) [128].
Many studies have focused on treatment with 1,25(OH)2D3 to alter immune function in CKD and ESRD patients. 1,25(OH)2D3, when used in HD patients with secondary hyperparathyroidism, is able to enhance Th2 cell differentiation [30] and decrease IL-6 expression [129]. It also can attenuate inflammatory and oxidative stress in HD patients [130]. Among dialysis patients, a low serum level of 25(OH)D is correlated with a high panel of reactive T cell values, which means that vitamin D deficiency is related to a poor posttransplant outcome [131]. When there is acute kidney injury, vitamin D deficiency seems to predispose individuals towards an increased risk of sepsis, endothelial dysfunction and also prevents the healing of renal ischemia-reperfusion injury via the TLR, NF-κB, and the RAAS pathway [132]. In HD patients, treatment with vitamin D and its analogues is able to reduce platelet activating factor/thrombin activity and metabolism as well as lower serum IL-6, IL-8, IL-1β, and TNF-α levels, all of which are inflammatory markers [133, 134]. In terms of its noncalciotropic effects, 1,25(OH)2D3 is able to significantly alter the behavior of T cells; this favors the development of tolerance and a reduction in proinflammatory activity, while at the same time ameliorating renal fibrosis and slowing down the development of proteinuria (Figure 4).
Figure 4.
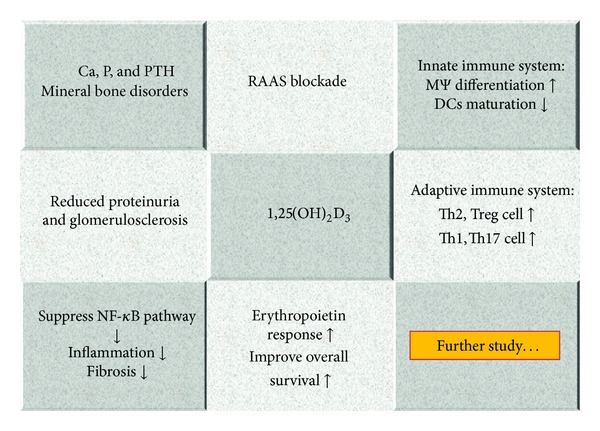
Overview of biological functions of vitamin D in clinical nephrology.
7. Conclusions
As nephrologists, we are continually looking for ways to improve the immune system of patients and patient outcome. The broad tissue distribution of 1α-hydroxylase and vitamin D receptor has established a role for 1,25(OH)2D3 in the pathophysiology of many diseases and this has provided a therapeutic role for the 1,25(OH)2D3. Growing evidence indicates that the usefulness of vitamin D extends beyond its classical role in maintenance of mineral homeostasis and, in this context, the present use of active vitamin D includes the treatment of secondary hyperparathyroidism in CKD. Moreover, vitamin D deficiency is common among CKD patients and in fact may contribute to deterioration in their kidney function. In addition to the traditional supplementation of CKD patients with 1,25(OH)2D3, it is possible that, by assessing and reducing any 25(OH)D deficiency and treating secondary hyperparathyroidism, physicians may be able to adequately fuel both the renal and extra-renal pathways of 1,25(OH)2D3 synthesis. This will maintain both the classical and nonclassical functions of vitamin D and ultimately influence the clinical outcomes of this high-risk group of patients.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American Journal of Kidney Diseases. 2002;39(2):S1–S266. [PubMed] [Google Scholar]
- 2.Uhlig K, Berns JS, Kestenbaum B, et al. KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the diagnosis, evaluation, and treatment of CKD-mineral and bone disorder (CKD-MBD) American Journal of Kidney Diseases. 2010;55(5):773–799. doi: 10.1053/j.ajkd.2010.02.340. [DOI] [PubMed] [Google Scholar]
- 3.Jeffery LE, Burke F, Mura M, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. Journal of Immunology. 2009;183(9):5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knight JA, Lesosky M, Barnett H, Raboud JM, Vieth R. Vitamin D and reduced risk of breast cancer: a population-based case-control study. Cancer Epidemiology Biomarkers and Prevention. 2007;16(3):422–429. doi: 10.1158/1055-9965.EPI-06-0865. [DOI] [PubMed] [Google Scholar]
- 5.Chiang KC, Yeh CN, Hsu JT, et al. MART-10, a novel vitamin D analog, inhibits head and neck squamous carcinoma cells growth through cell cycle arrest at G0/G1 with Upregulation of p21 and p27 and Downregulation of Telomerase. The Journal of Steroid Biochemistry and Molecular Biology. 2013;138:427–434. doi: 10.1016/j.jsbmb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Wu K, Feskanich D, Fuchs CS, Willett WC, Hollis BW, Giovannucci EL. A nested case-control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. Journal of the National Cancer Institute. 2007;99(14):1120–1129. doi: 10.1093/jnci/djm038. [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E. Strengths and limitations of current epidemiologic studies: vitamin D as a modifier of colon and prostate cancer risk. Nutrition reviews. 2007;65(8):S77–S79. doi: 10.1111/j.1753-4887.2007.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 8.Skinner HG, Michaud DS, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Vitamin D intake and the risk for pancreatic cancer in two cohort studies. Cancer Epidemiology Biomarkers and Prevention. 2006;15(9):1688–1695. doi: 10.1158/1055-9965.EPI-06-0206. [DOI] [PubMed] [Google Scholar]
- 9.Yin L, Mena JM, Chen T, Schottker B, Arndt V, Brenner H. Circulating 25-hydroxyvitamin D serum concentration and total cancer incidence and mortality: a systematic review and meta-analysis. Preventive Medicine. 2013;57(6):753–764. doi: 10.1016/j.ypmed.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 10.Segaert S, Ropke M. The biological rationale for use of vitamin d analogs in combination with corticosteroids for the topical treatment of plaque psoriasis. Journal of Drugs in Dermatology. 2013;12(8):e129–e137. [PubMed] [Google Scholar]
- 11.Ala-Houhala MJ, Karppinen T, Vahavihu K, et al. Narrow-band ultraviolet B treatment boosts serum 25-hydroxyvitamin D in patients with psoriasis on oral vitamin D supplementation. Acta Dermato-Venereologica. 2013 doi: 10.2340/00015555-1685. [DOI] [PubMed] [Google Scholar]
- 12.Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5(7):2502–2521. doi: 10.3390/nu5072502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holick MF. Vitamin D deficiency. The New England Journal of Medicine. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 14.Heaney RP. Vitamin D in health and disease. Clinical Journal of the American Society of Nephrology. 2008;3(5):1535–1541. doi: 10.2215/CJN.01160308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Badr W, Martin KJ. Vitamin D and kidney disease. Clinical Journal of the American Society of Nephrology. 2008;3(5):1555–1560. doi: 10.2215/CJN.01150308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zehnder D, Bland R, Walker EA, et al. Expression of 25-hydroxyvitamin D3-1α-hydroxylase in the human kidney. Journal of the American Society of Nephrology. 1999;10(12):2465–2473. doi: 10.1681/ASN.V10122465. [DOI] [PubMed] [Google Scholar]
- 17.Hewison M, Zehnder D, Chakraverty R, Adams JS. Vitamin D and barrier function: a novel role for extra-renal 1α-hydroxylase. Molecular and Cellular Endocrinology. 2004;215(1-2):31–38. doi: 10.1016/j.mce.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Dietary Reference Intakes for Calcium. Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC, USA: National Academy of Sciences; 1997. [PubMed] [Google Scholar]
- 19.Daiger SP, Schanfield MS, Cavalli Sforza LL. Group specific component (Gc) proteins bind vitamin D and 25 hydroxyvitamin D. Proceedings of the National Academy of Sciences of the United States of America. 1975;72(6):2076–2080. doi: 10.1073/pnas.72.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haddad JG. Plasma vitamin D-binding protein (Gc-globulin): multiple tasks. Journal of Steroid Biochemistry and Molecular Biology. 1995;53(1-6):579–582. doi: 10.1016/0960-0760(95)00104-8. [DOI] [PubMed] [Google Scholar]
- 21.Chen K-S, DeLuca HF. Cloning of the human 1α,25-dihydroxyvitamin D-3 24-hydroxylase gene promoter and identification of two vitamin D-responsive elements. Biochimica et Biophysica Acta. 1995;1263(1):1–9. doi: 10.1016/0167-4781(95)00060-t. [DOI] [PubMed] [Google Scholar]
- 22.Ohyama Y, Ozono K, Uchida M, et al. Identification of a vitamin D-responsive element in the 5’-flanking region of the rat 25-hydroxyvitamin D3 24-hydroxylase gene. The Journal of Biological Chemistry. 1994;269(14):10545–10550. [PubMed] [Google Scholar]
- 23.Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney International. 2007;71(1):31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 24.Martinez I, Saracho R, Montenegro J, Llach F. A deficit of calcitriol synthesis may not be the initial factor in the pathogenesis of secondary hyperparathyroidism. Nephrology Dialysis Transplantation. 1996;11(3):22–28. doi: 10.1093/ndt/11.supp3.22. [DOI] [PubMed] [Google Scholar]
- 25.White KE, Evans WE, O’Riordan JLH, et al. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nature Genetics. 2000;26(3):345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 26.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. Journal of the American Society of Nephrology. 2005;16(7):2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 27.Larsson T, Nisbeth U, Ljunggren O, Jüppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney International. 2003;64(6):2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 28.González EA, Sachdeva A, Oliver DA, Martin KJ. Vitamin D insufficiency and deficiency in chronic kidney disease: a single center observational study. American Journal of Nephrology. 2004;24(5):503–510. doi: 10.1159/000081023. [DOI] [PubMed] [Google Scholar]
- 29.LaClair RE, Hellman RN, Karp SL, et al. Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States. American Journal of Kidney Diseases. 2005;45(6):1026–1033. doi: 10.1053/j.ajkd.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Lang CL, Wang MH, Hung KY, Chiang CK, Lu KC. Altered molecular repertoire of immune system by renal dysfunction in the elderly: is prediction and targeted prevention in the horizon? EPMA Journal. 2013;4(1):p. 17. doi: 10.1186/1878-5085-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zehnder D, Landray MJ, Wheeler DC, et al. Cross-sectional analysis of abnormalities of mineral homeostasis, vitamin D and parathyroid hormone in a cohort of pre-dialysis patients: the Chronic Renal Impairment in Birmingham (CRIB) study. Nephron. 2007;107(3):c109–c116. doi: 10.1159/000108652. [DOI] [PubMed] [Google Scholar]
- 32.Andress DL. Vitamin D in chronic kidney disease: a systemic role for selective vitamin D receptor activation. Kidney International. 2006;69(1):33–43. doi: 10.1038/sj.ki.5000045. [DOI] [PubMed] [Google Scholar]
- 33.Koenig KG, Lindberg JS, Zerwekh JE, Padalino PK, Cushner HM, Copley JB. Free and total 1,25-dihydroxyvitamin D levels in subjects with renal disease. Kidney International. 1992;41(1):161–165. doi: 10.1038/ki.1992.22. [DOI] [PubMed] [Google Scholar]
- 34.Saha H. Calcium and vitamin D homeostasis in patients with heavy proteinuria. Clinical Nephrology. 1994;41(5):290–296. [PubMed] [Google Scholar]
- 35.Mattila C, Knekt P, Männistö S, et al. Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care. 2007;30(10):2569–2570. doi: 10.2337/dc07-0292. [DOI] [PubMed] [Google Scholar]
- 36.Qazi RA, Martin KJ. Vitamin D in kidney disease: pathophysiology and the utility of treatment. Endocrinology and Metabolism Clinics of North America. 2010;39(2):355–363. doi: 10.1016/j.ecl.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Armbrecht HJ, Zenser TV, Davis BB. Effect of age on the conversion of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 by kidney of rat. The Journal of Clinical Investigation. 1980;66(5):1118–1123. doi: 10.1172/JCI109941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matkovits T, Christakos S. Variable in vivo regulation of rat vitamin D-dependent genes (osteopontin, Ca,Mg-adenosine triphosphatase, and 25-hydroxyvitamin D3 24-hydroxylase): implications for differing mechanisms of regulation and involvement of multiple factors. Endocrinology. 1995;136(9):3971–3982. doi: 10.1210/endo.136.9.7649106. [DOI] [PubMed] [Google Scholar]
- 39.Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I. Disturbances of acquired immunity in hemodialysis patients. Seminars in Dialysis. 2007;20(5):440–451. doi: 10.1111/j.1525-139X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 40.Fleming SJ, Moran DM, Cooksley WGE, Faoagali JL. Poor response to a recombinant hepatitis B vaccine in dialysis patients. Journal of Infection. 1991;22(3):251–257. doi: 10.1016/s0163-4453(05)80007-6. [DOI] [PubMed] [Google Scholar]
- 41.Kreft B, Klouche M, Kreft R, Kirchner H, Sack K. Low efficiency of active immunization against diphtheria in chronic hemodialysis patients. Kidney International. 1997;52(1):212–216. doi: 10.1038/ki.1997.322. [DOI] [PubMed] [Google Scholar]
- 42.Matas AJ, Simmons RL, Kjellstrand CM, Buselmeier TJ, Najarian JS. Increased incidence of malignancy during chronic renal failure. The Lancet. 1975;1(7912):883–886. doi: 10.1016/s0140-6736(75)91684-0. [DOI] [PubMed] [Google Scholar]
- 43.Girndt M, Sester U, Sester M, Kaul H, Kohler H. Impaired cellular immune function in patients with end-stage renal failure. Nephrology Dialysis Transplantation. 1999;14(12):2807–2810. doi: 10.1093/ndt/14.12.2807. [DOI] [PubMed] [Google Scholar]
- 44.Sterling KA, Eftekhari P, Girndt M, Kimmel PL, Raj DS. The immunoregulatory function of vitamin D: implications in chronic kidney disease. Nature Reviews Nephrology. 2012;8(7):403–412. doi: 10.1038/nrneph.2012.93. [DOI] [PubMed] [Google Scholar]
- 45.Tzanno-Martins C, Futata E, Jorgetti V, Duarte AJS. Restoration of impaired T-cell proliferation after parathyroidectomy in hemodialysis patients. Nephron. 2000;84(3):224–227. doi: 10.1159/000045581. [DOI] [PubMed] [Google Scholar]
- 46.Vanholder R, De Smet R, Hsu C, Vogeleere P, Ringoir S. Uremic toxicity: the middle molecule hypothesis revisited. Seminars in Nephrology. 1994;14(3):205–218. [PubMed] [Google Scholar]
- 47.Sung CC, Liao MT, Lu KC, Wu CC. Role of vitamin D in insulin resistance. Journal of Biomedicine and Biotechnology. 2012;2012 doi: 10.1155/2012/634195.634195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hung K-C, Wu C-C, Chen H-S, et al. Serum IL-6, albumin and co-morbidities are closely correlated with symptoms of depression in patients on maintenance haemodialysis. Nephrology Dialysis Transplantation. 2011;26(2):658–664. doi: 10.1093/ndt/gfq411. [DOI] [PubMed] [Google Scholar]
- 49.Lim WH, Kireta S, Leedham E, Russ GR, Coates PT. Uremia impairs monocyte and monocyte-derived dendritic cell function in hemodialysis patients. Kidney International. 2007;72(9):1138–1148. doi: 10.1038/sj.ki.5002425. [DOI] [PubMed] [Google Scholar]
- 50.Viaene L, Evenepoel P, Meijers B, Vanderschueren D, Overbergh L, Mathieu C. Uremia suppresses immune signal-induced CYP27B1 expression in human monocytes. American Journal of Nephrology. 2012;36(6):497–508. doi: 10.1159/000345146. [DOI] [PubMed] [Google Scholar]
- 51.Lisowska KA, Debska-Ślizień A, Jasiulewicz A, Heleniak Z, Bryl E, Witkowski JM. Hemodialysis affects phenotype and proliferation of CD4-positive T lymphocytes. Journal of Clinical Immunology. 2012;32(1):189–200. doi: 10.1007/s10875-011-9603-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Descamps-Latscha B, Herbelin A, Anh Thu Nguyen ATN, et al. Balance between IL-1β, TNF-α, and their specific inhibitors in chronic renal failure and maintenance dialysis: relationships with activation markers of T cells, B cells, and monocytes. Journal of Immunology. 1995;154(2):882–892. [PubMed] [Google Scholar]
- 53.Fernández-Fresnedo G, Ramos MA, González-Pardo MC, De Francisco ALM, López-Hoyos M, Arias M. B lymphopenia in uraemia is related to an accelerated in vitro apoptosis and dysregulation of Bcl-2. Nephrology Dialysis Transplantation. 2000;15(4):502–510. doi: 10.1093/ndt/15.4.502. [DOI] [PubMed] [Google Scholar]
- 54.Yoon J-W, Gollapudi S, Pahl MV, Vaziri ND. Naïve and central memory T-cell lymphopenia in end-stage renal disease. Kidney International. 2006;70(2):371–376. doi: 10.1038/sj.ki.5001550. [DOI] [PubMed] [Google Scholar]
- 55.Litjens NHR, Huisman M, Van Den Dorpel M, Betjes MGH. Impaired immune responses and antigen-specific memory CD4+ T cells in hemodialysis patients. Journal of the American Society of Nephrology. 2008;19(8):1483–1490. doi: 10.1681/ASN.2007090971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Effros RB. Replicative senescence of CD8 T cells: effect on human ageing. Experimental Gerontology. 2004;39(4):517–524. doi: 10.1016/j.exger.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 57.Böhler T, Canivet C, Nguyen PNL, et al. Cytokines correlate with age in healthy volunteers, dialysis patients and kidney-transplant patients. Cytokine. 2009;45(3):169–173. doi: 10.1016/j.cyto.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 58.Libetta C, Rampino T, Dal Canton A. Polarization of T-helper lymphocytes toward the Th2 phenotype in uremic patients. American Journal of Kidney Diseases. 2001;38(2):286–295. doi: 10.1053/ajkd.2001.26092. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, Hua G, Zhang X, Tong R, Du X, Li Z. Regulatory T cells/T-helper cell 17 functional imbalance in uraemic patients on maintenance haemodialysis: a pivotal link between microinflammation and adverse cardiovascular events. Nephrology. 2010;15(1):33–41. doi: 10.1111/j.1440-1797.2009.01172.x. [DOI] [PubMed] [Google Scholar]
- 60.Chen D, Huang X, Yang M, Gan H, Gunawan EJ, Tang W. Treg/Th17 functional disequilibrium in Chinese uremia on hemodialysis: a link between calcification and cardiovascular disease. Renal Failure. 2012;34(6):697–702. doi: 10.3109/0886022X.2012.672155. [DOI] [PubMed] [Google Scholar]
- 61.Schindler R, Linnenweber S, Schulze M, et al. Gene expression of interleukin-1β during hemodialysis. Kidney International. 1993;43(3):712–721. doi: 10.1038/ki.1993.102. [DOI] [PubMed] [Google Scholar]
- 62.Girndt M, Kohler H, Schiedhelm-Weick E, Schlaak JF, Meyer Zum Buschenfelde K-H, Fleischer B. Production of interleukin-6, tumor necrosis factor α and interleukin-10 in vitro correlates with the clinical immune defect in chronic hemodialysis patients. Kidney International. 1995;47(2):559–565. doi: 10.1038/ki.1995.70. [DOI] [PubMed] [Google Scholar]
- 63.Beg AA. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends in Immunology. 2002;23(11):509–512. doi: 10.1016/s1471-4906(02)02317-7. [DOI] [PubMed] [Google Scholar]
- 64.Stenvinkel P, Barany P, Heimburger O, Pecoits-Filho R, Lindholm B. Mortality, malnutrition, and atherosclerosis in ESRD: what is the role of interleukin-6? Kidney International, Supplement. 2002;61(80):S103–S108. doi: 10.1046/j.1523-1755.61.s80.19.x. [DOI] [PubMed] [Google Scholar]
- 65.Honda H, Qureshi AR, Heimburger O, et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. American Journal of Kidney Diseases. 2006;47(1):139–148. doi: 10.1053/j.ajkd.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 66.Pachaly MA, Do Nascimento MM, Suliman ME, et al. Interleukin-6 is a better predictor of mortality as compared to C-reactive protein, homocysteine, pentosidine and advanced oxidation protein products in hemodialysis patients. Blood Purification. 2008;26(2):204–210. doi: 10.1159/000117438. [DOI] [PubMed] [Google Scholar]
- 67.Chiang C-K, Hsu S-P, Pai M-F, et al. Plasma interleukin-18 levels in chronic renal failure and continuous ambulatory peritoneal dialysis. Blood Purification. 2005;23(2):144–148. doi: 10.1159/000083620. [DOI] [PubMed] [Google Scholar]
- 68.Ishizuka T, Nitta K, Yokoyama T, et al. Increased serum levels of interleukin-12 may be associated with Th1 differentiation in hemodialysis patients. Nephron. 2002;90(4):503–504. doi: 10.1159/000054742. [DOI] [PubMed] [Google Scholar]
- 69.Cohen SD, Phillips TM, Khetpal P, Kimmel PL. Cytokine patterns and survival in haemodialysis patients. Nephrology Dialysis Transplantation. 2010;25(4):1239–1243. doi: 10.1093/ndt/gfp625. [DOI] [PubMed] [Google Scholar]
- 70.Grad R. Cod and the consumptive: a brief history of cod-liver oil in the treatment of pulmonary tuberculosis. Pharmacy in history. 2004;46(3):106–120. [PubMed] [Google Scholar]
- 71.Abe E, Miyaura C, Tanaka H, et al. 1 alpha,25-dihydroxyvitamin D3 promotes fusion of mouse alveolar macrophages both by a direct mechanism and by a spleen cell-mediated indirect mechanism. Proceedings of the National Academy of Sciences of the United States of America. 1983;80(18):5583–5587. doi: 10.1073/pnas.80.18.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanaka H, Abe E, Miyaura C. 1α,25-Dihydroxyvitamin D3 induces differentiation of human promyelocytic leukemia cells (HL-60) into monocyte-macrophages but not into granulocytes. Biochemical and Biophysical Research Communications. 1983;117(1):86–92. doi: 10.1016/0006-291x(83)91544-9. [DOI] [PubMed] [Google Scholar]
- 73.Koeffler HP, Amatruda T, Ikekawa N. Induction of macrophage differentiation of human normal and leukemic myeloid stem cells by 1,25-dihydroxyvitamin D3 and its fluorinated analogues. Cancer Research. 1984;44(12 I):5624–5628. [PubMed] [Google Scholar]
- 74.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clinic Proceedings. 2006;81(3):353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 75.Sharma OP. Hypercalcemia in granulomatous disorders: a clinical review. Current Opinion in Pulmonary Medicine. 2000;6(5):442–447. doi: 10.1097/00063198-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 76.Stoffels K, Overbergh L, Bouillon R, Mathieu C. Immune regulation of 1α-hydroxylase in murine peritoneal macrophages: unravelling the IFNγ pathway. Journal of Steroid Biochemistry and Molecular Biology. 2007;103(3-5):567–571. doi: 10.1016/j.jsbmb.2006.12.091. [DOI] [PubMed] [Google Scholar]
- 77.Esteban L, Vidal M, Dusso A. 1α-Hydroxylase transactivation by γ-interferon in murine macrophages requires enhanced C/EBPβ expression and activation. Journal of Steroid Biochemistry and Molecular Biology. 2004;89-90:131–137. doi: 10.1016/j.jsbmb.2004.03.092. [DOI] [PubMed] [Google Scholar]
- 78.Wang T-T, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-Dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. Journal of Immunology. 2004;173(5):2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 79.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB Journal. 2005;19(9):1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 80.White JH. Vitamin D metabolism and signaling in the immune system. Reviews in Endocrine and Metabolic Disorders. 2012;13(1):21–29. doi: 10.1007/s11154-011-9195-z. [DOI] [PubMed] [Google Scholar]
- 81.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 82.Ramanathan B, Davis EG, Ross CR, Blecha F. Cathelicidins: microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes and Infection. 2002;4(3):361–372. doi: 10.1016/s1286-4579(02)01549-6. [DOI] [PubMed] [Google Scholar]
- 83.Hewison M. Antibacterial effects of vitamin D. Nature Reviews Endocrinology. 2011;7(6):337–345. doi: 10.1038/nrendo.2010.226. [DOI] [PubMed] [Google Scholar]
- 84.Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. Journal of Clinical Virology. 2011;50(3):194–200. doi: 10.1016/j.jcv.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jeng L, Yamshchikov AV, Judd SE, et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. Journal of Translational Medicine. 2009;7, article 28 doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gombart AF, Bhan I, Borregaard N, et al. Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clinical Infectious Diseases. 2009;48(4):418–424. doi: 10.1086/596314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang HY, Lu KC, Lee HS, et al. Role of the functional Toll-Like receptor-9 promoter polymorphism (-1237T/C) in increased risk of end-stage renal disease: a case-control study. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0058444.e58444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cohen-Lahav M, Shany S, Tobvin D, Chaimovitz C, Douvdevani A. Vitamin D decreases NFκB activity by increasing IκBα levels. Nephrology Dialysis Transplantation. 2006;21(4):889–897. doi: 10.1093/ndt/gfi254. [DOI] [PubMed] [Google Scholar]
- 89.Cohen-Lahav M, Douvdevani A, Chaimovitz C, Shany S. The anti-inflammatory activity of 1,25-dihydroxyvitamin D3 in macrophages. Journal of Steroid Biochemistry and Molecular Biology. 2007;103(3-5):558–562. doi: 10.1016/j.jsbmb.2006.12.093. [DOI] [PubMed] [Google Scholar]
- 90.Sadeghi K, Wessner B, Laggner U, et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. European Journal of Immunology. 2006;36(2):361–370. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- 91.Adorini L, Penna G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Human Immunology. 2009;70(5):345–352. doi: 10.1016/j.humimm.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 92.Ghoreishi M, Bach P, Obst J, Komba M, Fleet JC, Dutz JP. Expansion of antigen-specific regulatory T cells with the topical vitamin D analog calcipotriol. Journal of Immunology. 2009;182(10):6071–6078. doi: 10.4049/jimmunol.0804064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Penna G, Amuchastegui S, Giarratana N, et al. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. Journal of Immunology. 2007;178(1):145–153. doi: 10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- 94.Ferreira GB, van Etten E, Verstuyf A, et al. 1,25-Dihydroxyvitamin D3 alters murine dendritic cell behaviour in vitro and in vivo . Diabetes/Metabolism Research and Reviews. 2011;27(8):933–941. doi: 10.1002/dmrr.1275. [DOI] [PubMed] [Google Scholar]
- 95.Adorini L, Penna G, Giarratana N, Uskokovic M. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting allograft rejection and autoimmune diseases. Journal of Cellular Biochemistry. 2003;88(2):227–233. doi: 10.1002/jcb.10340. [DOI] [PubMed] [Google Scholar]
- 96.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-Dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221(4616):1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 97.Hewison M. An update on vitamin D and human immunity. Clinical Endocrinology. 2012;76(3):315–325. doi: 10.1111/j.1365-2265.2011.04261.x. [DOI] [PubMed] [Google Scholar]
- 98.Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. Journal of Nutrition. 1995;125(supplement 6):1704S–1708S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- 99.Van Belle TL, Gysemans C, Mathieu C. Vitamin D in autoimmune, infectious and allergic diseases: a vital player? Best Practice and Research. 2011;25(4):617–632. doi: 10.1016/j.beem.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 100.Cantorna MT. Mechanisms underlying the effect of vitamin D on the immune system. Proceedings of the Nutrition Society. 2010;69(3):286–289. doi: 10.1017/S0029665110001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Palmer MT, Lee YK, Maynard CL, et al. Lineage-specific effects of 1,25-dihydroxyvitamin D3 on the development of effector CD4 T cells. The Journal of Biological Chemistry. 2011;286(2):997–1004. doi: 10.1074/jbc.M110.163790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HFJ, O’Garra A. 1α,25-Dihydroxyvitamin D3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. Journal of Immunology. 2001;167(9):4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 103.Overbergh L, Decallonne B, Waer M, et al. 1α,25-dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524-543) Diabetes. 2000;49(8):1301–1307. doi: 10.2337/diabetes.49.8.1301. [DOI] [PubMed] [Google Scholar]
- 104.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. Journal of Pharmacology and Experimental Therapeutics. 2008;324(1):23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 105.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 106.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunological Reviews. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 107.Rudensky AY. Regulatory T cells and Foxp3. Immunological Reviews. 2011;241(1):260–268. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. Journal of Experimental Medicine. 2002;195(5):603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gorman S, Kuritzky LA, Judge MA, et al. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. Journal of Immunology. 2007;179(9):6273–6283. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 110.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1α,25-Dihydroxyvitamin D3-binding macromolecules in human B lymphocytes: effects on immunoglobulin production. Journal of Immunology. 1986;136(8):2734–2740. [PubMed] [Google Scholar]
- 111.Lemire JM, Adams JS, Sakai R, Jordan SC. 1α,25-Dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. The Journal of Clinical Investigation. 1984;74(2):657–661. doi: 10.1172/JCI111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen S, Sims GP, Xiao XC, Yue YG, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. Journal of Immunology. 2007;179(3):1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 113.Patel NM, Gutiérrez OM, Andress DL, Coyne DW, Levin A, Wolf M. Vitamin D deficiency and anemia in early chronic kidney disease. Kidney International. 2010;77(8):715–720. doi: 10.1038/ki.2009.551. [DOI] [PubMed] [Google Scholar]
- 114.Saab G, Young DO, Gincherman Y, Giles K, Norwood K, Coyne DW. Prevalence of vitamin D deficiency and the safety and effectiveness of monthly ergocalciferol in hemodialysis patients. Nephron. 2007;105(3):c132–c138. doi: 10.1159/000098645. [DOI] [PubMed] [Google Scholar]
- 115.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49(5):1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 116.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney International. 2007;72(8):1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 118.Melamed ML, Eustace JA, Plantinga L, et al. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney International. 2006;70(2):351–357. doi: 10.1038/sj.ki.5001542. [DOI] [PubMed] [Google Scholar]
- 119.Teng M, Wolf M, Ofsthun MN, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. Journal of the American Society of Nephrology. 2005;16(4):1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 120.Kuhlmann A, Haas CS, Gross M-L, et al. 1,25-Dihydroxyvitamin D3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. American Journal of Physiology. 2004;286(3):F526–F533. doi: 10.1152/ajprenal.00316.2003. [DOI] [PubMed] [Google Scholar]
- 121.Xiao H-Q, Shi W, Liu S-X, et al. Podocyte injury is suppressed by 1,25-dihydroxyvitamin D3 via modulation of transforming growth factor-β1/bone morphogenetic protein-7 signalling in puromycin aminonucleoside nephropathy rats. Clinical and Experimental Pharmacology and Physiology. 2009;36(7):682–689. doi: 10.1111/j.1440-1681.2008.05133.x. [DOI] [PubMed] [Google Scholar]
- 122.Matsui I, Hamano T, Tomida K, et al. Active vitamin D and its analogue, 22-oxacalcitriol, ameliorate puromycin aminonucleoside-induced nephrosis in rats. Nephrology Dialysis Transplantation. 2009;24(8):2354–2361. doi: 10.1093/ndt/gfp117. [DOI] [PubMed] [Google Scholar]
- 123.Szeto C-C, Chow K-M, Kwan BC-H, Chung K-Y, Leung C-B, Li PK-T. Oral calcitriol for the treatment of persistent proteinuria in Immunoglobulin a nephropathy: an uncontrolled trial. American Journal of Kidney Diseases. 2008;51(5):724–731. doi: 10.1053/j.ajkd.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 124.Burgess ED, Hawkins RG, Watanabe M. Interaction of 1,25-dihydroxyvitamin D and plasma renin activity in high renin essential hypertension. American Journal of Hypertension. 1990;3(12 I):903–905. doi: 10.1093/ajh/3.12.903. [DOI] [PubMed] [Google Scholar]
- 125.Li YC, Kong J, Wei M, Chen Z-F, Liu SQ, Cao L-P. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. The Journal of Clinical Investigation. 2002;110(2):229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang Y, Deb DK, Kong J, et al. Long-term therapeutic effect of vitamin D analog doxercalciferol on diabetic nephropathy: strong synergism with AT1 receptor antagonist. American Journal of Physiology. 2009;297(3):F791–F801. doi: 10.1152/ajprenal.00247.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tan X, He W, Liu Y. Combination therapy with paricalcitol and trandolapril reduces renal fibrosis in obstructive nephropathy. Kidney International. 2009;76(12):1248–1257. doi: 10.1038/ki.2009.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guijarro C, Egido J. Transcription factor-κB (NF-κB) and renal disease. Kidney International. 2001;59(2):415–424. doi: 10.1046/j.1523-1755.2001.059002415.x. [DOI] [PubMed] [Google Scholar]
- 129.Lu K-C, Tseng C-F, Wu C-C, et al. Effects of calcitriol on type 5b tartrate-resistant acid phosphatase and interleukin-6 in secondary hyperparathyroidism. Blood Purification. 2006;24(5-6):423–430. doi: 10.1159/000094899. [DOI] [PubMed] [Google Scholar]
- 130.Wu C-C, Chang J-H, Chen C-C, et al. Calcitriol treatment attenuates inflammation and oxidative stress in hemodialysis patients with secondary hyperparathyroidism. Tohoku Journal of Experimental Medicine. 2011;223(3):153–159. doi: 10.1620/tjem.223.153. [DOI] [PubMed] [Google Scholar]
- 131.Sawinski D, Uribarri J, Peace D, et al. 25-OH-vitamin D deficiency and cellular alloimmunity as measured by panel of reactive T cell testing in dialysis patients. American Journal of Transplantation. 2010;10(10):2287–2295. doi: 10.1111/j.1600-6143.2010.03264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Braun AB, Christopher KB. Vitamin d in acute kidney injury. Inflamm Allergy Drug Targets. 2013;12(4):262–272. doi: 10.2174/18715281113129990044. [DOI] [PubMed] [Google Scholar]
- 133.Verouti SN, Tsoupras AB, Alevizopoulou F, Demopoulos CA, Iatrou C. Paricalcitol effects on activities and metabolism of platelet activating factor and on inflammatory cytokines in hemodialysis patients. The International Journal of Artificial Organs. 2013;36(2):87–96. doi: 10.5301/ijao.5000187. [DOI] [PubMed] [Google Scholar]
- 134.Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. Journal of the American Society of Nephrology. 2010;21(2):353–361. doi: 10.1681/ASN.2009040451. [DOI] [PMC free article] [PubMed] [Google Scholar]


